Supplemental Digital Content is available in the text
Abstract
We performed an observational prospective monocentric study in patients living with HIV (PLWH) diagnosed with COVID-19. Fifty-four PLWH developed COVID-19 with 14 severe (25.9%) and five critical cases (9.3%), respectively. By multivariate analysis, age, male sex, ethnic origin from sub-Saharan Africa and metabolic disorder were associated with severe or critical forms of COVID-19. Prior CD4+ T cell counts did not differ between groups. No protective effect of a particular antiretroviral class was observed.
In France, the Paris area, where more than 51 000 patients are living with HIV (PLWH), was highly affected by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) pandemic between February and May 2020. However, in most series, PLWH usually account for less than 1% of patients requiring hospitalization [1,2]. Only a few cases series are available concerning coronavirus disease 2019 (COVID-19) occurring in PLWH [1,3–5]. Several risk factors have been well described as associated with severe forms of COVID-19, such as age, male sex, metabolic disorders, cardiovascular and chronic respiratory diseases, while the role of immunosuppression remains unclear [2,6–11]. As PLWH are often carriers of this comorbidities, our objectives were to determine which factors most likely involved in the development of severe forms of COVID-19 in PLWH.
We performed an observational prospective monocentric cohort study of PLWH diagnosed with SARS-CoV2 infection from 1 March to 30 April 2020. Consecutive PLWH taken in care in our department and having developed COVID-19 clinical symptoms and/or hospitalized for COVID-19 in our hospital were included in the study. Data were collected through telephone interviews or from the hospital charts on a questionnaire filled at least 14 days after the first symptoms. Ethics demand of approval was registered with number 2020-A01550-39 with favorable response number 20053-67751.
Qualitative variables were described as numbers and percentages, and quantitative variables as the median and interquartile range (IQR). To assess potential differences between the three ‘moderate’, ‘severe’ and ‘critical’ cases, the Kruskal–Wallis and chi-squared tests were used, as appropriate. Given the small number of critical diseases, we pooled the ‘critical’ and ‘severe’ patients. The Wilcoxon-Mann–Whitney test, chi-squared or Fisher's exact tests were used to compare quantitative and qualitative variables between the ‘moderate’ and ‘severe/critical’ groups, respectively. Risk factors associated with severe and critical disease were determined using multivariable logistic regression models. In order to identify new independent predictors, we adjusted for age and sex. Statistical significance was considered for P value less than 0.05. As ethnic origin and BMI were highly correlated, we could not test those two variables into the same model. Analyses were performed using Stata software (version 14.2; StataCorp, College Station, Texas, USA).
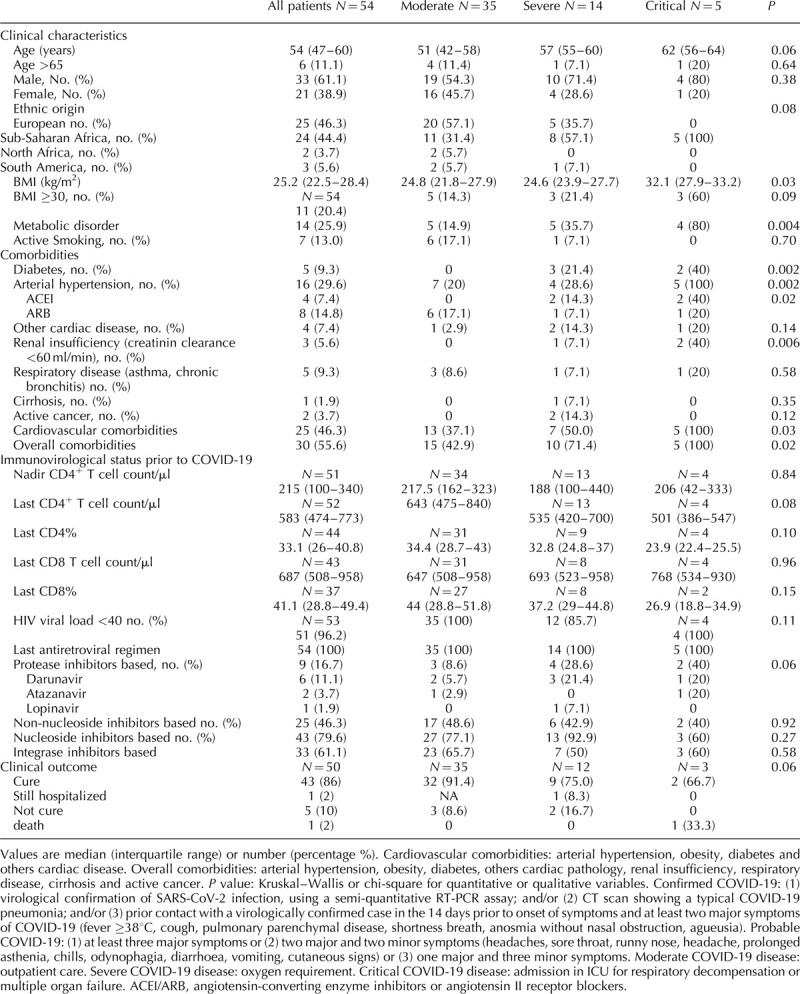
