Supplemental Digital Content is available in the text
Keywords: coronavirus disease 2019, HIV, prospective observational trial, severe acute respiratory syndrome coronavirus 2
Abstract
Background:
It is unclear how characteristics, risk factors, and incidence of coronavirus disease 2019 (COVID-19) in people living with HIV (PLWH) differ from the general population.
Methods:
Prospective observational single-center cohort study of adult PLWH reporting symptoms of COVID-19. We assessed clinical characteristics, risk factors for COVID-19 diagnosis and severity, and standardized incidence rate ratio for COVID-19 cases in PLWH cohort and in Barcelona.
Results:
From 1 March 2020 to 10 May 2020, 53 out of 5683 (0.9% confidence interval 0.7–1.2%) PLWH were diagnosed with COVID-19. Median age was 44 years, CD4+ T cells were 618/μl and CD4+/CD8+ was 0.90. All but two individuals were virologically suppressed. Cough (87%) and fever (82%) were the most common symptoms. Twenty-six (49%) were admitted, six (14%) had severe disease, four (8%) required ICU admission, and two (4%) died. Several laboratory markers (lower O2 saturation and platelets, and higher leukocytes, creatinine, lactate dehydrogenase, C reactive protein, procalcitonin, and ferritin) were associated with COVID-19 severity. No HIV or antiretroviral-related factors were associated with COVID-19 diagnosis or severity. Standardized incidence rate ratios of confirmed or confirmed/probable COVID-19 in PLWH were 38% (95% confidence interval 27–52%, P < 0.0001) and 33% (95% confidence interval 21–50%, P < 0.0001), respectively relative to the general population.
Conclusion:
PLWH with COVID-19 did not differ from the rest of the HIV cohort. Clinical presentation, severity rate, and mortality were not dependent on any HIV-related or antiretroviral-related factor. COVID-19 standardized incidence rate was lower in PLWH than in the general population. These findings should be confirmed in larger multicenter cohort studies.
Introduction
Risk for severe coronavirus disease 2019 (COVID-19) increases with age, male sex, and comorbidities [1–3]. Although individuals with immunosuppression have higher fatality rates [4], there is no clear evidence for a higher COVID-19 infection rate or different disease course in people living with HIV (PLWH) than in the general population [5–10]. In hospitalized patients with COVID-19, less than 1% involved PLWH and HIV did not adversely impact survival [3,11,12].
Low (<200/μl) CD4+ cell counts or not being on antiretroviral therapy have been suggested as contributing factors for a higher severity of COVID-19 in PLWH [13,14]. Moreover, other general factors may be over-represented in PLWH. In Spain more than three quarters of PLWH are men [15], and among PLWH over 50 years of age more than 50% have comorbidities [16].
Alternatively, some factors in PLWH might be protective for COVID-19. Antiretroviral therapy was suggested as a protective factor during the severe acute respiratory syndrome epidemic in 2003 [17]. Some antiretroviral agents such as lopinavir/ritonavir are currently being tested for COVID-19 in major trials such as RECOVERY (ClinicalTrials.gov: NCT04381936) or SOLIDARITY (ISRCTN83971151). In-vitro studies have shown that some nucleoside reverse transcriptase inhibitors used as anti-HIV therapy may inhibit the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA-dependent RNA polymerase [18,19]. Moreover, the persistent immune dysregulation that accompanies HIV infection despite effective antiretroviral therapy could play a role in preventing the cytokine storm that is characteristic of severe COVID-19 [20], although this point remains speculative.
We aimed to assess the clinical characteristics and outcome, the risk factors, and the incidence of COVID-19 in a large cohort of PLWH.
Methods
Study design and participants
Prospective observational study carried out at Hospital Clinic of Barcelona (Spain). All consecutive PLWH 18 years or older reporting symptoms suggestive of COVID-19 from 1 March 2020 to 10 May 2020 were included. A check-list of COVID-19 symptoms was assessed in each patient [1,21–24]. Demographics, HIV characteristics, and COVID-19-related clinical, laboratory, radiology, treatment, and outcome data were obtained from the hospital database. The study was approved by the local Research Ethics Committee (HCB/2020/0331) and individuals provided informed consent.
