Abstract
Most natural product biosynthetic gene clusters that can be observed bioinformatically are silent. This insight has prompted the development of several methodologies for inducing their expression. One of the more recent methods, termed reporter-guided mutant selection (RGMS), entails creation of a library of mutants that is then screened for the desired phenotype via reporter gene expression. Herein, we apply a similar approach to Burkholderia thailandensis and, using transposon mutagenesis, mutagenize three strains, each carrying a fluorescent reporter in the malleilactone (mal), capistruin (cap), or an unidentified ribosomal peptide (tomm) gene cluster. We show that even a small library of <500 mutants can be used to induce expression of each cluster. We also explore the mechanism of activation and find that inhibition of pyrimidine biosynthesis is linked to the induction of the mal cluster. Both a transposon insertion into pyrF as well as small molecule-mediated inhibition of PyrF triggers malleilactone biosynthesis. Our results pave the way toward the broad application of RGMS and related approaches to Burkholderia spp.
Keywords: biosynthesis, Burkholderia thailandensis, natural products, silent gene cluster, transposon mutagenesis
Graphical Abstract
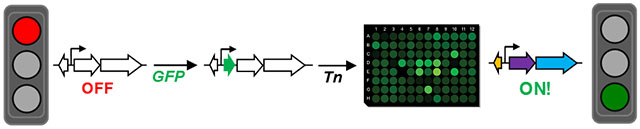
Silent biosynthetic gene clusters provide a treasure trove of new natural products. Herein, transposon mutagenesis is used in conjunction with fluorescent reporters to activate silent clusters, revealing nucleotide limitation as a key trigger for natural product biosynthesis in Burkholderia thailandensis.
Microbial natural products remain a source of inspiration for chemists and biologists alike. The vast diversity of their structures and the specificity of interactions with their target sites are by now well-established.[1,2] Perhaps less-appreciated is the recent realization that the ~300,000 natural products reported thus far merely represent the tip of the iceberg, and that microbes harbor an untapped source of new secondary metabolites.[3–6] Indeed, microbial genome sequences that have accumulated over the past decade indicate a much larger potential for natural product biosynthesis than was previously imagined. Genomes of prolific producers routinely reveal >25 biosynthetic gene clusters (BGCs) for which a cognate secondary metabolite cannot be isolated.[4–6] While challenges associated with detection of secondary metabolites may explain this phenomenon in some cases, the current consensus is that the majority of BGCs are expressed at low levels in laboratory cultures. Referred to as “silent” or “cryptic”, they outnumber those that are constitutively expressed by a factor of 5–10.[4,5] The presence of silent BGCs opens two major areas of inquiry: (1) how can they be activated and what kinds of compounds do they produce, and (2) how are they regulated and what conditions lead to their induction.
Several methods have been developed mostly to address the first question, including heterologous expression, insertion of constitutive or inducible promoters, co-culture, ribosome engineering, and high-throughput elicitor screening (HiTES).[7–14] Recently, the utility of modulating the expression of regulatory proteins either rationally or by leveraging random mutagenesis approaches has been demonstrated.[15–17] Guo et al. showed that silent BGCs can be activated in Streptomyces spp. using a reporter-guided UV mutagenesis approach referred to as RGMS (reporter-guided mutant selection), which led to the identification of a new glycosylated gaudimycin derivative.[17] Subsequently, transposon (Tn) mutagenesis was employed to connect genes, often unsuspected ones, to the production of a secondary metabolite in Streptomyces albus and Streptomyces coelicolor.[18,19] We recently expanded this strategy to Burkholderia thailandensis using phenotype-guided Tn mutagenesis to activate the expression of a silent type I polyketide synthase, thus enabling the discovery of the novel thailandenes.[20] Herein, we build on these results and implement reporter-guided Tn mutagenesis in B. thailandensis for inducing silent BGCs and identifying their underlying regulatory pathways. We demonstrate activation of three silent BGCs and in one case establish pyrimidine nucleotide limitation as a cue for induction of secondary metabolism.
B. thailandensis has emerged as a model system for exploring the production and regulation of silent secondary metabolite gene clusters.[5,21] It is a genetically tractable β-proteobacterium that carries ~25 BGCs, most of which are lowly-expressed. Currently, quorum sensing (QS) and ScmR are the key regulatory pathways implicated in controlling BGCs,[16,22,23] including those of malleilactone,[24–27] burkholdac,[28] and acybolin.[29] To globally explore pathways that are involved in activation of silent BGCs in B. thailandensis, we set out to implement the approach shown (Fig. 1). A reporter gene (lacZ or eGFP) is inserted downstream of a silent promoter or within a silent cluster to provide an expression read-out. Subsequently, a Tn carrying a tetracycline-resistance marker (TetR) is introduced to create a library of mutants. Those exhibiting resistance to Tet and enhanced LacZ activity or fluorescence are then examined further to determine the site of Tn insertion and production of the desired cryptic metabolite.