A total of 54 PLWH with COVID-19 were included for analysis, of whom 61.1% were men. Median age was 54 (IQR: 47–60) years. Prior to COVID-19, all were treated by combined antiretroviral drugs and 51 out of 53 (96.2%) had RNA HIV less than 40 copies/ml with last median CD4+ T cell counts at 583 cells/μl (IQR: 474–773). Compared with the whole out-patient population of 4000 PLWH, the proportion of COVID-19 patients was higher in those from sub-Saharan Africa origin (44.6 vs. 29.7%) and did not differ in patients treated with a protease inhibitor (16.7 vs. 19.7%). The diagnosis of COVID-19 was confirmed in 38 cases (70.3%) and judged to be probable according to the association of clinical criteria in 16 cases (29.6%). All cases were followed-up for at least 14 days and for a median duration of 29 days (29–45 days). Clinical course of COVID-19 was moderate in 35 cases (64.8%), severe in 14 cases (25.9%) and critical in five cases (9.3%). One patient died. As summarized in Table 1, patients with critical COVID-19 tended to be older than those with severe disease, and those with a moderate disease (P = 0.06). In severe and critical forms, 13 out of 19 patients were from sub-Saharan Africa origin vs. 11 out of 35 in moderate form. Those who developed severe or critical disease had more frequently metabolic disorders (P = 0.004), hypertension (P = 0.002) and/or renal insufficiency (P = 0.006). By multivariate analysis, increased age, male sex and being from sub-Saharan Africa origin were independently associated with severe and critical forms of COVID-19 in PLWH. Furthermore, the presence of a metabolic disorder (obesity and/or diabetes) was independently associated with the occurrence of severe and critical forms. There was no significant difference (for data prior COVID-19), between severe/critical disease and moderate/minor disease for CD4+ T cell count, proportion of patients with HIV RNA less than 40 copies/ml or receiving a protease inhibitor based treatment.
Table 1.
Comparison of moderate vs. severe and critical forms of COVID-19 in patients living with HIV (n = 54).
As in the general population, the severity of COVID-19 in PLWH was associated with older age, male sex and metabolic disorders, such as obesity and diabetes [2,6–11]. We also found that patients who originated from sub-Saharan Africa possibly were at an increased risk of disease severity. Black ethnicity has already been suggested to be associated with poor outcome of COVID-19 in at least two studies [12,13]. One explanation for this finding could be a higher frequency of metabolic disorders and social deprivation in this population as observed in the UK [13]. Poor outcome could be related to particular genetic traits in these populations. It is very important to confirm (or not) these data and explain this finding, especially as epidemiological trends raise fears that the COVID-19 epidemic is expanding in Africa [14,15]. On the basis of low level of evidence-based data, international recommendations state that PLWH are not at higher risk than the general population, provided that CD4+ T cell count are greater than 200 cells/μl and viral replication is controlled by antiretroviral treatment [16,17]. Our study did not allow us to assess the risk linked to immune deficiency, especially as all patients were taking antiretroviral treatment. We found a trend for lower CD4+ T cell counts in severe forms as suggested in a previous study based on low-level evidence [4]. As elevated IL-6 levels are associated with older age and higher BMI in PLWH, higher viral replication and low nadir CD4+ T cell count could be associated with poor outcome [18]. Larger prospective cohort studies, including patients under and not yet taking antiretroviral drugs, are warranted in order to fully assess the determinants of health outcomes in PLWH with COVID-19. Finally, we did not find any protective effect of protease inhibitors, but these results may be biased by the fact that protease inhibitors are usually prescribed as second-line antiretrovirals. Larger studies are also needed on this topic.