Procedures and definitions
According to the hospital COVID-19 Standard Operating Procedure (SOP), criteria for admission included respiratory rate more than 20 bpm, room air oxygen saturation less than 95%, and any comorbidity; having CD4+ T cell counts less than 350/μl was also a criterion for PLWH admission. PLWH had no restrictions regarding therapeutic resources including ICU admission. SARS-CoV-2, influenza A and B, and syncytial respiratory virus were assessed in nasal and pharyngeal swabs by real-time reverse-transcriptase–PCR (RT-PCR). ELISA IgM/IgA/IgG blood test performed 3 weeks or longer after the onset of the symptoms was used to confirm COVID-19 whenever SARS-CoV-2 RT-PCR had not been done.
Confirmed and probable COVID-19 cases were defined according to the European Centre for Disease Prevention and Control (ECDC) Case Definition for COVID-19 [25]. The degree of severity of COVID-19 was defined according to the American Thoracic Society guidelines for community-acquired pneumonia [26]. Last CD4+ T-cell count and plasma HIV RNA (viral load) were the most recent values registered at the clinical database, usually within the last 6 months period.
Statistical analysis
We calculated absolute frequencies and proportions for categorical variables and median and interquartile range (IQR) or mean and SD for quantitative variables. No imputation was made for missing data. Fisher's exact or Chi-squared and Wilcoxon Rank Sum tests were used for comparisons. Standardized incidence rates of COVID-19 were estimated along with 95% confidence interval (CI) using a Poisson regression model with robust standard errors. The standardized incidence rate ratio represents the ratio of the number of observed COVID-19 cases in the cohort of PLWH to the number that would be expected if this cohort had the same age and sex-specific rates as in Barcelona. The number of new COVID-19 cases during the period of study was obtained from the Government of Catalonia registry [27] and the age and sex of Barcelona population from the Statistical Institute of Catalonia [28]. All tests were two-tailed and the significance level was set at 0.05. We used REDCap for data storage and STATA (version 15; Stata Corp LLC, College Station, Texas, USA) for statistical analyses.
Results
People living with HIV with symptoms suggestive of coronavirus disease 2019
By 10 May 2020, 62 PLWH out of 5683 (1.09%) in the cohort had contacted the hospital reporting symptoms suggestive of COVID-19. Nine patients tested negative for SARS-CoV-2 RT-PCR and alternative causes were: pneumococcal pneumonia (n = 2), syphilis (n = 2), urinary tract infection (n = 2), tuberculosis (n = 1), acute tonsillitis (n = 1), and diabetic ketoacidosis (n = 1). COVID-19 was confirmed in 42 cases and probable in 11 cases. No other viral respiratory infections concomitant to SARS-CoV-2 were found.
Clinical characteristics of coronavirus disease 2019 people living with HIV and outcome
Characteristics of COVID-19 PLWH are summarized in Table 1. Median age was 44 years; 15 (28%) were older than 50 years and only five (9%) older than 65 years. Eight (15%) were healthcare workers and 17 (46%) had a recent history of a close contact with COVID-19 patients. Twenty-three (43%) patients had at least one comorbidity, 16 (31%) were current smokers, and 11 (21%) were recreational drug users in the context of group sex. Median last CD4+ T-cell counts and CD4+/CD8+ ratio were 618 cells/μl and 0.90, respectively. Eight (15%) and two (4%) individuals had CD4+ T-cell counts below 350 and 200 cells/μl, respectively. All but two individuals were on antiretroviral therapy and those treated were virologically suppressed. Forty-four (83%) patients were receiving triple therapy: 15 (28%) a boosted-protease inhibitor-regimen (all of them cobicistat-boosted darunavir), and 35 (66%) a tenofovir-containing regimen.
Table 1.
Characteristics of coronavirus disease 2019 patients.
