Abstract
In 2020 the whole world focused on antivirus drugs towards SARS-CoV-2. Most of the researchers focused on drugs used in other viral infections or malaria. We have not seen such mobilization towards one topic in this century. The whole situation makes clear that progress needs to be made in antiviral drug development. The first step to do it is to characterize the potential antiviral activity of new or already existed drugs on the market. Phenothiazines are antipsychotic agents used previously as antiseptics, anthelminthics, and antimalarials. Up to date, they are tested for a number of other disorders including the broad spectrum of viruses. The goal of this paper was to summarize the current literature on activity toward RNA-viruses of such drugs like chlorpromazine, fluphenazine, perphenazine, prochlorperazine, and thioridazine. We identified 49 papers, where the use of the phenothiazines for 23 viruses from different families were tested. Chlorpromazine, fluphenazine, perphenazine, prochlorperazine, and thioridazine possess anti-viral activity towards different types of viruses. These drugs inhibit clathrin-dependent endocytosis, cell-cell fusion, infection, replication of the virus, decrease viral invasion as well as suppress entry into the host cells. Additionally, since the drugs display activity at nontoxic concentrations they have therapeutic potential for some viruses, still, further research on animal and human subjects are needed in this field to verify cell base research.
Keywords: Phenothiazines, Neuroleptic drugs, Antiviral activity, RNA viruses
Graphical abstract
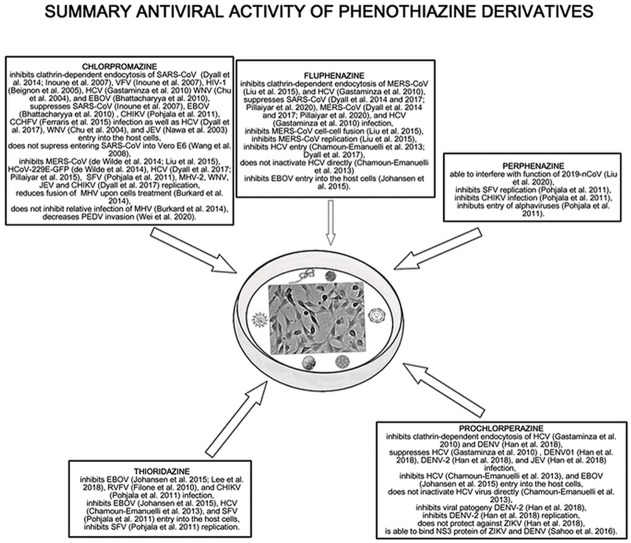
Diagrammatic summary of phenothiazine derivatives antiviral activity. All of the phenothiazines (chlorpromazine, fluphenazine, perphenazine, prochlorperazine, and thioridazine) possess antiviral activity towards RNA viruses. These drugs inhibit clathrin-dependent endocytosis, cell-cell fusion, infection, replication of the virus, decrease viral invasion as well as suppress entry into the host cells.
Highlights
-
•
Phenothiazines possess antiviral activity towards RNA viruses.
-
•
An antiviral activity can be achieved below toxic serum concentration.
-
•
Phenothiazines are characterized by multidirectional points of action.
1. Introduction
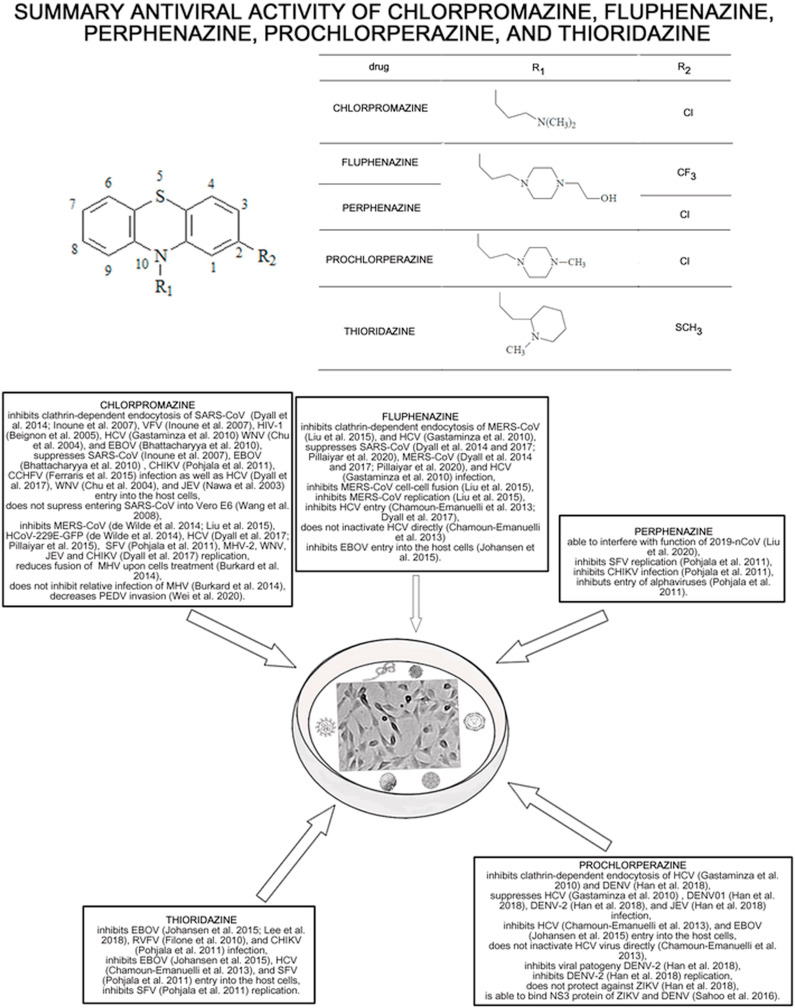
Chlorpromazine, fluphenazine, perphenazine, prochlorperazine, and thioridazine are phenothiazines and with the exception of thioridazine, they are widely used in the treatment of psychosis, schizophrenia, and bipolar disorders (Hendouei et al., 2019). Phenothiazine derivatives show anti-cancer, viral, bacterial, fungal, protozoa, prion activities (Hendouei et al., 2019; Varga et al., 2017). The main antidepressant effect of phenothiazines is related to the effect on dopamine receptor D2 and alpha-2 adrenergic receptor (α2). Noteworthy, the effect with D2 and α1 are associated with side effects such as extrapyramidal side-effects (akathisia, tardive dyskinesia, parkinsonism, acute dystonic reactions) and sympatholytic side-effects (hypotension, orthostatic hypotension, reflex tachycardia, dizziness, myosis, sexual dysfunction) (Varga et al., 2017). Phenothiazine derivatives may disturb the binding process of the virus to the cell membrane, blocking the entry of the virus or inhibiting the DNA replication by intercalating DNA bases, which might be useful in antiviral therapy (Varga et al., 2017).
Viral infections still represent a major global public health problem. Up to date, antiviral drugs are used only for treating less than 10 viral infections. Unfortunately, there are still no effective enough drugs against some pathogenic viruses, for example, Zika (ZIKV), Ebola (EBOV), or severe acute respiratory syndrome (SARS). The drugs are intended to be safe for human use, as most of the targeted viral proteins are not present in humans, except the viral polymerase which is partly similar to their human counterparts. On the other hand, proteins of different species or even genotypes of virus not often share structural similarity, as well as the ability of viruses to mutate during replication cause that the antiviral drug which targets a specific viral protein is not always effective against another virus. Noteworthy, there is a lack of effective antiviral drugs on the market (Ji and Li, 2020). The extremely high mutation rates of RNA viruses may lead to drug resistance induction and circumvent vaccine-induced immunity (Dinesh et al., 2020). The lack of broad-spectrum antiviral drugs may have catastrophic consequences in disease emergencies. Only some drugs (favipiravir, ribavirin, cidofovir, and brincidofovir) have broad-spectrum properties (Adalja and Inglesby, 2019). RNA viruses cause several notable diseases in humans. Based on the type of RNA molecule there are 3 types of RNA viruses: single-stranded (ss) RNA, double-stranded (ds) RNA, and circular RNA (circRNA). Additionally, ssRNA viruses can be classified based on the sense of nucleic acid (plus (+) and minus (−)) (Dinesh et al., 2020).
Since other reviews showed anti-viral activity for chlorpromazine and thioridazine is usually described (Varga et al., 2017), in this review, we focused on chlorpromazine, fluphenazine, perphenazine, prochlorperazine, and thioridazine as possible candidates for use in anti-viral therapy. Thus, the assays mainly based on the activity of the selected phenothiazine derivatives against RNA viruses, some in vivo studies were analyzed.