Figure 1.
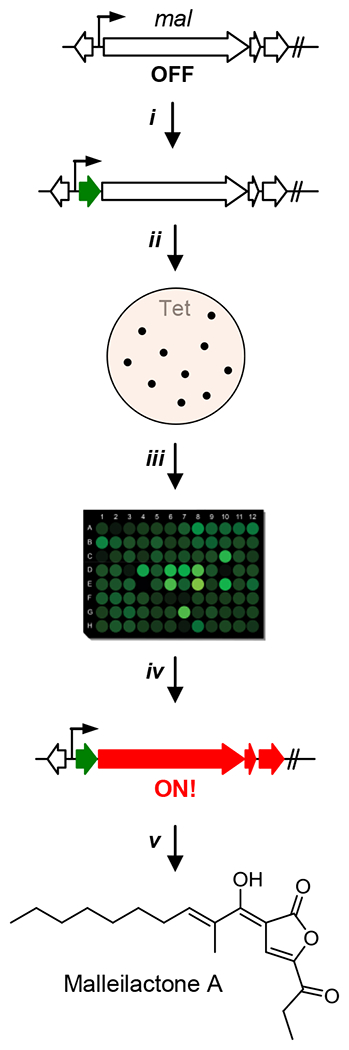
Workflow for reporter-guided Tn mutant selection. A reporter gene such as GFP (shown in green) is introduced into a silent BGC (i). The resulting strain is subjected to Tn mutagenesis and Tn-carrying strains isolated on selective media (ii). Fluorescence emission is measured as a proxy for expression of the silent cluster (iii). Appropriate mutant(s) that show enhanced expression of the BGC are used to characterize the cryptic metabolite (iv, v).
Our initial attempts to validate this approach used EZ-Tn5 mutagenesis in the sequence-defined in-frame lacZ insertion library in B. thailandensis pioneered by the Manoil group.[30] Because lacZ is encoded within a T8 transposon, subsequent mutagenesis led to frequent removal of the T8-derived reporter gene, rather than random insertion of Tn5. We therefore turned to genetically-encoded fluorescence proteins as reporters.[31] We first insured that their expression could be monitored rapidly by inserting Burkholderia-optimized eGFP, eYFP, or RFP into the bactobolin gene cluster (bta) as translational and transcriptional fusions (see SI, Tables S1, S2). The bta cluster is QS-activated and therefore expressed at high cell densities.[32–35] Growth of bta-eGFPx2, bta-eYFPx2 and bta-RFPx2 followed by imaging by fluorescence microscopy showed this to be the case (Fig. 2A–C). The double reporter-constructs increased signal-to-noise and allowed us to easily monitor fluorescence emission of bacterial cultures in grown in LB using a plate reader, thus simplifying the expression read-out. Next a double reporter cassette was inserted into three silent BGCs coding for malleilactone (mal),[24–27] capistruin (cap),[36,37] and a yet-uncharacterized putative TOMM (thiazole-oxazole modified microcin, tomm) (Fig. 3A).[38] With mal and cap, we generated translational fusions, while with tomm a transcriptional fusion was introduced directly downstream of an identifiable promoter that precedes tommR. We used different fluorophores for each locus (mal-eGFPx2, cap-YFPx2, Ptomm-RFPx2), with the idea of conducting this screen simultaneously with multiple unknown silent clusters in the future, if the method was successful.
Figure 2.