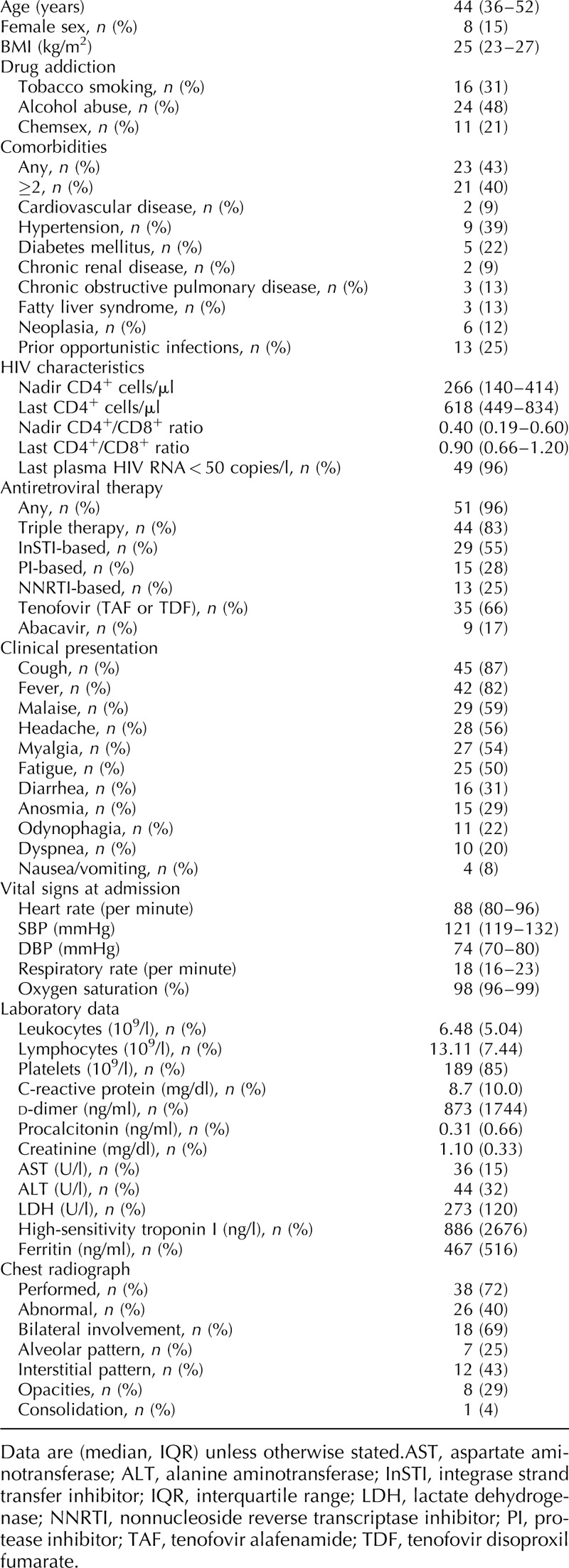
The median (IQR) time from the onset of the symptoms to medical consultation was 4 (3–7) days. Cough (n = 45, 87%) and fever (n = 42, 82%) were the most common symptoms, while gastrointestinal symptoms including nausea or vomiting (n = 4, 8%) or diarrhea (n = 16, 31%) were rare. Of 38 (72%) patients with a chest radiograph at admission, 26 (70%) had bilateral involvement (n = 18, 69%) or interstitial pattern in (n = 12, 43%) as the most common radiological features.
Twenty-six (49%) patients were admitted. The median (IQR) duration of hospitalization was 4 days (1–9). Four (7.5%) patients required ICU admission and two (4%) died. Among the PLWH admitted (n = 26, 49%), all but two received any specific COVID-19 therapy: 21 received triple therapy with a combination of lopinavir/ritonavir, azithromycin, and hydroxychloroquine, and five received dual therapy with a combination of two of the previous three drugs. Two (8%) individuals also received IFN-1β, seven (28%) systemic glucocorticoids, or eight (32%) tociluzimab. Mechanical ventilation was used in two patients and extracorporeal membrane oxygenation in one patient. Of the PLWH not admitted to hospital (n = 27, 51%), only one received specific COVID-19 treatment (azithromycin and hydroxychloroquine) and none switched their antiretroviral regimen.