2. The antiviral activity of chlorpromazine, fluphenazine, perphenazine, prochlorperazine, and thioridazine towards RNA viruses
2.1. Chlorpromazine
2.1.1. Corona viruses (CoVs)
Coronaviruses are pleomorphic or spherical viruses that have 80–160 nm diameter size (Sahin et al., 2020) and characterized by bears club-shaped projections of glycoproteins on its surface (Prajapat et al., 2020). The viruses have one among the largest single positive-stranded RNA genome (size range between 26.2 and 31.7 kb, positive sense) from all the RNA viruses, which is covered by an enveloped structure. Because genetic material of the virus is susceptible of frequent mutations, it may form new strains with alteration in virulence - up till now 7 strains of human CoVs: 229E, NL63, OC43, HKU1, Middle East respiratory syndrome (MERS)-CoV, severe acute respiratory syndrome (SARS)-CoV, and 2019-novel coronavirus (nCoV) are known. Only 3 from 7 strains have been found to have highly pathogenic (SARS-CoV, MERS-CoV, and 2019-nCoV). 4 strains (229E, NL63, OC43, HKU1) are known as non-severe (SARS)-like coronaviruses (Prajapat et al., 2020). The viral genome encodes four key structural proteins: spike (S), envelope (E), membrane (M), and nucleocapsid (N) proteins 3–5. S proteins play a vital role in membrane penetration, and together with E and M proteins is a part of the viral membrane. S protein is responsible for receptor-binding and in consequence entering host cells, making it a potential target for therapeutic purposes. The M and E proteins are responsible for virus envelope construction, and the N protein plays a role in RNA synthesis. The predominant receptors are human angiotensin-converting enzyme 2 (ACE2) and human dipeptidyl peptidase 4 (DPP4 or CD26), respectively for SARS and MERS-CoV (Prajapat et al., 2020, Song et al., 2019).
Till the end of January 2020, 2519 laboratory-confirmed cases of MERS-CoV were reported globally with 866 deaths (case-fatality rate: 34.3%). Most of the cases were reported in Saudi Arabia (2121 cases) (emro.who, 2020). In the case of COVID-19 2, 626,321 cases were confirmed globally with 181,938 deaths (Coronaviruse, 2020). 8098 reported cases and 774 deaths in 2002–2004 due to the SARS pandemic was reported by the National Health Service (NHS). SARS cases were not reported anywhere in the world since 2004 (Nhs, 2020).
Inoue et al. (2007) analyzed the impact of chlorpromazine on the clathrin-dependent endocytosis of coronavirus by expression angiotensin-converting enzyme 2 (ACE2). Chlorpromazine might interfere with clathrin-mediated endocytosis, by inhibition of clathrin and AP2 transportation on the cell membrane, due to its cationic amphiphilic properties. The Authors treated cells with different concentrations of chlorpromazine for 1 h then infected the cells with the indicated pseudoviruses: SARS-CoV-(HIV), VSV(HIV), or A-MLV(HIV). Chlorpromazine in the concentration of 20 μM inhibited SARS-CoV and VSV infection efficiency (the observed infectivity was 14 and 42%, respectively), while in the case of A-MLV infection was unaffected by the drug concentration. The Authors showed that not only the clathrin-dependent pathway, but also caveolae and/or lipid rafts participate in SARS-CoV entry into host cells. Moreover, the incubation of COS7 cells with the wild-type ACE2 or ACE2-Δtail with chlorpromazine and infected with SARS-CoV showed that the drug-induced suppression of SARS-CoV infection to the host cells. The Authors suggest that the obtained results “indicate that the cytoplasmic domain of the ACE2 is not essential for the clathrin-dependent entry of SARS-CoV, which suggests that there is a possible coreceptor for the ACE2, which interacts with the AP2/clathrin complex” (Inoue et al., 2007). In opposite to Inoue results, Wang et al. (2008) showed that SARS-CoV can enter Vero E6 cells despite chlorpromazine treatment. Those results suggest that the drug did not significantly inhibit virus entry as well as that SARS-CoV infects host cells not only in the absence of clathrin-mediated endocytosis but also in a caveolin-independent manner (Wang et al., 2008). de Wilde et al. (2014) showed that chlorpromazine in low molecular concentrations significantly inhibits MERS-CoV replication in vitro (in the concentration of 12 μM complete inhibition of MERS-CoV infected cells was observed). Furthermore, chlorpromazine inhibited HCoV-229E-GFP replication in a dose-dependent manner as well as affect an early and a post-entry stage of the MERS-CoV replication cycle in Vero and Huh7 cells. Based on the obtained results, it is unlikely, that endocytosis with clatrine is integrated into the antiviral mechanism (de Wilde et al., 2014). The EC50 value was calculated to be 4.9 μM, 8.8 μM, and 2.5 μM for MERS-CoV, SARS-CoV, and HCoV-229E-GFP, respectively (de Wilde et al., 2014; Pillaiyar et al., 2020). Dyall et al. (2014) analyzed the antiviral activity of chlorpromazine against MERS-CoV and SARS-CoV. The obtained EC50 values were 9.51 and 12.97 μM for MERS and SARS coronaviruses, respectively (Dyall et al., 2014). Since chlorpromazine possesses inhibitory activity of viral entry by clathrin-mediated endocytosis (prevent the formation of clathrin-coated pits at the cell membrane), it may be used as a potential broad-spectrum inhibitor not only against West Nile virus (WNV) but also MERS-CoV and SARS-CoV, which used clathrin-mediated endocytosis for entry to host cells (Dyall et al., 2014). The ability of chlorpromazine to the inhibition of cell-cell fusion of MERS-CoV was also analyzed by Liu et al. (2015). The authors showed, that the drug inhibits MERS-CoV replication (IC50 = 8.80 or 9.51 μM), cell-cell fusion, and clathrin-mediated endocytosis (Liu et al., 2015). The IC50 values of chlorpromazine inhibiting cell-cell fusion and clathrin-mediated endocytosis were shown in Table 1 .
Table 1.
Summarized IC50 values of phenothiazines inhibiting cell-cell fusion and clathrin-mediated endocytosis.
| Virus |
Drug |
IC50 |
Source |
|
|---|---|---|---|---|
| cell-cell fusion [μM] | clathrin-mediated endocytosis [μM] | |||
| MERS-CoV | Chlorpromazine | 7.24 ± 2.55 | 23.33 ± 2.89 | Liu et al. (2015) |
| Fluphenazine | 15 ± 4.33 | 3.23 ± 2.79 | Liu et al. (2015) | |
MERS - Middle East respiratory syndrome.
Unfortunately, the assay performer by Wahlbeck et al. (1997) showed that chlorpromazine, thioridazine, and perphenazine did not affect the mean cerebrospinal fluid ACE level in schizophrenic patients despite the reduction of the neuroleptic medication. The schizophrenic patients had a high level of ACE (Wahlbeck et al., 1997).
In the case of dipeptidyl-aminopeptidase, Chikuma et al. (1987) showed that chlorpromazine increase PZ-peptidase and collagenase-like peptidase activity, and the strength of the effect was highly dose-dependent with no influence on leucine aminopeptidase and post-proline cleaving enzyme activities in the osteoblastic MC3T3-E1 cells. Moreover, the drug in the concentration of 10 μg/ml enhanced the activity of PZ-peptidase, collagenase-like peptidase, and dipeptidyl-aminopeptidase for 72 h after treatment, which is involved in collagen degradation. Besides, the drug specifically inhibits collagen synthesis in clonal osteoblasts (Chikuma et al., 1987). Dipeptidyl-peptidase 4 interacts with the MERS-CoV spike protein, therefore the ability of chlorpromazine to increase dipeptidyl-aminopeptidase activity is not a desirable characteristic for MERS-CoV treatment. Despite this fact, it can not be rolled out that the drug is not useless in MERS-CoV treatment.
2.1.2. Human immunodeficiency virus 1 (HIV-1)
Human immunodeficiency virus 1 belongs to Retroviridae with a genome consisting of two identical single-stranded RNA (German Advisory Committee Blood, 2016). The target protein for HIV-1 is reverse transcriptase (Dinesh et al., 2020).
Beignon et al. (2005) showed that chlorpromazine inhibits clathrin-coated pit–mediated endocytosis and stops IFN-α secretion using HIV-1. It suggests that endocytosis is desired for HIV-1 to stimulate human plasmacytoid dendritic cells (pDCs) (Beignon et al., 2005).
2.1.3. Hepatitis C virus (HCV) and mouse hepatitis virus (MHV)
The hepatitis C virus is an RNA virus with a single positive-stranded RNA genome. The virus belongs to the Flaviviridae family and has 50 μm in diameter. The HCV nucleocapsid is capsulated with two wrapping proteins (E1 and E2) and can encode 6 nonstructural proteins (nonstructural 3/4A (NS3/4A), NS5A, and NS5B) which are the targets of direct-acting antiviral drugs (Puchades and Berenguer, 2018). NS3/4 protease is also a target protein for HCV (Dinesh et al., 2020).
Moreover, 71 million cases of Hepatitis C virus (HCV) and 399,000 cases of death were confirmed globally by WHO (Hepatitis, 2020).
