Abstract
Metabolic syndrome comprises a cluster of metabolic disorders related to the development of cardiovascular disease and type 2 diabetes mellitus. In latter years, plant secondary metabolites have become of special interest because of their potential role in preventing and managing metabolic syndrome. Sesquiterpene lactones constitute a large and diverse group of biologically active compounds widely distributed in several medicinal plants used for the treatment of metabolic disorders. The structural diversity and the broad spectrum of biological activities of these compounds drew significant interests in the pharmacological applications. This review describes selected sesquiterpene lactones that have been experimentally validated for their biological activities related to risk factors of metabolic syndrome, together with their mechanisms of action. The potential beneficial effects of sesquiterpene lactones discussed in this review demonstrate that these substances represent remarkable compounds with a diversity of molecular structure and high biological activity, providing new insights into the possible role in metabolic syndrome management.
Keywords: Sesquiterpene lactones, Medicinal plants, Metabolic syndrome, Hypoglycemic, Hypolipidemic, Antidiabetic
Abbreviations: ACE, angiotensin I-converting enzyme; AMPK, activated protein kinase; APOC3, apolipoprotein C3; AT, adipose tissue; CAT, catalase; COX-2, cyclooxygenase 2; CVD, cardiovascular disease; FFA, free fatty acids; FN, fibronectin; G6Pase, glucose-6-phosphatase; GK, glucokinase; GPx, glutathione peroxidase; GSH, reduced glutathione; HDL-C, high-density lipoproteins-cholesterol; IFN-γ, interferon gamma; IL-1β, interleukin 1 beta; IL-6, interleukin 6; iNOS, inducible nitric oxide synthase; IR, insulin resistance; JNK, c-Jun N-terminal kinases; LDL-C, low-density lipoprotein-cholesterol; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinases; MCP-1, monocyte chemoattractant protein 1; MetS, metabolic syndrome; NF-κB, nuclear factor kappa B; NO, nitric oxide; ROS, reactive oxygen species; SLns, sesquiterpene lactones; SOD, superoxide dismutase; STAT1, signal transducer and activator of transcription 1; STZ, streptozotocin; T2DM, type 2 diabetes mellitus; TBARS, thiobarbituric acid reactive substances; TC, total cholesterol; TG, triglycerides; TGF-β1, transforming growth factor beta; TLRs, Toll-like receptor; TNF-α, tumor necrosis factor alpha; VLDL, very-low-density lipoprotein
1. Introduction
Metabolic syndrome (MetS) refers to a cluster of risk factors related to the development of cardiovascular disease (CVD) and type 2 diabetes mellitus (T2DM) (Alberti et al., 2009). This pathological condition represents a public health concern in most nations due to its association with mortality caused by the cardiovascular and metabolic complications (Ju et al., 2017). Indeed, MetS is recognized as a risk factor that influences progression and prognosis of coronavirus disease 2019, the infectious disease caused by the most recently discovered coronavirus SARS-CoV-2 (Costa et al., 2020). Lifestyle modifications remain the primary component of MetS´s risk factors reduction and pharmacological therapies are indicated in special situations to improving more than one factor (Larsen et al., 2018). In latter years, secondary plant metabolites have become of special interest in the scientific community because of their potential role in preventing and managing MetS (Cicero and Colletti, 2016; Francini-Pesenti et al., 2019). Many compounds, such as sesquiterpene lactones (SLns), have been isolated from medicinal plants and their hypoglycemic, anti-inflammatory, and antioxidative properties coupled with their capacity to modulate key cellular functions have been confirmed by in vitro and in vivo methods (Chadwick et al., 2013; Chaturvedi et al., 2016; Alonso et al., 2018). Despite these effects are linked to the pathogenesis of MetS, the role of these compounds to avoid its progression is not well documented compared to many other compounds such as polyphenols into which a great deal of research has been conducted. Therefore, in order to provide relevant information regarding the potential benefits of SLns in preventing and managing MetS, this review addresses plant-derived SLns that are potentially responsible for the positive effect in improving risk factors associated with MetS.
2. Search strategy
An electronic literature search was conducted on SLns with hypoglycemic and/or hypolipidemic effects and isolated from plants used in traditional medicine for the same purposes. The search was done in databases of Google Scholar, Science Direct, and SciFinder until June 2020 using the keyword sesquiterpene lactones and following terms: metabolic syndrome, hypoglycemic, hypolipidemic, antidiabetic, antihypertensive, and medicinal plants. Additional information was identified from references located in the retrieved articles. Although many SLns were screened in some of the studies, only those considered active by the authors are included in this review. The plants included in this review were selected based on their ethnomedicinal records. For each species, the currently accepted name in the online “The Plant List” database has been checked and the reported name has been replaced with the current one. Compound class, plant source, biological activity and important aspects related with MetS are summarized in the Table 1 .
Table 1.
Sesquiterpene lactones with potential positive effects on MetS, biological effects and mechanisms implicated (structures illustrated in Figs. 1 and 2).
| No. | Compound | Class | Plant source | Biological effects | Mechanisms implicated | Refs. |
|---|---|---|---|---|---|---|
| 1 | Enhydrin | Melampolide | Smallanthus sonchifolius | Hypoglycemic (in vivo) | Post-prandial glucose levels (↓) Inhibition of α-glucosidase | Genta et al., 2010; Serra-Barcellona et al., 2017 |
| 2 | Polymatin A | Melampolide | Smallanthus macroscyphus | Hypoglycemic (in vivo) | Post-prandial glucose levels (↓) | Serra-Barcellona et al., 2014 |
| 3 | 20-dehydroeucannabinolide | Heliangolide | Helianthus annuus | Antidiabetic (in vivo) Antioxidant (in vitro) | Fasting blood glucose level (↓) DPPH radical and NO scavenging activities | Onoja et al., 2020 |
| 4 | Eremanthin | Guaianolide | Costus speciosus | Hypoglycemic, and hypolipidemic (in vivo) Antioxidant (in vivo) | Blood glucose levels (↓), HbA1c (↓), plasma insulin (↑), tissue glycogen (↑) TC, TG and LDL-C (↓); HDL-C (↑) TBARS levels (↓), GSH (↑), SOD, CAT and GPx (↑) in brain, liver, heart, kidney, and pancreas | Eliza et al., 2009a, 2009b, 2010 |
| 5 | Costunolide | Germacrolide | Costus speciosus | |||
| 6 | Alantolactone | Eudesmanolide | Inula helenium | Antiinflammatory-associated to glucose intolerance and IR (in vitro) Antiinflamatory- obesity-induced IR (in vitro) Attenuates lipid accumulation (in vitro) Antiinflamatory- associated to diabetic nephropathy (in vivo) | STAT3 inhibitor. Inhibition of the TLR4 gene expression. Inhibition of TLR4-JNK signaling. IL-6 and MCP-1 (↓). APOC3 expression at both mRNA and protein levels (↓) TNF-α and IL-6 (↓) in diabetic kidney. Serum creatinine and urea nitrogen levels (↓). | Kim et al., 2017a, 2017b; Yang et al., 2018; Zhu et al., 2020 |
| 7 | Tirotundin | 3,10-epoxygermacranolide | Tithonia diversifolia | Antidiabetic (in vitro) | Dual PPARα/γ agonists | Lin, 2012 |
| 8 | Tagitinin A | |||||
| 9 | Tagitinin G | Anti-hyperglycemic (in vitro) | Glucose uptake in 3T3-L1 adipocytes (↑). | Zhao et al., 2012 | ||
| 10 | Tagitinin I | |||||
| 11 | 1β-hydroxydiversifolin-3-O-methyl ether | |||||
| 12 | 1β-hydroxytirotundin-3-O-methyl ether | |||||
| 13 | Micheliolide | Guaianolide | Magnolia compressa | Anti-inflammatory- associated to diabetic nephropathy (in vitro) Anti-hepatic steatosis (in vivo and in vitro) |
Suppressed the glucose-stimulated degradation of IκBα and the subsequent activation of NF-κB in rat glomerular mesangial cells. MCP-1, TGF-β1 and FN (↓) PPAR-γ expression (↑) AMPK/mTOR signaling pathway (↑) NF-кB signaling pathway (↓) |
Jia et al., 2013; Zhong et al., 2018 |
| 14 | Byrsonine A | Guaianolide dimer | Byrsonima crassifolia | Hypoglycemic, hypolipidemic and antioxidant (in vivo) | Blood glucose levels (↓), serum insulin and pancreatic insulin levels (↑) G6Pase activity (↓) and GK activity (↑) TC, TG, LDL-C and VLDL (↓); HDL-C (↑) TBARS levels (↓), SOD, CAT and GPx (↑) in liver, kidneys, and pancreas TNF-α levels (↓). |
Gutiérrez and Ramirez, 2016 |
| 15 | Byrsonine B | |||||
| 16 | Lactucain C | Guaianolide dimer | Lactuca indica | Antidiabetic (in vivo) | Blood glucose levels (↓) | Hou et al., 2003 |
| 17 | 8-deoxylactucin | Guaianolide | Cichorium intybus | Anti-inflammatory | Inhibition of NF-kB activation. COX-2 inhibition. |
Cavin et al., 2005 |
| 18 | Artemisinin | Cadinanolide | Artemisia annua | Vascular protection (in vivo) Antidiabetic (in vivo) |
MCP-1, IFN-γ, IL-6 and TNF-α (↓) Inhibition of atherosclerotic plaque formation Promote the conversion of pancreatic glucagon-producing α cells to insulin-secreting β cells |
Cao et al., 2020; Li et al., 2017 |
| 19 | Scoporanolide | Guaianolide | Artemisia scoparia | Antihypertensive (in vivo) | Inhibition of plasma ACE activity | Cho et al., 2016 |
| 20 | Estafiatin | |||||
| 21 | Cumambrin A | Guaianolide | Chrysanthemum boreale | Antihypertensive (in vivo) Vasorelaxant (ex vivo) |
Normalization of blood pressure | Hong et al., 1999, 2005 |
Reduction (↓); increment (↑); thiobarbituric acid reactive substances (TBARS); reduced glutathione (GSH); superoxide dismutase (SOD); catalase (CAT); glutathione peroxidase (GPx); insulin resistance (IR); Toll-like receptor 4 (TLR4); c-Jun N-terminal kinases (JNK); interleukin 6 (IL-6); monocyte chemoattractant protein 1 (MCP-1); Apolipoprotein C3 (APOC3); AMP-activated protein kinase (AMPK); peroxisome proliferator-activated receptors α and γ (PPARα/γ); nuclear factor kappa B (NF-κB); transforming growth factor beta (TGF-β1); fibronectin (FN); glucose-6-phosphatase (G6Pase); glucokinase (GK); cyclooxygenase 2 (COX-2); interferon-gamma (IFN-γ); tumor necrosis factor α (TNF-α); angiotensin I-converting enzyme (ACE).
3. Metabolic syndrome
MetS is defined as a cluster of risk factors such as raised blood pressure, atherogenic dyslipidemia, raised fasting glucose, and central obesity, related to the development of CVD and T2DM (Alberti et al., 2009). Since the World Health Organization (WHO) reported the first formalized definition of the MetS, the diagnostic criteria have been sequentially developed by several public health organizations (Engin, 2017). Nowadays, the most recognized criterion for the clinical diagnosis of MetS (Alberti et al., 2009) is based on identifying at least three of the following risk factors: (1) elevated waist circumference (population and country-specific definitions determined by local organizations); (2) triglycerides (TG) ≥ 150 mg/Dl (<1.0 mmol/L) or on drug treatment for elevated triglycerides; (3) high-density lipoproteins-cholesterol (HDL-C) <40 mg/dL (<1.0 mmol/L) in men or <50 mg/dL (<1.3 mmol/L) in women or on drug treatment for reduced HDL-C; (4) blood pressure ≥130/85 mmHg or on antihypertensive medication treatment, and/or a history of hypertension; and (5) fasting glucose >100 mg/dL (>5.6 mmol/L) or on drug treatment for elevated glucose.