Factors associated with severity among people living with HIV with coronavirus disease 2019
There were six (11%) severe COVID-19 cases. They had more commonly neoplasia (n = 4, 67% vs. n = 2, 4%; P = 0.0008) among comorbidities, more commonly dyspnea (n = 5, 83% vs. n = 5, 11%; P = 0.0006) and less commonly headache (n = 0, 0% vs. n = 28, 64%; P = 0.0047) at presentation, lower median (IQR) O2 saturation (93% (93–96) vs. 98% (96–99); P = 0.0121), lower median (IQR) platelets [135 (80–163–13.04) vs. 5.93 (4.10–7.29) × 109/l; P = 0.0489], higher median (IQR) leukocytes (8.91 (6–51–13.04) vs. 5.93 (4.10–7.29) × 109/L; P = 0.0489), higher creatinine [1.45 (1.30–1.63) vs. 1.01 (0.85–1.13) mg/dl; P = 0.0062], higher lactate dehydrogenase [332 (316–357) vs. 239 (172–270) U/l; P = 0.0232], higher median (IQR) C reactive protein [16.99 (5.46–30.00) vs. 3.01 (0.45–10.21) mg/dl; P = 0.0186], higher procalcitonin [0.72 (0.12–1.63) vs. 0.03 (0.03–0.08) ng/ml; P = 0.0016], and higher ferritin [905 (595–1101) vs. 200 (84–311) ng/ml; P = 0.0020]. We were unable to find any HIV or antiretroviral factors significantly associated with COVID-19 severity.
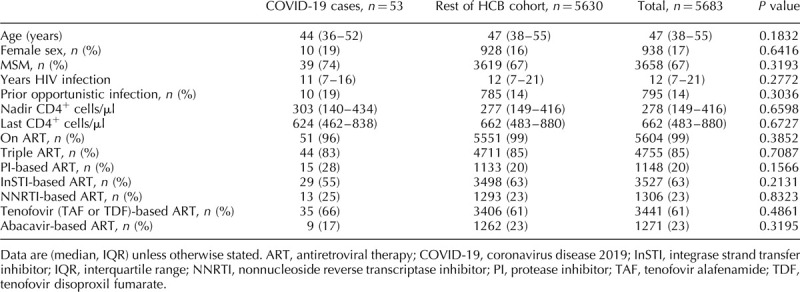
Factors associated with coronavirus disease 2019 in the people living with HIV cohort
We did not identify any demographic, HIV, or antiretroviral factor significantly associated with the diagnosis of COVID-19 in PLWH (Table 2).
Table 2.
Factors associated with severe coronavirus disease 2019 in the Hospital Clinic Barcelona people living with HIV cohort.
Incidence of coronavirus disease 2019 in the people living with HIV cohort compared with the incidence of coronavirus disease 2019 in the city of Barcelona
The standardized incidence rates of confirmed COVID-19 were 107 (95% CI 72–141) cases per 10 000 in the PLWH cohort and 282 (282–282) cases per 10 000 Barcelona inhabitants. The standardized incidence rate ratio was 0.38 (95% CI 0.27–0.52, P < 0.0001) indicating that confirmed COVID-19 cases in the PLWH cohort were 62% lower than those in Barcelona.
Similarly, the standardized incidence rates of confirmed/probable COVID-19 cases were 136 (95% CI 78–193) cases per 10000 PLWH and 417 (417–417) cases per 10 000 Barcelona inhabitants. The standardized incidence rate ratio was 0.33 (95% CI 0.21–0.50, P < 0.0001), which means that confirmed/probable COVID-19 cases in the PLWH cohort were 67% lower than those in Barcelona.
Discussion
To our knowledge, this is the first prospective study assessing clinical characteristics and outcome, risk factors, and incidence of symptomatic COVID-19 in a large cohort of PLWH. During the COVID-19 epidemics in Barcelona (Spain), approximately 1% of PLWH sought help for clinical symptoms suggestive of COVID-19. Although the vast majority of them had COVID-19 confirmed, 15% had other causes that may also affect PLWH.
Clinical characteristics of COVID-19 did not differ from those already described in the general population [1,3,11,12,23,29,30]. However, cough, fever, bilateral lung infiltrates, and lymphocytopenia should prompt differential diagnosis with opportunistic diseases such as Pneumocystis jiroveci, mycobacterial or cryptoccocal pneumonia. The 4% rate of mortality we saw in PLWH is similar to that reported for the general population aged 50–59 years in patients with COVID-19 admitted to a major Spanish hospital [12].
Because anti-COVID-19 therapy was protocolized in the SOP, in general PLWH received similar anti-COVID-19 therapy as other non-HIV-infected patients. This meant that antiretroviral therapy was temporarily switched to a regimen that included lopinavir/ritonavir unless otherwise contraindicated. We are unable to provide further data on the efficacy of this strategy against COVID-19 in PLWH. As in the general population, some PLWH with a worse clinical evolution also received other drugs including IFN-1β, glucocorticoids, and tociluzimab according to SOP [31,32].