Gastaminza et al., (2010) suggested that chlorpromazine inhibits clathrin-dependent endocytosis of the HCV, which is also enveloped, positive-stranded RNA virus as coronaviruses (Gastaminza et al., 2010). Chlorpromazine as a cationic amphiphilic drug inhibits HCV infection during virus-host cell fusion (intercalate into the cholesterol-rich domains of the host cell membrane and increases membrane fluidity) (Dyall et al., 2017). The anti-HCV activity of chlorpromazine was also analyzed by Chamoun-Emanuelli et al. (2013). The obtained IC50 value was 1.47 ± 0.32 μM and a therapeutic index (CC50/IC50) was 6 (Chamoun-Emanuelli et al., 2013). Moreover, the drug is capable to inhibit the replication hepatitis C virus (Dyall et al., 2017; Pillaiyar et al., 2015) and mouse hepatitis virus-2 (MHV-2) (Pillaiyar et al., 2015).
Burkard et al. (2014) showed that chlorpromazine did not inhibit relative infection of the mouse hepatitis virus, which is used as a model to study CoV infections. Moreover, the drug significantly reduced the fusion of MHV upon treatment of cells (Burkard et al., 2014).
2.1.4. Flaviviruses
The Japanese encephalitis virus (JEV), dengue virus (DENV), West Nile virus (WNV), and Zika virus (ZIKV) are flaviviruses, which has a positive-sense, single-stranded RNA genome (size about 11 kB long) and 50 nm virion size (Filgueira and Lannes, 2019). The WNV genome encodes structural and nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) which are very important for intracellular virus replication (Chu and Ng, 2004). The target protein for ZIKV is MTase (N-terminal of NS5) (Dinesh et al., 2020). DENV NS5 was found in the infected cell nucleus. Therefore, NS5 nuclear localization is essential for infection. It has been reported for DENV, WNV, and ZIKV. The role of NS5 has the potential to suppress the antiviral response, as it can impact mRNA splicing (Yang et al., 2019).
Furthermore, due to another WHO report from the 2019 year, about 68,000 cases of Japanese encephalitis virus (JEV) are estimated each year globally, with about 13,600–20,400 lethal infections (Dengue, 2020). In the case of dengue virus (DENV) in the 2015 year, 3,312,040 cases and 4032 deaths were noticed (Japanese, 2020). 6640 cases of Zika virus (ZIKV) and 1 case of death were reported in the North, Central, and South America in the 2019 year (Paho, 2020). 463 cases and 50 deaths caused by WNV were reported in the European Union, as reported by the European Centre for Disease Prevention and Control (ECDPC) (europa, 2020).
Chlorpromazine activity on the infectious entry of WNV was measured by Chu and Ng (2004). In the case of WNV chlorpromazine inhibits clathrin-mediated endocytic pathway by accumulating clathrin and Adaptor Protein Complex-2 (AP-2) in endosomal compartments, what prevents clathrin-coated pits formation. Moreover, the effect of clathrin-mediated endocytosis-disrupting drugs on WNV entry showed a time-dependent increase antigen formulation (Chu and Ng, 2004).
Chlorpromazine anti-JEV activity by using the vertebrate cell line Vero was analyzed by Nawa et al. (2003). The drug caused inhibition of the Japanese encephalitis virus and the number of JEV antigen-positive cells was about 80% lower in comparison to the untreated culture. The percentage of JEV antigen-positive cells was 60% (control samples) and 11% in chlorpromazine (10 μg/ml) treated cultures.
Moreover, chlorpromazine did not influence JEV bounding but stoped its transportation into endosomes. Noteworthy, the inhibition of JEV binding to Vero cells was not observed in the concentration range 2–5 to 20 μg/ml (data were not shown by the Authors). Furthermore, the Authors showed that chlorpromazine in the concentration of 10 μg/ml affects the distribution of clathrin within a cell and inhibits the uptake of JEV in Vero cells with no influence on progeny viral protein when the infection was established if the drug was present at an early stage of infection. (Nawa et al., 2003). Moreover, chlorpromazine can inhibit the replication of the WNV and JEV viruses (Dyall et al., 2017).
Li et al. (2020) showed that chlorpromazine in the non-toxic concentrations in T98G cells inhibits ZIKV cell infection in a dose-dependent manner without influencing JEV copies of viral RNA (Li et al., 2020).
2.1.5. Ebola virus (EBOV)
The Ebola virus is a part of the Filoviridae family with a single negative-sensed RNA genome (size 19 Kb). 7 proteins with multiple morphological forms can be found in the virus (Kimura et al., 2015). The virus uses the virus-like particle (VLP) expressing EBOV glycoprotein (GP1,2) and containing a β-lactamase (BlaM) for entering into host cells, which makes them an interesting target for antiviral therapy (Johansen et al., 2015). 5 different species of EBOV have been identified (EBOV-Z, EBOV-S, EBOVIC, EBOV-B, and Reston ebolavirus). Noteworthy, the virulence of each species may differ markedly from the others (Kimura et al., 2015). Glycoprotein (VP30) is a target protein of EBOV (Dinesh et al., 2020).
The WHO report from March 31, 2020 about the Ebola virus (EBOV) in the Democratic Republic of the Congo informs about 3310 confirmed cases with 2273 deaths (Ebola, 2020).
The impact of chlorpromazine on the infection of the EBOV virus was analyzed by Bhattacharyya et al. (2010). The Authors showed the ability of the drug for significant inhibition of replication-competent EBOV infection - chlorpromazine in the concentration of 10 μg/ml inhibits clathrin-mediated endocytosis. Moreover, the analysis of EBOV envelope glycoprotein (EbGP) infectivity in HMEC, HeLa, Vero, and 293T cell lines suggests that the observed inhibition after chlorpromazine treatment is not restricted to cell type but is present in different cell types. Furthermore, the effect of chlorpromazine on GFP-expressing replication-competent EBOV Zaire (ZEBOV-GFP) showed dose-dependent infections decrease with complete inhibition at a concentration of 10 μg/ml. Interestingly, the analysis of the infection potential of ZEBOV-GFP in HeLa cells and Vero-E6 cells showed that the inhibitory effect of chlorpromazine on EBOV was not restricted to Vero-E6 cells (Bhattacharyya et al., 2010).
2.1.6. Porcine epidemic diarrhea virus (PEDV)
The porcine epidemic diarrhea virus is an enveloped Alphacoronavirus, which has 28 kb single-stranded, positive-sense RNA genome. The PEDV genome like Coronaviruses also encodes four key viral structural proteins (S, E, M, and N). The glycoprotein S is responsible for PEDV binding to host receptors and triggering virus-host membrane fusion. The structural proteins M and E are responsible for the construction of envelopes, while N protein is multifunctional and is involved among others in viral replication. The porcine epidemic diarrhea virus (PEDV) causing high mortality rates in newborn pigs, may lead to high economic losses in the pork industry (Hou and Wang, 2019).
Wei et al. (2020) analyzed the impact of chlorpromazine on the infection of alphacoronavirus - porcine epidemic diarrhea virus (PEDV). The cytotoxicity results showed that the concentration of 30 μM and 50 μM did not affect the viability of the Vero and the IPEC-J2 cells, respectively. The qRT-PCR results showed a significant decrease of PEDV invasion after chlorpromazine treatment (10 and 30 μM for Vero cells as well as 30 and 50 μM for IPEC-J2 cells). Moreover, chlorpromazine pre-treatment (to prevent clathrin assembly and further block clathrin-mediated endocytosis) and siRNA-mediated knockdown of clathrin heavy chain as well as EPS 15 (the critical component of clathrin-coated pits by interacting with adaptor protein 2) significantly reduce the invasion rate of PEDV into cells (Wei et al., 2020).
2.1.7. Chikungunya virus (CHIKV) and Semliki Forest virus (SFV)
The Chikungunya virus is enveloped alphavirus with a diameter of 60–70 nm and a single-stranded, positive-sense, linear RNA genome. CHIKV genome contains 2 open reading frames (ORF). The first one encodes the non-structural proteins (nsP1, 2, 3, and 4), while the next one is responsible for the structural proteins (capsid, E3, E2,6K, and E1). Nonstructural proteins make up 2/3 of the genome, while structural proteins 1/3 of it. E3 proteins carry a signal peptide and are important for targeting the structural polyprotein for initial processing. E3 is also very important for the stabilization and maturation of the E2 glycoprotein, which is a key receptor binding protein for CHIKV. The E1 protein is a class II viral fusion protein mediating the fusion of the virus outermost layer with the host endosome after endocytosis, what release of the nucleocapsid. Moreover, E1, E2, and E3 proteins can be potential targets in new CHIKV therapy (Wong and Chu, 2018).
According to the Pan American Health Organization (PAHO) 2.5 million cases of Chikungunya virus (CHIKV) and 631 deaths were reported in the North, Central, and South America, except Canada, Cuba, and Chile, where the cases of the virus were not observed (Lima et al., 2019). The death rate of the Crimean-Congo hemorrhagic virus (CCHFV), a zoonotic viral illness, is from 5% to 30% in more than 30 countries worldwide (Jeeva et al., 2019).
