Abstract
The aim of this study is to analyze the concentrations of cytokines in tear of hospitalized COVID-19 patients compared to healthy controls. Tear samples were obtained from 41 healthy controls and 62 COVID-19 patients. Twenty-seven cytokines were assessed: interleukin (IL)-1b, IL-1RA, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL9, IL-10, IL-12, IL-13, IL-15, IL-17, eotaxin, fibroblast growth factor basic, granulocyte colony-stimulating factor (G-CSF), granulocyte-monocyte colony-stimulating factor (GM-CSF), interferon (IFN)-γ, interferon gamma-induced protein, monocyte chemo-attractant protein-1, macrophage inflammatory protein (MIP)-1a, MIP-1b, platelet-derived growth factor (PDGF), regulated on activation normal T cell expressed and secreted, tumor necrosis factor-α and vascular endothelial growth factor (VEGF). In tear samples of COVID-19 patients, an increase in IL-9, IL-15, G-CSF, GM-CSF, IFN-γ, PDGF and VEGF was observed, along with a decrease in eotaxin compared to the control group (p < 0.05). A poor correlation between IL-6 levels in tear and blood was found. IL-1RA and GM-CSF were significantly lower in severe patients and those who needed treatment targeting the immune system (p < 0.05). Tear cytokine levels corroborate the inflammatory nature of SARS-CoV-2.
Keywords: Cytokine, COVID, Tear, Inflammation
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the coronavirus disease 2019 (COVID-19). COVID-19 is considered a pandemia by the World Health Organization and is having a great impact globally. It causes a wide range of clinical manifestations that vary from mild symptoms like fever, cough and asthenia to severe complications, that can eventually lead to death(Guan et al., 2020).
Cytokines play a significant role as essential mediators of the inflammatory response during a viral infection. An adequate, fast and well-coordinated innate immune response is crucial as the first line of defense against viruses(Ye et al., 2020). SARS-CoV-2 not only triggers an innate immune system response that releases cytokines and chemokines, but also activates the adaptive immune system by which T and B cells also release inflammatory cytokines(Crisci et al., 2020).
COVID-19 patients present a characteristic cytokine profile in serum, which has been investigated by many groups. Huang et al., (2020) reported higher blood levels of interleukin (IL)-1β, IL-1RA (IL-1 receptor antagonist), IL-7, IL-8, IL-9, IL-10, fibroblast growth factor basic (FGF basic), granulocyte colony-stimulating factor (G-CSF), granulocyte-monocyte colony-stimulating factor (GM-CSF),interferon (IFN)-γ, interferon gamma-induced protein (IP10), monocyte chemo-attractant protein-1 (MCP-1), macrophage inflammatory protein (MIP)-1A, MIP-1B, platelet-derived growth factor (PDGF), tumor necrosis factor (TNF)-α, and vascular endothelial growth factor (VEGF) in patients infected with SARS-CoV-2 compared healthy adults. In Chi et al.'s series(Chi et al., 2020), in addition to those found by Huang et al., higher serum levels of IL-2, IL-2Rα, IL-6, IL-13, IL-15, IL-17, IL-18 and IFN-α2 were detected in symptomatic patients in comparison to controls. Eotaxin levels were lower in symptomatic cases than healthy controls.
This intense cytokine secretion particularly occurs in severe and critical patients, being key in disease progression and the ultimate death of patients infected with SARS-CoV-2(Henderson, 2020; Ruscitti et al., 2020; Ye et al., 2020). Liu et al., (2020) reported that IL-1RA, IL-1α, IL-2, IL-4, IL-7, IL-10, IL-12, IL- 17, IP-10, PDGF, TNF-α, IFN-γ, GM-CSF, G-CSF and hepatocyte growth factor (HGF) were associated with lung injury and disease severity. Huang et al.’s (Huang et al., 2020) comparison between critical and non-critical patients showed that IL-2, IL-7, IL-10, G-CSF, IP10, MCP-1, MIP-1A, and TNF-α concentrations were higher in the plasma ICU (intensive care unit) patients than non-ICU patients. Yang et al., (2020) also identified IP10, MCP-3, and IL-1RA as biomarkers for severity and worse outcomes of the disease.
Hence, effectively suppressing the cytokine storm may serve as an important mechanism to prevent the aggravation of patients with COVID-19. In this regard, targeting treatment to reduce cytokine levels, such as glucocorticoids, intravenous immunoglobulins, tocilizumab (anti-IL-6) and anakirna (anti-IL-1 receptor), may improve prognosis(Soy, 2020).