4. Pathogenesis of MetS
The mechanisms underlying pathogenesis of MetS have not been completely explored, but obesity-induced adipocyte dysfunction and inflammation is associated with the progression of insulin resistance and metabolic disorders (Klöting and Blüher, 2014). Body fat mass accumulation in obesity depends on different factors including the relationship of energy intake to energy expenditure and body energy storage (Gomez-Hernandez et al., 2016). Adipose tissue (AT) plays an important role as an energy storage organ, as well as an endocrine organ produces adipokines such as leptin, adiponectin, monocyte chemoattractant protein 1 (MCP1), tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6), which circulate and regulate systemic metabolism and inflammation. The cell type composition of AT includes adipocytes, fibroblasts, macrophages, stromal cells, monocytes and preadipocytes (Ràfols, 2014). On further AT expansion, hypertrophy of adipocytes and increased secretion of macrophage chemoattractants occurs, including the secretion of MCP-1, which recruits additional macrophages. These actions in turn result in local inflammatory state, enhanced basal lipolysis, increasing the leakage of free fatty acids (FFA) and a dysregulated secretion of several pro-inflammatory adipokines (Longo et al., 2019; Xu et al., 2019). Subsequently, these adverse signals reach metabolic tissues (e.g. liver, pancreatic islets, and skeletal muscle) and modify inflammatory responses as well as glucose and lipid metabolism, thereby contributing to a global metabolic effect of insulin resistance.
Chronic low-grade inflammation in obesity results from the activation of various inflammatory mechanisms through nuclear factor kappa B (NF-κB) and c-Jun N-terminal kinases (JNK) pathways. These pathways represent important modulators of cytokine gene expression downstream of Toll-like receptors (TLRs) in insulin target cells (Catrysse and van Loo, 2017; Rogero and Calder, 2018). NF-κB is an important transcription factor involved in different processes of the immune and inflammatory responses. It is composed of p50 and p65 subunits and is found in the cytoplasm in complex with inhibitory proteins of the IκB family. Cytokines and lipopolysaccharide (LPS) can stimulate cell surface receptors including Toll-like receptor (TLR4) to initiate a signaling cascade that converge on the activation of the inhibitor of κB kinase (IKK) complex (Baker et al., 2011). The IKK complex phosphorylates IκBα and induces its degradation, leading to the release and nuclear translocation of NF-κB to promote transcription of target genes such as TNF-α, interleukin-1beta (IL-1β), IL-6, IL-8, cyclooxygenase 2 (COX-2) and inducible nitric oxide synthase (iNOS) (Knab et al., 2014; Ruan et al., 2011).
On the other hand, JNK are members of the mitogen-activated protein kinases (MAPK) that mediate cellular responses to a variety of intra- and extracellular stresses (Zeke et al., 2016). In the context of obesity, JNK pathways can be activated by proinflammatory cytokines, FFA and reactive oxygen species (ROS), and regulate several nuclear and extra-nuclear substrates, specially the transcription factor activator protein 1 (AP1) which controls the expression of proinflammatory genes and protein synthesis (e.g. TNF-α, IL-1β, IL-6 and IL-8) (Pal et al., 2016; Feng et al., 2020). Deregulated activation of NF-κB and JNK pathways results in increased transcription of IL-6 and TNF-α, which reduce the sensitivity of insulin target cells towards insulin. Thus, chronically activated NF-κB and JNK pathways leads to the promotion of insulin resistance (Yung and Giacca, 2020).
Additionally, increased flux of FFA to the liver in the insulin resistant state stimulates production TG and secretion in the form of very-low-density lipoprotein (VLDL). The resulting hypertriglyceridemia leads to lower HDL‐C levels and normal or slightly elevated low-density lipoprotein-cholesterol (LDL-C) levels (Iqbal et al., 2018). This is related to the typical dyslipidemic profile in MetS.
Consistent with a potential role in the pathogenesis of MetS, SLns interfering with these processes described above could be useful to prevent the onset of insulin resistance and the risk of T2DM and CVD in obese individuals.
5. Sesquiterpene lactones
SLns represent a diverse group of terpenoids with more than 5000 different elucidated structures. They are particularly diversified in the Compositae (Asteraceae) family, but also occurring in Apiaceae, Illiciaceae, Magnoliaceae, Solanaceae, and Euphorbiaceae families (Padilla-Gonzalez et al., 2016). Numerous species of these families are used in traditional medicine and SLns have been described as their primary active constituents. These compounds possess several biological activities such as anti-inflammatory, antidiabetic, antimalarial, anti-proliferative, anti- parasitic and antimicrobial (Merfort, 2011; Chadwick et al., 2013; Chaturvedi et al., 2011).
SLns are characterized by the presence of a γ-lactone ring. The lactone ring can be fused to the remaining skeleton in either a cis or trans configuration, being most common the trans configuration (Fischer et al., 1979; Fischer, 1990; Sülsen and Martino, 2018). Based on their carbocyclic skeleton, SLns are mainly classified in four major groups: germacranolides (10-membered ring), eudesmanolides (6–6 bicyclic compounds), guaianolides and pseudoguaianolides (5–7 bicyclic compounds) and many subtypes of SLns according to their skeletal arrangement (Adekenov, 2017; Padilla-Gonzalez et al., 2016). In many cases, the γ-lactone ring contains an exocyclic double bond conjugated to the carbonyl group (α-methylene-γ-lactone) but in some cases the exocyclic methylene is reduced or the double bond can be endocyclic (Martínez et al., 2012; Padilla-Gonzalez et al., 2016; Sülsen et al., 2018). It has been well documented that biological activity of the most common types of SLns is mainly attributed to formation of covalent union between the α,β-unsaturated group in the exo-methylene-γ-lactone and nucleophilic biological targets (e.g. free cysteine sulfhydryl) resulting in alkylation through Michael-type addition (Schmidt, 2006). This chemical reaction induces steric and chemical changes in enzymes, receptors or transcriptional factors leading to a series of cellular events that culminate in diverse biologic responses. The number of alkylating structural elements present on the molecule define differences between the activity of each SLn. Another structural influences on the biological effects are the molecular size, hydrophobicity, chemical environment and the presence of other functional groups (e.g., epoxy groups, hydroxyls or hydroxyls esterified with acetate, propionate, isobutyrate, angelate, epoxyangelate, and benzoate) (Chaturvedi, 2011; Ivanescu et al., 2015; Padilla-Gonzalez et al., 2016; Sülsen et al., 2018).
The structural diversity and the broad spectrum of biological activities drew significant interests in the pharmacological applications of SLns (Moujir et al., 2020). Many SLns have proved to have a significant anti-inflammatory, hypoglycemic and hypolipidemic activity therefore making them attractive for MetS therapy (Chaturvedi, 2011; Chaturvedi et al., 2016).
6. SLns isolated from medicinal plants and their potential role for MetS treatment
6.1. Enhydrin from Smallanthus sonchifolius (Poepp.) H.Rob
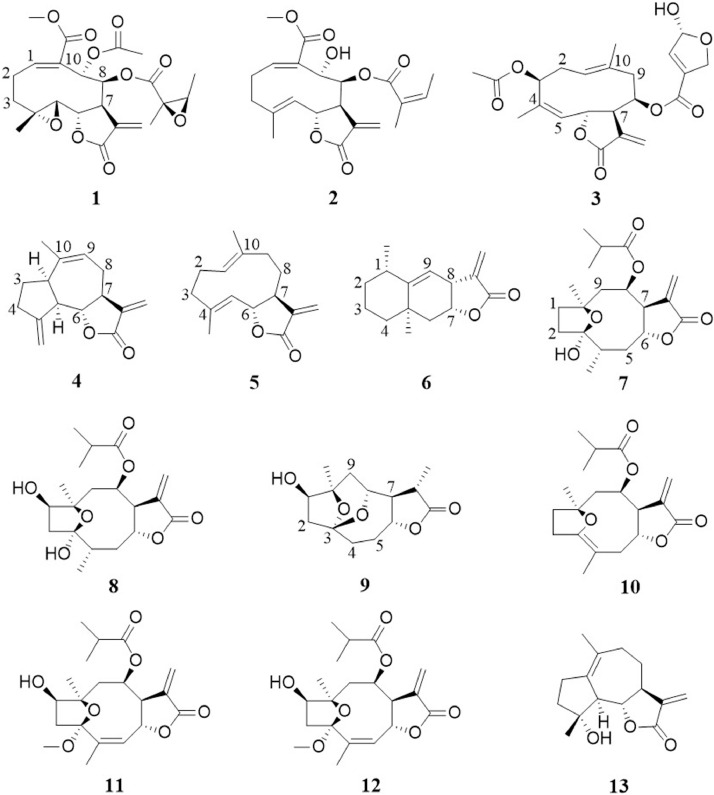
Smallanthus sonchifolius (Poepp.) H.Rob., commonly known as yacon, is a perennial herbaceous plant native to the Andean regions of South America (Caetano et al., 2016). Yacon is consumed as a safety dietary supplement and because of its low glucose content and high fructooligosaccharide levels putative antidiabetic effects were suggested (Delgado et al., 2013). Indeed, this suggestion is supported by the hypoglycemic (Aybar et al., 2001; Baroni et al., 2008) and hypolipidemic (Miura et al., 2004; Habib et al., 2011) reported activities. Also, a potential use in metabolic disorders could be proposed based on the anti-inflammatory and antioxidant properties (Feltenstein et al., 2004; Sousa et al., 2015). Besides the above mentioned evidences, enhydrin (1), a melampolide (cis,trans-germacranolide) and the major sesquiterpene lactone component in leaves of S. sonchifolius, has proven to be effective reducing post-prandial glucose levels and a useful compound for blood glucose control by the induction of a late increase in plasma insulin in streptozotocin (STZ)-induced diabetic rats (Genta et al., 2010). More recently, in vivo and in vitro experiments showed that enhydrin (1) is effective in the management of post-prandial hyperglycemia through inhibition of α-glucosidase in the small intestine (Serra-Barcellona et al., 2017). The inhibition of this enzyme has been linked to the presence of the α,β-unsaturatedγ-lactone ring system (Yin et al., 2014), similar to the intact exo-methylene-γ-lactone group in 1 (Fig. 1 ). Thus, the anti- α-glucosidase activity of enhydrin could be derived from this interaction. In addition, the alkylation of nucleophilic sites of factors involved in early stages of the inflammatory cascade described above allows to relate the anti-inflammatory properties reported for 1 with the presence of the exo-methylene-γ-lactone ring system (Feltenstein et al., 2004; Ma et al., 2007).
Fig. 1.
Chemical structures of compounds 1-13.
Regarding toxicological studies, it has been reported that a single oral administration of enhydrin (1) at doses of 1.6, 4 and 8 mg/kg body weight (b.w.) did not caused deaths or acute toxic effects within 7 days in male and female rats (Genta et al., 2010). In acute study in rats, there were no deaths or signs of toxicity observed after single oral administration of 1 at any dose level up to the highest dose tested (0.32 g/kg b.w.) within 14 days. In subchronic studies, after oral administration for 90 days at daily doses of 0.4, 0.8 and 8.0 mg/kg b.w., did not caused hematological, biochemical and histological alterations in rats (Serra et al., 2012).
6.2. Polymatin A from Smallanthus macroscyphus (Baker ex Baker) A. Grau
Smallanthus macroscyphus (Heliantheae, Asteraceae), is a perennial herb commonly known as ‘‘wild yacon’’ native from the South American region comprising from southern Bolivia to northwestern Argentina. This species is closely related to S. sonchifolius and possibly with similar antidiabetic properties. Polymatin A (2) is the main sesquiterpene lactone isolated from S. macroscyphus (De Pedro et al., 2003). This melampolide exerts an effective inhibition of post-prandial blood glucose peak and hypoglycemic activity in STZ-diabetic rats probably by the stimulation of insulin release or due to an insulin-like effect (Serra-Barcellona et al., 2014). Due to the close relationship in the polymatin A (2) and enhydrin (1) structures, the melampolide 2 could be effective in the management of postprandial hyperglycemia through inhibition of α-glucosidase. However, no studies of these effects were found.
In contrast to the C4–C5 epoxy group in enhydrin (1), polymatin A (2) presents a double bond (Fig. 1). C4–C5 epoxy groups in melampolides such as enhydrin have been reported to hinder the ability of these compounds to inhibit NF-κB DNA binding responsible to cytokines expression (Schorr et al., 2007) commonly observed in inflammatory response. Conversely, the effect is favored by the presence of a double bond between C-4–C-5 in other melampolides (Schorr et al., 2007). Hence, hopeful results could be expected in future studies to explore anti-inflammatory activity in polymatin A.
