Abstract
Background:
Studies examining the role of factor V Leiden among patients at higher risk of atherothrombotic events, such as those with established coronary heart disease (CHD) are lacking. Given that coagulation is involved in the thrombus formation stage upon atherosclerotic plaque rupture, we hypothesized that factor V Leiden may be a stronger risk factor for atherothrombotic events in patients with established CHD.
Methods:
We performed an individual-level meta-analysis including 25 prospective studies (18 cohorts, 3 case-cohorts, 4 randomized trials) from the GENIUS-CHD consortium involving patients with established CHD at baseline. Participating studies genotyped factor V Leiden status and shared risk estimates for the outcomes of interest using a centrally developed statistical code with harmonized definitions across studies. Cox-regression models were used to obtain age and sex adjusted estimates. The obtained estimates were pooled using fixed-effect meta-analysis. The primary outcome was composite of myocardial infarction and CHD death. Secondary outcomes included any stroke, ischemic stroke, coronary revascularization, cardiovascular mortality and all-cause mortality.
Results:
The studies included 69,681 individuals of whom 3,190 (4.6%) were either heterozygous or homozygous (n=47) carriers of factor V Leiden. Median follow-up per study ranged from 1.0 to 10.6 years. A total of 20 studies with 61,147 participants and 6,849 events contributed to analyses of the primary outcome. Factor V Leiden was not associated with the combined outcome of myocardial infarction and CHD death (hazard ratio, 1.03; 95% CI, 0.92 – 1.16; I2 = 28%; P-heterogeneity = 0.12). Subgroup analysis according to baseline characteristics or strata of traditional cardiovascular risk factors did not show relevant differences. Similarly, risk estimates for the secondary outcomes including stroke, coronary revascularization, cardiovascular mortality and all-cause mortality were close to identity.
Conclusions:
Factor V Leiden was not associated with increased risk of subsequent atherothrombotic events and mortality in high-risk participants with established and treated CHD. Routine assessment of factor V Leiden status is unlikely to improve atherothrombotic events risk stratification in this population.
Keywords: Factor V Leiden, thrombosis, genetics, association studies, prognosis, single nucleotide polymorphism, coronary artery disease, recurrent myocardial infarction, secondary prevention
INTRODUCTION
Factor V Leiden, is a genetic variant leading to alteration of the inactivation site of factor V, which in turn leads to activated protein C resistance and a prothrombotic state.1 Affecting almost 5% of the Caucasian population,2 carriers of factor V Leiden have a 4-fold higher risk of venous thromboembolism.3 However, the risk of arterial atherothrombotic events, such as myocardial infarction or stroke, conferred by the presence of this variant is less certain.4–10 Association analyses of factor V Leiden with atherothrombotic events have been mostly conducted in case-control studies and limited to the occurrence of a first cardiovascular event in young or disease free individuals at low risk of adverse outcomes.4–10
In contrast, studies examining the role of factor V Leiden among patients at higher risk of atherothrombotic events, such as those with prior events or established coronary heart disease (CHD) are lacking. In such individuals, who may have a greater atheroma burden and more vulnerable plaques, we hypothesized that carriage of Factor V Leiden could manifest as a greater risk of subsequent atherothrombotic events, given the role of coagulation cascade in the acute thrombus formation following plaque rupture or erosion. Furthermore, the synergistic interaction between factor V Leiden and traditional cardiovascular risk factors as reported in some previous studies,11–13 suggests the possibility of a greater effect in populations with greater atheroma burden. Given the high prevalence of Factor V Leiden, if an association exists, this could support screening of patients with established cardiovascular disease for considering use of anticoagulant therapies,14 for a targeted precision medicine approach to treatment.
We therefore assessed the association of the factor V Leiden polymorphism with subsequent atherothrombotic events including mortality, in individuals with established CHD using an individual-level data meta-analysis of twenty-five prospective studies from the Genetics of Subsequent (GENIUS) CHD Consortium.
METHODS
In accordance with Transparency and Openness Promotion Guidelines, the authors declare that all summary level data used for meta-analysis are available within the article and its online supplementary files. Individual participant level data for each study were not collected through the federated analysis approach employed by the consortium and will therefore not be made available. Further details and contact information are available at www.genius-chd.org.
Study selection criteria
The GENIUS-CHD consortium is an international collaboration of prospective studies selectively including individuals with established coronary heart disease at baseline and following them for future subsequent CHD events.15
The primary criteria for inclusion in the consortium are studies that recruited individuals with: (1) established CHD, defined as a history of or presence at baseline of acute coronary syndrome, or of coronary artery disease as evidenced by any revascularization procedure such as percutaneous coronary intervention or coronary bypass surgery, or a significant (50%) coronary artery plaque at angiography affecting any major epicardial vessel, (2) availability of prospective follow-up and ascertainment of at least one clinical cardiovascular outcome including all-cause mortality, and (3) availability of samples or biomarkers or in-silico genotyping data. Full details about the GENIUS-CHD Consortium have been published elsewhere.15
A short description of the individual studies from the consortium, participating in this specific analysis of factor V Leiden are listed in the supplemental material. Participating studies received local institutional review board approval and included participants who had provided informed consent at the time of enrolment. The central analysis sites also received waivers from their local institutional review board for collating and analyzing summary level data from the participating studies.
