Abstract
Diffusion basis spectrum imaging (DBSI) models diffusion-weighted MRI signals as a combination of discrete anisotropic diffusion tensors and a spectrum of isotropic diffusion tensors. Here we report the histopathological correlates of DBSI in the biopsied brain tissue of a patient with an inflammatory demyelinating lesion typical of multiple sclerosis (MS). Increased radial diffusivity (marker of demyelination), decreased fiber fraction (apparent axonal density), elevated non-restricted isotropic fraction (marker of vasogenic edema), but unchanged axial diffusivity (marker of integrity of residual axons) seen in the lesion appeared consistent with histopathological findings of inflammatory demyelination with relative axonal sparing. Our report supports the application of DBSI as a biomarker in human studies of MS.
Introduction
Multiple sclerosis (MS) is a pathologically heterogeneous disease with varying degrees of demyelination, inflammation, and axonal injury or loss.1 An unmet need remains for developing pathologically specific, non-invasive quantitative neuroimaging biomarkers of central nervous system (CNS) pathologies in MS.2 Diffusion basis spectrum imaging (DBSI) models diffusion-weighted magnetic resonance imaging (MRI) signals as a combination of discrete anisotropic diffusion tensors (representing the integrity of axon fibers and myelin while resolving for crossing fibers), and a spectrum of isotropic diffusion tensors (reflecting the extra-axonal non-anisotropic environment associated with inflammation and tissue loss).3,4 Details of DBSI and its accuracy in capturing white matter (WM) pathologies have been described in tissue phantoms, spinal cord and optic nerves of mice with experimental allergic encephalomyelitis, the corpus callosum of mice with cuprizone-induced demyelination, and autopsied MS spinal cord specimens.4–7 Here for the first time we report the histopathological correlates of DBSI in the biopsied brain tissue of a living person with inflammatory demyelinating lesions consistent with MS. We also compared the DBSI findings with those of diffusion tensor imaging (DTI), an earlier diffusion method used to study WM which is confounded by inflammation and crossing fibers.8,9
Methods
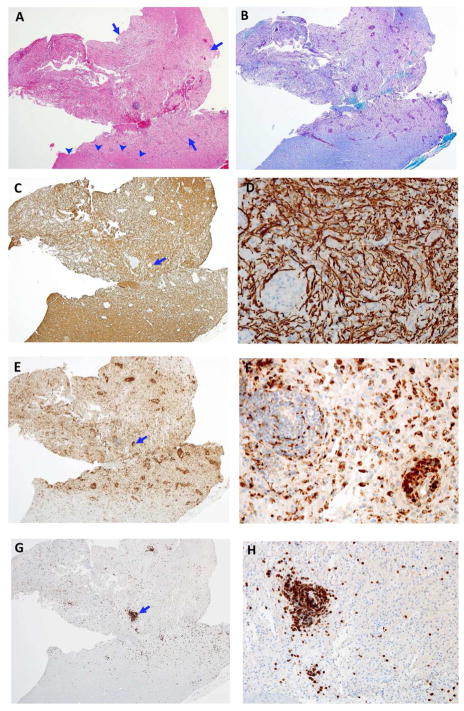
A 53-year-old man presented with seizures. Brain MRI demonstrated several WM T2-weighted MRI abnormalities including a large right frontal lesion with surrounding edema (Figure 1-A). Gadolinium was not administered due to renal insufficiency. Due to the atypical presentation and tumefactive right frontal WM lesion, brain biopsy was performed. To visualize the pathological processes, a combination of histologic and immunohistochemical stains was used including Luxol Fast Blue (for myelin), and antibodies to neurofilament protein (for axons), CD68 (for macrophages/microglia), and CD3 (for T cells) (Figure 2).