Tears are accessible and non-invasive sources from which biomarkers can be obtained. Recently, tear analysis has proven to be a useful biomarker in many ocular surface and systemic diseases(ten Berge et al., 2019; Burgos-Blasco et al., 2020). Diabetic patients present a significant increase in the number of peaks when electrophoretic patterns from tear proteins when analyzed. This changes in protein patterns strongly correlated with the duration of the diabetic disease(Grus et al., 2002). Tear protein levels can also be upregulated or downregulated in breast cancer patients, as described by Böhm et al. They hypothezised that different biological processes may be altered in discrete fluids such as tears that are located far away from the original site(Böhm et al., 2012). However, tear cytokines in systemic diseases have not been fully assessed, nor has been correlation of blood and tear cytokines.
SARS-CoV-2 has been recently detected in tears of COVID-19 patients, suggesting that the ocular surface and the tears might represent a potential route for SARS-CoV- 2 infection(Güemes-Villahoz et al., 2020). The exact route of entry is unknown and various theories including direct entry, through the nasolacrimal drainage system of the lacrimal gland have been proposed. To enter and infect cells, SARS-CoV-2 employs the angiotensin-converting enzyme 2 receptor (ACE2), which has been identified in the cornea, the conjunctiva, the retina and the choroid. Viral RNA detection in retinal biopsies supports the theory of direct entry of the virus in the eye, either through the ocular surface or through the hematogenous route(Casagrande et al., 2020; Güemes-Villahoz et al., 2020).
The main purpose of this study is to analyze cytokine levels in tears of hospitalized COVID-19 patients compared to healthy controls. Other objectives of this study were to assess the correlation and concordance between the values of IL-6 in tears and in serum of COVID-19 patients and to evaluate any association with clinical variables. To the best of our knowledge, this is the first study in the literature that analyzes the cytokine profile of tears of COVID-19 hospitalized patients and could provide valuable insight on immune system involvement.
2. Methods
2.1. Patients
This is a cross-sectional study and has been conducted at the Hospital Clinico San Carlos in Madrid, Spain, a tertiary hospital that attends patients within the Madrid metropolitan area. Patients that had been admitted to the hospital due to laboratory-confirmed COVID-19 diagnosis were recruited. Healthy patients from a previous study performed in 2018 were used as controls.
This study has been approved by the Clinical Research Ethics Committee and has been conducted in accordance with the Helsinki Declaration. Informed consent was obtained from all patients.
The general admission criteria according to the hospital's protocol included: 1) <50 years of age without comorbidities with bilateral pneumonia, or unilateral pneumonia with respiratory failure (saturation <96% and respiratory rate> 20); or 2) > 50 years of age or patients with comorbidity: with pneumonia, respiratory failure (saturation <96% and respiratory rate> 20), or laboratory/clinical severity (arterial blood gas, hemogram, D-dimer, C reactive protein (CRP), procalcitonin, lactate dehydrogenase (LDH), transaminases).
Inclusion criteria for the patients were: over 18 years of age; patient with positive reverse transcriptase–polymerase chain reaction (RT-PCR) test from nasopharyngeal swab for SARS-CoV-2, hospitalization because of COVID-19, and ability to give verbal consent. Patients admitted to the intensive care unit, as well as those unable or unwilling to give verbal consent were excluded from the study.
For the control group, the inclusion criteria considered were as follows: over 40 years of age; absence of ophthalmological pathology, absence of systemic inflammatory or infectious diseases and no medications with known influence on immunological factors.
The exclusion criteria for both groups were ocular surgery or an ocular laser procedure in the previous six months; concomitant ophthalmological pathology; and topical treatment. Patients who had been hospitalized for less than 10 days were included, that is, at the start of hospitalization, so as to be able to correlate with treatment and prognosis.
To select patients, all the patients with hospitalization in the previous 10 days in the COVID unit were selected and checked for inclusion and exclusion criteria. After determining which patients could be included, they were informed about the study and if they consented, a sample was taken. The same technique was repeated every week recruiting consecutively the patients until enough patients had been included.
2.2. Data collection
Clinical charts, laboratory findings, and chest x-rays for all the patients included in the study were reviewed. Demographic, clinical, laboratory, and radiological characteristics, along with treatment and outcomes were obtained from the electronic medical records. Two researchers independently reviewed the data to double-check the collected data.
Patients were classified according to the severity. Moderate cases had fever, respiratory tract symptoms, and radiological abnormalities, and the severe cases had at least 1 of the following: (1) respiration rate ≥ 30 times/min, (2) oxygen saturation at rest ≤ 93%, (3) arterial Pao2/Fio2 ≤ 300 mmHg, and (4) respiratory failure, shock, or other organ failure requiring intensive care unit treatment. No mild or critical cases were included in this study.