Outcomes
The primary outcome of interest was a composite of CHD death or myocardial infarction, whichever came first (CHD death/myocardial infarction) during follow-up. Myocardial infarction included both ST-segment elevation and non-ST segment elevation myocardial infarction. Secondary outcomes consisted of non-fatal myocardial infarction, any stroke, ischemic stroke, coronary revascularization by means of percutaneous coronary intervention or bypass surgery, cardiovascular mortality and mortality from any cause.
Exposure variable definition
Factor V Leiden was defined as the presence of a single nucleotide mutation; G-to-A substitution at nucleotide 1691 in the factor V (factor V R506Q) gene (single-nucleotide polymorphism rs6025), documented by individual genotyping assays or direct DNA sequencing using various commercially available whole genome or targeted sequencing kits.
Statistical analysis
Analyses were performed in two stages. First, individual studies participating within the GENIUS-CHD Consortium and with available genotype data, evaluated the association between factor V Leiden and subsequent events assuming a dominant genetic model and using time to event Cox proportional hazards regression. All analyses were adjusted for age and sex and were performed using shared statistical scripts and harmonized datasets, under a federated analysis approach, as described previously.15 Study-specific summary estimates were then shared with the study coordination centers (University College London, London, UK, and University Medical Center Utrecht, Utrecht, the Netherlands) for meta-analysis.
Differences in baseline characteristics by factor V Leiden status were assessed using z-value based approach of mean/proportion differences in participants with and without factor V Leiden in each study. Study-level hazard ratios for the association between factor V Leiden and the primary outcome and their corresponding standard errors were pooled in an inverse variance weighted fixed-effect meta-analysis. Estimates of random-effects meta-analysis were also reported in the forest plots. Between-study variance in the random-effects meta-analysis was calculated with the Restricted Maximum Likelihood approach. Heterogeneity was quantified using a χ2 test for heterogeneity and the I2 statistic. Study-level effect-modification by baseline characteristics and follow-up length were evaluated with random-effects meta-regression analysis. Global P values for study-level effect-modification were based on a χ2 omnibus test.
In addition to the overall analyses, to assess consistency of effects, stratified estimates according to type of baseline CHD at enrollment (acute coronary syndrome, coronary artery disease with and without prior myocardial infarction) were assessed. Factor V Leiden association with the primary outcome was also stratified on patient-level characteristics measured at baseline, including: age (< or ≥ 65 years), sex, hypertension (physician diagnosed or treated), type 2 diabetes (physician diagnosed or treated), body mass index (BMI, categorized as <18.5; 18.5 to <25; 25 to <30; ≥30.0 kg/m2), statin use, antiplatelet drugs use. Studies contributing to only one level of these strata were excluded from the stratified analyses to simplify comparison. P values for the differences across the levels of the stratifying factor were calculated using a Wald test. A P value of less than 0.05 was considered statistically significant. All analyses were conducted using the R software package.16
RESULTS
Twenty-five studies contributed to this analysis and included 69,681 individuals with established CHD. Of the 25 studies, 18 were cohort studies, 3 case-cohort and 4 randomized clinical trials (Tables I–II in the supplement). The majority of participants who were included were male (73%) and Caucasian (92%), with a mean age ranging from 60–71 years per study. Prevalence of traditional cardiovascular risk factors (i.e., hypertension, hyperlipidemia, diabetes mellitus, and current smoking) across the studies was as expected for a CHD population (Table II in the supplement).
Among the participants, 3,190 (4.6%) were heterozygous and 47 (0.07%) homozygous carriers of factor V Leiden (Table II in the supplement). The median follow-up ranged from 1.0 to 10.6 years per study. Whereas the majority of studies had data on all outcomes, some studies had data on only one outcome (Table III in the supplement).
Association of factor V Leiden with primary outcome
A total of 20 studies with 61,147 participants and 6,849 events contributed to the age and sex- adjusted associations with the primary outcome (i.e. composite of myocardial infarction and CHD death). Factor V Leiden was not associated with the primary outcome (hazard ratio, 1.03; 95% CI, 0.92 – 1.16) and showed absence of significant heterogeneity (I2 = 28%; P-heterogeneity = 0.12) in the overall CHD population (Figure 1 and Figure I in the supplement). In subgroup analysis of patients by type of CHD at baseline, among those with acute coronary syndrome and with or without prior myocardial infarction, the associations of factor V Leiden with the primary outcome were close to identity (Figure 1 and Figures II–IV in the supplement).
Figure 1. Pooled associations of factor V Leiden with the primary outcome in patients with overall and subtypes of baseline CHD.