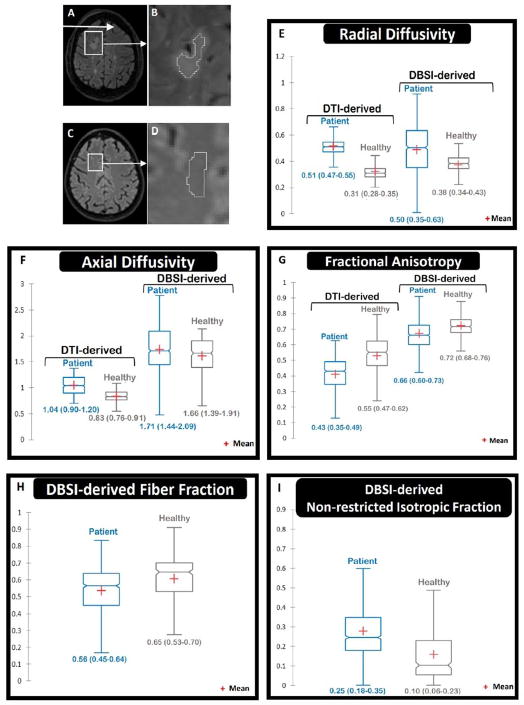
Figure 1.
A & B FLAIR image shows the region of interest (ROI) in the biopsied lesion (excluding the actual biopsy site, and separated by a two-voxel layer to avoid possible artifacts). Other disseminated white matter hyperintensities are seen in the FLAIR image, too. C & D. A comparable ROI in the right frontal white matter of a healthy control was identified. In panels E to I, the central horizontal line in each box plot shows medians. The vertical thickness of each box represents the interquartile range (IQR). Numbers below each box show median (1st quartile – 3rd quartile). Notches show the 95% confidence intervals (CI) around medians (non-overlapping CIs represent statistical significance). The lower and upper whiskers show the lowest and highest values within 1.5 IQR of the lower and higher quartiles, respectively. E. Both DTI-derived and DBSI-derived radial diffusivity (RD) were increased in the lesion compared to the healthy control (consistent with demyelination). F. DTI-derived axial diffusivity (AD) was increased in the lesion (confounded by isotropic fraction such as edema); whereas DBSI-derived AD was similar to the healthy control (consistent with preserved integrity of residual axons). G. Both DTI-derived and DBSI-derived fractional anisotropy were decreased (consistent with impaired white matter integrity). H. DBSI-derived fiber fraction was modestly decreased in the lesion compared to the healthy control (consistent with relatively reduced axonal content). I. DBSI non-restricted isotropic fraction was elevated in the lesion (consistent with edema).
Figure 2.
Pathology of the biopsied right frontal lesion. A. This image of a hematoxylin & eosin (H&E, 4X magnification) stained neurosurgical specimen shows patchy pallor of the white matter (between arrows), an edge of normal white matter (arrowheads) and perivascular lymphocytic cuffing. B. Image of the same area as A stained with luxol fast blue-periodic acid-schiff (LFB-PAS, 4X magnification) stain for myelin shows loss of myelin involving the pale area of image A compared to adjacent white matter. C. An adjacent section demonstrates relative sparing of neurofilament stained axons in the area lacking myelin (same magnification). This confirms a demyelinative process, i.e. near complete loss of myelin with relative axonal preservation (neurofilament immunohistochemistry [NF IHC]). D. Higher magnification of a demyelinated area of C (arrow) shows large numbers of normal axons (NF IHC). E. The same area as A, B, and C demonstrates large numbers of perivascular and parenchymal macrophages (CD68 IHC). F. Higher magnification of the area marked with arrow in E shows perivascular and parenchymal macrophages. G. The same area as A, B, C, and E shows numerous CD3+ T cells (CD3 IHC). H. Higher magnification of the area marked with arrow in G shows a large number of CD+3 T cells.
Diffusion-weighted imaging (DWI) was performed three weeks after the biopsy, prior to administration of corticosteroids or any disease modifying therapy. Diffusion data were collected on a 3 Tesla TIM Trio (Siemens) scanner with a 32-channel head coil at 2×2×2 mm3 resolution in the axial plane with repetition time/echo time=10,000/120 ms, and employing a 99-direction diffusion-weighting scheme (maximum b value=2200 s/mm2) with a total acquisition time of 16 minutes, as described.3,4 Voxel-wise DBSI and DTI metrics were calculated from six contiguous slices of the biopsied right frontal lesion. The region of interest (ROI) within the lesion (Figure 1-B) was compared with an ROI in the same frontal lobe area of an age-matched healthy control (Figure 1-C and 1-D). The boundary of the site of needle insertion (dark area in Figure 1-A) was separated from the ROI by a two-voxel layer to avoid possible artifacts from blood products, or possible damage to the immediately surrounding area by the biopsy needle placement. DTI-derived single-tensor metrics included radial diffusivity (RD), axial diffusivity (AD), and fractional anisotropy (FA). DBSI-derived multi-tensor metrics included RD, AD, FA, fiber fraction (FF), non-restricted isotropic fraction, and restricted isotropic fraction.3 Whereas the DTI-derived metrics were based on single-tensor analysis, the DBSI-derived RD, AD, FA, fiber fraction (FF), non-restricted isotropic fraction, and restricted isotropic fraction were extracted from multi-tensor modeling to resolve intra-voxel underlying structure and pathology components.3 As previously reported,3,4 in the settings of acute inflammation and tissue loss commonly seen in MS, DTI-derived axon/myelin injury metrics are confounded by infiltrating cells and extracellular water.