To correlate with laboratory data, blood test results from the day the tear sample were used. IL-6 analysis was also performed on the same day as the tear sample. Regarding anti-inflammatory treatment, glucocorticoids, intravenous immunoglobulins and/or anti-cytokine therapies such as tocilizumab and anakinra were noted.
2.3. Tear sample and cytokine determination
The tear samples (3–5 μl) were collected with a 10 μl capillary tube. The tube was carefully placed in the inferior conjunctival fornix so as not to cause reflex stimulation. Samples from both eyes were obtained with the same capillary tube. In the laboratory, the samples were stored at −80 °C. All procedure followed infection control and prevention measures according to the hospital's protocol.
The cytokine levels in the tear samples were analyzed using the Bio-Plex Pro Human Cytokine 27-Plex Immunoassay kit (Bio-Rad Laboratories, Hercules, CA, USA). The concentration of 27 pro-inflammatory cytokines were determined: IL-1b, IL-1RA, IL- 2, IL-4, IL-5, IL-6, IL-7, IL-8, IL9, IL-10, IL-12, IL-13, IL-15, IL-17, eotaxin, FGFbasic, G-CSF, GM-CSF, IFN-γ, IP-10, MCP-1, MIP)-1A, MIP-1B, PDGF, regulated on activation, normal T cell expressed and secreted (RANTES), TNF-α and VEGF.
Cytokine concentrations were quantified by interpolating the fluorescence intensities emitted measured with a Luminex MAGPIX reader (Luminex Corporation, Austin, TX, USA) against their standard curves. Bio-Plex ManagerTM software (Bio-Rad Laboratories, Hercules, CA, USA) was used to calculate the cytokine levels.
2.4. Statistics
The data were processed and analyzed statistically using IBM SPSS Statistics for Mac, version 21.0 (IBM Corp., Armonk, NY, USA). Continuous data is presented as mean and standard deviation, while categorical data is presented as frequencies and percentages. For categorical variables, frequency distributions were calculated and compared using the chi-square test.
A Shapiro-Wilk test was performed to understand the distribution of the value across the cohort. To analyze the differences in the concentrations of each cytokine between the different subgroups a Mann Whitney U test was performed. The correlation between IL-6 in blood and tear was determined by Spearman correlation. A p-value < 0.05 was considered to be statistically significant.
3. Results
The overall study population included 103 subjects: 62 COVID-19 patients (124 eyes) with laboratory-confirmed diagnosis of SARS-CoV-2 infection admitted at the hospital and 41 historic controls (82 eyes).
Of the 584 patients in the COVID-unit, the first consecutive 80 patients that met the inclusion and exclusion criteria and consented to participation in the study were consecutively included. 18 patients presented too little tear and there was not enough sample for analysis; 9 patients did not give verbal consent; 5 patients with previous history of ocular surgery or concomitant treatment were excluded: 1 patient had received intravitreal dexamethasone implant due to diabetic macular edema 5 weeks prior, 1 patient had received antiVEGF treatment due to age related macular degeneration 1 week prior; 1 patient underwent phacoemulsification surgery 2 months prior and 2 patients were using intraocular pressure IOP-lowering therapy.
The main clinical characteristics found on the COVID-19 patients included are shown in Table 1 . Among the 41 control subjects included, 16 patients (39%) were male and the median age was 66.9 years (SD 16.0 years). No statistically significant difference in the sex and age was found between both groups (p > 0.05).
Table 1.
COVID-19 patients’ characteristics.