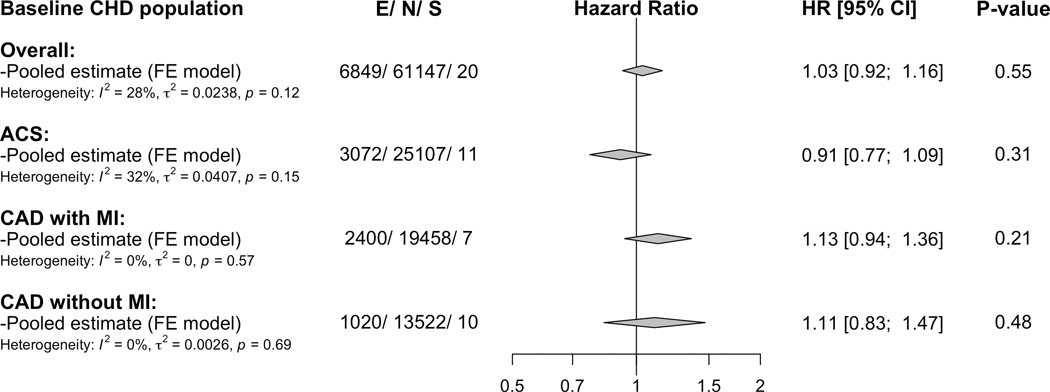
CHD= coronary heart disease, ACS= acute coronary syndrome, CAD=coronary artery disease, MI=myocardial infarction, E=number of the primary outcome (i.e., composite of myocardial infarction and CHD death), N= total number of included participants, S= number of studies contributing to the pooled estimates, HR= hazard ratio, and CI= confidence interval. Estimates are adjusted for sex and age and are based on fixed-effect meta-analysis.
Sensitivity analyses
The associations of factor V Leiden with the primary outcome stratified by sex, age (≥65 versus <65 years) and traditional cardiovascular risk factors including hypertension, diabetes mellitus, and overweight/obesity were similar (P for difference ≥0.07) (Figure 2). Stratification by statin use and antiplatelet agents use also did not reveal any significant differences (Figure 2). Further comparison by study-level features such as study-type, CHD type, follow-up duration and proportion of history of myocardial infarction revealed no clear differences (Figure 3).
Figure 2. Pooled associations of factor V Leiden with the primary outcome across traditional cardiovascular risk factors strata in the overall CHD population.
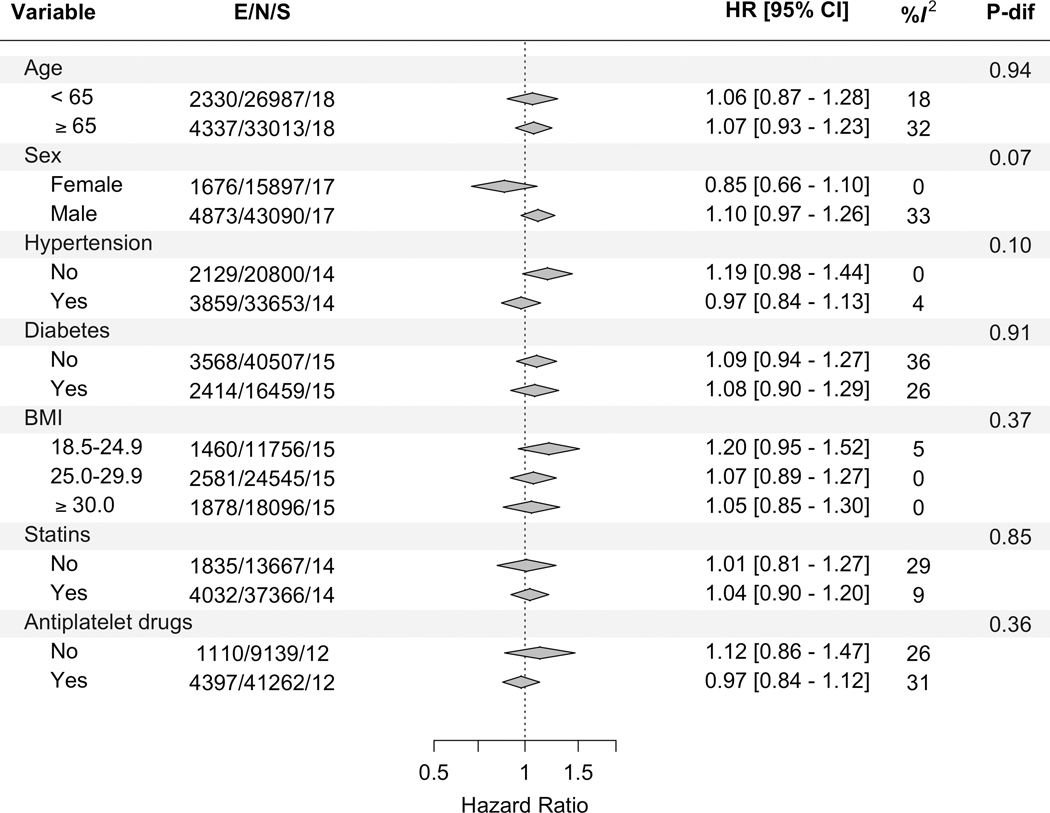
BMI= body-mass index, E=number of the primary outcome (i.e., composite of myocardial infarction and CHD death), N= total number of included participants, S= number of contributing studies, HR= hazard ratio, CI= confidence interval, and P-dif= P for difference across the strata of the variable. Estimates are adjusted for sex and age, when appropriate, and are based on fixed-effect meta-analysis.
Figure 3. Association between study-level characteristics and the log-hazard ratios for the primary outcome in the overall CHD population.