Acute oral toxicity of polymatin A (2) in normal healthy rats at doses assayed (0.7, 1.4 and 2.8 g dried powder/kg b.w.) do not produced deaths or acute toxic effect (changes in behavior or posture, presence of convulsions or occurrence of secretions) within 14 days. The doses were well tolerated and did not produce adverse nutritional effect. No gastrointestinal symptom such as diarrhea or constipation were observed at the doses assayed. Volume, pH and urine specific gravity were within normal ranges. No nitrites, protein or blood were detected in the urine samples of the animal groups treated (Serra-Barcellona et al., 2014).
In subchronic studies, polymatin A (2) was orally administered to Wistar rats for 90 days at daily doses of 7, 14 and 28 mg/kg b.w. No toxicity signs or deaths were observed. There were no changes in the behavior, body or organ weights, hematological, biochemical or urine parameters of the rats. No histopathological lesions were observed in the examined organs. The results indicate that polymatin A (2) from S. macroscyphus leaves may be considered as nontoxic substance at a wide range of doses (Serra-Barcellona., 2016).
6.3. 20-dehydroeucannabinolide from Helianthus annuus Linn
The common sunflower, Helianthus annuus Linn (Asteraceae), is a well-known plant with edible seeds, flower petals and tender leaf petioles. This plant was proposed to offer a variety of medical benefits (Lim, 2014; Guo et al., 2017). To provide a scientific explanation for its use in Nigerian traditional medicine, Onoja and Anaga (2014) reported a hypoglycemic effect on the fasting blood glucose level in alloxan-induced diabetic rats after a single dose of the methanolic extract of H. annuus leaves. This study led to the recent isolation of the heliangolide 20-dehydroeucannabinolide (3) (Fig. 1). (Onoja et al., 2020). Heliangolide SLns represent an isomeric form of germacranolides with a cyclodecadiene skeleton and double bonds at C1=C10 and C4=C5, which stereochemical configurations are trans, cis respectively (Sülsen and Martino, 2018). The heliangolide 3 reduced the fasting blood glucose level at dose of 0.2 mmol/kg in alloxan-induced diabetic rats (Onoja et al., 2020). The authors suggested that this sesquiterpene lactone might have reducing effects in glucose absorption based on the heliangolide derivative structure, which presents an intact exo-methylene-γ-lactone group. As mentioned above, α,β-unsaturated γ-lactone ring system has an important relation in the inhibition of α-glucosidase activity, thus the heliangolide 3 may be inhibiting this enzyme similar to enhydrin (1).
In addition to blood glucose regulation, it has also been demonstrated the positive effects of hydromethanolic leaf extract of H. annuus L. on other components of MetS, including reduced LDL-C and TG levels as well as hepatoprotective and antilipid peroxidation activities of alloxan-induced diabetic rats (Onoja et al., 2018). Since 20-dehydroeucannabinolide (3) is one of the major components of the extract, potential activity of this molecule in managing dyslipidemia may be proposed. However, additional studies are needed to ascertain the hypolipidemic property of this compound.
No in vivo toxicity studies on 20-dehydroeucannabinolide (3) have been reported to date.
6.4. Costunolide and eremanthin from Costus speciosus (J. Koenig) Sm
Costus speciosus (J. Koenig) Sm is a plant used in traditional medicine in South Asia to treat diabetic patients by eating one leaf of this species per day to regulate blood glucose levels (Waisundara et al., 2015). Also, the hypoglycemic effect of C. speciosus root crude extracts have been reported (Daisy et al., 2008). Eremanthin (4) (Fig. 1) is a guaianolide sesquiterpene lactone present in C. speciosus and other plants such as Pterodon pubescens (Benth.) Benth., Eremanthus elaeagnus (Mart. ex DC.) Sch.Bip. and Laurus nobilis L. (Eliza et al., 2009a; Waisundara et al., 2015). Another sesquiterpene lactone reported to be present in C. speciosus is the germacrolide (a trans,trans-germacranolide) costunolide (5) (Fig. 1) (Eliza et al., 2009b), which has been also isolated from Magnolia spp, Laurus nobilis and Saussurea costus (Falc.) Lipsch (Koo et al., 2001).
Studies in STZ-induced diabetic rats showed that oral administration of 4 and 5 decreased plasma glucose level, glycosylated hemoglobin (HbA1c), total cholesterol (TC), TG, LDL-C and at the same time markedly increased plasma insulin, tissue glycogen and HDL-C (Eliza et al., 2009a, 2009b). The specific mechanism of eremanthin (4) bioactivity is not completely characterized, but the experimental model used in both works allowed the authors to suggest that this molecule might exert an insulin-like effect on peripheral tissues by either promoting glucose uptake metabolism, by inhibiting hepatic gluconeogenesis or by absorption of glucose into the muscle and adipose tissues through the stimulation of a regeneration process and revitalization of the remaining β cells (Eliza et al., 2009a, 2009b).
In addition, costunolide (5) has shown to suppress LPS-induced NF-κB activation that leads to the suppression of iNOS and a consequent nonproduction of NO. This activity was stronger than the observed for parthenolide, a germacranolide sesquiterpene lactone (Koo et al., 2001). Thus, the anti-inflammatory activity exhibited by 5 serve as a promising and expanding strategy for treatment of metabolic disease-associated inflammation.
On the other hand, the possible inhibition of inflammatory processes has not been studied in eremanthin (4). However, this compound has a rigid skeleton and an intact exo-methylene-γ-lactone group, previously related to anti-inflammatory properties (Simonsen et al., 2013). Additionally, the absence of free hydroxyl groups in 4 could help to improve anti-inflammatory properties since an increasing number of free hydroxyl groups are reported to diminish NF-κB inhibition activity (Simonsen et al., 2013). Taking together these structural characteristics, we suggest that eremathin (4) is a candidate to be tested for inhibition of inflammatory processes related to metabolic diseases.
Acute oral toxicity of eremanthin (4) and costunolide (5) in normal healthy male Wistar rats at doses assayed (10, 20, 40, 80 and 160 mg/kg b.w.) do not produced behavioral changes on the rats and mortality was not observed during acute toxicity test (10 days) (Eliza et al., 2010).
Finally, the antioxidant activity of eremanthin (4) and costunolide (5) has been demonstrated by significantly decreasing of the thiobarbituric acid reactive substances (TBARS) and lipid peroxidation markers levels as well as by increasing reduced glutathione (GSH) levels and enzymatic antioxidants (Eliza et al., 2010). This activity could indicate a protective effect of both on oxidative stress in diabetes.
6.5. Alantolactone from Inula spp
Inula (Asteraceae) is one of the most popular herbs in Traditional Chinese Medicine. This genus comprises more than one hundred species widely distributed throughout Asia, Africa, and Europe, and many of these have been used to treat a wide range of diseases such as bronchitis, diabetes, obesity, hypertension, and inflammation (Seca et al., 2014). Extracts of Inula spp. such as Inula britannica L., Inula viscosa [the accepted name is Dittrichia viscosa (L.) Greuter.], Inula racemosa Hook.f., Inula helenium L., etc., have been documented for their potential effects on some components of MetS, especially hypoglycemic, hypolipidemic and antioxidant activities (Kobayashi et al., 2002; Zeggwagh et al., 2006; Shan et al., 2006; Ajani et al., 2009) as well as inhibition of α-glucosidase enzyme (Orhan et al., 2017). Regarding bioactive secondary metabolites, SLns represent the largest group of compounds occurring in Inula spp. Many of these compounds were isolated from plants mentioned above and demonstrated to exert diverse biological activities (Seca et al., 2014; Wang et al., 2014). The anti-inflammatory mechanisms of some SLns have been related to their influence on directly inhibit the MAPK and block the activation of NF-κB, thus achieving the prevention of pro-inflammatory signaling (Han et al., 2001; Park et al., 2013).
Alantolactone (6) (Fig. 1) is the most known eudesmanolide present in several Inula species, including Inula helenium L. used for hypoglycemic and antiobesity purposes (Seca et al., 2014; Wang et al., 2014). This compound has been extensively studied for its antitumor, antioxidant and anti-inflammatory effects (Moujir et al., 2020). Regarding anti-inflammatory activity, the eudesmanolide 6 suppresses NO, PGE2 and TNF-α production in LPS-stimulated RAW 264.7 cells by modulating the activity of the NF-κB and MAPK signaling pathways (Chun et al., 2012), and it also suppresses TNF-α and interferon gamma (IFN-γ)-induced production of chemokines by blocking the Signal Transducer and Activator of Transcription 1 (STAT1) phosphorylation in HaCaT cells (Lim et al., 2015).
As previously mentioned, it is recognized the fact that TLRs are responsible for the activation of inflammatory pathways in obesity state. In vitro trials confirmed that alantolactone (6) prevents the increase of IL-6 levels and regulates macrophage infiltration by reduction of MCP-1 concentrations via inhibition of the TLR4-JNK pathway in both, lean (adipocytes) and obese (adipocyte-macrophage) states (Kim et al., 2017a, 2017b).
Besides the effect on adipocytes, alantolactone (6) can also act in skeletal muscle and liver cells. In this context, the compound 6 inhibits IL-6-induced insulin-stimulated glucose intolerance and insulin resistance in the skeletal muscle through blockade the activation of STAT3 and the abnormal expression of TLR4 (Kim et al., 2017a, 2017b). In L02 cells, alantolactone (6) also inhibits oxLDL-induced lipid accumulation trough regulating the apolipoprotein C3 (APOC3) expression partly by decreasing the activation of STAT3 (Yang et al., 2018). Thus, may be relevant to explore further its role in managing metabolic complications on e.g. modulating hepatic IL-6/STAT3 signaling in high-fat diet-induced metabolic disorder in animal models, since the physiological metabolic response to IL-6 depends on the specific source of IL-6 in vivo (Han et al., 2020)
Concerning to in vivo experiments, no hypoglycemic effect was observed on STZ-induced diabetic mice after oral administration of alantolactone. However, inflammation and renal abnormalities were suppressed via inhibition of NF-κB gene expression and the high glucose-induced overexpression of pro-inflammatory cytokines and macrophage adhesion in renal NRK-52E cells were inhibited. (Zhu et al., 2020). These results led the authors to propose a beneficial use for the treatment of diabetic neuropathy. Taking together all evidences mentioned for alantolactone (6) activity, it is probably that this compound could reduce some components of MetS by the regulation of inflammatory processes.
Alantolactone (6) did not induced significant hepatotoxicity and nephrotoxicity after daily administration (100 mg/kg b.w.) for 5 weeks in mice (Khan et al., 2012). This compound also is a contact allergen, and in vitro is cytotoxic in cancer cells and induces apoptosis (Tisserand and Young, 2014).
6.6. 3,10-Epoxygermacranolides from Tithonia diversifolia (Hemsl.) A. Gray
Tithonia diversifolia (Hemsl) A. Gray, commonly known as tree marigold or Mexican sunflower (Asteraceae: Heliantheae), is a shrub-like perennial or annual invasive plant, native from North and Central America. Traditionally, its leaves are widely used by indigenous people for treating a wide spectrum of diseases, including diabetes mellitus. Several in vitro and in vivo studies have stated the antioxidant antidiabetic, hypolipidemic and antiobesity effects of T. diversifolia (Ajao and Moteetee, 2017; Tagne et al., 2018). Germacranolides, eudesmanolides and guaianolides are some of the major components occurring in this plant (Ajao and Moteetee, 2017; Tagne et al., 2018) and because of their previously described activity, they apparently are involved in the important spectrum of bioactivity reported for T. diversifolia. Tirotundin (7) and tagitinin A (8) (Fig. 1) isolated from the ethyl acetate soluble fraction of T. diversifolia are related to the activation of peroxisome proliferator-activated receptors (PPARs) (Lin, 2012). PPARs are members of nuclear hormone receptors superfamily involved in the metabolic regulation of lipid and lipoprotein levels, blood glucose, and abdominal adiposity. In mammals, three isoforms of PPARs have been recognized namely PPAR-α, PPAR-β/δ and PPAR-γ. Activation of PPAR-α by hypolipidemic fibrate class of drugs decreases TG levels and raises HDL-C in dyslipidemic individuals, whereas activation of PPAR-γ by antidiabetic thiazolidinedione agents causes insulin sensitization to enhance glucose metabolism (Botta et al., 2018). Currently, dual PPARα/γ agonists, which stimulate both PPARα and PPAR-γ isoforms to similar extents, are gaining popularity since it is believed that they are able to ameliorate the unwanted side effects of selective PPAR-α and PPAR-γ agonists; and may also be used to treat dyslipidemia and T2DM simultaneously (Balakumar et al., 2019). Tirotundin (7) and tagitinin A (8) have been suggested being dual PPARα/γ agonists after the evaluation of their agonistic activity against PPARs employing a transient transfection reporter assay in HepG2 cells (Lin et al., 2012).