We were unable to identify any HIV or antiretroviral factors associated with COVID-19 severity. As reported in the general population [32], several laboratory parameters best distinguished between severe and nonsevere COVID-19 in PLWH. These results suggest that HIV characteristics including CD4+, CD4+/CD8+ ratio, or plasma viral load, or the type of antiretroviral therapy do not seem to play a major influence on the severity of COVID-19 in PLWH. In addition, we were unable to identify any epidemiological, HIV, or antiretroviral factor associated with the diagnosis of COVID-19 in PLWH.
The incidence of COVID-19 seen in this cohort of PLWH was much lower than that of the general population adjusted by age and sex living the same geographical area. This finding needs further confirmation in larger cohort studies. Whether it may be due to HIV, antiretroviral therapy, or other factors should be subject of further research.
The current study is limited by the low number of PLWH diagnosed with symptomatic COVID-19. Only symptomatic PLWH were included in this study, but we now know that asymptomatic COVID-19 is not unusual [33]; we are systematically performing ELISA IgM/IgA/IgG blood tests to know the real incidence of COVID-19 in the PLWH cohort. This cohort of PLWH had universal standardized HIV and COVID-19 care and this situation may not be extrapolated to other settings. The lack of identification of HIV or antiretroviral factors associated with a higher risk or severity of COVID-19 in PLWH does not rule out that they may exist. Although probable cases in the general population fulfilled the ECDC Case Definition for COVID-19 [24], they could be due to causes other than SARS-CoV-2 that were not so thoroughly investigated as we did in the PLWH cohort and therefore the comparison of incidences between PLWH and the general population should be taken with caution (Supplementary Figure).
In summary, COVID-19 in PLWH had similar clinical characteristics and outcome but a lower incidence than in the general population. We were unable to identify any major role of HIV or antiretroviral factors on the risk or severity. These findings should be confirmed in larger multicenter cohort studies.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Supplementary Material
Footnotes
Josep M. Miro, Esteban Martinez, and Jose L. Blanco have equivalent merit as senior authors.
The COVID-19 in HIV Investigators are listed in the Supplementary Appendix 2.
References
- 1.Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–1720.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis 2020; 94:91–95.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323:2052–2059.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang W, Su B, Pang L, Qiao L, Feng Y, Ouyang Y, et al. High-dimensional immune profiling by mass cytometry revealed immunosuppression and dysfunction of immunity in COVID-19 patients. Cell Mol Immunol 2020; 17:650–652.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanco JL, Ambrosioni J, Garcia F, Martinez E, Soriano A, Mallolas J, et al. COVID-19 in patients with HIV: clinical case series. Lancet HIV 2020; 7:e314–e316.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Härter G, Spinner CD, Roider J, Bickel M, Krznaric I, Grunwald S, et al. COVID-19 in people living with human immunodeficiency virus: a case series of 33 patients. Infection 2020; 1–6.. [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Q, Chen T, Zhang H. Recovery from COVID-19 in two patients with coexisted HIV infection. J Med Virol 2020; 10.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karmen-Tuohy S, Carlucci PM, Zacharioudakis IM, Zervou FN, Rebick G, Klein E, et al. Outcomes among HIV-positive patients hospitalized with COVID-19. medRxiv 2020; [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gervasoni C, Meraviglia P, Riva A, Giacomelli A, Oreni L, Minisci D, et al. Clinical features and outcomes of HIV patients with coronavirus disease 2019. Clin Infect Dis 2020; ciaa579.doi:10.1093/cid/ciaa579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vizcarra P, Perez-Elias MJ, Quereda C, Moreno A, Vivancos MJ, Dronda F, et al. Description of COVID-19 in HIV-infected individuals: a single centre, prospective cohort. Lancet HIV 2020; S2352-3018(20)30164-8. [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 16,749 hospitalised UK patients with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol. medRxiv 2020; 369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borobia AM, Carcas AJ, Arnalich F, Alvarez-Sala R, Montserrat J, Quintana M, et al. A cohort of patients with COVID-19 in a major teaching hospital in Europe. J Clin Med 2020; 9:E1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. What to Know About HIV and COVID-19. Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention. Content source: National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases. [Accesssed 26 June 2020] [Google Scholar]