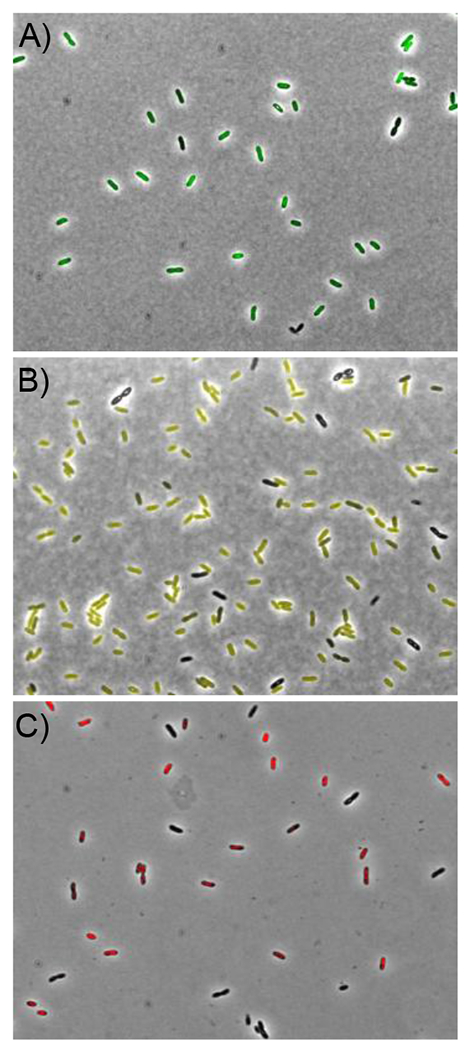
Secondary metabolite gene expression detected with fluorescence reporters. Expression of the QS-activated bta cluster with a double reporter cassette containing eGFP (A), eYFP (B), or RFP (C).
Figure 3.
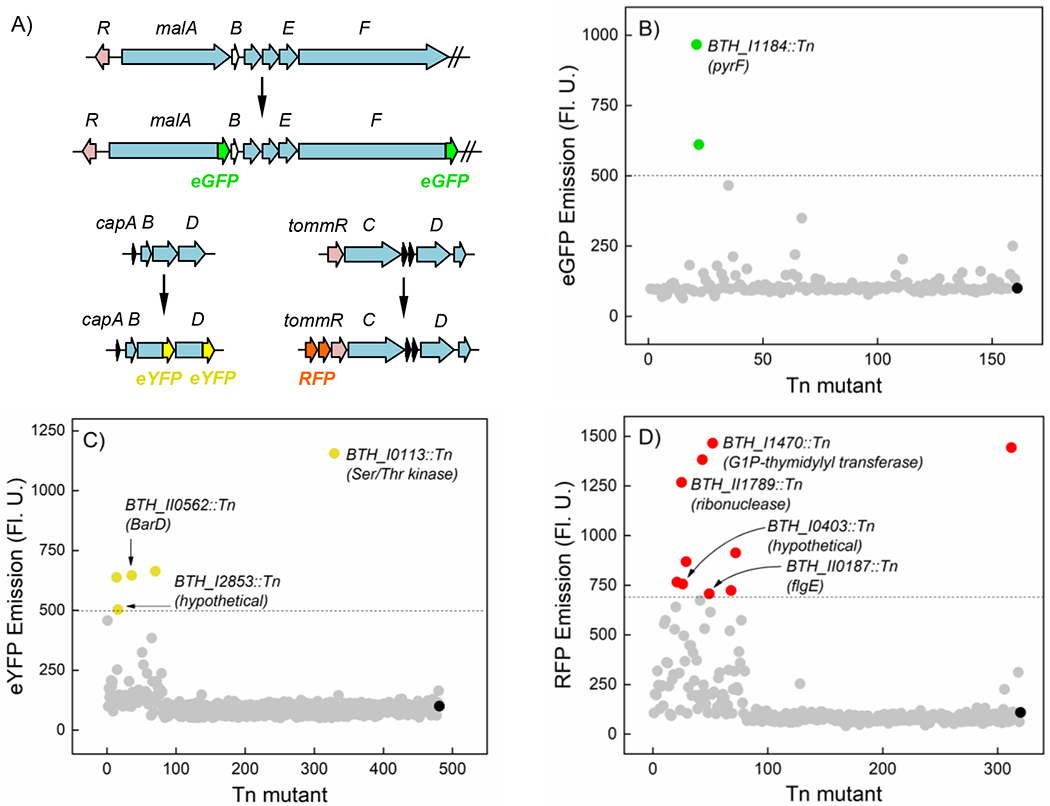
Reporter-guided Tn mutant selection in B. thailandensis. (A) Reporter constructs generated to induce the silent mal, cap, and tomm BGCs. Gene are color-coded as follows: blue, biosynthetic enzyme; purple, regulatory protein; black, precursor peptide, white, gene of unknown function. (B-D) Activation of the mal (B), cap (C), and tomm (D) BGCs. The fluorescence output is normalized to the unmutagenized reporter which is shown as a block sphere in each case. Mutants above the dotted lines were designated as hits. Mutants in which the site of Tn insertion was successfully identified using arbitrary PCR are labelled.