On the other hand, the germacranolides tagitinin G (9), tagitinin I (10), 1β-hydroxydiversifolin-3-O-methyl ether (11) and 1β-hydroxytirotundin-3-O-methyl ether (12) (Fig. 1) isolated from the aerial parts of T. diversifolia were found to significantly elevate glucose uptake in 3T3-L1 adipocytes without any toxicity (Zhao et al., 2012). The authors suggested that these compounds had PPARγ agonist activity on glucose uptake, similar to pioglitazone, a PPAR-γ agonist used as a control compound in the study. No in vivo acute, subchronic or chronic toxicity studies on 3,10-epoxygermacranolides described above have been reported to date.
The increasing abundance of Tithonia diversifolia in conservation and agricultural areas over the past ten years in South Africa has been of concern. This plant is considered as a scrub resulting in the initiation of a biological control program against its propagation (Simelane et al., 2011). Therefore, utilization for medicinal purposes of this invasive plant could be important before major eradication takes place.
6.7. Micheliolide from Magnolia compressa (Maxim.) Sarg
The genus Magnolia, previously known as Michellia, reported to exert various biological effects, including anti-cancer, anti-anxiety, antidepressant, antioxidant and anti-inflammatory (Lee et al., 2011). In later years, Magnolia species and their components have been related to ameliorate characters of obesity and diabetes, such as hyperglycemia, hyperlipidemia, and other complications (Zhao et al., 2016). Since terpenoids are one of the principal substantial compounds in Magnolia species, SLns may be related with its medicinal properties (Sun et al., 2015). Anticancer activity investigation lead to the isolation of the guaianolide micheliolide (13) (Fig. 1) from M. compressa (Maxim.) Sarg. (Ogura et al., 1978). This compound has also been obtained in a semi-synthetic way from a BF3-mediated rearrangement of parthenolide (Castañeda-Acosta et al., 1993; Zhai et al., 2012), presenting enhanced stability and remarkable anti-inflammatory effect by suppressing activation of the NF-κB through inhibition of IκBα degradation as well as for the inhibition of p65 translocation to the nucleus (Viennois et al., 2014). The compound also influenced the MAPK and PI3K/Akt signaling pathways in LPS-stimulated BV-2 cells (Sun et al., 2017). Further, an in vitro assay has revealed that 13 attenuate the high glucose-stimulated activation of NF-κB, the degradation of IκBα, and the expression of MCP-1 in rat mesangial cells (Jia et al., 2013).
In addition, the dimethylamino Michael adduct of 13 known as dimethylaminomicheliolide is a pro-drug of this sesquiterpene lactone approved for clinical trials for glioblastoma treatment (Guo et al., 2019). This compound protects the kidneys against proteinuria, renal failure, histopathological injury, and inflammation by suppressing activation of the NF-κB signaling pathway in db/db mice (Liu et al., 2019). These results showed that dimethylaminomicheliolide intervention mitigated diabetic kidney disease in db/db mice without directly affecting hyperglycemia.
Furthermore, it has been demonstrated that micheliolide (13) alleviates hepatic steatosis in obese db/db mice, and the molecular mechanisms driving the therapeutic effects of this compound might involve PPAR-γ upregulation to consequently inhibit NF-κB mediated inflammation and activate AMPK/mTOR-dependent autophagy (Zhong et al., 2018). Thus, it will be interesting to future work evaluate if this effect in hepatic steatosis impacts glucose metabolism. No in vivo toxicity studies on micheliolide (13) have been reported to date.
6.8. Byrsonines A and B from Byrsonima crassifolia (L.) Kunth
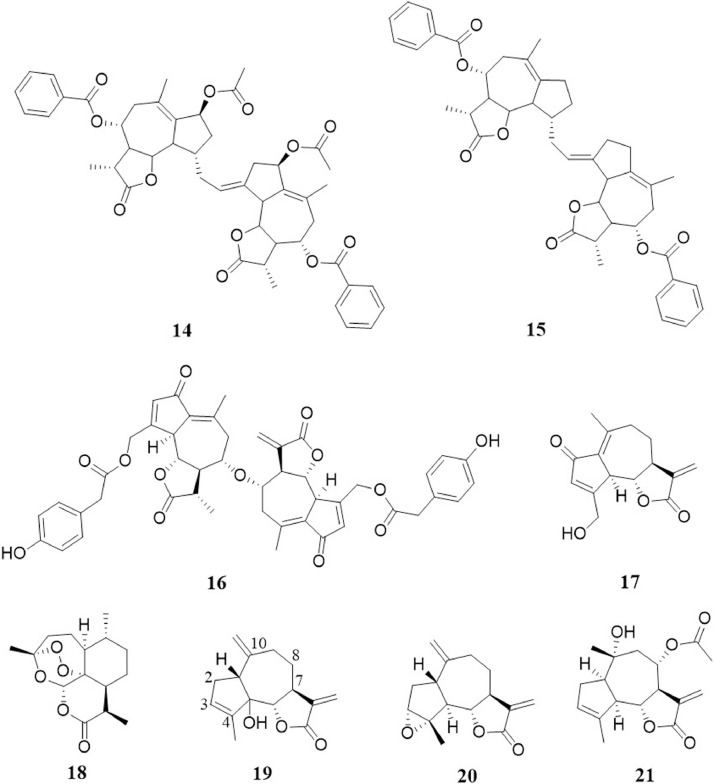
Byrsonima crassifolia (Malpighiaceae), commonly known as “nanche”, is a tropical tree widely distributed in Mexico, Central and South America. This tree is commonly harvested, both for its edible fruit and for its health benefits (Béjar and Malone, 1993; Duarte, 2011). In folk medicine, the fruit or bark infusion have been used as hypoglycemic remedy (Andrade-Cetto and Heinrich, 2005) and according to ethnomedicinal reports, the antihyperglycemic activity of hexane and chloroform extracts from fruits and seeds of B. crassifolia in STZ-induced diabetic rats was reported (Perez-Gutierrez et al., 2010). The phytochemical analysis of antihyperglycemic extract from the seed of B. crassifolia revealed that byrsonines A (14) and B (15) (Fig. 2 ), two dimeric guaianolides, are responsible for the antioxidant, hypoglycemic and hypolipidemic activities (Gutiérrez and Ramirez, 2016). In particular, it has been proposed that the antihyperglycemic activity of these compounds may involve both pancreatic and extra pancreatic mechanisms, based on the observed increase in serum insulin level and the reduction of hepatic glucose output via decreasing glucose-6-phosphatase (G6Pase) activity and increasing glucokinase activity (Gutiérrez and Ramirez, 2016). Although the mechanisms underlying the antihyperglycemic activity of 14 and 15 have not been completely explored, this effect could likely be ascribed to the activation AMP-activated protein kinase (AMPK). Activation of the AMPK pathway in the liver regulates glucose homeostasis by inhibiting hepatic glucose production and downregulating the expression of gluconeogenic genes, including G6Pase gene expression. Targeting AMPK by natural products demonstrated considerable success in lowering blood glucose levels (Joshi et al., 2019). Nevertheless, more experimental research is needed to substantiate this claim.
Fig. 2.
Chemical structures of compounds 14-21
On the other hand, the induction of antioxidant enzymes in hepatic, renal, and pancreas tissues after the administration of 14 and 15, indicates a positive effect of these molecules in improving glycemic control in STZ-induced diabetic rats (Gutiérrez and Ramirez, 2016), since oxidative stress takes an important role in the pathogenesis and progression of diabetic tissue injury. In this sense, the reported improvement in insulin resistance by byrsonines A and B can be related to the observed increase in the HDL-C levels and the decrease in TC, TG, LDL-C, and VLDL (Gutiérrez and Ramirez, 2016). Additionally, TNF-α levels were also decreased after treatment of byrsonines A and B in STZ-induced diabetic rats.
The bioactivity described for 14 and 15 makes them one of the most active guaianolides on different components of MetS reported at this time. As described above, another molecule of this family with activity on different components of MetS is eremathin. However, byrsonines A and B (14, 15) seems to be more effective, which could be due to their dimeric structure that provides two possible Michaels acceptors. Dimeric SLns have been found to be more potently cytotoxic than their monomers toward human cancer cells, indicating that the antitumor potential of SLns are improved by the presence of additional α-methylene-γ-lactone ring (Singh et al., 2011;Ren et al., 2016). This phenomenon could be studied in 14 and 15 for MetS treatment due to their extended activity reviewed here. Moreover, pharmacokinetics studies are needed to understand either byrsonines suffer biotransformation reactions and act as monomeric molecules or if they can show biological activity in their original dimeric forms. No in vivo toxicity studies on byrsonines A (14) and B (15) have been reported to date.
6.9. Lactucain from Lactuca indica Linn
Various species of the genus Lactuca are globally important edible plants containing valuable nutrients such as polyphenols, sterols, vitamins, minerals, and dietary fiber. L. sativa L., Lactuca indica L. and L. serriola L. (syn. L. scariola L.) have been used in the treatment of diabetes mellitus, hypertension, and cardiac diseases (Eddouks et al., 2002; Subramoniam, 2016). In vivo experiments of some Lactuca species shown the ability to reduce several metabolic risk factors, especially hyperglycemia, TG and TC (Nicolle et al., 2004; Salih, 2019). SLns are commonly found in Lactuca species and are represented as subgroups such as lactucin-type guaianolides and the eudesmanolide-type. The latter is commonly found in some species of Lactuca such as L. sativa L. var. anagustata, L. saligna L. and L. canadensis L. (Han et al., 2010; Kisiel and Gromek, 1993; Michalska et al., 2013).
The dimeric guaianolide lactucain C (16) (Fig. 2), isolated from L. indica Linn. showed moderate lowering of plasma glucose in STZ-diabetic rats (Hou et al., 2003). Nevertheless, there are no evidences about the possible mechanism of action and no further research has been made about this compound. As mentioned before, dimeric SLns could show an important activity in components of MetS derived from their double α,β-unsaturated γ-lactone ring. Because of its hypoglycemic properties and chemical structure, 16 is also a candidate to further studies that analyze deeply its effect in blood glucose levels and some other MetS´s components. No in vivo toxicity studies on luctucain C (16) have been reported to date.
6.10. 8-Deoxylactucin from Cichorium intybus L
Cichorium intybus, commonly known as chicory, was historically cultivated by the ancient Egyptians as a vegetable crop, a coffee substitute, and a medicinal plant. Nowadays, it is appreciated for its bitter taste and widely used in India as a traditional treatment for diabetes mellitus (Al-Snafi, 2016; Pushparaj et al., 2007). The antidiabetic, hypolipidemic, hepatoprotective, antioxidant and anti-inflammatory effects of C. intybus have been widely studied (Chandra and Sk, 2016). This broad spectrum of biological activities has been attributed to its high content of phytochemical constituents, being SLns the most abundant in roots and the responsible of the bitterness of leaves (Al-Snafi, 2016).
8-deoxylactucin (17) (Fig. 2) is a guaianolide found as a major component of chicory root and it is also common in species of Lactuca. It has been demonstrated that 17 possess anti-inflammatory activity by inhibiting the nuclear transcription factor NF-κB and as a selective inhibitor of COX-2 (Cavin et al., 2005). An important structural characteristic of 17 is the presence of α-methylene-γ-lactone group and α,β-unsaturated cyclopentenone. Compounds that possess these structures, such as Helenalin, can react with sulfhydryl group of Cys38 in NF-kB and prevent DNA binding (Lyß et al., 1998; Widen et al., 2017). Thus, 8-deoxylactucin (17) could interact with NF-κB by a similar mechanism. No in vivo toxicity studies on 8-deoxylactucin (17) have been reported to date.