Inhibition of Semliki Forest virus entry by chlorpromazine was analyzed by Pohjala et al. (2011). The obtained results showed that the drug IC50 for SFV replication is 15.7 μM, while IC50 for BHK cell viability is 67.3 μM using reporter gene Renilla luciferase, Rluc screening assay as an analysis method. Moreover, the drug decreased Rluc activity and inhibited Chikungunya virus-Rluc (CHIKV-Rluc) infection (IC50 = 39.4 μM), and do not influence CHIKV replicon formation. It suggests that chlorpromazine can decrease entry and replication of the alphavirus and that SFV is a safe model for anti-CHIKV screening (Pohjala et al., 2011). Chlorpromazine is also able to inhibit the replication of CHIKV (Dyall et al., 2017).
2.1.8. Crimean-Congo hemorrhagic virus (CCHFV)
The Crimean-Congo hemorrhagic virus is a Nairovirus composed of three segments (large (L), medium (M), and small (S)) of the negative-sense single-stranded RNA genome. N protein is a key protein responsible for transcription and replication of the viral genome. It suggests that the N protein can be a new target for CCHFV infection treatment (Jeeva et al., 2019).
In vitro, anti-Crimean-Congo hemorrhagic virus efficacy of chlorpromazine was analyzed by Ferraris et al. (2015). The drug inhibited CCHFV strains 86–07 and 87–07 and the obtained IC50 values were 10.6 ± 0.031 and 15.8 ± 0.051 μM for 86–07 and 87–07 using Vero E6 cells, respectively. In the case of 87–07 and Huh7 cells, the IC50 value was 4.3 μM. It suggests that the drug was more effective towards Huh7 than in Vero E6 cells. Furthermore, the caveolae-1 pathway of Huh7 cells was missing, which strongly suggests the involvement of clathrin-mediated endocytosis. Interestingly, chlorpromazine possesses the antiviral effect when it is used as pre-treatment, during the infection, or post-infection (EC50 = 16.3 ± 0.008, 6.7 ± 0.002, and 8.5 ± 0.004 μM, respectively for pre-treatment, concurrent and permanent conditions). Moreover, a significant synergistic antiviral activity after the ribavirin/chlorpromazine combination treatment was observed (Ferraris et al., 2015).
2.2. Fluphenazine
2.2.1. Corona viruses (CoVs)
Fluphenazine possesses antiviral activity towards MERS-CoV (EC50 = 5.86 μM) and SARS-CoV (EC50 = 21.43 μM) (Dyall et al., 2017; Pillaiyar et al., 2020). As in the case of chlorpromazine, the antiviral activity of fluphenazine was analyzed by Dyall et al. (2014). The obtained EC50 values were 5.87 and 21.43 μM for MERS-CoV and SARS-CoV, respectively (Dyall et al., 2014). Liu et al., (2015) showed that fluphenazine moderate inhibits MERS-CoV protein-mediated cell-cell fusion. The IC50 value was about 29 μM. It suggests that the inhibitor of the ABL-1 pathway interesting material for new antivirus drugs since the pathway is crucial for viral replication. Moreover, the drug inhibits MERS-CoV replication (IC50 = 5.86 μM) and cell-cell fusion stronger than chlorpromazine as well as disrupts clathrin-mediated endocytosis (Liu et al., 2015). The IC50 values of fluphenazine inhibiting cell-cell fusion and clathrin-mediated endocytosis were shown in Table 1.
2.2.2. Hepatitis C virus (HCV)
Gastaminza et al., (2010) based on NCC library compounds showed that fluphenazine caused a reduction in HCV infection (EC50 = 0.5 ± 0.2 μM, LD50 = 14.5 ± 5.5 μM) as well as inhibits clathrin-dependent endocytosis of the hepatitis C virus (HCV) (Gastaminza et al., 2010). Fluphenazine as chlorpromazine is a cationic amphiphilic drug, which inhibits HCV entry at virus-host cell fusion (Dyall et al., 2017). The anti-HCV activity of fluphenazine was also analyzed by Chamoun-Emanuelli et al. (2013). The obtained IC50 value was 0.37 ± 0.01 μM and a therapeutic index (CC50/IC50) was 15.3. Fluphenazine the most significant inhibits a post attachment step of HCV entry after adding the temperature shift to 37 °C even after CD81 antibody inactivation, which suggests, that the point of action is independent of CD81 binding, probably during a fusion stage. Moreover, fluphenazine exhibits the strongest fusion inhibition of HCVpp-liposome in vitro in a dose-dependent manner. Furthermore, samples treated with fluphenazine prior to infection with HCVcc were similar to the control, which suggests that the drug does not inhibit HCV entry directly (Chamoun-Emanuelli et al., 2013). Banda et al. (2019) showed that fluphenazine inhibits GT2-derived viral strains what seems to be linked with the regulation of the fusion that is related to their E1 protein properties (Banda et al., 2019).
2.2.3. Ebola virus (EBOV)
Antiviral activity towards the Ebola virus (EBOV) of fluphenazine was analyzed by Johansen et al. (2015). The concentration of fluphenazine which caused 50% inhibition of host viability (without virus) were 5.54 ± 0.19 and 3.05 ± 1.68 μM for uninfected Vero E6 and HepG2 host cells, respectively. Interestingly, the EC50 value for viral infection was not calculated. The Authors also showed that the fluphenazine concentration of 10 μM strongly (>90%) inhibits EBOV-VLP entry into SNB19 cells (Johansen et al., 2015).
2.3. Perphenazine
2.3.1. Corona viruses (CoVs)
Coronavirus key enzyme for replication is the main protease (Mpro), which highly similar in sequences and 3D structures in this family. Thus, the main protease is considered as an attractive target for the design of anti-coronaviral drugs. Based on the DrugBank database, Liu and Wang (2020) identified perphenazine as the 1 from 10 commercial medicines as a potential inhibitor of 2019-nCoV Mpro, which can bind the pocket site of the virus. As the Authors suggest the drug can form two hydrogen bonds with Asn28 and Asn119, which makes it theoretically “capable to bind to the pocket formed by these amino acids and interfere with the function of 2019-nCoV Mpro” (Liu and Wang, 2020). Noteworthy, the analysis was performed in silico, thus further experiments are very crucial to validate the efficacy of perphenazine.
Maes et al. (1996) found that chlorpromazine lower DPP 4 activity in major depression and higher DPP 4 activity in schizophrenia patients than in normal volunteers in contrast to Chikuma et al. (1987). Subchronic use of antidepressants or antipsychotic agents (perphenazine 8–48 mg/day) does not have an influence on the alterations in DPP 4 is depressed (33.6 ± 10.4 U/l before treatment and 37.5 ± 9.0 U/l after treatment) or schizophrenic patients (44.8 ± 9.1 U/l before treatment and 45.6 ± 10.2 U/l after treatment), respectively (Maes et al., 1996). It suggests that perphenazine is a much better candidate against MERS-CoV.
2.3.2. Chikungunya virus (CHIKV) and Semliki Forest virus (SFV)
Inhibition of Semliki Forest virus (SFV) entry also by perphenazine was analyzed by Pohjala et al. (2011). The obtained results showed that the drug IC50 for SFV replication is 25.1 μM, while IC50 for BHK cell viability is 155.0 μM using reporter gene Renilla luciferase, Rluc screening assay. Moreover, the drug decreased Rluc activity and inhibited Chikungunya virus-Rluc (CHIKV-Rluc) infection (IC50 = 48.1 μM), and did not influence CHIKV replicon formation. It suggests that perphenazine can decrease entry and replication of the alphavirus and that SFV is a safe model for anti-CHIKV screening (Pohjala et al., 2011).
2.4. Prochlorperazine
2.4.1. Hepatitis C virus (HCV)
Gastaminza et al., (2010) based on NCC library compounds showed that prochlorperazine like fluphenazine cause a reduction in HCV infection (EC50 = 1.3 ± 0.7 μM, LD50 = 29.3 ± 12.3 μM) as well as inhibits clathrin-dependent endocytosis of the hepatitis C virus (HCV) (Gastaminza et al., 2010). The anti-HCV activity of prochlorperazine was also analyzed by Chamoun-Emanuelli et al. (2013). The obtained IC50 value was 0.92 ± 0.11 μM and a therapeutic index (CC50/IC50) was 8.3. Infectivity of HCVcc samples pretreated with prochlorperazine was also as in the case of fluphenazine similar to the control (non-pretreated) results what suggests that the drug does not inhibit HCV entry with direct inactivation (Chamoun-Emanuelli et al., 2013).
2.4.2. Ebola virus (EBOV)
Antiviral activity towards the EBOV of prochlorperazine was analyzed by Johansesn et al. (2015). The concentration of the drug which caused 50% inhibition of host viability (without virus) were 5.96 ± 0.42 and 3.59 ± 0.19 μM for Vero E6 and HepG2 host cells, respectively. Interestingly the EC50 value for viral infection was not calculated. The Authors also showed that the prochlorperazine concentration of 10 μM strongly (>90%) inhibits EBOV-VLP entry into SNB19 cells (Johansesn et al., 2015).