- 14.COVID-19 & HIV. BHIVA, DAIG, EACS, GESIDA & Polish Scientific AIDS Society. Statement on risk of COVID-19 for people living with HIV (PLWH) (Last Updated May 25, 2020). Available at https://www.eacsociety.org/home/covid-19-and-hiv.html. [Accesssed 26 June 2020]. [Google Scholar]
- 15.Centro Nacional de Epidemiología – Instituto de Salud Carlos III/Plan Nacional sobre el Sida – D.G. de Salud Pública CeICNdE-I Encuesta Hospitalaria de pacientes con infección por el VIH. Resultados 2018. Análisis de la evolución 2003–2018. 2019; Available from: https://www.mscbs.gob.es/ciudadanos/enfLesiones/enfTransmisibles/sida/vigilancia/InformeEncuestaHospitalaria2018_def.pdf. [Accesssed 26 June 2020]. [Google Scholar]
- 16.del Amo J, Campbell C, Navarro G, Segura F, Suarez I, Teira R, et al. VIH en España 2017: políticas para una nueva gestión de la cronicidad más allá del control virológico [HIV in Spain 2017: policies for a new management of chronicity beyond virological control]. Rev Esp Salud Publica 2018; 92:e201809062. [PMC free article] [PubMed] [Google Scholar]
- 17.Chen XP, Cao Y. Consideration of highly active antiretroviral therapy in the prevention and treatment of severe acute respiratory syndrome. Clin Infect Dis 2004; 38:1030–1032.. [DOI] [PubMed] [Google Scholar]
- 18.Jockusch S, Tao C, Li X, Anderson TK, Chien M, Kumar S, et al. Triphosphates of the two components in DESCOVY and TRUVADA are inhibitors of the SARS-CoV-2 polymerase. bioRxiv 2020; [Online ahead of print]. [Google Scholar]
- 19.Ju J, Li X, Kumar S, Jockusch S, Chien M, Tao C, et al. Nucleotide analogues as inhibitors of SARS-CoV polymerase. bioRxiv 2020; [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laurence J. Why aren’t people living with HIV at higher risk for developing severe coronavirus disease 2019 (COVID-19)?. AIDS Patient Care STDS 2020; 34:247–248.. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. Coronavirus disease (COVID-19) Pandemic. Coronavirus. Symptoms. In: https://www.who.int/health-topics/coronavirus#tab=tab_3. [Google Scholar]
- 22.National Center for Immunization and Respiratory Diseases (NCIRD) DoVD. Symptoms of Coronavirus. Available from: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. [Updated Page last reviewed: 20 March 2020]. [Google Scholar]
- 23.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–1062.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. Global surveillance for COVID-19 caused by human infection with COVID-19 virus: interim guidance. In: https://www.who.int/publications/i/item/global-surveillance-for-covid-19-caused-by-human-infection-with-covid-19-virus-interim-guidance. [Accesssed 9 June 2020]. [Google Scholar]
- 26.Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200:e45–e67.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.(AQuAS) AdQiASdC Mapa interactiu de casos per municipi. 2020; Available from: http://aquas.gencat.cat/ca/actualitat/ultimes-dades-coronavirus/mapa-per-municipis/.[5 May 2020]. [Google Scholar]
- 28.Catalonia SIoCGo Població. Per sexe i edat quinquennal. 2020; Available from: http://www.idescat.cat/pub/?id=pmh&n=9548&geo=mun:080193&lang=en. [Accesssed 9 June 2020]. [Google Scholar]
- 29.Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope 2020; 130: 1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 2020; 277:2251–2261.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jean SS, Lee PI, Hsueh PR. Treatment options for COVID-19: the reality and challenges. J Microbiol Immunol Infect 2020; 53:436–443.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Felsenstein S, Herbert JA, McNamara PS, Hedrich CM. COVID-19: immunology and treatment options. Clin Immunol 2020; 215:108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao Z, Xu Y, Sun C, Wang X, Guo Y, Qiu S, et al. A systematic review of asymptomatic infections with COVID-19. J Microbiol Immunol Infect 2020; 10.1016/j.jmii.2020.05.001. doi:10.1016/j.jmii.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