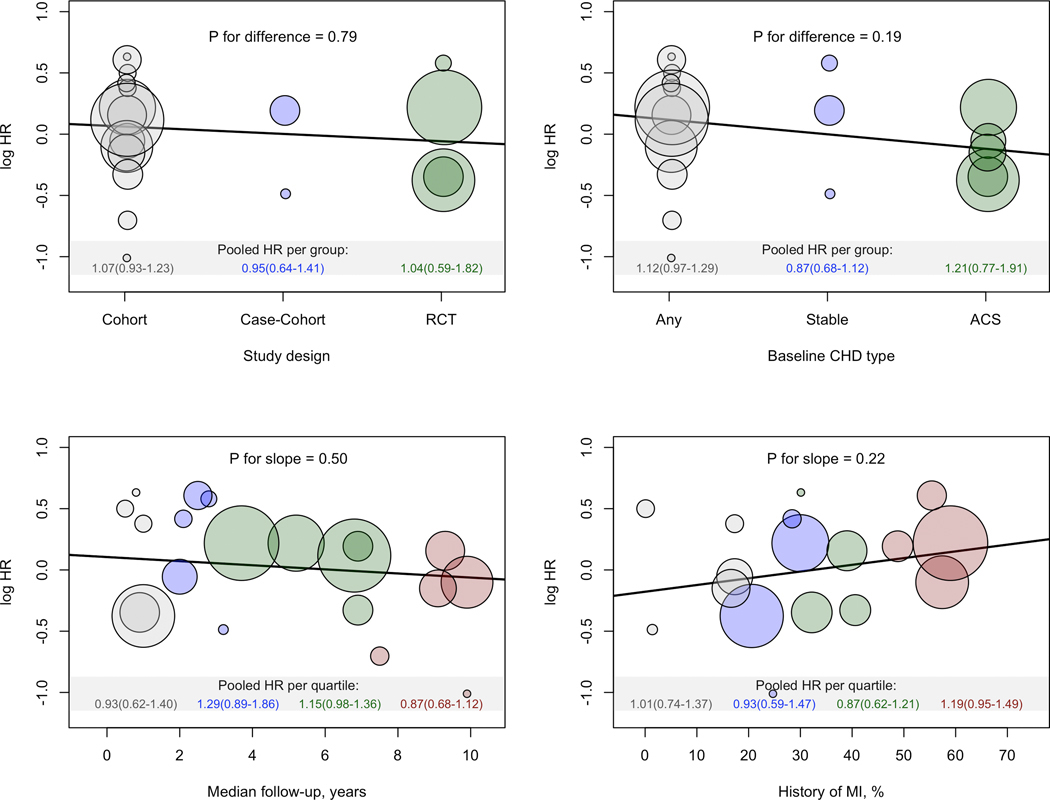
The middle of each bubble represents the log-hazard ratio of the primary outcome from the individual studies against the study-characteristics shown on the x-axis. The sizes of the bubbles are proportional to the inverse of the standard-errors of the log-hazard ratios.
Study level associations between factor V Leiden and the primary outcome (Figure I in the supplement) revealed a significant but paradoxically inverse risk association (hazards ratio, 0.69; 95% CI 0.49–0.96) for the Platelet Inhibition and Patient Outcomes (PLATO) study, a randomized controlled trial of ticagrelor plus aspirin versus clopidogrel plus aspirin. A similar but non-significant nominal association (hazards ratio, 0.80; 95% CI 0.5–1.28) was observed for the Clopidogrel in the Unstable Angina to Prevent Recurrent Events (CURE) study, a randomized controlled trial of clopidogrel plus aspirin versus aspirin alone. We therefore conducted a post-hoc analysis in both studies, to assess for a potential interaction between dual antiplatelet drugs use (i.e., aspirin plus a P2Y12 inhibitor clopidogrel or ticagrelor) and factor V Leiden status. Based on the combined PLATO and CURE trials data, while there was a nominal association towards a greater protective benefit in factor V Leiden carriers taking dual antiplatelet agents (hazard ratio, 0.67; 95%CI, 0.50–0.92), compared to aspirin alone (hazard ratio, 0.97; 95% CI, 0.50–1.89), the statistical test for interaction was non-significant (P interaction = 0.33; Figure V in the supplement).
Association of factor V Leiden with secondary outcomes
Pooled estimates for the secondary outcomes including non-fatal myocardial infarction, any stroke, ischemic stroke, coronary revascularization, cardiovascular mortality and all-cause mortality, accompanied by heterogeneity statistics, number of events, number of participants and studies are shown in Figure 4. In line with the primary outcome, the associations of factor V Leiden with the various secondary outcomes were close to identity and non-significant with low heterogeneity (I2 ≤32%; P-heterogeneity ≥0.10). The estimates of the individual studies contributing to these pooled estimates are shown in the Figures VI–XI in the supplement.
Figure 4. Pooled associations of factor V Leiden with the secondary outcomes in the overall CHD population.