6.11. Artemisinin from Artemisia spp
Artemisinin (18) (Fig. 2) is a cadinanolide endoperoxide sesquiterpene lactone originally isolated from Artemisa annua Linn in 1972 (Tu, 2016). Nowadays, 18 and their derivatives are part of the protocols for malaria treatment (Bridgford et al., 2018). Experimental studies have also established their effectiveness as an anti-inflammatory, antioxidant, anti-tumor, and vascular protection agent (Kim et al., 2015; Jiang et al., 2016; Efferth, 2017). Regarding to vascular protection, Cao et al. (2020) demonstrated that oral administration of artemisinin (18) effectively alleviated inflammatory response (MCP-1, IFN-γ, IL-6 and TNF-α), elevated macrophage autophagy, suppressed foamy macrophage transformation, and thereby inhibiting atherosclerotic plaque formation in high-fat diet treated ApoE−/− mice. In addition, 18 has been discovered to be a potential therapeutic agent for ameliorating type 1 diabetes because of its ability to promote the conversion of pancreatic glucagon-producing α cells to insulin-secreting β cells in rats (Li et al., 2017). Nevertheless, the widespread application of artemisinin as an anti-malarial drug could contributed to the selection of resistant strains of the etiologic agent Plasmodium, thus its use to combating non-communicable human diseases would raise the risk for this selection and lead to an important drug resistance development. Some authors have suggested that unless necessary, artemisinin (18) should be replaced by other therapeutic agents for the treatment of versatile types of human diseases (Dong-Sheng et al., 2017).
It is important to note that some species of Artemisia have been traditionally used in treatment of diabetes such as A. dracunulus L., A. minor Jacq. ex Besser, A. pallens Wall. ex D.C., A. sphaerocephala Krasch and A. herba-alba Asso, (Mohamed et al., 2010; Subramoniam, 2016). Several eudesmanolides and guaianolides have been isolated from A. herba-alba, all of them having in common the absence of the α-methylene in the γ-lactone ring as in artemisinin (18) structure. Indeed the ethanol, chloroform and water extracts of this plant have showed important hypoglycemic activity (Mohamed et al., 2010).
Animal experiments show considerable toxicity after the application of artemisinin family (neurotoxicity, embryotoxicity, genotoxicity, hemato- and immunotoxicity, cardiotoxicity, nephrotoxicity, and allergic reactions), but large clinical studies and meta-analyzes did not show serious human side effects, although proper monitoring of adverse effects in developing countries may not be a trivial task. There is a paucity of large-scale clinical trials adequate to detect rare but significant toxicity. The lesson learned from animal and human studies is that long-term availability rather than short-term peak concentrations of artemisinin cause toxicity (Efferth and Kaina, 2010). Thus, the observation of the toxicity of artemisinin derivatives in animals, but not in humans, is most likely due to different pharmacokinetic profiles after different routes of administrations.
6.12. Scoporanolide and estafiatin from Artemisia scoparia Waldst. & Kit
Artemisia scoparia Waldst. & Kit. (redstem wormwood) is widely distributed in Southwest Asia and central Europe. This species has been reported to possess anti-obesity (Richard et al., 2014) and hypolipidemic (Boudreau et al., 2018) properties. A. scoparia ethanolic extract reduces non-alcoholic fatty liver disease by enhancing hepatic insulin and AMP-AMPK signaling in diet-induced obese mice (Wang et al., 2013). A. scoparia hot water extract reduces blood pressure in spontaneously hypertensive rats via the inhibition of plasma angiotensin I-converting enzyme (ACE) activity (Cho et al., 2015). The phytochemical analysis of this extract revealed that seven SLns contribute to the antihypertensive effect inhibition of ACE activity, highlighting the activity of scoporanolide (19) and estafiatin (20) (Fig. 2) (Cho et al., 2016) showing significantly higher ACE inhibitory activities than the other isolated SLns. No in vivo toxicity studies on scoporanolide (19) and estafiatin (20) have been reported to date.
6.13. Cumambrin A from Chrysanthemum boreale (Makino) Makino
The guaianolide cumambrin A (21) (Fig. 2), isolated from Chrysanthemum boreale (Makino) Makino, has shown a pharmacological effect on the normalization of blood pressure in spontaneously hypertensive rats (Hong et al., 1999). Ex vivo study demonstrate that this compound is a potent relaxant of aortic smooth muscle at a concentration of 5 × 10−5 M (Hong et al., 2005). No in vivo toxicity studies on cumambrin A (21) have been reported to date.
7. Concluding remarks
The potential beneficial effects of SLns on MetS´s risk factors discussed in this review clearly demonstrate that these substances represent remarkable compounds with a diversity of molecular structure and biological activity. These beneficial effects include normalization of blood glucose levels, improvement of blood lipid profiles, anti-inflammatory activity, improvement of insulin sensitivity and normalization of blood pressure. The underlying molecular targets mediating these effects have not been completely understood. Regardless of that, it can be noticed that at least six molecular targets or pathways could explain the effect of SLns on components of MetS: (a) inhibition of α-glucosidase; (b) inhibition of TLR4 signaling; (c) inhibition of NF-κB pathway; (d) dual PPARα/γ agonists; (e) AMPK agonist activity and (f) regeneration and revitalization of the remaining β cells.
Furthermore, the evidence from published literature demonstrates that some SLns belonging to guaianolides like eremanthin (4) and germacranolides such as costunolide (5) and guaianolide dimers byrsonines A and B (14, 15), show multifunctional benefits due to their action on many MetS´s risk factors. These observations suggest that structural specificity may be a key for understanding the mechanisms of action of SLns. Looking forward, the effects of SLns on MetS should be explained based on the molecular targets and should be related to biochemical mechanisms. MetS has become a major public health problem worldwide and represents a common clinical condition in countries with a high incidence of obesity and western dietary patterns. Natural products, such as SLns, are attractive drug candidates and more attention must be paid to their potential use in the treatment and prevention of MetS.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
This work was partially supported by the Secretaría de Investigación y Posgrado, Instituto Politécnico Nacional. ASG was CONACyT (267721) and IPN-BEIFI fellow. Julio C. Ontiveros thanks CONACyT for funding CONACyT project No. 1069 “Cátedras CONACyT”.
Edited by AM Viljoen
References
- Adekenov S.M. Sesquiterpene lactones with unusual structure. Their biogenesis and biological activity. Fitoterapia. 2017;121:16–30. doi: 10.1016/j.fitote.2017.05.017. [DOI] [PubMed] [Google Scholar]
- Ajani H.B., Patel H.P., Shah G.B., Acharya S.R., Shah S.K. Evaluation of antidiabetic effect of methanolic extract of Inula racemosa root in rats. Pharmacologyonline. 2009;129:118–129. [Google Scholar]
- Ajao A.A., Moteetee A.N. Tithonia diversifolia (Hemsl) A. Gray. (Asteraceae: Heliantheae), an invasive plant of significant ethnopharmacological importance: A review. South African Journal of Botany. 2017;113:396–403. doi: 10.1016/j.sajb.2017.09.017. [DOI] [Google Scholar]
- Alberti K.G., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., Fruchart J.C., James W.P.T., Loria C.M., Smith S.C., Jr. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- Alonso M.R., Anesini C.A., Martino R.F. In: Sesquiterpene Lactones. Advances in Their Chemistry and Biological Aspects. Sülsen V.P., Martino V.S., editors. Springer; Cham: 2018. Anti-inflammatory activity; pp. 325–346. [DOI] [Google Scholar]
- Al-Snafi A.E. Medical importance of Cichorium intybus-A review. IOSR Journal of Pharmacy. 2016;6(3):41–56. http://www.iosrphr.org/papers/v6i3/E0634156.pdf [Google Scholar]
- Andrade-Cetto A., Heinrich M. Mexican plants with hypoglycaemic effect used in the treatment of diabetes. Journal of Ethnopharmacology. 2005;99(3):325–348. doi: 10.1016/j.jep.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Aybar M.J., Riera A.N.S., Grau A., Sanchez S.S. Hypoglycemic effect of the water extract of Smallantus sonchifolius (yacon) leaves in normal and diabetic rats. Journal of Ethnopharmacology. 2001;74(2):125–132. doi: 10.1016/S0378-8741(00)00351-2. [DOI] [PubMed] [Google Scholar]
- Baker R.G., Hayden M.S., Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metabolism. 2011;13(1):11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakumar P., Mahadevan N., Sambathkumar R. A contemporary overview of PPARα/γ dual agonists for the management of diabetic dyslipidemia. Current Molecular Pharmacology. 2019;12(3):195–201. doi: 10.2174/1874467212666190111165015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroni S., Suzuki-Kemmelmeier F., Caparroz-Assef S.M., Cuman R.K.N., Bersani-Amado C.A. Effect of crude extracts of leaves of Smallanthus sonchifolius (yacon) on glycemia in diabetic rats. Revista Brasileira de Ciências Farmacêuticas. 2008;44(3):521–530. doi: 10.1590/S1516-93322008000300024. [DOI] [Google Scholar]
- Béjar E., Malone M.H. Pharmacological and chemical screening of Byrsonima crassifolia, a medicinal tree from Mexico. Part I. Journal of Ethnopharmacology. 1993;39(2):141–158. doi: 10.1016/0378-8741(93)90029-5. [DOI] [PubMed] [Google Scholar]
- Botta M., Audano M., Sahebkar A., Sirtori C.R., Mitro N., Ruscica M. PPAR agonists and metabolic syndrome: an established role? International Journal of Molecular Sciences. 2018;19(4):1197. doi: 10.3390/ijms19041197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau A., Richard A.J., Burrell J.A., King W.T., Dunn R., Schwarz J.M., Ribnicky D.M., Rood J., Salbaum J.M., Stephens J.M. An ethanolic extract of Artemisia scoparia inhibits lipolysis in vivo and has antilipolytic effects on murine adipocytes in vitro. American Journal of Physiology. Endocrinology and Metabolism. 2018;315(5):E1053–E1061. doi: 10.1152/ajpendo.00177.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgford J.L., Xie S.C., Cobbold S.A., Pasaje C.F.A., Herrmann S., Yang T., Gillett D.L., Dick L.R., Ralph S.A., Dogovski C., Spillman N.J., Tilley L. Artemisinin kills malaria parasites by damaging proteins and inhibiting the proteasome. Nature Communications. 2018;9:3801. doi: 10.1038/s41467-018-06221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano B.F.R., De Moura N.A., Almeida A.P.S., Dias M.C., Sivieri K., Barbisan L.F. Yacon (Smallanthus sonchifolius) as a food supplement: health-promoting benefits of fructooligosaccharides. Nutrients. 2016;8(7):436. doi: 10.3390/nu8070436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q., Du H., Fu X., Duan N., Liu C., Li X. Artemisinin attenuated atherosclerosis in high-fat diet-fed ApoE-/- mice by promoting macrophage autophagy through the AMPK/mTOR/ULK1 pathway. Journal of Cardiovascular Pharmacology. 2020;75(4):321–332. doi: 10.1097/FJC.0000000000000794. [DOI] [PubMed] [Google Scholar]
- Castañeda-Acosta J., Fischer N.H., Vargas D. Biomimetic transformations of parthenolide. Journal of Natural Products. 1993;56(1):90–98. doi: 10.1021/np50091a013. [DOI] [PubMed] [Google Scholar]