2.4.3. Flaviviruses
In vitro and in vivo antiviral activity of prochlorperazine towards the Dengue virus (DENV) was analyzed by Simanjuntak et al. (2015). The cytotoxicity analysis in human kidney (HEK293T), lung (A549), microglia (CHME3), monocytic (THP-1) cells, and mouse neuroblastoma (N18) cells showed that prochlorperazine in the concentration up to 30 μM did not influence significantly cell viability, cell proliferation, or cytotoxicity. The drug inhibits protein expression and viral progeny in HEK293T DENV-2 infected cells, with an EC50 of 88 nM. Moreover, the drug inhibits the replication of the Dengue virus serotype 2 (DENV-2) at noncytotoxic doses (EC50 = 137 nM) in a dose-dependent manner. The drug inhibits also infection of DENV-1, DENV-2, and Japanese encephalitis virus. The authors showed that the drug blocks DENV entry through clathrin-mediated endocytosis. DENV-2 was located with clathrin on the cell membrane without cell penetration after prochlorperazine treatment (30 min, 20, and 30 μM) (Simanjuntak et al., 2015). Interestingly, the in vivo experiment performed by the Authors, using the Stat1−/− mice challenged with DENV-2, showed that prochlorperazine dimaleate in the concentration of 5 mg/kg body weight/day protected the animals against death caused by DENV-2. Interestingly, 90% of vehicle control mice died, while the drug in the concentration of 1 mg drug/kg body weight/day only delayed animal mortality. Noteworthy, the authors admit that it is possible to reach the equivalent of 5 mg/kg body weight/day for mice in human (0.405 mg/kg/day). Thus, the dose for a 60-kg person would be 24.3 mg/day, what is in line with the clinical dose of prochlorperazine dimaleate recommended to prevent nausea and vomiting (5–10 mg/dose, 2–3 times daily) as well as for treating nausea and vomiting (20 mg followed by 10 mg 2 h later if required) However, detailed pharmacokinetic studies and antiviral tests in humans are still required (Simanjuntak et al., 2015).
Han et. Al (2017) showed that prochlorperazine dimaleate did not achieve 100% protection against the Zika virus (ZIKV) at optimal concentrations. The drug was effective only over a narrow range of concentrations with CC50 = 10.91 ± 0.95 μM (Han et al., 2018).
Noteworthy, potential inhibitors of the NS3 protein of ZIKV were analyzed by Sahoo et al. (2016). The Authors showed that prochlorperazine can bind the NS3 protein of ZIKV and DENV. In the case of ZIKV, the binding energy is -5.5 kcal/mol and the H-bond interaction is possible by the Tyr161, while in the case of DENV the binding energy is -6.0 kcal/mol and the H-bond interaction was not observed (Sahoo et al., 2016).
2.5. Thioridazine
2.5.1. Ebola virus (EBOV)
As in the case of fluphenazine and prochlorperazine, antiviral activity towards the EBOV of thioridazine was also analyzed by Johansesn et al. (2015). The concentration of the drug which caused 50% inhibition of host viability (without virus) were 6.24 ± 0.79 and 2.06 ± 0.12 μM for Vero E6 and HepG2 host cells, respectively. In opposite to fluphenazine and prochlorperazine, the EC50 value for viral infection was 10.2 ± 9.06 and 21.6 ± 0.78 μM for Vero E6 and HepG2 host cells, respectively. The Authors also showed that the thioridazine concentration of 10 μM strongly (>90%) inhibits EBOV-VLP entry into SNB19 cells [45]. According to the table published by Lee et. Al (2018) thioridazine possess anti-EBOV activity with EC50 = 1.45 ± 0.26 μM [57].
2.5.2. Hepatitis C virus (HCV)
The anti-HCV activity of thioridazine was analyzed by Chamoun-Emanuelli et al. (2013). The obtained IC50 value was 0.78 ± 0.31 μM and a therapeutic index (CC50/IC50) was 6.8. Thioridazine like other phenothiazines also inhibits HCV entry (Chamoun-Emanuelli et al., 2013).
2.5.3. Chikungunya virus (CHIKV) and Semliki Forest virus (SFV)
Inhibition of Semliki Forest virus (SFV) entry also by thioridazine was analyzed by Pohjala et al. (2011). The obtained results showed that the drug IC50 for SFV replication is 14.9 μM and 19.3 μM, while IC50 for BHK cell viability is 179.4 μM, respectively to the analysis method (reporter gene Renilla luciferase, Rluc screening assay or CPE reduction and virus production assays). In the case of Sindbis virus (SINV) the IC50 of viral replication is 37.3 μM using CPE reduction. Moreover, the drug decreased Rluc activity, inhibited Chikungunya virus-Rluc (CHIKV-Rluc) infection (IC50 = 71.5 μM), and had no influence on CHIKV replicon formation. It suggests that thioridazine can decrease entry and replication of the alphavirus and that SFV is a safe model for anti-CHIKV screening (Pohjala et al., 2011).
2.5.4. Rift Valley fever virus (RVFV)
The Rift Valley fever virus (RVFV) is a Phlebovirus, with RNA, which can be divided into three fragments. Large (L) responsible for RNA polymerase, medium (M) encodes structural glycoproteins Gn and Gc (mediate binding and entry via receptors), and small (S) responsible for nucleoprotein of the single-stranded RNA genome. The structural glycoproteins play a crucial role in virus entry to the cell. The 78-kDa glycoprotein is able to form a complex with the Gc glycoprotein and may constitute a target for the immune system. The RVFV causes zoonotic disease affecting both ruminants and humans, which is endemic to the African continent and occurs also in the Middle East (Mansfield et al., 2015).
Filone et al. (2010) showed that thioridazine inhibits the Rift Valley fever virus in 293T cells (IC50 = 26.2 μM). Noteworthy, IC50 values were calculated only for non-toxic compounds. Furthermore, they found that the drug also inhibited RVFV MP12 infection in the HeLa and Vero cells (Filone et al., 2010).
3. Discussion
The World Health Organization (WHO) published a list of diseases that pose a population health risk. Viruses such as COVID-19, Crimean-Congo hemorrhagic fever, Ebola virus disease, Rift Valley fever, Zika, Middle East respiratory syndrome coronavirus (MERS-CoV), and Severe Acute Respiratory Syndrome (SARS) were listed as the one with the urgent need for more effective countermeasures (Who, 2020).
The increasing number of people suffering from virus diseases requires the development of new and effective methods of treatment. Two main approaches are considered in the field. The first longer approach is based on developing anti-viral drug de-novo in opposite to the second approach, which is based on drug repositioning or drug repurposing. In the second case, the safety data and needed assays are already in place, so it may save a year of new drug development (Mani et al., 2019). In the case of antipsychotic drugs such as phenothiazine derivatives, the novel antiviral activity of chlorpromazine, fluphenazine, perphenazine, prochlorperazine, and thioridazine were observed and confirmed by several research groups toward different types of viruses, both in vitro and in vivo.
All the researcher groups which analyzed the antiviral activity of chlorpromazine, fluphenazine, perphenazine, prochlorperazine, and thioridazine observed decrease viability of host cells after treatment. The summarized IC50 values of the analyzed drugs towards different types of viruses were shown in Table 2 . In case of chlorpromazine towards MERS-CoV and HCoV-229E-GFP, prochlorperazine towards HCV, and thioridazine towards HCV and EBOV the obtained IC50 and EC50 values are lower than the upper range of toxic concentration observed in human plasma, which is up to 2 μg/ml (i.e. 5.63 μM), 0.1 μg/ml (i.e. 0.20 μM), 1 μg/ml (i.e. 2.47 μM), 1 μg/ml (i.e.1.65 μM), and 5 μg/ml (i.e.12.28 μM) for chlorpromazine, fluphenazine, perphenazine, and prochlorperazine, and thioridazine respectively (Schulz and Schmoldt, 2003; Winek et al., 2001).
Table 2.
Summarized EC50 and IC50 values of chlorpromazine, fluphenazine, perphenazine, prochlorperazine, and thioridazine towards different types of viruses.
| Virus | Drug | EC50 or IC50 [μM] | Source |
|---|---|---|---|
| MERS-CoV | Chlorpromazine | EC50 = 4.9 | de Wilde et al. (2014); Pillaiyar et al. (2020) |
| EC50 = 9.51 | Dyall et al. (2014) | ||
| Fluphenazine |
EC50 = 5.86 |
Dyall et al. (2017); Pillaiyar et al. (2020) |
|
| SARS-CoV | Chlorpromazine | EC50 = 8.8 | de Wilde et al. (2014);Pillaiyar et al. (2020) |
| EC50 = 12.97 | Dyall et al. (2014) | ||
| Fluphenazine |
EC50 = 21.43 |
Dyall et al. (2017); Pillaiyar et al. (2020) |
|
| HCoV-229E-GFP |
Chlorpromazine |
EC50 = 2.5 |
de Wilde et al. (2014), Pillaiyar et al. (2020) |
| HCV | Chlorpromazine | IC50 = 1.47 ± 0.32 | Chamoun-Emanuelli et al. (2013) |
| Fluphenazine | EC50 = 0.5 ± 0.2 | Gastaminza et al. (2010) | |
| IC50 = 0.37 ± 0.01 | Chamoun-Emanuelli et al. (2013) | ||
| Prochlorperazine | EC50 = 1.3 ± 0.7 | Gastaminza et al. (2010) | |
| IC50 = 0.92 ± 0.11 | Chamoun-Emanuelli et al. (2013) | ||
| Thioridazine |
IC50 = 0.78 ± 0.31 |
Chamoun-Emanuelli et al. (2013) |
|
| EBOV | Thioridazine | EC50 = 1.45 ± 0.26 | Lee et al. (2018) |
CoVs - corona viruses, EBOV - Ebola virus, GFP – green fluorescence protein, HCoV – human corona viruses, HCV - Hepatitis C virus, MERS - Middle East respiratory syndrome, SARS - severe acute respiratory syndrome.