| Patient characteristics | All patients (n = 62) | Moderate patients (n = 35) | Severe patients (n = 27) | p |
|---|---|---|---|---|
| Sociodemographic data | ||||
| Sex | ||||
| Male. N (%) | 33 (53) | 18 (51) | 15 (56) | 0.747 |
| Female. N (%) | 29 (47) | 17 (49) | 12 (44) | 0.747 |
| Age. Years (SD) | 69.7 (16.9) | 68.5 (18.5) | 71.3 (14.9) | 0.500 |
| Race | ||||
| Caucasic. N (%) | 52 (84) | 28 (80) | 24 (89) | 0.345 |
| Hispanic. N (%) | 10 (16) | 7 (20) | 3 (11) | 0.345 |
| Medical history | ||||
| AH. N (%) | 35 (56) | 18 (51) | 17 (63) | 0.364 |
| DM. N (%) | 19 (31) | 9 (26) | 10 (37) | 0.338 |
| DL. N (%) | 32 (52) | 17 (49) | 15 (56) | 0.585 |
| Chronic obstructive pulmonary disease. N (%) | 14 (23) | 9 (26) | 5 (19) | 0.502 |
| Cardiovascular disease. N (%) | 22 (35) | 11 (31) | 11 (41) | 0.447 |
| Chronic renal disease. N (%) | 7 (11) | 2 (6) | 5 (19) | 0.114 |
| Chronic liver disease. N (%) | 3 (5) | 2 (6) | 1 (4) | 0.715 |
| Malignancy. N (%) | 6 (10) | 2 (6) | 4 (15) | 0.229 |
| Clinical data | ||||
| Pneumonia. N (%) | 42 (68) | 21 (60) | 21 (78) | 0.138 |
| Hemoglobin. g/dL (SD) | 12.3 (2.2) | 12.4 (2.4) | 12.1 (1.9) | 0.516 |
| Leucocytes.103/μL (SD) | 6.4 (2.6) | 6.2 (2.1) | 6.7 (3.1) | 0.499 |
| Neutrophils. 103/μL (SD) | 4.5 (2.6) | 3.9 (1.9) | 5.3 (3.2) | 0.064 |
| Linfocytes.103/μL (SD) | 1.3 (0.8) | 1.5 (0.7) | 1.0 (0.8) | 0.009** |
| CPR. mg/dL (SD) | 4.4 (6.2) | 1.6 (1.9) | 7.9 (8.0) | <0.001** |
| Ferritin. ng/mL (SD) | 581.0 (1324.6) | 299.3 (336.6) | 946.2 (1929.5) | 0.096 |
| D-dimer. ng/mL (SD) | 1397.1 (2622.9) | 1381.0 (3131.7) | 1417.4 (1851.2) | 0.955 |
| Fibrinogen. mg/dL (SD) | 580.0 (186.4) | 532.0 (177.1) | 642.1 (182.9) | 0.021* |
| LDH. U/L (SD) | 512.2 (206.6) | 460.3 (155.7) | 579.4 (245.2) | 0.033* |
| IL-6. pg/mL (SD) | 31.3 (50.5) | 10.8 (15.0) | 53.2 (64.7) | 0.002** |
| Treatment | ||||
| Hydroxychloroquine. N (%) | 41 (66) | 22 (63) | 19 (70) | 0.535 |
| Ritonavir/lopinavir. N (%) | 12 (19) | 5 (14) | 7 (26) | 0.141 |
| Glucocorticoids. N (%) | 19 (31) | 4 (11) | 15 (56) | <0.001** |
| Tocilizumab. N (%) | 4 (6) | 0 (0) | 4 (15) | 0.019 |
| Anti-inflammatory treatment. N (%) | 22 (35) | 5 (14) | 17 (63) | <0.001** |
| Clinical outcome | ||||
| Recovery. N (%) | 57 (92) | 35 (100) | 22 (81) | 0.007** |
| Death. N (%) | 5 (8) | 0 (0) | 5 (19) | 0.007** |
SD: Standard deviation; AT: Arterial hypertension; DM: Diabetes mellitus; DL: dislypidemia; CRP: C-reactive protein; LDH: lactate dehydrogenase; IL-6: interleukin 6.
Mean days from onset of symptoms to hospitalization were 8.6 days (SD 6.2 days), while mean days from hospitalization to tear sampling was 3.7 days (SD 2.0 days).
IL-1b, IL-2, IL-5, IL-7, IL-10, IL-12, IL-13, FGFb, MCP-1, MIP-1b and TNF-α were detected in fewer than 40% of samples and therefore were not included in further analysis. Shapiro-Wilk test revealed that the remaining variables were not normally distributed.
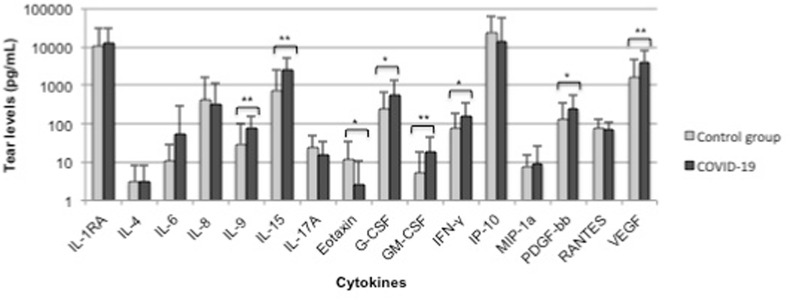
Table 2 depicts the cytokine levels found in COVID-19 patients compared to the control group. A significant increase in IL-9, Il-15, G-CSF, GM-CSF, IFN-γ, PDGF and VEGF, as well as a decrease in eotaxin in tears of COVID-19 patients was found (Fig. 1 ).
Table 2.