Each of the three reporter strains was then subjected to Tn mutagenesis, in which a modified Tn8, carrying a tetracycline resistance marker (TetR), was inserted using conjugation with the appropriate E. coli donor strain.[39] The transformants were selected on Tet-agar plates and a total of 200–500 colonies arrayed into 96-well plates. Subsequently, the plates were grown for a defined period and absorbance at 600 nm (OD600) and fluorescence emission were measured as a proxy for growth and expression of the chosen cluster, respectively. Results for all three clusters are shown (Fig. 3B–3D). All three clusters were expressed at low levels in the absence of any mutation. Within the small library generated, growth defects were rare as less than 10% of the mutants exhibited an OD600 value >2-fold lower (or higher) than the unmutagenized reporter strain. Even with a relatively small number of transformants, we could observe induction of each gene cluster, which we arbitrarily defined as a >5-fold (mal, cap) or >7-fold (tomm) expression in the transfor-mant, as determined by fluorescence emission spectroscopy. With the mal cluster, we found 2 hits from 160 transformants. Likewise, with cap and tomm we found 5 hits (from 480) and 10 hits (from 320), respectively, suggesting that activation of silent pathways via Tn mutagenesis was fairly efficient.
To verify that each cluster was in fact induced, selected hit(s) were assessed by RT-qPCR and/or HPLC-MS. The product of the mal cluster is known, but insertion of the eGFP reporter genes rendered the strain deficient in malleilactone production, as experiments with trimethoprim, a proven inducer of the mal cluster,[40] demonstrated. We therefore transplanted the Tn from the selected strain into a wt background using established methods.[41] Comparison of the secondary metabolomes of wt and the Tn-carrying strain demonstrated 26-fold enhanced malleilactone production (Fig. 4A). For the cap cluster, three mutants were examined by RT-qPCR, revealing moderate upregulation in capB relative to wt for two strains (Fig. 4B). All three Tn mutants did produce capistruin, as determined by HPLC-Qtof-MS (Fig. 4C). In contrast, no capistruin was detected in the wt strain. Lastly, mutants that exhibited increased expression of the tomm cluster were further verified by RT-qPCR. Four selected mutants showed a 2–4.5-fold upregulation of tommR relative to wt (Figs. 3A, 4D). The product of the tomm cluster is not yet known. Repeated attempts to identify it proved unsuccessful. Nonetheless, our results verify that all three silent clusters were induced using the workflow described in Fig. 1, and that a combination of reporter screening and Tn mutagenesis can activate silent BGCs in a targeted fashion in B. thailandensis.
Figure 4.
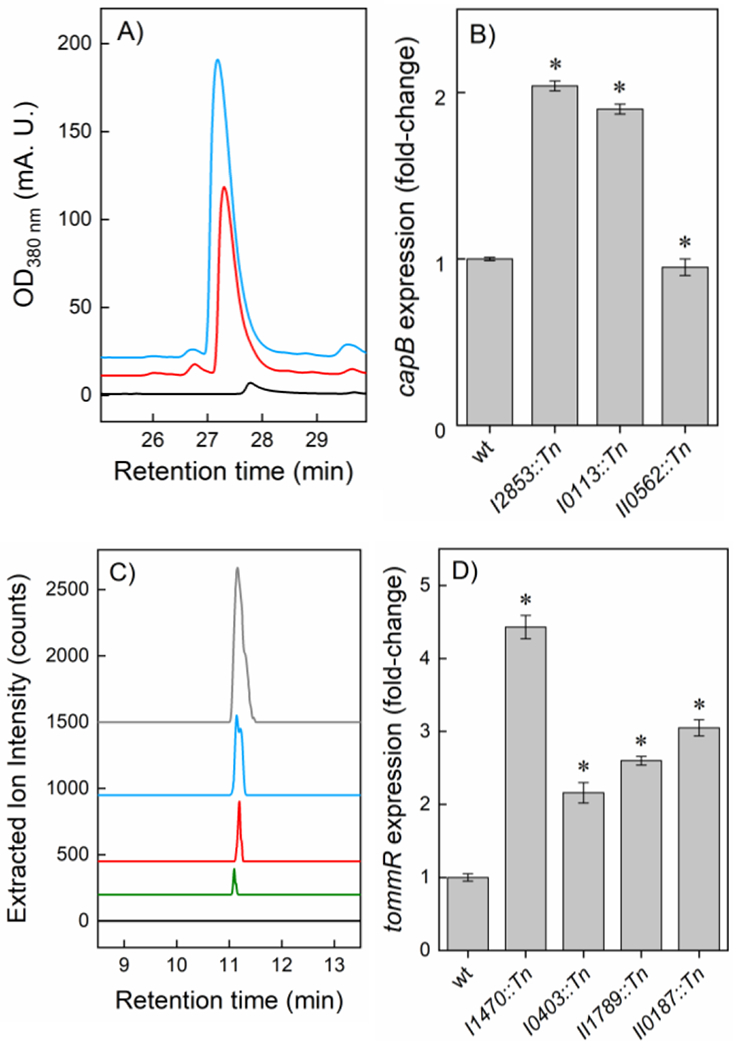