The summarizes the antiviral activity of chlorpromazine, fluphenazine, perphenazine, prochlorperazine, and thioridazine towards different RNA viruses were shown in Fig. 1 .
Fig. 1.
The summary of the antiviral effect of chlorpromazine, fluphenazine, perphenazine, prochlorperazine, and thioridazine towards different types of RNA viruses.
Chlorpromazine inhibits the clathrin-mediated endocytosis influencing the transport of clathrin and AP2, which is one of the possible ways of viral entry into the host cells. Caveolae and/or lipid rafts precipitate are also used by viruses (e.g. SARS-CoV) for entering into the cells. Moreover, the drug inhibits MERS-CoV (Inoue et al., 2007; de Wilde et al., 2014; Dyall et al., 2014), SARS-CoV (Inoue et al., 2007; Wang et al., 2008), HCoV-229E-GFP (de Wilde et al., 2014; Pillaiyar et al., 2020), HCV (Chamoun-Emanuelli et al., 2013; Dyall et al., 2017; Gastaminza et al., 2010), MHV (Burkard et al., 2014), CHIKV (Dyall et al., 2017) and SFV (Pohjala et al., 2011) replication in vitro in low molecular concentrations. Thus, chlorpromazine can be used as a potential broad-spectrum inhibitor of viruses using the clathrin-mediated endocytosis such as WNV (Chu and Ng, 2004; Dyall et al., 2014), HIV-1 (Beignon et al., 2005), HCV (Chamoun-Emanuelli et al., 2013; Dyall et al., 2017; Gastaminza et al., 2010), EBOV (Bhattacharyya et al., 2010; Dyall et al., 2017), JEV (Dyall et al., 2017; Nawa et al., 2003), HCoV-229E-GFP (de Wilde et al., 2014; Pillaiyar et al., 2020), MERS-CoV (Dyall et al., 2014; Inoue et al., 2007; de Wilde et al., 2014), and SARS-CoV (Inoue et al., 2007; Wang et al., 2008). The summarized concentrations of the analyzed drugs inhibiting RNA viruses replication were shown in Table 3 . Moreover, the drug inhibits PEDV (Wei et al., 2020) invasion. Noteworthy, the antiviral activity of chlorpromazine towards HCV is very promising as the obtained by Chamoun-Emanuelli et al. (2013) results (IC50 = 1.47 ± 0.32 μM and CC50/IC50 = 6) (Chamoun-Emanuelli et al., 2013) since both values are lower or very near of the toxic human plasma concentration. A similar situation was observed in the case of CCHFV treatment by Ferraris et al. (2015). The stronger effect of the drug towards Huh7 than Vero E6 cells was noticed. The IC50 value for 87–07 strain and Huh7 cells was 4.3 μM, which is in the range of toxic human plasma concentration (Ferraris et al., 2015). On the other hand, the role of chlorpromazine in JEV treatment is debatable since in the concentration range 2.5–20 μg/ml inhibition of viral binding to Vero cells was not observed, while the drug in the concentration of 10 μg/ml affects the clathrin distribution within a cell with inhibition of JEV uptake (Nawa et al., 2003).
Table 3.
Summarized concentrations of phenothiazines inhibiting RNA virus replication.
| Virus | Drug | Concentration inhibiting viral replication [μM] | Source |
|---|---|---|---|
| DENV-2 | Prochlorperazine | EC50 = 137 nM | Simanjuntak et al. (2015) |
| MERS-CoV | Chlorpromazine | 12 μM (complete inhibition) | (Wilde et al. 2014) |
| SINV | Thioridazine | IC50 = 37.3 μM | Pohjala et al. (2011) |
| SFV | Chlorpromazine | IC50 = 15.7 μM | Pohjala et al. (2011) |
| Perphenazine | IC50 = 25.1 μM | Pohjala et al. (2011) | |
| Thioridazine | IC50 = 14.9 μM and 19.3 μM | Pohjala et al. (2011) | |
| ZEBOV-GFP | Chlorpromazine | 10 μg/ml i.e. 31.36 μM (complete inhibition) | Bhattacharyya et al. (2010) |
DENV-2 - Dengue virus serotype 2, MERS - Middle East respiratory syndrome, SFV - Semliki Forest virus, SINV - Sindbis virus, ZEBOV - GFP-expressing replication-competent EBOV Zaire.
Fluphenazine also possesses antiviral activity towards MERS-CoV and SARS-CoV (Dyall et al., 2017; Pillaiyar et al., 2020), which also suggests potential inhibitor activities for viruses using the clathrin-mediated endocytosis. The drug inhibits also HCV (Chamoun-Emanuelli et al., 2013; Dyall et al., 2017) and EBOV (Johansen et al., 2015) entry into the host cells. Based on the EC50 values obtained by Dyall et al. (2014) chlorpromazine is more efficient towards SARS-CoV than fluphenazine, while fluphenazine is more potent against MERS-CoV than chlorpromazine (Dyall et al., 2014). Moreover, the drug inhibits MERS-CoV replication and cell-cell fusion. The IC50 values of the analyzed drugs inhibiting cell-cell fusion and clathrin-mediated endocytosis were shown in Table 1. Interestingly, a comparison of the IC50 values for MERS-CoV replication and cell-cell fusion and clathrin-mediated endocytosis obtained by Liu et al. (2015) and Dyall et al. (2014) show that in all cases fluphenazine is more effective than chlorpromazine.
Prochlorperazine possesses anti-EBOV (Johansen et al., 2015), DENV-2 (Simanjuntak et al., 2015), HCV (Chamoun-Emanuelli et al., 2013; Gastaminza et al., 2010) activity, in case of HCV, entry inhibition does not inactivate the virus directly (Chamoun-Emanuelli et al., 2013). The EC50 values obtained by Gastaminza et al. (2010) and Chamoun-Emanuelli et al. (2013) suggest that fluphenazine is about 2.5 times more efficient towards HCV than prochlorperazine, but the concentration of prochlorperazine is in the range of human plasma concentration. Moreover, the drug is more effective in HCV treatment than chlorpromazine, but much less effective than fluphenazine. The most promising are results obtained by Simanjuntak et al. (2015) which showed that prochlorperazine in nM concentration inhibits protein expression, viral progeny, replication of DENV-2. The drug also reduces infection of DENV-1, DENV-2, and Japanese encephalitis virus as well as inhibits DENV entry through clathrin-mediated endocytosis (Simanjuntak et al., 2015). Moreover, the in vivo results suggest that the concentration which protected the animals against death caused by DENV-2 can obtain since it is similar to the dose used in nausea and vomiting prevent and/or treating. On the other hand, prochlorperazine is not effective in ZIKV treatment (Han et al., 2018).
Thioridazine possesses anti-EBOV (Johansesn et al., 2015; Lee et al., 2018), HCV (Chamoun-Emanuelli et al., 2013), SFV (Pohjala et al., 2011), and RVFV (Filone et al., 2010) activity. In the case of EBOV comparison of the results obtained by Johansesn et al. (2015) show that fluphenazine, prochlorperazine, and thioridazine strongly inhibit EBOV entry into the host cells. Moreover, thioridazine was more cytotoxic towards host cells than fluphenazine and prochlorperazine. Chamoun-Emanuelli et al. (2013) show that thioridazine is less potent towards HCV than fluphenazine but more potent than chlorpromazine and prochlorperazine. It can be explained by the chemical structure of the phenothiazines, which is presented in Fig. 1. Piperazine ring phenothiazines are more potent than those without it (prochlorperazine and fluphenazine > chlorpromazine), also the presence of a propanol group on the piperazine ring and a trifluoromethyl group at position 2 (fluphenazine) increases the anti-HCV potency. Noteworthy the thioridazine concentration caused by anti-HCV activity is in the range of therapeutic human plasma concentration. It makes thioridazine a possible candidate for effective HCV treatment. The results obtained by Pohjala et al. (2011) show that chlorpromazine, perphenazine, and thioridazine decrease entry and replication of SFV. Moreover, the obtained IC50 for SFV replication showed that thioridazine inhibits the viral replication a little bit stronger than chlorpromazine and about 2 times stronger than perphenazine.