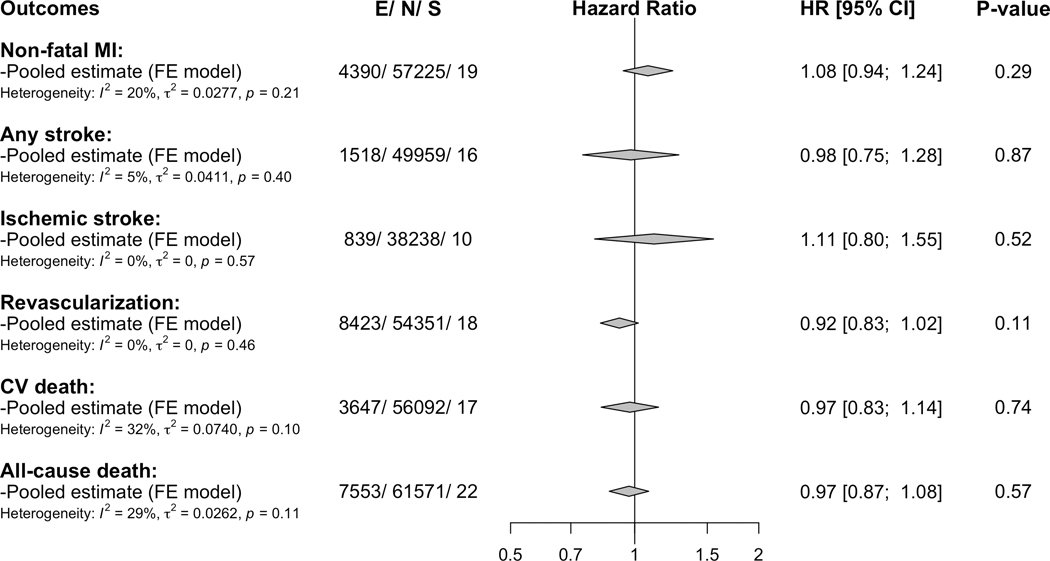
MI= myocardial infarction, CV= cardiovascular, E=number of the outcome, N= total number of included participants, S= number of contributing studies, HR= hazard ratio and CI= confidence interval. Estimates are adjusted for sex and age and are based on fixed-effect meta-analysis. For the estimates of the individual studies contributing to these pooled estimates see Supplemental Figures VI–XI in the Supplement.
DISCUSSION
In this large-scale individual-level data meta-analysis, we did not find evidence of an association between factor V Leiden and subsequent or recurrent atherothrombotic event risk in nearly 70,000 patients with established CHD. Furthermore, in subgroup analyses no statistically significant interactions to suggest differences by type of baseline CHD, study level or patient level factors were found. These findings suggest there is limited value in assessing factor V Leiden status for risk stratification once CHD is established and treated.
Prior studies on the association of the factor V Leiden polymorphism with atherothrombotic outcomes including myocardial infarction and stroke, have been limited to case-control studies or to the occurrence of a first event in asymptomatic individuals.4–10 They showed contradictory results ranging from no risk to a ~20% relative risk increase for myocardial infarction or stroke, as summarized in several meta-analyses.4–6, 8 In prospective cohort studies, factor V Leiden was not associated with incident myocardial infarction or stroke.6, 9, 10 Concordant with these cohort studies, but studying only those with established CHD, who are at high risk for atherothrombotic events, we found no association of factor V Leiden with subsequent myocardial infarction, stroke, coronary revascularization or mortality. There were no significant subgroup effects, including follow-up duration, study design, and baseline history of myocardial infarction. Furthermore, in contrast to prior studies that identified a greater risk association of factor V Leiden with stroke compared to myocardial infarction,17 we did not find such heterogeneity in analysis of our secondary outcomes.
There could be several reasons for our findings. First, it is possible that factor V Leiden has no impact on the risk of atherothrombotic events and development of acute thrombosis in relation to plaque rupture or erosion in the arterial system. Second, the present analysis may have been underpowered to detect a small association and our results are therefore prone to type 2 error. However, the 95% confidence interval for the primary outcome (95%CI, 0.92 – 1.16) excludes any clinically relevant association. Third, the effects of factor V Leiden may be masked in the presence of other substantial cardiovascular disease risk factors driving the risk of subsequent events, although a synergistic interaction of factor V Leiden with traditional cardiovascular risk factors has been reported.11–13
Importantly, medication usage, in particular antiplatelets and anticoagulants may blur any small elevated risk from factor V Leiden. We noted that the PLATO trial,18 enrolling only patients with acute coronary syndromes and comparing dual antiplatelet agents (i.e. aspirin plus ticagrelor versus aspirin plus clopidogrel), showed a significant inverse association of factor V Leiden with the primary outcome. A similar but non-significant trend was also noted for the CURE trial comparing clopidogrel plus aspirin versus aspirin alone. Combining these trial data, we found a trend favoring a paradoxically protective effect for carriers of factor V Leiden taking a P2Y12 inhibitor (i.e. clopidogrel or ticagrelor) plus aspirin, compared to aspirin alone, but without statistical evidence for an interaction, although this may be due to the low numbers of participants in the aspirin arm compared to the P2Y12 inhibitors groups. Indeed, a potential interaction of factor V Leiden with antiplatelet agents may be biologically plausible given that approximately 20% of human factor V is contained within platelet-granules, is released on platelet activation and is more haemostatically potent than circulating factor V.19, 20 Although a post hoc finding, this is hypothesis generating and warrants further assessment in existing trials of intensive dual antiplatelet and even combined antiplatelet and anticoagulant strategies,14 as it opens the intriguing pharmacogenomic possibility that factor V Leiden carriers may derive greater outcome benefit from intensive and prolonged rather than standard antiplatelet therapy. If confirmed, then assessment of factor V Leiden status could help personalize treatment decisions and improve net clinical benefit in high-risk patients.