Validation of Tn mutants. (A) HPLC-MS analysis of wt (black trace) and pyrF::Tn (red) reveals enhanced malleilactone production, similar to levels obtained with trimethoprim-treated wt cells (blue). (B) RT-qPCR analysis shows enhanced capB expression in the indicated Tn mutants. (C) HPLC-MS analysis of wt (black trace), BTH_I0113::Tn (green), BTH_II0562::Tn (red), and BTH_I2853::Tn (blue) reveals capistruin production only in the Tn mutants. A capistruin standard (gray) is also shown. (D) RT-qPCR analysis shows increased tommR expression in the indicated Tn mutants. Data in panels B and D represent the averages of two independent measurements ± standard error; p-values (*, <0.01) are indicated.
We next investigated the site of Tn insertion in selected hits to better understand the origin of induction of the mal, cap, and tomm clusters. Using arbitrary PCR, we identified pyrF as the site of Tn insertion in the mutant that induced the mal cluster most (Fig. 3B). PyrF catalyzes the final step in UMP biosynthesis,[42] a pathway that intersects with one-carbon metabolism, the inhibition of which by trimethoprim has been shown to induce malleilactone synthesis.[40] Examination of three mutants that increased cap expression identified a putative Ser/Thr kinase (BTH_I0113), a barD homolog (BTH_II0562), and a gene of unknown function (BTH_I2853) as genes that control or affect the production of capistruin (Fig. 3C). Lastly, examination of select mutants identified in the tomm screen revealed Tn insertion into ribonuclease T2 (BTH_II1789), a flagellar hook protein (FlgE), glucose-1-phosphate thymidylyltransferase (BTH_I1470), and a gene of unknown function (BTH_I0403), all previously not known to control secondary metabolism. How these genes contribute to the regulation of secondary metabolism remains to be determined. The results show that control over natural product biosynthesis is complex and that many unsuspected genes are involved in this process. Xu et al. recently reached a similar conclusion by examining genes that contribute to actinorhodin biosynthesis in Streptomyces coelicolor. [18]
The results above suggest a connection between pyrimidine nucleotide biosynthesis and malleilactone production. As independent corroboration, we conducted experiments with 5-fluoroorotic acid (5-FOA), a well-known competitive inhibitor of E. coli PyrF,[42] which is homologous to the enzyme in B. thailandensis. Treatment of wt B. thailandensis with 5-FOA revealed dose-dependent overproduction of malleilactone (Fig. 5A). Optimal production was observed at 100 µM 5-FOA, leading to 12-fold enhanced malleilactone biosynthesis. Moreover, much like trimethoprim, 5-FOA globally affected secondary metabolism as amplified production of numerous secondary metabolites – including several hydroxymethyl-quinoline (HMQ) derivatives[29,43] and thailandamide[44] – was also observed (Fig. 5A). These results provide a link between pyrimidine biosynthesis and malleilactone biogenesis that would have been difficult to predict a priori.
Figure 5.