- Catrysse L., van Loo G. Inflammation and the metabolic syndrome: the tissue-specific functions of NF-κB. Trends in Cell Biology. 2017;27(6):417–429. doi: 10.1016/j.tcb.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Cavin C., Delannoy M., Malnoe A., Debefve E., Touché A., Courtois D., Schilter B. Inhibition of the expression and activity of cyclooxygenase-2 by chicory extract. Biochemical and Biophysical Research Communications. 2005;327(3):742–749. doi: 10.1016/j.bbrc.2004.12.061. [DOI] [PubMed] [Google Scholar]
- Chadwick M., Trewin H., Gawthrop F., Wagstaff C. Sesquiterpenoids lactones: benefits to plants and people. International Journal of Molecular Sciences. 2013;14(6):12780–12805. doi: 10.3390/ijms140612780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra, K., Sk, J., 2016. Therapeutic potential of Cichorium Intybus in lifestyle disorders: a review. 9(3). https://innovareacademics.in/journals/index.php/ajpcr/article/view/11268
- Chaturvedi D. In: Opportunity, Challenge and Scope of Natural Products in Medicinal Chemistry. Tiwari V.K., Mishra B.B., editors. Research Signpost; Kerala: 2011. Sesquiterpene lactones: structural diversity and their biological activities; pp. 313–334. [Google Scholar]
- Chaturvedi D., Dwivedi P.K. In: Discovery and Development of Antidiabetic Agents from Natural Products: Natural Product Drug Discovery. Brahmachari G., editor. Elsevier; 2016. Recent developments on the antidiabetic sesquiterpene lactones and their semisynthetic analogues; pp. 185–208. [Google Scholar]
- Cho J.Y., Jeong S.J, Lee H.L., Park K.H., Hwang D.Y., Park S.Y., Lee Y.G., Moon J.H., Ham K.S. Sesquiterpene lactones and scopoletins from Artemisia scoparia Waldst. & Kit. and their angiotensin I-converting enzyme inhibitory activities. Food Science and Biotechnology. 2016;25(6):1701–1708. doi: 10.1007/s10068-016-0261-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J.Y., Park K.H., Hwang D.Y., Chanmuang S., Jaiswal L., Park Y.K., Park S.Y., Kim S.Y., Kim H.R., Moon J.H., Ham K.S. Antihypertensive effects of Artemisia scoparia waldst in spontaneously hypertensive rats and identification of Angiotensin I converting enzyme inhibitors. Molecules. 2015;20(11):19789–19804. doi: 10.3390/molecules201119657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J., Choi R.J., Khan S., Lee D.S., Kim Y.C., Nam Y.J., Lee D.U., Kim Y.S. Alantolactone suppresses inducible nitric oxide synthase and cyclooxygenase-2 expression by down-regulating NF-κB, MAPK and AP-1 via the MyD88 signaling pathway in LPS-activated RAW 264.7 cells. International Immunopharmacology. 2012;14(4):375–383. doi: 10.1016/j.intimp.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Cicero A.F., Colletti A. Role of phytochemicals in the management of metabolic syndrome. Phytomedicine. 2016;23(11):1134–1144. doi: 10.1016/j.phymed.2015.11.009. [DOI] [PubMed] [Google Scholar]
- Costa F.F., Rosário W.R., Ribeiro-Farias A.C., de Souza R.G., Duarte-Gondim R.S., Barroso W.A. Metabolic syndrome and COVID-19: an update on the associated comorbidities and proposed therapies. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2020;14(5):809–814. doi: 10.1016/j.dsx.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daisy P., Eliza J., Ignacimuthu S. Influence of Costus speciosus (Koen.) Sm. Rhizome extracts on biochemical parameters in streptozotocin induced diabetic rats. Journal of Health Science. 2008;54(6):675–681. doi: 10.1248/jhs.54.675. [DOI] [Google Scholar]
- De Pedro A., Cuenca M.D.R., Grau A., Catalán C.A.N., Gedris T.E., Herz W. Melampolides from Smallanthus macroscyphus. Biochemical Systematics and Ecology. 2003;31(9):1067–1071. doi: 10.1016/S0305-1978(03)00064-4. [DOI] [Google Scholar]
- Delgado G.T.C., Tamashiro W.M.D.S.C., Junior M.R.M., Pastore G.M. Yacon (Smallanthus sonchifolius): a functional food. Plant Foods for Human Nutrition. 2013;68:222–228. doi: 10.1007/s11130-013-0362-0. [DOI] [PubMed] [Google Scholar]
- Dong-Sheng Y., Yan-Ping C., Li-Li T., Shui-Qing H., Chang-Qing L., Qi W., Qing-Ping Z. Artemisinin: A panacea eligible for unrestrictive use? Frontiers in Pharmacology. 2017;8:737. doi: 10.3389/fphar.2017.00737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte O. In: Yahia E.M., editor. Vol. 4. Woodhead Publishing; 2011. Nance (Byrsonima crassifolia (L.) Kunth) pp. 44–52e. (Postharvest Biology and Technology of Tropical and Subtropical Fruits). [DOI] [Google Scholar]
- Eddouks M., Maghrani M., Lemhadri A., Ouahidi M.-.L., Jouad H. Ethnopharmacological survey of medicinal plants used for the treatment of diabetes mellitus, hypertension and cardiac diseases in the south-east region of Morocco (Tafilalet) Journal of Ethnopharmacology. 2002;82(2–3):97–103. doi: 10.1016/S0378-8741(02)00164-2. [DOI] [PubMed] [Google Scholar]
- Efferth T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Seminars in Cancer Biology. 2017;46:65–83. doi: 10.1016/j.semcancer.2017.02.009. [DOI] [PubMed] [Google Scholar]
- Efferth T., Kaina B. Toxicity of the antimalarial artemisinin and its dervatives. Critical Reviews in Toxicology. 2010;40(5):405–421. doi: 10.3109/10408441003610571. [DOI] [PubMed] [Google Scholar]
- Eliza J., Daisy P., Ignacimuthu S. Antioxidant activity of costunolide and eremanthin isolated from Costus speciosus (Koen ex. Retz) Sm. Chemico-Biological Interactions. 2010;188:467–472. doi: 10.1016/j.cbi.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Eliza J., Daisy P., Ignacimuthu S., Duraipandiyan V. Normo-glycemic and hypolipidemic effect of costunolide isolated from Costus speciosus (Koen ex. Retz.)Sm. in streptozotocin-induced diabetic rats. Chemico-Biological Interactions. 2009;179(2-3):329–334. doi: 10.1016/j.cbi.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Eliza J., Daisy P., Ignacimuthu S., Duraipandiyan V. Antidiabetic and antilipidemic effect of eremanthin from Costus speciosus (Koen.) Sm., in STZ-induced diabetic rats. Chemico-Biological Interactions. 2009;182:67–72. doi: 10.1016/j.cbi.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Engin A. In: Engin A.B., Engin A., editors. Vol. 960. Springer; Cham: 2017. The definition and prevalence of obesity and metabolic syndrome; pp. 1–17. (Obesity and Lipotoxicity. Advances in Experimental Medicine and Biology). [DOI] [PubMed] [Google Scholar]
- Feltenstein M.W., Schühly W., Warnick J.E., Fischer N.H., Sufka K.J. Anti-inflammatory and anti-hyperalgesic effects of sesquiterpene lactones from Magnolia and Bear's foot. Pharmacology Biochemistry and Behavior. 2004;79(2):299–302. doi: 10.1016/j.pbb.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Feng J., Lu S., Ou B., Liu Q., Dai J., Ji C., Zhou H., Huang H., Ma Y. The role of JNk signaling pathway in obesity-driven insulin resistance. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2020;13:1399–1406. doi: 10.2147/DMSO.S236127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer N.H. In: Biochemistry of the Mevalonic Acid Pathway to Terpenoids. Towers G.H.N., Stafford H.A., editors. Springer; Boston, MA: 1990. Sesquiterpene lactones: biogenesis and biomimetic transformations; pp. 161–201. [DOI] [Google Scholar]
- Fischer N.H., Olivier E.J., Fischer H.D. In: Herz W., Grisebach H., Kirby G.W., editors. Vol. 38. Springer; Vienna: 1979. The biogenesis and chemistry of sesquiterpene lactones; pp. 47–320. (Fortschritte Der Chemie organischer Naturstoffe / Progress in the Chemistry of Organic Natural Products). [DOI] [Google Scholar]
- Francini-Pesenti F., Spinella P., Calò L.A. Potential role of phytochemicals in metabolic syndrome prevention and therapy. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2019;12:1987–2002. doi: 10.2147/DMSO.S214550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genta S.B., Cabrera W.M., Mercado M.I., Grau A., Catalán C.A., Sánchez S.S. Hypoglycemic activity of leaf organic extracts from Smallanthus sonchifolius: constituents of the most active fractions. Chemico-Biological Interactions. 2010;185:143–152. doi: 10.1016/j.cbi.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Gomez-Hernandez A., Beneit N., Diaz-Castroverde S., Escribano O. Differential role of adipose tissues in obesity and related metabolic and vascular complications. International Journal of Endocrinology. 2016;2016 doi: 10.1155/2016/1216783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Xue Q., Liu K., Ge W., Liu W., Wang J., Zhang M., Li Q., Cai D., Shan C., Zhang C., Liu X., Li J. Dimethylaminomicheliolide (DMAMCL) Suppresses the Proliferation of Glioblastoma Cells via Targeting Pyruvate Kinase 2 (PKM2) and Rewiring Aerobic Glycolysis. Frontiers in Oncology. 2019;9:993. doi: 10.3389/fonc.2019.00993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S., Ge Y., Jom K.N. A review of phytochemistry, metabolite changes, and medicinal uses of the common sunflower seed and sprouts (Helianthus annuus L.) Chemistry Central Journal. 2017;11:95. doi: 10.1186/s13065-017-0328-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez R.M.P., Ramirez A.M. Hypoglycemic Effects of sesquiterpene lactones from Byrsonima crassifolia. Food Science and Biotechnology. 2016;25(4):1135–1145. doi: 10.1007/s10068-016-0182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib N.C., Honoré S.M., Genta S.B., Sánchez S.S. Hypolipidemic effect of Smallanthus sonchifolius (yacon) roots on diabetic rats: biochemical approach. Chemico-Biological Interactions. 2011;194:31–39. doi: 10.1016/j.cbi.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Han J.W., Lee B.G., Kim Y.K., Yoon J.W., Jin H.K., Hong S., Lee H.Y., Lee K.R., Lee H.W. Ergolide, sesquiterpene lactone from Inula britannica, inhibits inducible nitric oxide synthase and cyclo-oxygenase-2 expression in RAW 264.7 macrophages through the inactivation of NF-κB. British Journal of Pharmacology. 2001;133(4):503–512. doi: 10.1038/sj.bjp.0704099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M.S., White A., Perry R.J., Camporez J.P., Hidalgo J., Shulman G.I., Davis R.J. Regulation of adipose tissue inflammation by interleukin 6. Proceedings of the National Academy of Sciences. 2020;117(6):2751–2760. doi: 10.1073/pnas.1920004117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y.F., Cao G.X., Gao X.J., Xia M. Isolation and characterisation of the sesquiterpene lactones from Lactuca sativa L var. anagustata. Food Chemistry. 2010;120(4):1083–1088. doi: 10.1016/j.foodchem.2009.11.056. [DOI] [Google Scholar]
- Hong Y.G., Yang M.S., Pak Y.B. Effect of Cumambrin A treatment on blood pressure in spontaneously hypertensive rats. Korean Journal of Pharmacognosy. 1999;30(2):226–230. http://kpubs.org/article/articleMain.kpubs?articleANo=HKSOBF_1999_v30n2_226 [Google Scholar]
- Hong Y.G., Yang M.S., Pak Y.B. Effect of Cumambrin A on the relaxation of rat aorta. Korean Journal of Pharmacognosy. 2005;36(1):17–20. http://kpubs.org/article/articleMain.kpubs?articleANo=HKSOBF_2005_v36n1s140_17 [Google Scholar]
- Hou C.C., Lin S.J., Cheng J.T., Hsu F.L. Antidiabetic dimeric guianolides and a lignan glycoside from Lactuca indica. Journal of Natural Products. 2003;66(5):625–629. doi: 10.1021/np0205349. https://pharmacologyonline.silae.it/files/archives/2009/vol3/013.HARESH.pdf [DOI] [PubMed] [Google Scholar]
- Iqbal J., Al Qarni A., Hawwari A., Alghanem A.F., Ahmed G. Metabolic syndrome, dyslipidemia and regulation of lipoprotein metabolism. Current Diabetes Reviews. 2018;14(5):427–433. doi: 10.2174/1573399813666170705161039. [DOI] [PubMed] [Google Scholar]