A further explanation for our overall neutral findings, as with all studies on disease progression is the impact of selection bias, as described previously.21 Selection biases may include survival bias, with loss of more severe phenotypic manifestations of factor V Leiden not entering the cohort for study. Alternatively, index event bias may be at play. Indeed, the association of heterozygous factor V Leiden with the risk of recurrent venous thromboembolism (odds-ratio 1.4) is also much weaker than its association with a first venous thromboembolism (odds-ratio 4.2),3, 22 indicating either selection biases or potential effect-modification by disease-status or treatment effects may attenuate associations in the second event context.
Among the known hereditary thrombophilic defects, factor V Leiden is generally considered as a moderate prothrombotic risk factor.23 However, one of the strongest known hereditary thrombophilic defects (i.e., antithrombin deficiency) has also failed to demonstrate a significant association with risk of atherothrombotic events,24 leaving gaps in our understanding as to why hypercoagulable defects fail to associate with atherothrombotic event risk. In contrast anticoagulant drugs targeting the coagulation cascade seem to be at least as effective, for prevention and treatment of atherothrombotic events, as the widely used antiplatelet agent (i.e., aspirin).14, 25–27
Our study has some limitations. First, although care was taken to harmonize the definitions across studies, it is possible that residual differences remained among studies. Second, detailed patient level information on the concurrent use of antiplatelet agents, anticoagulant drugs, dual versus single antiplatelet agents use and its duration was not available in most of the cohorts. This, along with the absence of data on other P2Y12 inhibitors such as prasugrel within the Consortium, limited further exploration of the possible interaction between factor V Leiden and clopidogrel/ticagrelor we found in the CURE and PLATO studies. It is also possible that treatment with any antiplatelet agent may have attenuated overall findings and given most patient with established CHD are on aspirin, this could account for our findings. Importantly, if risk from factor V Leiden is indeed attenuated by aspirin use, the value of measuring the genotype among those on aspirin remains questionable in any case. Third, we lacked sufficient data on incident venous thromboembolism events among patients with established CHD to demonstrate a suitable positive control association with factor V Leiden in this setting. Finally, our results may not be generalizable to non-Caucasian populations given that in most studies primarily Caucasian individuals were enrolled.
In conclusion, the prothrombotic hereditary coagulation defect, factor V Leiden, does not show a clear association with increased risk of subsequent atherothrombotic events or mortality, among high-risk individuals with established and treated CHD. Routine assessment of factor V Leiden status is unlikely to improve risk stratification in this population. However, whether there is pharmacogenomic value for factor V Leiden status guiding intensive antiplatelet therapy warrants further study.
Supplementary Material
CLINICAL PERSPECTIVE:
What Is New?
In a large-scale individual-level data meta-analysis of 25 studies recruiting nearly 70K patients with established CHD, factor V Leiden was not associated with an increased risk of further atherothrombotic events or death compared to non-carriers.
A post hoc analysis however suggests that factor V Leiden carriers with established CHD may gain greater protection from subsequent CHD death or myocardial infarction from dual antiplatelet therapy compared to non-carriers.
What Are the Clinical Implications?
The routine assessment of factor V Leiden genotype to improve risk stratification in secondary prevention settings is unlikely to be of value and is not recommended.
Further work is required to understand if there may instead be a pharmacogenomic role for factor V Leiden status, to help personalize treatment with intensive antiplatelet therapy.
Acknowledgements:
VT, FS, RM, KD and MH helped develop the analysis scripts, curate and manage the data at the coordinating sites. BKM performed the meta-analysis of the collected estimates and drafted the paper. RSP and FWA coordinated analyses and development of the GENIUS-CHD Consortium. All authors took part in the interpretation of the data, and all authors provided critical revisions of the manuscript for important intellectual content.