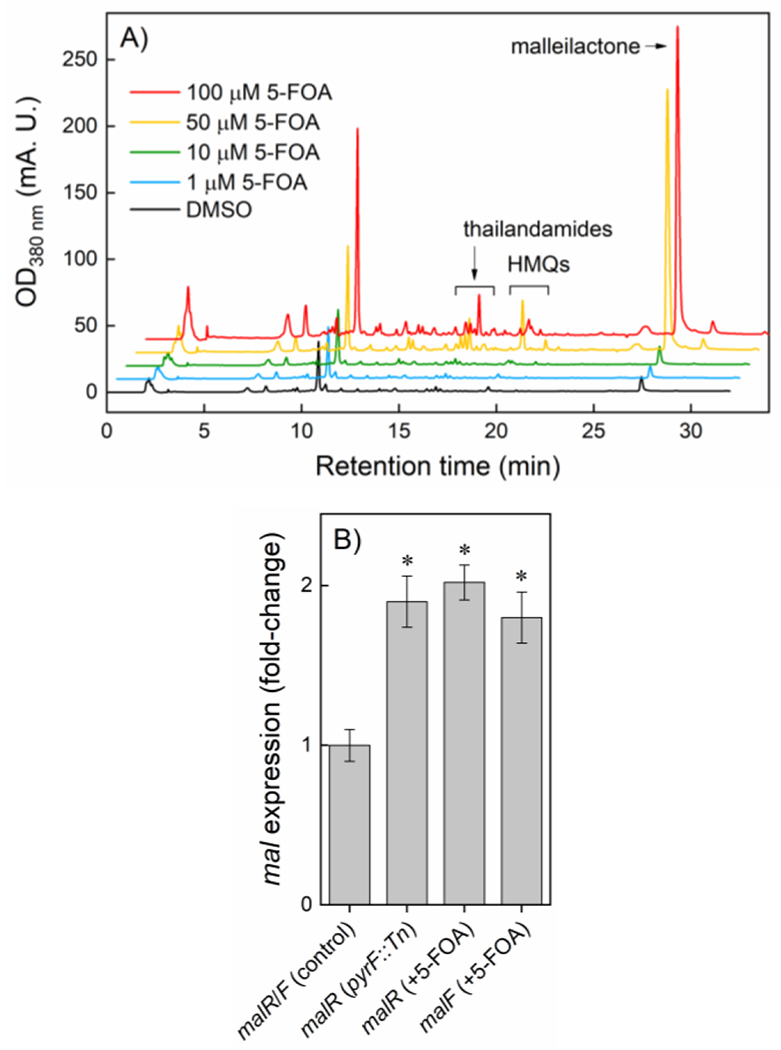
5-Fluoroorotic acid broadly activates secondary metabolism in B. thailandensis. (A) HPLC-MS analysis of B. thailandensis treated with DMSO (control) or with 5-FOA at the concentrations indicated. Identified metabolite groups are labeled. (B) RT-qPCR analysis shows induction of malR after inhibition of PyrF either by gene deletion or by 5-FOA. Data represent the averages of three independent measurements ± standard error; p-values (*, <0.01) are indicated.
Trimethoprim induces the mal cluster by activating malR, an ‘orphan’ LuxR regulator that serves as a positive transcriptional regulator.[40,45] We examined the involvement of malR in the activation of mal using RT-qPCR and found its expression to be ~2-fold enhanced in pyrF::Tn relative to wt (Fig. 5B). Moreover, addition of 5-FOA to wt cells also resulted in ~2-fold up-regulation of malR and malF (Fig. 5B). At the same time, a markerless malR deletion mutant (ΔmalR) failed to trigger malleilactone synthesis in response to 5-FOA (Fig. S1). Thus, inhibition of pyrimidine biosynthesis by 5-FOA leads to enhanced malR expression, which in turn induces the mal cluster. These results consolidate the link between pyrimidine biosynthesis and cryptic metabolism in B. thailandensis.
In summary, we report the implementation of RGMS for induction of silent BGCs in B. thailandensis. This approach was previously used in Streptomyces sp. R435, where UV mutagenesis along with a promoter-reporter construct led to activation of a gaudimycin gene cluster.[17] Meanwhile Xu et al. and Ahmed et al. used Tn mutagenesis with either a compound-specific colorimetric read-out (actinorhodin, S. coelicolor) or a reporter gene in S. albus, respectively, to investigate genes involved in the regulation of secondary metabolite biosynthesis.[18,19] We have similarly used Tn mutagenesis along with cluster-specific reporter constructs to induce silent BGCs. By applying the method to three different BGCs, we demonstrate the cluster-agnostic feature of this approach. Following up on one Tn mutant, we show that alteration of pyrimidine biosynthesis, either via gene deletion or by small molecule inhibition, results in upregulation of the mal BGC. Our previous work showed that Tmp and sulfonamide antibiotics, inhibitors of one-carbon metabolism, also result in malleilactone biosynthesis.[45] Thus, using two very different approaches, we have converged on the same primary metabolite network, the inhibition of which is tied to induction of secondary metabolism. In the current study, we used a small number of Tn mutants to activate the selected operons. Exhaustive Tn mutagenesis could identify all genes that are involved in regulating, or indirectly affecting the expression of, these and other silent BGCs. RGMS is an addition to the cadre of approaches available for gaining further insights into the hidden metabolome of B. thailandensis. Much like HiTES, reporter-guided Tn mutagenesis can address both the chemistry, i.e. small molecule products, and biology, i.e. regulatory networks, that underlie silent BGCs. And much like HiTES, read-outs other than transcriptional/translational reporters may be employed, for example bioactivity assays or mass spectrometry.[46–49] Coupling these additional read-outs to Tn mutagenesis is currently underway.