Cytokine levels in tear of COVID-19 patients compared to controls.
| Cytokines | Control group (n = 41) | COVID-19 (n = 62) | p |
|---|---|---|---|
| IL-1RA | 10219.12 ± 20072.36 | 12497.95 ± 18423.12 | 0.562 |
| IL-4 | 3.16 ± 5.17 | 3.11 ± 5.13 | 0.960 |
| IL-6 | 10.71 ± 17.28 | 54.87 ± 245.28 | 0.163 |
| IL-8 | 423.47 ± 1222.22 | 331.45 ± 777.89 | 0.670 |
| IL-9 | 30.02 ± 73.43 | 79.91 ± 74.15 | 0.001** |
| IL-15 | 742.02 ± 1702.32 | 2575.88 ± 2726.65 | <0.001** |
| IL-17A | 24.43 ± 25.52 | 15.56 ± 18.94 | 0.061 |
| Eotaxin | 11.55 ± 22.33 | 2.68 ± 8.39 | 0.019* |
| G-CSF | 248.51 ± 427.92 | 543.19 ± 781.12 | 0.015* |
| GM-CSF | 5.1 ± 13.83 | 18.42 ± 27.33 | 0.002** |
| IFN-γ | 77.21 ± 110.71 | 154.65 ± 197.1 | 0.012* |
| IP-10 | 24494.77 ± 37384.68 | 13882.07 ± 42887.93 | 0.187 |
| MIP-1a | 7.82 ± 7.47 | 8.73 ± 18.75 | 0.732 |
| PDGF | 127.58 ± 237.44 | 242.75 ± 310.92 | 0.036* |
| RANTES | 75.4 ± 52.21 | 71.39 ± 41.89 | 0.681 |
| VEGF | 1571.6 ± 3146.13 | 4141.32 ± 4387.04 | 0.001** |
Data are expressed as mean (pg/mL) ± standard deviation (SD). * (p < 0.05); ** (p < 0.01).
Fig. 1.
Tear cytokine levels in controls and COVID-19 patients. Data are expressed as mean (pg/mL) + standard deviation (SD). * (p < 0.05); ** (p < 0.01).
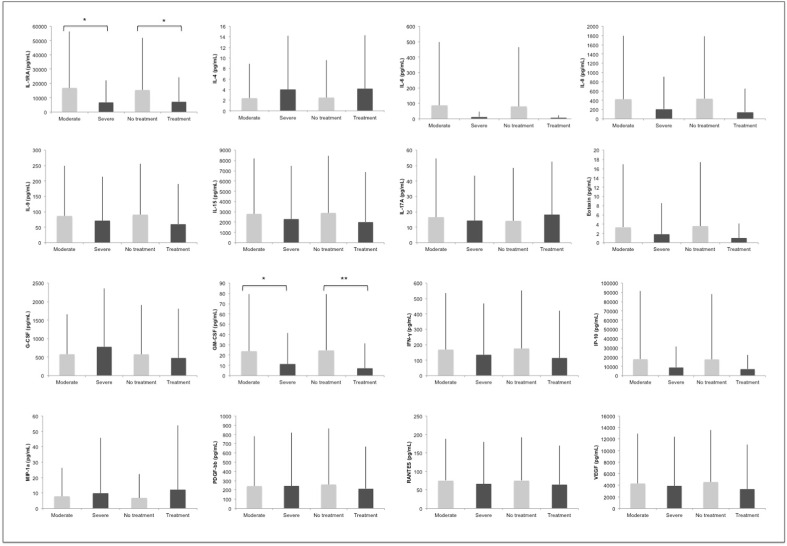
When comparing IL-6 blood and tear levels, the correlation (r = -.94) was not statistically significant (p = 0.489). Fig. 2 depicts the association between cytokine tear levels and severity of the disease and need for specific treatment targeting hypercytokinemia. IL-1RA and GM-CSF were significantly lower in severe patients and those who needed treatment targeting the immune system (p < 0.05).
Fig. 2.
Cytokine differences by severity and need for treatment targeting the release of cytokines. Moderate patients are compared to severe patients. No treatment is compared to need for specific treatment. Data are expressed as mean (pg/mL) + standard deviation (SD). * (p < 0.05); ** (p < 0.01).
The correlation of cytokine tear levels with laboratory findings: neutrophils/lymphocytes ration, CPR, ferritin, D-dimer and LDH showed no statistically significant differences when corrected for multiple comparisons. Neither did comparison for cytokine tear levels between sex.