Results were displayed using notched box plots representing median and 95% confidence intervals of image voxels from the defined ROI for each diffusion metric. Non-overlapping notches between boxes indicate that the corresponding medians differ. Formal hypothesis testing was not performed, given the single sample. The Institutional Review Board at Washington University in St Louis approved the study.
Results
Figure 1E – I show the DBSI- and DTI-derived metrics in the biopsied lesion (Figure 1A–B) and in normal WM of the corresponding region in a healthy subject (Fig. 1C–D). Compared to the healthy subject, both DTI- and DBSI-derived RD were significantly increased (consistent with demyelination) in the biopsied lesion (Figure 1E). DBSI-derived AD in the biopsied lesion did not appear different compared to the healthy control (Figure 1F), however, DTI-derived AD in the lesion was higher than that of the healthy control. The DBSI finding was consistent with preserved integrity of residual axons, whereas the DTI result likely reflected confounding effects of increased water content in the setting of inflammation. DBSI-derived non-restricted isotropic fraction was increased (Figure 1I) a finding consistent with edema. Both DTI-derived and DBSI-derived FA were significantly decreased in the biopsied lesion compared to the healthy control (Figure 1G). DBSI-derived FF (apparent axonal density) was decreased in the biopsied lesion compared to the healthy control (Figure 1H), suggesting reduced axonal content in the lesion.
Microscopic images of the biopsied lesion (Figure 2) showed regions of reduced LFB stain (demyelination) with relatively less reduction in neurofilament stain (relative axonal loss). Increased cellular content was also present (Figure 2A). Only infrequent signs of axonal injury (spheroids) were observed (Figure 2D). Overall the DBSI metrics of increased radial diffusivity, unchanged axial diffusivity, mildly decreased fiber fraction, and increased non-restricted isotropic fraction (Figure 1. E–I) were consistent with the histopathology findings of an inflammatory demyelinating lesion with relative axonal sparing (Figure 2).
Discussion
Based on animal data and human autopsy results,4,5,7 increased RD and decreased AD are indicators of demyelination and axonal injury, respectively, and an increased proportion of non-restricted isotropic diffusion is a marker of vasogenic edema or loss of axonal fibers. Compared to healthy control tissue, DBSI-derived metrics in this biopsied lesion demonstrated increased RD, decreased FF, and elevated non-restricted isotropic fraction, but with normal AD. This pattern was consistent with inflammatory demyelination with relative axonal sparing that was observed by histopathology. The mildly decreased FF in the lesion was in accord with the relative preservation of axons seen histologically.
In this study, DBSI was more consistent with the underlying WM pathologies found in the biopsy than DTI. DTI metrics, both RD and AD, are known to be impacted by the presence of inflammation and vasogenic edema.10 This confounding effect led to falsely increased DTI-derived AD. In contrast, DBSI-derived AD was not increased. DBSI results were more in accordance with histological neurofilament staining (Fig. 2C). In addition, DTI-derived FA was underestimated due to averaging in the effects of increased isotropic diffusion from vasogenic edema. In contrast, DBSI models multiple intra-voxel pathologies and structures to estimate the extent of vasogenic edema and tissue loss, represented by the non-restricted isotropic diffusion–tensor component.3,4
This study provides evidence supporting the feasibility of DBSI to differentiate the coexisting pathologies in acute MS lesions in human brain in vivo. DBSI metrics better reflected the actual histological findings than DTI because DBSI models multiple intra-voxel structural and pathological components using multiple anisotropic and isotropic tensors,3,4 whereas DTI parameters represent “averaged” data within imaging voxels. Several advanced diffusion MR methods have been developed to address the limitations of DTI. Much can be learned from comparison of such advanced methods. Our study suggests that DBSI is a promising non-invasive biomarker of MS neuropathology.