Supplementary Material
Acknowledgements
We thank the National Institutes of Health (grant no. DP2-AI-124786 to M.R.S.), and the Uehara Memorial Foundation Postdoctoral Fellowship (to A.Y.) for financial support.
Footnotes
Experimental Section
Full experiment details are provided in the Supporting Information.
Conflict of Interest
The authors declare no conflict of interest.
References
- [1].Clardy J, Walsh C, Nature 2004, 432, 829–837. [DOI] [PubMed] [Google Scholar]
- [2].Newman D, Cragg GM, J. Nat. Prod. 2016, 79, 629–661. [DOI] [PubMed] [Google Scholar]
- [3].Buckingham J, The Dictionary of Natural Products, CRC Press, London, 2019. [Google Scholar]
- [4].Nett M, Ikeda H, Moore BS, Nat. Prod. Rep. 2009, 26, 1362–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liu X, Cheng YQ, J. Ind. Microbiol. Biotechnol. 2014, 41, 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Baltz RH, J. Ind. Microbiol. Biotechnol. 2017, 44, 573–588. [DOI] [PubMed] [Google Scholar]
- [7].Teijaro CN, Adhikari A, Shen B, J. Ind. Microbiol. Biotechnol. 2019, 46, 433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lee N, Hwang S, Lee Y, Cho S, Palsson B, Cho B-K, J. Microbiol. Biotechnol. 2019, 29, 667–686. [DOI] [PubMed] [Google Scholar]
- [9].Mao D, Okada BK, Wu Y, Xu F, Seyedsayamdost MR, Curr. Opin. Microbiol. 2018, 45, 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pettit RK, Appl. Microbiol. Biot. 2009, 83, 19–25. [DOI] [PubMed] [Google Scholar]
- [11].Okada BK, Seyedsayamdost MR, FEMS Microbiol. Rev. 2017, 41, 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ochi K, Hosaka T, Appl. Microbiol. Biotechnol. 2013, 97, 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hug JJ, Bader CD, Remškar M, Cirnski K, Müller R, Antibiotics 2018, 7, E44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhu H, Sandiford SK, van Wezel GP, J. Ind. Microbiol. Biotechnol. 2014, 41, 371–386. [DOI] [PubMed] [Google Scholar]
- [15].Laureti L, Song L, Huang S, Corre C, Leblond P, Challis GL, Aigle B, Proc. Natal. Acad. Sci. USA 2011, 108, 6258–6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mao D, Bushin LB, Moon K, Wu Y, Seyedsayamdost MR, Proc. Natl. Acad. Sci. USA 2017, 114, E2920–E2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Guo F, Xiang S, Li L, Wang B, Rajasärkkä J, Gröndahl-Yli-Hannuksela K, Ai G, Metsä-Ketelä M, Yang K, Metab. Eng. 2015, 28, 134–142. [DOI] [PubMed] [Google Scholar]
- [18].Xu Z, Wang Y, Chater KF, Ou HY, Xu HH, Deng Z, Tao M, Appl. Environ. Microbiol. 2017, 83, e02889–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ahmed Y, Rebets Y, Tokovenko B, Brötz E, Luzhetskyy A, Sci. Rep. 2017, 7, 9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Park J-D, Moon K, Miller C, Rose J, Xu F, Ebmeier CC, Jacobsen JR, Mao D, Old W, DeShazer D, Seyedsayamdost MR, ACS Chem. Biol. 2019, In Press. DOI: 10.1021/acschembio.9b00883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Brett PJ, DeShazer D, Woods DE, Int. J. Syst. Bacteriol. 1998, 48, 317–320. [DOI] [PubMed] [Google Scholar]
- [22].Majerczyk C, Brittnacher M, Jacobs M, Armour CD, Radey M, Schneider E, Phattarasokul S, Bunt R, Greenberg EP, J. Bacteriol. 2014, 196, 1412–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Majerczyk CD, Brittnacher MJ, Jacobs MA, Armour CD, Radey MC, Bunt R, Hayden HS, Bydalek R, Greenberg EP, J. Bacteriol. 2014, 196, 3862–3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Biggins JB, Ternei MA, Brady SF, J. Am. Chem. Soc. 2012, 134, 13192–13195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Franke J, Ishida K, Hertweck C, Angew. Chem. Int. Ed. 2012, 51, 11611–11615. [DOI] [PubMed] [Google Scholar]
- [26].Trottmann F, Franke J, Richter I, Ishida K, Cyrulies M, Dahse HM, Regestein L, Hertweck C, Angew. Chem. Int. Ed. 2019, 58, 14129–14133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Note, the structure of the active form of malleilactone and the primary product of mal, termed malleicyprol, has recently been disclosed with an unusual cyclopropanol moiety (Ref. 26). Because we detect the ring-opened form, the structure of malleilactone is shown in Fig. 1.