The link between the in vitro and in vivo anti-viral effect of prochlorperazine suggests that the drug in a therapeutic concentration can achieve an antiviral effect towards DENV and that phenothiazines can be repurposed from an antipsychotic drug to an anti-viral drug. Moreover, the in vivo results suggest that chlorpromazine may be potentially used in the anti-HCV and CCHFV therapy, but it is not efficient in CCHFV treatment since inhibition of viral binding to the Vero cells was not observed. On the other hand prochlorperazine role in HCV treatment is debatable, as the drug is not effective in ZIKV treatment, but it is very promising in DENV treatment. Thus, further investigations are needed to determine the fragments in phenothiazines responsible for the antiviral effect.
4. Conclusion
Chlorpromazine, fluphenazine, perphenazine, prochlorperazine, and thioridazine possess anti-viral activity towards different types of viruses. These drugs inhibit clathrin-dependent endocytosis, cell-cell fusion, infection, replication of the virus, decrease viral invasion as well as suppress entry into the host cells. The drugs display activity at nontoxic concentrations and may have the potential for therapeutic use. Moreover, the animal in vivo study showed that the antiviral activity of prochlorperazine can be effective in the therapeutic concentration. Despite in vitro and in vivo antipsychotic drugs may lead to side effects. Thus, many experiments are important to precise the molecular mechanism for antiviral effects of phenothiazines and reduce a concentration to reasonable dose and/or generate derivatives to diminish the side effects of antiviral therapy.
Funding
The manuscript received no funding.
Author contributions
Michał Otręba conducted the literature search, selection process, data extraction, data analysis, results interpretation, and wrote the manuscript for submission. Leon Kośmider conducted results interpretation and wrote the manuscript for submission. Anna Rzepecka-Stojko wrote the manuscript for submission. All authors participated in the research design, discussions, and approved the manuscript for submission.
Declaration of competing interest
The authors declare no conflict of interest.
References
- Adalja A., Inglesby T. Broad-spectrum antiviral agents: A crucial pandemic tool. Expert Rev. Anti Infect. Ther. 2019;17:467–470. doi: 10.1080/14787210.2019.1635009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banda D.H., Perin P.M., Brown R.J.P., Todt D., Solodenko W., Hoffmeyer P., Sahu K.Kumar, Houghton M., Meuleman P., Müller R., Kirschning A., Pietschmann T. A central hydrophobic E1 region controls the pH range of hepatitis C virus membrane fusion and susceptibility to fusion inhibitors. J. Hepatol. 2019;70:1082–1092. doi: 10.1016/j.jhep.2019.01.033. [DOI] [PubMed] [Google Scholar]
- Beignon A.S., McKenna K., Skoberne M., Manches O., DaSilva I., Kavanagh D.G., Larsson M., Gorelick R.J., Lifson J.D., Bhardwaj N. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor– viral RNA interactions. J. Clin. Invest. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S., Warfield K.L., Ruthel G., Bavari S., Aman M.J., Hope T.J. Ebola virus uses clathrin-mediated endocytosis as an entry pathway. Virology. 2010;401:18–28. doi: 10.1016/j.virol.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkard C., Verheije M.H., Wicht O., van Kasteren S.I., van Kuppeveld F.J., Haagmans B.L., Pelkmans L., Rottier P.J., Bosch B.J., de Haan C.A. Coronavirus cell entry occurs through the endo-/lysosomal pathway in a proteolysis-dependent manner. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamoun-Emanuelli A.M., Pecheur E.I., Simeon R.L., Huang D., Cremer P.S., Chen Z. Phenothiazines inhibit hepatitis C virus entry, likely by increasing the fluidity of cholesterol-rich membranes. Antimicrob. Agents Chemother. 2013;57:2571–2581. doi: 10.1128/AAC.02593-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikuma T., Ishii Y., Kato T., Kurihara N., Hakeda Y., Kumegawa M. Effect of chlorpromazine on PZ-peptidase and several other peptidase activities in cloned osteoblastic cells (MC3T3-E1) Biochem. Pharmacol. 1987;36:4319–4324. doi: 10.1016/0006-2952(87)90678-2. [DOI] [PubMed] [Google Scholar]
- Chu J.J., Ng M.L. Infectious entry of West Nile virus occurs through a clathrin-mediated endocytic pathway. J. Virol. 2004;78:10543–10555. doi: 10.1128/JVI.78.19.10543-10555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wilde A.H., Jochmans D., Posthuma C.C., Zevenhoven-Dobbe J.C., van Nieuwkoop S., Bestebroer T.M., van den Hoogen B.G., Neyts J., Snijder E.J. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob. Agents Chemother. 2014;58:4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinesh D.C., Tamilarasan S., Rajaram K., Bouřa E. Antiviral drug targets of single-stranded RNA viruses causing chronic human diseases. Curr. Drug Targets. 2020;21:105–124. doi: 10.2174/1389450119666190920153247. [DOI] [PubMed] [Google Scholar]
- Dyall J., Coleman C.M., Hart B.J., Venkataraman T., Holbrook M.R., Kindrachuk J., Johnson R.F., Olinger G.G., Jr., Jahrling P.B., Laidlaw M., Johansen L.M., Lear-Rooney C.M., Glass P.J., Hensley L.E., Frieman M.B. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob. Agents Chemother. 2014;58:4885–4893. doi: 10.1128/AAC.03036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall J., Gross R., Kindrachuk J., Johnson R.F., Olinger G.G., Jr., Hensley L.E., Frieman M.B., Jahrling P.B. Middle East respiratory syndrome and severe acute respiratory syndrome: Current therapeutic options and potential targets for novel therapies. Drugs. 2017;77:1935–1966. doi: 10.1007/s40265-017-0830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraris O., Moroso M., Pernet O., Emonet S., Rembert A.Ferrier, Paranhos-Baccalà G., Peyrefitte C.N. Evaluation of Crimean-Congo hemorrhagic fever virus in vitro inhibition by chloroquine and chlorpromazine, two FDA approved molecules. Antivir. Res. 2015;118:75–81. doi: 10.1016/j.antiviral.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filgueira L., Lannes N. Review of emerging Japanese Encephalitis Virus: New aspects and concepts about entry into the brain and inter-cellular spreading. Pathogens. 2019;8:111. doi: 10.3390/pathogens8030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filone C.M., Hanna S.L., Caino M.C., Bambina S., Doms R.W., Cherry S. Rift valley fever virus infection of human cells and insect hosts is promoted by protein kinase c epsilon. PloS One. 2010;5 doi: 10.1371/journal.pone.0015483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastaminza P., Whitten-Bauer C., Chisari F.V. Unbiased probing of the entire hepatitis C virus life cycle identifies clinical compounds that target multiple aspects of the infection. Proc. Natl. Acad. Sci. U.S.A. 2010;107:291–296. doi: 10.1073/pnas.0912966107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German Advisory Committee Blood (Arbeitskreis Blut) Subgroup ‘assessment of pathogens transmissible by blood’. Human immunodeficiency virus (HIV) Transfus. Med. Hemotherapy. 2016;43:203–222. doi: 10.1073/pnas.0912966107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., Mesplède T., Xu H., Quan Y., Wainberg M.A. The antimalarial drug amodiaquine possesses anti-ZIKA virus activities. J. Med. Virol. 2018;90:796–802. doi: 10.1002/jmv.25031. [DOI] [PubMed] [Google Scholar]
- Hendouei N., Saghafi F., Shadfar F., Hosseinimehr S.J. Molecular mechanisms of anti-psychotic drugs for improvement of cancer treatment. Eur. J. Pharmacol. 2019;856:172402. doi: 10.1016/j.ejphar.2019.05.031. [DOI] [PubMed] [Google Scholar]
- Hou Y., Wang Q. Emerging highly virulent porcine epidemic diarrhea virus: Molecular mechanisms of attenuation and rational design of live attenuated vaccines. Int. J. Mol. Sci. 2019;20:5478. doi: 10.3390/ijms20215478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data/historical (accessed April 2020)
- http://www.emro.who.int/pandemic-epidemic-diseases/mers-cov/mers-situation-update-january-2020.html (accessed April 2020)
- https://www.nhs.uk/conditions/sars/ (accessed April 2020)
- https://www.paho.org/data/index.php/en/?option=com_content&view=article&id=524:zika-weekly-en&Itemid=352 (accessed April 2020)
- https://www.who.int/activities/prioritizing-diseases-for-research-and-development-in-emergency-contexts (accessed April 2020)