Acknowledgements
The COVID-19 ID Team: Dominique Salmon, Laurence Weiss, Jean-Paul Viard, Laurence Slama, Juliette Pavie, Marina Karmochkine, Dominique Batisse, Nicolas Etienne, Patricia Brazille, Laurent Finkielsztejn, Gabriela Spiridon, Benjamin Silbermann, Olivier Zak dit Zbar, Philippe Blanche, Rafael Usubillaga, Mohamed Meghadecha, Myriam Kalambay, Nicolas Carlier, Jean-Pierre Morini, Odile Launay, Thu Huyen Nguyen, Hassan Joumaa, Valérie Lebaut, Marie Pierre Pietri and Marie Laure Lucas, Dorsaf Slama, Valérie Anne Letembet
DS, NE, FC, LW and JPV conceived and designed the study. All the members of the COVID-19 ID Team were responsible for patient inclusion, clinical follow-up and helped to write the manuscript. RU, MM, MK and VL collected the data. FC and LS took responsibility for the integrity of the data and the accuracy of the data analysis. All authors critically revised the manuscript and gave final approval for the final version.
This study was not sponsored by any external financial support.
All the authors have read and agreed with the study's content. No authors have financial or personal conflicts. Neither the work nor any part of its essential substance, tables or figures have been or will be published or submitted to another scientific journal or are being considered for publication elsewhere.
Conflicts of interest
None.
SDC Table2:
Supplementary Material
References
- 1.Blanco JL, Ambrosioni J, Garcia F, Martínez E, Soriano A, Mallolas J, et al. COVID-19 in patients with HIV: clinical case series. Lancet HIV 2020; 7:e314–e316.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323:2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gervasoni C, Meraviglia P, Riva A, Giacomelli A, Oreni L, Minisci D, et al. Clinical features and outcomes of HIV patients with coronavirus disease 2019. Clin Infect Dis 2020; ciaa579.doi:10.1093/cid/ciaa579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Childs K, Post FA, Norcross C, Ottaway Z, Hamlyn E, Quinn K, et al. Hospitalized patients with COVID-19 and HIV: a case series. Clin Infect Dis 2020; ciaa657.doi:10.1093/cid/ciaa657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vizcarra P, Pérez-Elías MJ, Quereda C, Moreno A, Vivancos MJ, Dronda F, et al. Description of COVID-19 in HIV-infected individuals: a single-centre, prospective cohort. Lancet HIV 2020; S2352-3018(20)30164-8. doi:10.1016/S2352-3018(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–1720.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323:1239. [DOI] [PubMed] [Google Scholar]
- 8.Wu JT, Leung K, Bushman M, Kishore N, Niehus R, de Salazar PM, et al. Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat Med 2020; 26:506–510.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis 2020; 94:91–95.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020; 46:846–848.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dietz W, Santos-Burgoa C. Obesity and its implications for COVID-19 mortality. Obesity 2020; 28:1005–11005.. [DOI] [PubMed] [Google Scholar]
- 12.Millett GA, Jones AT, Benkeser D, Baral S, Mercer L, Beyrer C, et al. Assessing differential impacts of COVID-19 on black communities. Ann Epidemiol 2020; 47:37–44.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coronavirus (COVID-19) related deaths by ethnic group, England and Wales - Office for National Statistics [Internet]. [cited 3 June 2020]. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/coronavirusrelateddeathsbyethnicgroupenglandandwales/latest. [Accessed 29 July 2020]. [Google Scholar]
- 14.Pearson CA, Van Schalkwyk C, Foss AM, O’Reilly KM. SACEMA Modelling and Analysis Response Team, CMMID COVID-19 working group Projected early spread of COVID-19 in Africa through 1 June 2020. Euro Surveill 2020; 25:2000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells CR, Stearns JK, Lutumba P, Galvani AP. COVID-19 on the African continent. Lancet Infect Dis 2020; S1473-3099(20)30374-1. doi: 10.1016/S1473-3099(20)30374-1. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.GmbH B EACS-BHIVA statement 1 April [Internet]. EACSociety 2020; https://www.eacsociety.org/home/eacs-bhiva-statement-1-april.htmlhttps://www.eacsociety.org/home/eacs-bhiva-statement-1-april.html. [Accessed 29 July 2020]. [Google Scholar]
- 17.GmbH B COVID-19 and HIV [Internet]. EACSociety 2020; https://www.eacsociety.org/home/covid-19-and-hiv.htmlhttps://www.eacsociety.org/home/covid-19-and-hiv.html. [Accessed 29 July 2020]. [Google Scholar]
- 18.Hernández-Walias F, Ruiz-de-León MJ, Rosado-Sánchez I, Vázquez E, Leal M, Moreno S, et al. New signatures of poor CD4 cell recovery after suppressive antiretroviral therapy in HIV-1-infected individuals: involvement of miR-192, IL-6, sCD14 and miR-144. Sci Rep 2020; 10:2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