The GENIUS-CHD collaborators would like to express their immense gratitude to all patients who participated in each of the individual studies as well as the many personnel who helped with recruitment, collection, curation, management, and processing of the samples and data. Sponsors of the individual studies are listed here and study-acronyms are listed in the supplemental material: The Cleveland Clinic Genebank Study was supported in part by NIH grants R0133169, R01ES021801, R01MD010358, and R01ES025786, R01HL103866, R01DK106000, R01HL126827, P20HL113452.P01HL098055, P01HL076491, and R01HL103931. The LURIC study was supported by the 7th Framework Program (AtheroRemo, grant agreement number 201668 and RiskyCAD, grant agreement number 305739) of the European Union. INVEST-GENES was supported by the National Institute of Health Pharmacogenomics Research Network grant U01-GM074492, NIH R01 HL074730, University of Florida Opportunity Fund, BASF Pharma and Abbott Laboratories. FAST-MI (French Registry of Acute ST-Elevation or non- ST-elevation Myocardial Infarction) 2005 is a registry of the French Society of Cardiology, supported by unrestricted grants from Pfizer and Servier. Additional support was obtained from a research grant from the French Caisse Nationale d’Assurance Maladie. The Wellcome Trust United Kingdom Type 2 Diabetes Case Control Collection (supporting GoDARTS) was funded by the Wellcome Trust (072960/Z/03/Z, 084726/Z/08/Z, 084727/Z/08/Z, 085475/Z/08/Z, 085475/B/08/Z) and as part of the EU IMI-SUMMIT programme. The Finnish Cardiovascular Study (FINCAVAS) has been financially supported by the Competitive Research Funding of the Tampere University Hospital (Grant 9M048 and 9N035), the Finnish Cultural Foundation, the Finnish Foundation for Cardiovascular Research, the Emil Aaltonen Foundation, Finland, the Tampere Tuberculosis Foundation, EU Horizon 2020 (grant 755320 for TAXINOMISIS), and the Academy of Finland grant 322098. CABGenomics was supported by Stanton Shernan, C. David Collard, Amanda A. Fox/R01 HL 098601 NHLBI. The CDCS and PMI studies were funded by the Health Research Council and Heart Foundation of New Zealand. The TRIUMPH study was sponsored by the National Institutes of Health: Washington University School of Medicine SCCOR Grant P50 HL077113. The KRAKOW GENIUS Study was supported by a grant from the Polish Ministry of Science and Higher Education, no. NN402083939 and the National Science Centre, no. 2013/09/B/NZ5/00770. The ANGES has been financially supported by the Competitive Research Funding of the Tampere University Hospital (Grant 9M048 and 9N035), the Finnish Cultural Foundation, the Finnish Foundation for Cardiovascular Research, the Emil Aaltonen Foundation, Finland, the Tampere Tuberculosis Foundation, EU Horizon 2020 (grant 755320 for TAXINOMISIS), and the Academy of Finland grant 322098. The UCP studies were funded by the Netherlands Heart Foundation and the Dutch Top Institute Pharma Mondriaan Project. LIFE-Heart was funded by the Leipzig Research Center for Civilization Diseases (LIFE). LIFE is an organizational unit affiliated to the Medical Faculty of the University of Leipzig. LIFE is funded by means of the European Union, by the European Regional Development Fund and by funds of the Free State of Saxony within the framework of the excellence initiative. OHGS was funded in part by a Heart and Stroke Foundation grant.
Source of Funding:
The funder(s) of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The funder(s) of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. Funding and sponsors of all participating investigators not directly linked to this analysis are listed in the disclosure section and funding for the individual studies is acknowledged in the acknowledgement section. Dr. Mahmoodi received a VENI grant from the Dutch Research Council for investigating the role of factor V Leiden in the pathogenesis of myocardial infarction. Dr. Patel is funded by a British Heart Foundation Intermediate Fellowship (FS/14/76/30933). The GENIUS-CHD project was also supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
Disclosures:
Dr. Holmes has collaborated with Boehringer Ingelheim in research, and in accordance with the policy of The Clinical Trial Service Unit and Epidemiological Studies Unit (University of Oxford), did not accept any personal payment. He works in a unit that receives funding from the UK Medical Research Council and is supported by a British Heart Foundation Intermediate Clinical Research Fellowship (FS/18/23/33512) and the National Institute for Health Research Oxford Biomedical Research Centre. Dr. Schmidt is funded by BHF grant PG/18/5033837. Dr. Allayee was supported, in part, by NIH grants R01HL133169 and R01HL148110. Dr. Eriksson reports Institutional research grants from AstraZeneca and GlaxoSmithKline. Dr. Nelson is funded by the British Heart Foundation. Dr. Vilmundarson is supported by a graduate fellowship of the University of Ottawa Heart Institute. Dr. James has received grants from AstraZeneca, The Medicines Company, Swedish heart and lung foundation, Swedish research council, Janssen; personal fees from Bayer. Dr. Mordi is supported by an NHS Education of Scotland/Chief Scientist Office Postdoctoral Clinical Lectureship (PCL 17/07). Dr. Cresci is supported, in part, by the National Institutes of Health (Cresci R01 NR013396). Dr. Hagstrom declares being an expert committee member, lecture fees, and institutional research grant from Sanofi, and Amgen; institutional research grants from AstraZeneca, and GlaxoSmithKline; expert committee member and lecture fees NovoNordisk and Behringer. Dr. Held declares institutional research grant, advisory board member and speaker’s bureau from AstraZeneca; institutional research grants from Bristol-Myers Squibb Merck and Co, GlaxoSmithKline, Roche Diagnostics. Advisory board for Bayer and Boehringer Ingelheim. Dr. Palmer has received grant funding from the Wellcome Trust to develop the GoDARTS cohort. Dr. Akerblom has received institutional research grant and speakers fee from AstraZeneca, institutional research grant from Roche Diagnostics. Prof. Samani is funded by the British Heart Foundation and is a NIHR Senior Investigator. Dr. ten Berg reports receiving fees for board membership from AstraZeneca,consulting fees from AstraZeneca, Eli Lilly, and Merck, and lecture fees from Daiichi Sankyo and Eli Lilly, AstraZeneca, Sanofi and Accumetrics. Prof Wallentin reports institutional research grants, consultancy fees, lecture fees, and travel support from Bristol-Myers Squibb/Pfizer, AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim; institutional research grants from Merck and Co, Roche Diagnostics; consultancy fees from Abbott; and holds a patent EP2047275B1 licensed to Roche Diagnostics, and a patent US8951742B2 licensed to Roche Diagnostics.