4. Discussion
Cytokines are a key factor in determining the clinical course of SARS-CoV-2 infection. A dysregulated or an exaggerated immune response by SARS-CoV-2 infected cells plays an important role in the infection's pathogenesis. We analyzed the tear levels of 27 cytokines in a cohort of 62 patients including moderate and severe cases with laboratory-confirmed COVID-19 in Madrid, Spain. Our results show an increase in IL-9, IL-15, G-CSF, GM-CSF, IFN-γ, PDGF and VEGF in the tears of COVID-19 patients compared to controls, all which have been reported to be increased in serum of COVID-19 patients(Chi et al., 2020; Huang et al., 2020).
One of the most investigated cytokines in COVID-19 is IL-6, as it is one of the main mediators of the inflammatory and immune response. In fact, IL-6 levels at hospital admission are considered to have a good prognostic value for severe disease progression and/or in-hospital mortality(Grifoni et al., 2020). IL-6 can be more easily measured in blood than others cytokines and is therefore currently being used to evaluate hyperinflammation and the need for specific anti-inflammatory treatment in hospitalized COVID-19 patients. Because IL-6 is routinely measured in COVID-19 patients and correlation of tear and blood cytokines has not been thoroughly investigated in systemic diseases, we decided to analyze whether a correlation between serum and tear IL-6 levels exists. However, we found no such correlation, suggesting that although IL-6 can be produced in all tissues, it may be especially increased in blood.
As for other cytokines found to be increased in tears, colony-stimulating factors such as GM-CSF and G-CSF stimulate the proliferation of granulocytes and macrophages, contributing to the maintenance of innate immune homeostasis. Recent evidence suggests that GM-CSF also enhances proinflammatory cytokine release and CD4+ T cell differentiation(Shiomi and Usui, 2015). Interestingly, pathogenic Th1 cells with both IFN-γ and GM-CSF expression have been found in critical patients (Zhou et al., 2020). Our results support the role of GM-CSF in SARS-CoV-2 infection.
In addition, IFN-γ, a Th1 cytokine mainly secreted by T and NK cells, has been described to be elevated in COVID-19 patients(Henderson, 2020). There are conflicting results which describe a mild elevation of IFN-γ systemic levels in non-severe patients(Jamilloux et al., 2020). Our results reflect an increase in IFN-γ in tear of COVID-19 patients, but no association with severity was detected.
VEGF tear levels were also increased in our study compared to controls. VEGF presents an important pro-angiogenic, mitogenic and anti-apoptotic effect on endothelial cells(Maharaj and D'Amore, 2007). In COVID-19, vascular alterations have been linked to acute lung injury and acute respiratory distress syndrome(Ciceri, 2020). Hence, increased serum and tear levels of VEGF may be associated to the procoagulant state characteristic of COVID-19 patients by increasing vascular permeability and altering the hemostatic features of endothelial cells(Connors and Levy, 2020). VEGF secretion is stimulated by IL-6 and IL-1, which are elevated in COVID-19, also linking VEGF to the immune response(Moore and June 2020).
In the present study, COVID-19 patients had decreased levels of eotaxin-1. Interestingly, lower eotaxin levels have also been described in serum of COVID-19 patients(Chi et al., 2020).
Additionally, we assessed the relationship between severity, treatment and tear cytokine levels in COVID-19 patients. Our results report lower tear levels of IL1-RA and GM-CSF in severe patients compared to those with a moderate form of the disease. These cytokines also presented decreased levels in those patients who received treatment to target the cytokine storm. A possible association of GM-CSF with both CPR and LDH may exist, supporting its role in the inflammatory response to SARS-CoV-2, but no statistical significance was reached when correction for multiple comparisons was applied. The lack of correlation of more cytokines with severity, like those observed in blood, could be explained by the fact that patients with mild forms of the disease (not admitted to the hospital) and critically ill patients (no possibility of giving consent) were not included.
Cytokine ocular sources have not been fully elucidated, but it is believed that both inflammatory and structural (epithelial and fibroblast) cells act as local sources(Enríquez-de-Salamanca and Calonge, 2008). In corneal surgery, IL-6 increases have been suggested to originate from epithelial cells and keratocytes(Malecaze et al., 1997). Similarly, exposure of these cells to IL-1 or TNF-alpha was found to stimulate IL-8 synthesis(Cubitt et al., 1993). On the other hand, the retinal pigment epithelium can secrete cytokines, but under the control of cells, such as monocytes, present at a blood–retinal barrier breakdown, which could be possibly due to the vascular involvement of COVID-19(Wallace et al., 2004). Hence, tear cytokines may come directly from those present in the blood or be a result of a response to systemic cytokines. The latter would explain why no direct correlation was found between IL-6 tear and blood levels. However, there are not only many uncertainties about the origin of tear cytokines, but the investigations that exist are of ocular diseases and not systemic ones. Therefore, the different origins of these cytokines can only be hypothesized, but more studies are definitely needed in this regard.