- https://www.who.int/csr/don/02-April-2020-ebola-drc/en/ (accessed April 2020)
- https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200424-sitrep-95-covid-19.pdf?sfvrsn=e8065831_4 (accessed April 2020)
- https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed April 2020a)
- https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed April 2020b)
- https://www.who.int/news-room/fact-sheets/detail/japanese-encephalitis (accessed April 2020c)
- Inoue Y., Tanaka N., Tanaka Y., Inoue S., Morita K., Zhuang M., Hattori T., Sugamura K. Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. J. Virol. 2007;81:8722–8729. doi: 10.1128/JVI.00253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeeva S., Mir S., Velasquez A., Ragan J., Leka A., Wu S., Sevarany A.T., Royster A.D., Almeida N.A., Chan F., O'Brien L., Mir M.A. Crimean Congo hemorrhagic fever virus nucleocapsid protein harbors distinct RNA binding sites in the stalk and head domains. J. Biol. Chem. 2019;294:5023–5037. doi: 10.1074/jbc.RA118.004976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X., Li Z. Medicinal chemistry strategies toward host targeting antiviral agents. Med. Res. Rev. 2020 doi: 10.1002/med.21664. doi: 10.1002/med.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen L.M., DeWald L.E., Shoemaker C.J., Hoffstrom B.G., Lear-Rooney C.M., Stossel A., Nelson E., Delos S.E., Simmons J.A., Grenier J.M., Pierce L.T., Pajouhesh H., Lehár J., Hensley L.E., Glass P.J., White J.M., Olinger G.G. A screen of approved drugs and molecular probes identifies therapeutics with anti-Ebola virus activity. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.aaa5597. 290ra89. [DOI] [PubMed] [Google Scholar]
- Kimura H., Tsukagoshi H., Ryo A., Oda Y., Kawabata T., Majima T., Kozawa K., Shimojima M. Ebola virus disease: a literature review. J. Coast. Life Med. 2015;3:85–90. doi: 10.1016/S2221-1691(15)30341-5. [DOI] [Google Scholar]
- Lee N., Shum D., König A., Kim H., Heo J., Min S., Lee J., Ko Y., Choi I., Lee H., Radu C., Hoenen T., Min J.Y., Windisch M.P. High-throughput drug screening using the Ebola virus transcription- and replication-competent virus-like particle system. Antivir. Res. 2018;158:226–237. doi: 10.1016/j.antiviral.2018.08.013. [DOI] [PubMed] [Google Scholar]
- Li M., Zhang D., Li C., Zheng Z., Fu M., Ni F., Liu Y., Du T., Wang H., Griffin G.E., Zhang M., Hu Q. Characterization of Zika virus endocytic pathways in human glioblastoma cells. Front. Microbiol. 2020;11:242. doi: 10.3389/fmicb.2020.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima Neto A.S., Sousa G.S., Nascimento O.J., Castro M.C. Chikungunya-attributable deaths: A neglected outcome of a neglected disease. PLoS Neglected Trop. Dis. 2019;13(9) doi: 10.1371/journal.pntd.0007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Wang X.J. Potential inhibitors against 2019-nCoV coronavirus M protease from clinically approved medicines. J. Genet. Genomics. 2020;47:119–121. doi: 10.1016/j.jgg.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Xia S., Sun Z., Wang Q., Du L., Lu L., Jiang S. Testing of Middle East respiratory syndrome coronavirus replication inhibitors for the ability to block viral entry. Antimicrob. Agents Chemother. 2015;59:742–744. doi: 10.1128/AAC.03977-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M., De Meester I., Scharpe S., Desnyder R., Ranjan R., Meltzer H.Y. Alterations in plasma dipeptidyl peptidase IV enzyme activity in depression and schizophrenia: effects of antidepressants and antipsychotic drugs. Acta Psychiatr. Scand. 1996;93:1–8. doi: 10.1111/j.1600-0447.1996.tb10612.x. [DOI] [PubMed] [Google Scholar]
- Mani D., Wadhwani A., Krishnamurthy P.T. Drug repurposing in antiviral research: A current scenario. J. Young Pharm. 2019;11:117–121. doi: 10.1038/s42003-020-1088-9. [DOI] [Google Scholar]
- Mansfield K.L., Banyard A.C., McElhinney L., Johnson N., Horton D.L., Hernández-Triana L.M., Fooks A.R. Rift Valley fever virus: A review of diagnosis and vaccination, and implications for emergence in Europe. Vaccine. 2015;33:5520–5531. doi: 10.1016/j.vaccine.2015.08.020. [DOI] [PubMed] [Google Scholar]
- Nawa M., Takasaki T., Yamada K.I., Kurane I., Akatsuka T. Interference in Japanese encephalitis virus infection of Vero cells by a cationic amphiphilic drug, chlorpromazine. J. Gen. Virol. 2003;84:1737–1741. doi: 10.1099/vir.0.18883-0. [DOI] [PubMed] [Google Scholar]
- Pillaiyar T., Manickam M., Jung S.H. Middle east respiratory syndrome-coronavirus (MERS-CoV): An updated overview and pharmacotherapeutics. Med. Chem. 2015;5:361–372. doi: 10.4172/2161-0444.1000287. [DOI] [Google Scholar]
- Pillaiyar T., Meenakshisundaram S., Manickam M. Recent discovery and development of inhibitors targeting coronaviruses. Drug Discov. Today. 2020;25:668–688. doi: 10.1016/j.drudis.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohjala L., Utt A., Varjak M., Lulla A., Merits A., Ahola T., Tammela P. Inhibitors of alphavirus entry and replication identified with a stable Chikungunya replicon cell line and virus-based assays. PloS One. 2011;6 doi: 10.1371/journal.pone.0028923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajapat M., Sarma P., Shekhar N., Avti P., Sinha S., Kaur H., Kumar S., Bhattacharyya A., Kumar H., Bansal S., Medhi B. Drug targets for corona virus: A systematic review. Indian J. Pharmacol. 2020;52:56–65. doi: 10.4103/ijp.IJP_115_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchades, Renau L., Berenguer M. Introduction to hepatitis C virus infection: Overview and history of hepatitis C virus therapies. Hemodial. Int. 2018;22:S8–S21. doi: 10.1111/hdi.12647. [DOI] [PubMed] [Google Scholar]
- Sahin A.R., Erdogan A., Agaoglu P.Mutlu, Dineri Y., Cakirci A.Y., Senel M.E., Okyay R.A., Tasdogan A.M. 2019 Novel coronavirus (COVID-19) outbreak: A Review of the current literature. EJMO. 2020;4:1–7. doi: 10.14744/ejmo.2020.12220. [DOI] [Google Scholar]
- Sahoo M., Jena L., Daf S., Kumar S. Virtual screening for potential inhibitors of NS3 protein of Zika virus. Genomics Inform. 2016;14:104–111. doi: 10.5808/GI.2016.14.3.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz M., Schmoldt A. Therapeutic and toxic blood concentrations of more than 800 drugs and other xenobiotics. Pharmazie. 2003;58:447–474. [PubMed] [Google Scholar]
- Simanjuntak Y., Liang J.J., Lee Y.L., Lin Y.L. Repurposing of prochlorperazine for use against Dengue virus infection. J. Infect. Dis. 2015;211:394–404. doi: 10.1093/infdis/jiu377. [DOI] [PubMed] [Google Scholar]
- Song Z., Xu Y., Bao L., Zhang L., Yu P., Qu Y., Zhu H., Zhao W., Han Y., Qin C. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019;11:59. doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga B., Csonka Á., Csonka A., Molnár J., Amaral L., Spengler G. Possible biological and clinical applications of phenothiazines. Anticancer Res. 2017;37:5983–5993. doi: 10.21873/anticanres.12045. [DOI] [PubMed] [Google Scholar]
- Wahlbeck K., Ahokas A., Nikkilä H., Miettinen K., Rimón R. A longitudinal study of cerebrospinal fluid angiotensin-converting enzyme in neuroleptic-treated schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 1997;21:591–599. doi: 10.1016/s0278-5846(97)00034-1. [DOI] [PubMed] [Google Scholar]
- Wang H., Yang P., Liu K., Guo F., Zhang Y., Zhang G., Jiang C. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18:290–301. doi: 10.1038/cr.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X., She G., Wu T., Xue C., Cao Y. PEDV enters cells through clathrin-, caveolae-, and lipid raft-mediated endocytosis and traffics via the endo-/lysosome pathway. Vet. Res. 2020;51:10. doi: 10.1186/s13567-020-0739-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winek C.L., Wahba W.W., Winek C.L., Jr., Balzer T.W. Drug and chemical blood level data 2001. Forensic Sci. Int. 2001;122:107–123. doi: 10.1016/s0379-0738(01)00483-2. [DOI] [PubMed] [Google Scholar]
- Wong K.Z., Chu J.J.H. The interplay of viral and host factors in chikungunya virus infection: Targets for antiviral strategies. Viruses. 2018;10:294. doi: 10.3390/v10060294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.N.Y., Atkinson S.C., Fraser J.E., Wang C., Maher B., Roman N., Forwood J.K., Wagstaff K.M., Borg N.A., Jans D.A. Novel falvivirus antiviral that targets the host nuclear transport importin α/β1 heterodimer. Cells. 2019 doi: 10.3390/cells8030281. [DOI] [PMC free article] [PubMed] [Google Scholar]