- Ivanescu B., Miron A., Corciova A. Sesquiterpene lactones from Artemisia genus: biological activities and methods of analysis. Journal of Analytical Methods in Chemistry. 2015;2015 doi: 10.1155/2015/247685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Q.Q., Wang J.C., Long J., Zhao Y., Chen S.J., Zhai J.D., Wei L.B., Zhang Q., Chen Y., Long H.B. Sesquiterpene lactones and their derivatives inhibit high glucose-induced NF-κB activation and MCP-1 and TGF-β1 expression in rat mesangial cells. Molecules. 2013;18(10):13061–13077. doi: 10.3390/molecules181013061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Cen Y., Song Y., Li P., Qin R., Liu C., Zhao Y., Zheng J., Zhou H. Artesunate attenuated progression of atherosclerosis lesion formation alone or combined with rosuvastatin through inhibition of pro-inflammatory cytokines and pro-inflammatory chemokines. Phytomedicine. 2016;23(11):1259–1266. doi: 10.1016/j.phymed.2016.06.004. [DOI] [PubMed] [Google Scholar]
- Joshi T., Singh A.K., Haratipour P., Sah A.N., Pandey A.K., Naseri R., Juyal V., Farzaei M.H. Targeting AMPK signaling pathway by natural products for treatment of diabetes mellitus and its complications. Journal of Cellular Physiology. 2019;234(10):17212–17231. doi: 10.1002/jcp.28528. [DOI] [PubMed] [Google Scholar]
- Ju S.Y., Lee J.Y., Kim D.H. Association of metabolic syndrome and its components with all-cause and cardiovascular mortality in the elderly: a meta-analysis of prospective cohort studies. Medicine. 2017;96(45) doi: 10.1097/MD.0000000000008491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M., Yi F., Rasu A., Li T., Wang N., Gao H., Gao R., Ma T. Alantolactone induces apoptosis in glioblastoma cells via GSH depletion, ROS generation, and mitochondrial dysfunction. IUBMB Life. 2012;64(9):783–794. doi: 10.1002/iub.1068. [DOI] [PubMed] [Google Scholar]
- Kim W.S., Choi W.J., Lee S., Kim W.J., Lee D.C., Sohn U.D., Shin H.S., Kim W. Anti-inflammatory, antioxidant and antimicrobial effects of artemisinin extracts from Artemisia annua L. Korean Journal of Physiology & Pharmacology. 2015;19(1):21–27. doi: 10.4196/kjpp.2015.19.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Song K., Kim Y.S. Alantolactone improves palmitate-induced glucose intolerance and inflammation in both lean and obese states in vitro: Adipocyte and adipocyte-macrophage co-culture system. International Immunopharmacology. 2017;49:187–194. doi: 10.1016/j.intimp.2017.05.037. [DOI] [PubMed] [Google Scholar]
- Kim M., Song K., Kim Y.S. Alantolactone improves prolonged exposure of interleukin-6-induced skeletal muscle inflammation associated glucose intolerance and insulin resistance. Frontiers in Pharmacology. 2017;8:405. doi: 10.3389/fphar.2017.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisiel W., Gromek D. Sesquiterpene lactones from Lactuca saligna. Phytochemistry. 1993;34(6):1644–1646. doi: 10.1016/S0031-9422(00)90864-1. [DOI] [Google Scholar]
- Klöting N., Blüher M. Adipocyte dysfunction, inflammation and metabolic syndrome. Reviews in Endocrine and Metabolic Disorders. 2014;15(4):277–287. doi: 10.1007/s11154-014-9301-0. [DOI] [PubMed] [Google Scholar]
- Knab L.M., Grippo P.J., Bentrem D.J. Involvement of eicosanoids in the pathogenesis of pancreatic cancer: the roles of cyclooxygenase-2 and 5-lipoxygenase. World Journal of Gastroenterology. 2014;20:10729–10739. doi: 10.3748/wjg.v20.i31.10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Song Q.H., Hong T., Kitamura H., Cyong J.C. Preventative effects of the flowers of Inula britannica on autoimmune diabetes in C57BL/KsJ mice induced by multiple low doses of streptozotocin. Phytotherapy Research. 2002;16(4):377–382. doi: 10.1002/ptr.868. [DOI] [PubMed] [Google Scholar]
- Koo T.H., Lee J.H., Park Y.J., Hong Y.S., Kim H.S., Kim K.W., Lee J.J. A sesquiterpene lactone, costunolide, from Magnolia grandiflora inhibits NF-κB by targeting IκB phosphorylation. Planta Medica. 2001;67(02):103–107. doi: 10.1055/s-2001-11503. [DOI] [PubMed] [Google Scholar]
- Larsen J.R., Dima L., Correll C.U., Manu P. The pharmacological management of metabolic syndrome. Expert Review of Clinical Pharmacology. 2018;11(4):397–410. doi: 10.1080/17512433.2018.1429910. [DOI] [PubMed] [Google Scholar]
- Lee Y.J., Lee Y.M., Lee C.K., Jung J.K., Han S.B., Hong J.T. Therapeutic applications of compounds in the Magnolia family. Pharmacology & Therapeutics. 2011;130(2):157–176. doi: 10.1016/j.pharmthera.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Li J., Casteels T., Frogne T., Ingvorsen C., Honoré C., Courtney M., Huber K.V.M., Schmitner N., Kimmel R.A., Romanov R.A., Sturtzel C., Lardeau C.H., Klughammer J., Farlik M., Sdelci S., Vieira A., Avolio F., Briand F., Baburin I., Májek P., Pauler F.M., Penz T., Stukalov A., Gridling M., Parapatics K., Barbieux C., Berishvili E., Spittler A., Colinge J., Bennett K.L., Hering S., Sulpice T., Bock C., Distel M., Harkany T., Meyer D., Superti-Furga G., Collombat P., Hecksher-Sørensen J., Kubicek S. Artemisinins target GABA a receptor signaling and impair α cell identity. Cell. 2017;168(1–2):86–100. doi: 10.1016/j.cell.2016.11.010. e15https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H.S., Jin S.E., Kim O.S., Shin H.K., Jeong S.J. Alantolactone from Saussurea lappa exerts antiinflammatory effects by inhibiting chemokine production and STAT1 phosphorylation in TNF‐α and IFN‐γ‐induced in HaCaT cells. Phytotherapy Research. 2015;29(7):1088–1096. doi: 10.1002/ptr.5354. [DOI] [PubMed] [Google Scholar]
- Lim T.K. In: Edible Medicinal And Non-Medicinal Plants. Lim T.K, editor. Springer; Dordrecht: 2014. Helianthus annuus; pp. 372–396. [DOI] [Google Scholar]
- Lin H.R. Sesquiterpene lactones from Tithonia diversifolia act as peroxisome proliferator-activated receptor agonists. Bioorganic & Medicinal Chemistry Letters. 2012;22(8):2954–2958. doi: 10.1016/j.bmcl.2012.02.043. [DOI] [PubMed] [Google Scholar]
- Liu W., Chen X., Wang Y., Chen Y., Chen S., Gong W., Chen T., Sun L., Zheng C., Yin B., Li S., Luo C., Huang Q., Xiao J., Xu Z., Peng F., Long H. Micheliolide ameliorates diabetic kidney disease by inhibiting Mtdh-mediated renal inflammation in type 2 diabetic db/db mice. Pharmacological Research. 2019;150 doi: 10.1016/j.phrs.2019.104506. [DOI] [PubMed] [Google Scholar]
- Longo M., Zatterale F., Naderi J., Parrillo L., Formisano P., Raciti G.A., Beguinot F., Miele C. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. International Journal of Molecular Sciences. 2019;20(9):2358. doi: 10.3390/ijms20092358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyß G., Knorre A., Schmidt T.J., Pahl H.L., Merfort I. The anti-inflammatory sesquiterpene lactone helenalin inhibits the transcription factor NF-κB by directly targeting p65. Journal of Biological Chemistry. 1998;273(50):33508–33516. doi: 10.1074/jbc.273.50.33508. [DOI] [PubMed] [Google Scholar]
- Ma G., Khan S.I., Benavides G., Schühly W., Fischer N.H., Khan I.A., Pasco D.S. Inhibition of NF-κB-mediated transcription and induction of apoptosis by melampolides and repandolides. Cancer Chemotherapy and Pharmacology. 2007;60(1):35–43. doi: 10.1007/s00280-006-0344-0. [DOI] [PubMed] [Google Scholar]
- Martínez M.J.A., Del Olmo L.M.B., Ticona L.A., Benito P.B. In: Rahman A., editor. Vol. 37. Elsevier; Amsterdam: 2012. The Artemisia L. genus: a review of bioactive sesquiterpene lactones; pp. 43–65. (Studies in Natural Products Chemistry). [DOI] [Google Scholar]
- Merfort I. Perspectives on sesquiterpene lactones in inflammation and cancer. Current Drug Targets. 2011;12(11):1560–1573. doi: 10.2174/138945011798109437. [DOI] [PubMed] [Google Scholar]
- Michalska K., Szneler E., Kisiel W. Sesquiterpene lactones from Lactuca canadensis and their chemotaxonomic significance. Phytochemistry. 2013;90:90–94. doi: 10.1016/j.phytochem.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Miura T., Itoh Y., Ishida T. Hypoglycemic and hypolipidemic activity of the leaf of Samallanthus sonchifolius in genetically type 2 diabetic mice. Journal of Traditional Medicines. 2004;21(6):275–277. doi: 10.11339/jtm.21.275. [DOI] [Google Scholar]
- Mohamed A.E.H.H., El-Sayed M.A., Hegazy M.E., Helaly S.E., Esmail A.M., Mohamed N.S. Chemical constituents and biological activities of Artemisia herba-alba. Records of Natural Products. 2010;4(1):1–25. http://www.acgpubs.org/article/records-of-natural-products/2010/1-january-march/chemical-constituents-and-biological-activities-of-artemisia-herba-alba [Google Scholar]
- Moujir L., Callies O., Sousa P., Sharopov F., Seca A.M. Applications of sesquiterpene lactones: a review of some potential success cases. Applied Sciences. 2020;10(9):3001. doi: 10.3390/app10093001. [DOI] [Google Scholar]
- Nicolle C., Cardinault N., Gueux E., Jaffrelo L., Rock E., Mazur A., Amouroux P., Rémésy C. Health effect of vegetable-based diet: Lettuce consumption improves cholesterol metabolism and antioxidant status in the rat. Clinical Nutrition. 2004;23(4):605–614. doi: 10.1016/j.clnu.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Ogura M., Cordell G.A., Farnsworth N.R. Anticancer sesquiterpene lactones of Michelia compressa (magnoliaceae) Phytochemistry. 1978;17(5):957–961. doi: 10.1016/S0031-9422(00)88656-2. [DOI] [Google Scholar]
- Onoja S.O., Anaga A.O. Evaluation of the antidiabetic and antioxidant potentials of methanolic leaf extract of Helianthus annuus L. on alloxan-induced hyperglycemic rats. Comparative Clinical Pathology. 2014;23(5):1565–1573. doi: 10.1007/s00580-013-1824-3. [DOI] [Google Scholar]
- Onoja S.O., Nnadi C.O., Udem S.C., Anaga A.O. Potential antidiabetic and antioxidant activities of a heliangolide sesquiterpene lactone isolated from Helianthus annuus L. leaves. Acta Pharmaceutica. 2020;70(2):215–226. doi: 10.2478/acph-2020-0019. [DOI] [PubMed] [Google Scholar]
- Onoja S.O., Udem S.C., Anaga A.O. Hypoglycemic, antidyslipidemic, hepatoprotective and antilipid peroxidation activities of hydromethanol leaf extract of Helianthus annuus in alloxan-induced diabetic rats. Tropical Journal of Pharmaceutical Research. 2018;17(9):1817–1824. doi: 10.4314/tjpr.v17i9.20. [DOI] [Google Scholar]
- Orhan N., Gökbulut A., Orhan D.D. Antioxidant potential and carbohydrate digestive enzyme inhibitory effects of five Inula species and their major compounds. South African Journal of Botany. 2017;111:86–92. doi: 10.1016/j.sajb.2017.03.040. [DOI] [Google Scholar]
- Padilla-Gonzalez G.F., dos Santos F.A., Da Costa F.B. Sesquiterpene lactones: more than protective plant compounds with high toxicity. Critical Reviews in Plant Sciences. 2016;35(1):18–37. doi: 10.1080/07352689.2016.1145956. [DOI] [Google Scholar]
- Pal M., Febbraio M.A., Lancaster G.I. The roles of c‐Jun NH2‐terminal kinases (JNKs) in obesity and insulin resistance. The Journal of Physiology. 2016;594(2):267–279. doi: 10.1113/JP271457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.H., Kim M.J., Li Y., Park Y.N., Lee J., Lee Y.J., Kim S.G., Park H.J., Son J.K., Chang H.W., Lee E. Britanin suppresses LPS-induced nitric oxide, PGE2 and cytokine production via NF-κB and MAPK inactivation in RAW 264.7 cells. International Immunopharmacology. 2013;15(2):296–302. doi: 10.1016/j.intimp.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Perez-Gutierrez R.M., Muñiz-Ramirez A., Gomez Y.G., Ramírez E.B. Antihyperglycemic, antihyperlipidemic and antiglycation effects of Byrsonima crassifolia fruit and seed in normal and streptozotocin-induced diabetic rats. Plant Foods for Human Nutrition. 2010;65(4):350–357. doi: 10.1007/s11130-010-0181-5. [DOI] [PubMed] [Google Scholar]