Acknowledgments
Funding
This work was funded by a grant from the U.S. National Institutes of Health (PO1 NS059560, PI: A.H.C.).
Footnotes
Declaration of conflicting interests
A.S. is funded through a clinician scientist development award from the National Multiple Sclerosis Society (USA), and a clinical research training scholarship from the American Academy of Neurology. P.S., R.E.S., and K.T. report no conflicts of interest. R.T.N. has consulted for Alkermes, Acorda, Bayer, Biogen, EMD Serono, Genentech, Genzyme, Novartis, and Teva. S.K.S is currently funded by NIH U01EY025500, R01NS047592, P01NS059560, and NMSS RG 5258-A-5. He is a co-founder of DxGPS and may financially benefit if the company is successful in marketing its product(s) that is/are related to this research. A.H.C has been a paid consultant for: Biogen, EMD-Serono, Genzyme/Sanofi, Genentech/Roche, and Novartis. A.H.C. was funded in part by the Manny & Rosalyn Rosenthal – Dr. John L Trotter MS Center Chair in Neuroimmunology of Barnes-Jewish Hospital Foundation. Washington University may receive royalty income based on a technology licensed by Washington University to DxGPS LLC. That technology is evaluated in this research.
Contributor Information
Afsaneh Shirani, The John L. Trotter Multiple Sclerosis Center and Neuroimmunology Section, Department of Neurology, Washington University School of Medicine, St Louis, MO.
Peng Sun, Mallinckrodt Institute of Radiology, Department of Radiology, Washington University School of Medicine, St Louis, MO.
Robert E. Schmidt, Department of Pathology and Immunology, Washington University School of Medicine, St Louis, MO.
Kathryn Trinkaus, Biostatistics Shared Resource and Siteman Cancer Center, Washington University School of Medicine, St Louis, MO.
Robert T. Naismith, The John L. Trotter Multiple Sclerosis Center and Neuroimmunology Section, Department of Neurology, Washington University School of Medicine, St Louis, MO.
Sheng-Kwei Song, Mallinckrodt Institute of Radiology, Department of Radiology, Washington University School of Medicine, St Louis, MO.
Anne H. Cross, The John L. Trotter Multiple Sclerosis Center and Neuroimmunology Section, Department of Neurology, Washington University School of Medicine, St Louis, MO.
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–17. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 2.Fox RJ, Beall E, Bhattacharyya P, Chen JT, Sakaie K. Advanced MRI in multiple sclerosis: Current status and future challenges. Neurol Clin. 2011;29:357–380. doi: 10.1016/j.ncl.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cross AH, Song SK. A new imaging modality to non-invasively assess multiple sclerosis pathology. J Neuroimmunol. 2017;304:81–85. doi: 10.1016/j.jneuroim.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Sun P, Wang Q, et al. Differentiation and quantification of inflammation, demyelination and axon injury or loss in multiple sclerosis. Brain. 2015;138:1223–1238. doi: 10.1093/brain/awv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin TH, Chiang CW, Perez-Torres CJ, et al. Diffusion MRI quantifies early axonal loss in the presence of nerve swelling. J Neuroinflammation. 2017:14. doi: 10.1186/s12974-017-0852-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Wang Q, Haldar JP, et al. Quantification of increased cellularity during inflammatory demyelination. Brain. 2011;134:3587–3598. doi: 10.1093/brain/awr307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Cusick MF, Wang Y, et al. Diffusion basis spectrum imaging detects and distinguishes coexisting subclinical inflammation, demyelination and axonal injury in experimental autoimmune encephalomyelitis mice. NMR Biomed. 2014;27:843–852. doi: 10.1002/nbm.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 9.Naismith RT, Xu J, Tutlam NT, et al. Radial diffusivity in remote optic neuritis discriminates visual outcomes. Neurology. 2010;74:1702–1710. doi: 10.1212/WNL.0b013e3181e0434d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiang CW, Wang Y, Sun P, et al. Quantifying white matter tract diffusion parameters in the presence of increased extra-fiber cellularity and vasogenic edema. Neuroimage. 2014;101:310–319. doi: 10.1016/j.neuroimage.2014.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]