The following limitations should be considered when interpreting our findings. For the control group, a historical control group was used, since both PCR and serology do not have 100% sensitivity and present many false negatives. This was the only way to ensure that no asymptomatic patients nor false negative cases were accidentally included. They did not differ in age and sex from the studied group. Most COVID-19 cohorts present a high male prevalence of up to 80%, while our sample presents a 55% of male patients. Male patients present usually a more severe form of the disease and were therefore difficult to include in this study, where collaboration and verbal consent was needed.
To the best of our knowledge, no previous studies have evaluated the tear cytokine profile among hospitalized COVID-19 patients and compared it to healthy controls. There are few studies of tear cytokines in systemic diseases without ocular involvement and fewer correlating cytokines in tear and blood.
The importance of this study not only lies in the relevance of the ocular inflammatory reaction, but also the possible direct involvement of the virus. SARS-CoV-2 detection in tears and ocular fluids rises concern for the safety of ophthalmological procedures. Because of the proximity necessary for an ophthalmological examination, the exposure to tears and the risk of aerosol generation, ophthalmologists are at high risk for SARS-CoV-2 infection(Güemes-Villahoz et al., 2020). Several ophthalmological societies have issued guidelines with recommended protocols, all agreeing that it is safest to assume that any patient is potentially infected, especially in high risk areas. Aerosol generation has been evaluated during phacoemulsification and should also be considered(Darcy et al., 2020).
Hypercytokinemia with an uncontrolled proinflammatory response in SARS-CoV-2 plays an important role in the pathogenesis. SARS-CoV-2 infection is a potent inducer of proinflammatory cytokines in tear, some of them correlating with severity. We believe that the cytokine tear pattern in COVID-19 could serve as biomarkers to evaluate the disease severity. Further investigation are needed to get a full picture of the spectrum of tear cytokine levels in SARS-CoV-2 infection.
Data availability
No data available.
Funding
No funding received.
Declaration of competing interest
The authors declare that they have no conflicts of interests.
References
- Soy Mehmet, et al. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020;39(7):2085–2094. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Berge Josianne C., et al. Intraocular cytokine profile and autoimmune reactions in retinitis pigmentosa, age-related macular degeneration, glaucoma and cataract. Acta Ophthalmol. 2019;97(2):185–192. doi: 10.1111/aos.13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm Daniel, et al. Comparison of tear protein levels in breast cancer patients and healthy controls using a de Novo proteomic approach. Oncol. Rep. 2012;28(2):429–438. doi: 10.3892/or.2012.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Blasco Barbara, et al. Tear and aqueous humour cytokine profile in primary open-angle glaucoma. Acta Ophthalmol. 2020:1–5. doi: 10.1111/aos.14374. http://www.ncbi.nlm.nih.gov/pubmed/32043817 [DOI] [PubMed] [Google Scholar]
- Casagrande Maria, et al. 2020. “Detection of SARS-CoV-2 in Human Retinal Biopsies of Deceased COVID-19 Patients.” Ocular Immunology and Inflammation: 1–5.http://www.ncbi.nlm.nih.gov/pubmed/32469258 [DOI] [PubMed] [Google Scholar]
- Chi Ying, et al. Serum cytokine and chemokine profile in relation to the severity of coronavirus disease 2019 (COVID-19) in China. J. Infect. Dis. 2020;(1):1–9. doi: 10.1093/infdis/jiaa363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciceri Fabio, et al. “Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis.” Critical care and resuscitation. J. Aust. Acad. Critical Care Med. 2020;22(2):95–97. doi: 10.51893/2020.2.pov2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors Jean M., Levy Jerrold H. “Thromboinflammation and the hypercoagulability of COVID‐19. J. Thromb. Haemostasis: jth. 2020:14849. doi: 10.1111/jth.14849. https://onlinelibrary.wiley.com/doi/abs/10.1111/jth.14849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisci Carlos D., Ardusso Ledit R.F., Mossuz Antonela, Müller Leila. A precision medicine approach to SARS-CoV-2 pandemic management. Curr. Treat. Opt. Allergy. 2020:1–19. doi: 10.1007/s40521-020-00258-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubitt C.L., et al. IL-8 gene expression in cultures of human corneal epithelial cells and keratocytes. Invest. Ophthalmol. Vis. Sci. 1993;34(11):3199–3206. http://www.ncbi.nlm.nih.gov/pubmed/7691777 [PubMed] [Google Scholar]