- Pushparaj P.N., Low H.K., Manikandan J., Tan B.K.H., Tan C.H. Anti-diabetic effects of Cichorium intybus in streptozotocin-induced diabetic rats. Journal of Ethnopharmacology. 2007;111(2):430–434. doi: 10.1016/j.jep.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Ràfols M.E. Adipose tissue: cell heterogeneity and functional diversity. Endocrinología y Nutrición (English Edition) 2014;61(2):100–112. doi: 10.1016/j.endoen.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Ren Y., Yu J., Kinghorn A.D. Development of anticancer agents from plant-derived sesquiterpene lactones. Current Medicinal Chemistry. 2016;23(23):2397–2420. doi: 10.2174/0929867323666160510123255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard A.J., Fuller S., Fedorcenco V., Beyl R., Burris T.P., Mynatt R., Ribnicky D.M., Stephens J.M. Artemisia scoparia enhances adipocyte development and endocrine function in vitro and enhances insulin action in vivo. PLoS One. 2014;9(6):e98897. doi: 10.1371/journal.pone.0098897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogero M.M., Calder P.C. Obesity, inflammation, toll-like receptor 4 and fatty acids. Nutrients. 2018;10(4):432. doi: 10.3390/nu10040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan C.H., So S.P., Ruan K.H. Inducible COX-2 dominates over COX-1 in prostacyclin biosynthesis: mechanisms of COX-2 inhibitor risk to heart disease. Life Sciences. 2011;88:24–30. doi: 10.1016/j.lfs.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salih B.A. Effect of Lactuca serriola on β-Cell dysfunction and glucose tolerance induced by high sucrose fed in albino rats. Journal of Physics: Conference Series. 2019;1294(6):0–8. doi: 10.1088/1742-6596/1294/6/062092. [DOI] [Google Scholar]
- Schmidt T.J. In: Rahman A., editor. Vol. 33. Elsevier; Amsterdam: 2006. Structure-activity relationships of sesquiterpene lactones; pp. 309–392. (Studies in Natural Products Chemistry). Part Mhttps://doi.org/ [DOI] [Google Scholar]
- Schorr K., Merfort I., Da Costa F.B. A novel dimeric melampolide and further terpenoids from Smallanthus sonchifolius (Asteraceae) and the inhibition of the transcription factor NF-κB. Natural Product Communications. 2007;2(4):367–374. doi: 10.1177/1934578X0700200404. [DOI] [Google Scholar]
- Seca A.M.L., Grigore A., Pinto D.C.G.A., Silva A.M.S. The genus Inula and their metabolites: From ethnopharmacological to medicinal uses. Journal of Ethnopharmacology. 2014;154(2):286–310. doi: 10.1016/j.jep.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Serra B.C., Cabrera W.M., Honoré S.M., Mercado M.I., Sánchez A.S., Genta S.B. Safety assessment of aqueous extract from leaf Smallanthus sonchifolius and its main active lactone, enhydrin. Journal of Ethnopharmacology. 2012;144(2):362–370. doi: 10.1016/j.jep.2012.09.02. [DOI] [PubMed] [Google Scholar]
- Serra-Barcellona C., Aráoz M.V.C., Cabrera W.M., Habib N.C., Honoré S.M., Catalán C.A., Sánchez S.S. Smallanthus macroscyphus: a new source of antidiabetic compounds. Chemico-Biological Interactions. 2014;209:35–47. doi: 10.1016/j.cbi.2013.11.015. [DOI] [PubMed] [Google Scholar]
- Serra-Barcellona C., Habib N.C., Honore S.M., Sánchez S.S., Genta S.B. Enhydrin regulates postprandial hyperglycemia in diabetic rats by inhibition of α-glucosidase activity. Plant Foods for Human Nutrition. 2017;72(2):156–160. doi: 10.1007/s11130-017-0600-y. [DOI] [PubMed] [Google Scholar]
- Serra-Barcellona C., Honoré S.M., Cabrera W.M., Habib N.C., Genta S.B., Sánchez S.S. Polymatin A from Smallanthus macroscyphus leaves: A safe and promising antidiabetic compound. Journal of Applied Toxicology. 2016;36:1516–1525. doi: 10.1002/jat.3312. [DOI] [PubMed] [Google Scholar]
- Shan J.J., Yang M., Ren J.W. Anti-diabetic and hypolipidemic effects of aqueous-extract from the flower of Inula japonica in alloxan-induced diabetic mice. Biological and Pharmaceutical Bulletin. 2006;29(3):455–459. doi: 10.1248/bpb.29.455. [DOI] [PubMed] [Google Scholar]
- Simelane D.O., Mawela K.V., Fourie A. Prospective agents for the biological control of Tithonia rotundifolia (Mill.) S.F. Blake and Tithonia diversifolia (Hemsl.) A. Gray (Asteraceae) in South Africa. African Entomology. 2011;19(2):443–450. doi: 10.4001/003.019.0223. [DOI] [Google Scholar]
- Simonsen H.T., Weitzel C., Christensen S.B. In: Natural Products. Ramawat K., Mérillon J.M., editors. Springer; Berlin, Heidelberg: 2013. Guaianolide sesquiterpenoids: pharmacology and biosynthesis; pp. 3069–3098. [DOI] [Google Scholar]
- Singh N.P., Lai H.C., Park J.S., Gerhardt T.E., Kim B.J., Wang S., Sasaki T. Effects of artemisinin dimers on rat breast cancer cells in vitro and in vivo. Anticancer Research. 2011;31(12):4111–4114. http://ar.iiarjournals.org/content/31/12/4111.short [PubMed] [Google Scholar]
- Sousa S., Pinto J., Rodrigues C., Gião M., Pereira C., Tavaria F., Pintado M. Antioxidant properties of sterilized yacon (Smallanthus sonchifolius) tuber flour. Food Chemistry. 2015;188:504–509. doi: 10.1016/j.foodchem.2015.04.047. [DOI] [PubMed] [Google Scholar]
- Subramoniam A. CRC Press; Boca Raton: 2016. Plants With Anti-Diabetes Mellitus Properties; pp. 17–432. [DOI] [Google Scholar]
- Sülsen V.P., Catalán C.A.N., Martino V.S. In: Sesquiterpene Lactones: Advances in Their Chemistry and Biological Aspects. Sülsen V.P., Martino V.S., editors. Springer; Cham: 2018. Analytical procedures; pp. 119–135. [DOI] [Google Scholar]
- Sülsen V.P., Martino V.S. In: Sesquiterpene Lactones: Advances in Their Chemistry and Biological Aspects. Sülsen V.P., Martino V.S., editors. Springer; Cham: 2018. Overview; pp. 3–18. [DOI] [Google Scholar]
- Sun J., Wang Y., Fu X., Chen Y., Wang D., Li W., Xing S., Li G. Magnolia officinalis extract contains potent inhibitors against PTP1B and attenuates hyperglycemia in db/db mice. BioMed Research International. 2015 doi: 10.1155/2015/139451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z., Li G., Tong T., Chen J. Micheliolide suppresses LPS-induced neuroinflammatory responses. PLoS One. 2017;12(10) doi: 10.1371/journal.pone.0186592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagne A.M., Marino F., Cosentino M. Tithonia diversifolia (Hemsl.) A. Gray as a medicinal plant: a comprehensive review of its ethnopharmacology, phytochemistry, pharmacotoxicology and clinical relevance. Journal of Ethnopharmacology. 2018;220:94–116. doi: 10.1016/j.jep.2018.03.025. [DOI] [PubMed] [Google Scholar]
- Tisserand R., Young R. second ed. Elsevier Health Sciences; China: 2014. Essential Oil Safety. A Guide for Health Care Professionals. [Google Scholar]
- Tu Y. Artemisinin—A gift from Traditional Chinese Medicine to the world (Nobel Lecture) Angewandte Chemie International Edition. 2016;55:10210–10226. doi: 10.1002/anie.201601967. [DOI] [PubMed] [Google Scholar]
- Viennois E., Xiao B., Ayyadurai S., Wang L., Wang P.G., Zhang Q., Chen Y., Merlin D. Micheliolide, a new sesquiterpene lactone that inhibits intestinal inflammation and colitis-associated cancer. Laboratory Investigation. 2014;94(9):950–965. doi: 10.1038/labinvest.2014.89. [DOI] [PubMed] [Google Scholar]
- Waisundara V.Y., Watawana M.I., Jayawardena N. Costus speciosus and Coccinia grandis: Traditional medicinal remedies for diabetes. South African Journal of Botany. 2015;98:1–5. doi: 10.1016/j.sajb.2015.01.012. [DOI] [Google Scholar]
- Wang G., Qin J., Cheng X., Shen Y., Shan L., Jin H., Zhang W. Inula sesquiterpenoids: structural diversity, cytotoxicity and anti-tumor activity. Expert Opinion on Investigational Drugs. 2014;23(3):317–345. doi: 10.1517/13543784.2014.868882. [DOI] [PubMed] [Google Scholar]
- Wang Z.Q., Zhang X.H., Yu Y., Tipton R.C., Raskin I., Ribnicky D., Johnson W., Cefalu W.T. Artemisia scoparia extract attenuates nonalcoholic fatty liver disease in diet-induced obesity mice by enhancing hepatic insulin and AMPK signaling independently of FGF21 pathway. Metabolism. 2013;62(9):1239–1249. doi: 10.1016/j.metabol.2013.03.004. 2013https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widen J.C., Kempema A.M., Villalta P.W., Harki D.A. Targeting NF-κB p65 with a helenalin inspired bis-electrophile. ACS Chemical Biology. 2017;12(1):102–113. doi: 10.1021/acschembio.6b00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Li X., Adams H., Kubena K., Guo S. Etiology of metabolic syndrome and dietary intervention. International Journal of Molecular Sciences. 2019;20(1):128. doi: 10.3390/ijms20010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Zhao H., Ai H., Zhu H., Wang S., Bao Y., Li Y. Alantolactone suppresses APOC3 expression and alters lipid homeostasis in L02 liver cells. European Journal of Pharmacology. 2018;828:60–66. doi: 10.1016/j.ejphar.2018.03.021. [DOI] [PubMed] [Google Scholar]
- Yin Z., Zhang W., Feng F., Zhang Y., Kang W. α-Glucosidase inhibitors isolated from medicinal plants. Food Science and Human Wellness. 2014;3(3-4):136–174. doi: 10.1016/j.fshw.2014.11.003. [DOI] [Google Scholar]
- Yung J.H.M., Giacca A. Role of c-Jun N-terminal Kinase (JNK) in obesity and type 2 diabetes. Cells. 2020;9(3):706. doi: 10.3390/cells9030706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeggwagh N.A., Ouahidi M.L., Lemhadri A., Eddouks M. Study of hypoglycaemic and hypolipidemic effects of Inula viscosa L. aqueous extract in normal and diabetic rats. Journal of Ethnopharmacology. 2006;108(2):223–227. doi: 10.1016/j.jep.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Zeke A., Misheva M., Reményi A., Bogoyevitch M.A. JNK signaling: regulation and functions based on complex protein-protein partnerships. Microbiology and Molecular Biology Reviews. 2016;80(3):793–835. doi: 10.1128/MMBR.00043-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai J.D., Li D., Long J., Zhang H.L., Lin J.P., Qiu C.J., Zhang Q., Chen Y. Biomimetic semisynthesis of arglabin from parthenolide. Journal of Organic Chemistry. 2012;77(16):7103–7107. doi: 10.1021/jo300888s. [DOI] [PubMed] [Google Scholar]
- Zhao G., Li X., Chen W., Xi Z., Sun L. Three new sesquiterpenes from Tithonia diversifolia and their anti-hyperglycemic activity. Fitoterapia. 2012;83:1590–1597. doi: 10.1016/j.fitote.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Zhao X., Li F., Sun W., Gao L., Kim K.S., Kim K.T., Cai L., Zhang Z., Zheng Y. Extracts of Magnolia species-induced prevention of diabetic complications: A brief review. International Journal of Molecular Sciences. 2016;17(10) doi: 10.3390/ijms17101629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J., Gong W., Chen J., Qing Y., Wu S., Li H., Huang C., Chen Y., Wang Y., Xu Z., Liu W., Li H.Y., Long H. Micheliolide alleviates hepatic steatosis in db/db mice by inhibiting inflammation and promoting autophagy via PPAR-γ-mediated NF-кB and AMPK/mTOR signaling. International Immunopharmacology. 2018;59:197–208. doi: 10.1016/j.intimp.2018.03.036. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Ling Y., Wang X. Alantolactone mitigates renal injury induced by diabetes via inhibition of high glucose-mediated inflammatory response and macrophage infiltration. Immunopharmacology and Immunotoxicology. 2020;42(2):84–92. doi: 10.1080/08923973.2020.1725039. [DOI] [PubMed] [Google Scholar]