- [28].Biggins JB, Gleber CD, Brady SF, Org. Lett. 2011, 13, 1536–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Okada BK, Wu Y, Mao D, Bushin LB, Seyedsayamdost MR, ACS Chem. Biol. 2016, 11, 2124–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gallagher LA, Ramage E, Patrapuvich R, Weiss E, Brittnacher M, Manoil C, MBio 2013, 4, e00604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Barrett AR, Kang Y, Inamasu KS, Son MS, Vukovich JM, Hoang TT, Appl. Environ. Microbiol. 2008, 74, 4498–4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Duerkop BA, Varga J, Chandler JR, Peterson SB, Herman JP, Churchill ME, Parsek MR, Nierman WC, Greenberg EP, J. Bacteriol. 2009, 191, 3909–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Seyedsayamdost MR, Chandler JR, Blodgett JA, Lima PS, A Duerkop B, Oinuma K, Greenberg EP, Clardy J, Org. Lett. 2010, 12, 716–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Carr G, Seyedsayamdost MR, Chandler JR, Greenberg EP, Clardy J, Org. Lett. 2011, 13, 3048–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Amunts A, Fiedorczuk K, Truong TT, Chandler J, Greenberg EP, Ramakrishnan V, J. Mol. Biol. 2015, 427, 753–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Knappe TA, Linne U, Zirah S, Rebuffat S, Xie X, Marahiel MA, J. Am. Chem. Soc. 2008, 130, 11446–11454. [DOI] [PubMed] [Google Scholar]
- [37].Maksimov MO, Link AJ, J. Ind. Microbiol. Biotechnol. 2014, 41, 333–344. [DOI] [PubMed] [Google Scholar]
- [38].Burkhart BJ, Schwalen CJ, Mann G, Naismith JH, Mitchell DA, Chem. Rev. 2017, 117, 5389–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Blodgett JA, Oh DC, Cao S, Currie CR, Kolter R, Clardy J, Proc. Natl. Acad. Sci. USA 2010, 107, 11692–11697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Seyedsayamdost MR, Proc. Natl. Acad. Sci. USA 2014, 111, 7266–7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Thongdee M, Gallagher LA, Schell M, Dharakul T, Songsivilai S, Manoil C, Appl. Environ. Microbiol. 2008, 74, 2985–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Miller BG, Wolfenden R, Annu. Rev. Biochem. 2002, 71, 847–885. [DOI] [PubMed] [Google Scholar]
- [43].Vial L, Lépine F, Milot S, Groleau MC, Dekimpe V, Woods DE, Déziel E, J. Bacteirol 2008, 190, 5339–5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Nguyen T, Ishida K, Jenke-Kodama H, Dittmann E, Gurgui C, Hochmuth T, Taudien S, Platzer M, Hertweck C, Piel J, Nat. Biotechnol. 2008, 26, 225–233. [DOI] [PubMed] [Google Scholar]
- [45].Truong TT, Seyedsayamdost MR, Greenberg EP, Chandler JR, J. Bacteriol. 2015, 197, 3456–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Xu F, Wu Y, Zhang C, Davis KM, Moon K, Bushin LB, Seyedsayamdost MR, Nat. Chem. Biol. 2019, 15, 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Moon K, Xu F, Zhang C, Seyedsayamdost MR, ACS Chem. Biol. 2019, 14, 767–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Moon K, Xu F, Seyedsayamdost MR, Angew. Chem. Int. Ed. 2019, 58, 5973–5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pimentel-Elardo SM, Sørensen D, Ho L, Ziko M, Bueler SA, Lu S, Tao J, Moser A, Lee R, Agard D, Fairn G, Rubinstein JL, Shoichet BK, Nodwell JR, ACS Chem. Biol. 2015, 10, 2616–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