- Darcy Kieren, et al. Eye; London, England: 2020. Reducing Visible Aerosol Generation during Phacoemulsification in the Era of Covid-19.http://www.ncbi.nlm.nih.gov/pubmed/32591733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enríquez-de-Salamanca Amalia, Calonge Margarita. Cytokines and chemokines in immune-based ocular surface inflammation. Expet Rev. Clin. Immunol. 2008;4(4):457–467. doi: 10.1586/1744666X.4.4.457. http://www.ncbi.nlm.nih.gov/pubmed/20477574 [DOI] [PubMed] [Google Scholar]
- Grifoni Elisa, et al. Interleukin-6 as prognosticator in patients with COVID-19. J. Infect. 2020;(xxxx):9–10. doi: 10.1016/j.jinf.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grus F.H., et al. Changes in the tear proteins of diabetic patients. BMC Ophthalmol. 2002;2(1):4. doi: 10.1186/1471-2415-2-4. http://bmcophthalmol.biomedcentral.com/articles/10.1186/1471-2415-2-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Wei jie, et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020:1–13. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güemes Villahoz Noemi, Burgos-Blasco Barbara, Vilela Ana Arribi, et al. Detecting SARS-CoV-2 RNA in conjunctival secretions: is it a valuable diagnostic method of COVID-19? J. Med. Virol. 2020 doi: 10.1002/jmv.26219. http://www.ncbi.nlm.nih.gov/pubmed/32579256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güemes Villahoz Noemi, Burgos-Blasco Barbara, Vidal Villegas Beatriz, et al. Novel insights into the transmission of SARS-CoV-2 through the ocular surface and its detection in tears and conjunctival secretions: a review. Adv. Ther. 2020;37(10):4086–4095. doi: 10.1007/s12325-020-01442-7. http://link.springer.com/10.1007/s12325-020-01442-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson Lauren A., et al. On the alert for cytokine storm: immunopathology in COVID-19. Arthritis Rheumatol. 2020;72(7):1059–1063. doi: 10.1002/art.41285. 0–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., et al. Clinical features of patients infected with 2019 novel coronavirus in wuhan, China. Lancet. 2020;15–21(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. Febr(395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamilloux Yvan, et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun. Rev. 2020;19(7) doi: 10.1016/j.autrev.2020.102567. https://linkinghub.elsevier.com/retrieve/pii/S1568997220301294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Yingxia, et al. Elevated plasma levels of selective cytokines in COVID-19 patients reflect viral load and lung injury. Nat. Sci. Rev. 2020;7(6):1003–1011. doi: 10.1093/nsr/nwaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharaj Arindel S.R., D'Amore Patricia A. Roles for VEGF in the adult. Microvasc. Res. 2007;74(2–3):100–113. doi: 10.1016/j.mvr.2007.03.004. https://linkinghub.elsevier.com/retrieve/pii/S0026286207000283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malecaze F., et al. Interleukin-6 in tear fluid after photorefractive keratectomy and its effects on keratocytes in culture. Cornea. 1997;16(5):580–587. [PubMed] [Google Scholar]
- Moore John B., June Carl H. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474. doi: 10.1126/science.abb8925. https://www.sciencemag.org/lookup/doi/10.1126/science.abb8925 [DOI] [PubMed] [Google Scholar]
- Ruscitti Piero, Berardicurti Onorina, Iagnocco Annamaria, Giacomelli Roberto. Cytokine storm syndrome in severe COVID-19. Autoimmun. Rev. 2020;19(7) doi: 10.1016/j.autrev.2020.102562. https://linkinghub.elsevier.com/retrieve/pii/S1568997220301245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiomi Aoi, Usui Takashi. Pivotal roles of GM-CSF in autoimmunity and inflammation. Mediat. Inflamm. 2015 doi: 10.1155/2015/568543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace Graham R., et al. The role of chemokines and their receptors in ocular disease. Prog. Retin. Eye Res. 2004;23(4):435–448. doi: 10.1016/j.preteyeres.2004.04.004. https://linkinghub.elsevier.com/retrieve/pii/S1350946204000266 [DOI] [PubMed] [Google Scholar]
- Yang Yang, et al. Exuberant elevation of IP-10, MCP-3 and IL-1ra during SARS-CoV-2 infection is associated with disease severity and fatal outcome. medRxiv. 2020;2019 (December 2019): 2020.03.02.20029975. [Google Scholar]
- Ye Qing, Wang Bili, Mao Jianhua. “The pathogenesis and treatment of the ‘Cytokine storm’ in COVID-19. J. Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Yonggang, et al. 2020. Aberrant pathogenic GM-CSF+ T cells and inflammatory CD14+CD16+ monocytes in severe pulmonary syndrome patients of a new coronavirus. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data available.