Abbreviations and Acronyms:
- CHD
Coronary Heart Disease
Footnotes
REFERENCES:
- 1.Bertina RM, Koeleman BP, Koster T, Rosendaal FR, Dirven RJ, de Ronde H, van der Velden PA and Reitsma PH. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature. 1994;369:64–67. [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM, Miletich JP, Hennekens CH and Buring JE. Ethnic distribution of factor V Leiden in 4047 men and women. Implications for venous thromboembolism screening. JAMA. 1997;277:1305–1307. [PubMed] [Google Scholar]
- 3.Simone B, De Stefano V, Leoncini E, Zacho J, Martinelli I, Emmerich J, Rossi E, Folsom AR, Almawi WY, Scarabin PY, et al. Risk of venous thromboembolism associated with single and combined effects of Factor V Leiden, Prothrombin 20210A and Methylenetethraydrofolate reductase C677T: a meta-analysis involving over 11,000 cases and 21,000 controls. Eur J Epidemiol. 2013;28:621–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bentley P, Peck G, Smeeth L, Whittaker J and Sharma P. Causal relationship of susceptibility genes to ischemic stroke: comparison to ischemic heart disease and biochemical determinants. PLoS ONE. 2010;5:e9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamedani AG, Cole JW, Mitchell BD and Kittner SJ. Meta-analysis of factor V Leiden and ischemic stroke in young adults: the importance of case ascertainment. Stroke. 2010;41:1599–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juul K, Tybjaerg-Hansen A, Steffensen R, Kofoed S, Jensen G and Nordestgaard BG. Factor V Leiden: The Copenhagen City Heart Study and 2 meta-analyses. Blood. 2002;100:3–10. [DOI] [PubMed] [Google Scholar]
- 7.Mannucci PM, Asselta R, Duga S, Guella I, Spreafico M, Lotta L, Merlini PA, Peyvandi F, Kathiresan S and Ardissino D. The association of factor V Leiden with myocardial infarction is replicated in 1880 patients with premature disease. J Thromb Haemost. 2010;8:2116–2121. [DOI] [PubMed] [Google Scholar]
- 8.Ye Z, Liu EH, Higgins JP, Keavney BD, Lowe GD, Collins R and Danesh J. Seven haemostatic gene polymorphisms in coronary disease: meta-analysis of 66,155 cases and 91,307 controls. Lancet. 2006;367:651–658. [DOI] [PubMed] [Google Scholar]
- 9.Cushman M, Rosendaal FR, Psaty BM, Cook EF, Valliere J, Kuller LH and Tracy RP. Factor V Leiden is not a risk factor for arterial vascular disease in the elderly: results from the Cardiovascular Health Study. Thromb Haemost. 1998;79:912–915. [PubMed] [Google Scholar]
- 10.Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR and Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med. 1995;332:912–917. [DOI] [PubMed] [Google Scholar]
- 11.Doggen CJ, Cats VM, Bertina RM and Rosendaal FR. Interaction of coagulation defects and cardiovascular risk factors: increased risk of myocardial infarction associated with factor V Leiden or prothrombin 20210A. Circulation. 1998;97:1037–1041. [DOI] [PubMed] [Google Scholar]
- 12.Mahmoodi BK, Veeger NJ, Middeldorp S, Lijfering WM, Brouwer JL, Ten Berg J, Hamulyak K and Meijer K. Interaction of Hereditary Thrombophilia and Traditional Cardiovascular Risk Factors on the Risk of Arterial Thromboembolism: Pooled Analysis of Four Family Cohort Studies. Circ Cardiovasc Genet. 2016;9:79–85. [DOI] [PubMed] [Google Scholar]
- 13.Rosendaal FR, Siscovick DS, Schwartz SM, Beverly RK, Psaty BM, Longstreth WT Jr., Raghunathan TE, Koepsell TD and Reitsma PH. Factor V Leiden (resistance to activated protein C) increases the risk of myocardial infarction in young women. Blood. 1997;89:2817–2821. [PubMed] [Google Scholar]
- 14.Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N Engl J Med. 2017;377:1319–1330. [DOI] [PubMed] [Google Scholar]
- 15.Patel RS, Tragante V, Schmidt AF, McCubrey RO, Holmes MV, Howe LJ, Direk K, Akerblom A, Leander K, Virani SS, et al. Subsequent Event Risk in Individuals With Established Coronary Heart Disease. Circ Genom Precis Med. 2019;12:e002470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.R Development Core Team. R: A language and environment for statistical computing [computer program]. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 17.Maino A, Rosendaal FR, Algra A, Peyvandi F and Siegerink B. Hypercoagulability Is a Stronger Risk Factor for Ischaemic Stroke than for Myocardial Infarction: A Systematic Review. PLoS ONE. 2015;10:e0133523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. [DOI] [PubMed] [Google Scholar]
- 19.Duckers C, Simioni P, Spiezia L, Radu C, Dabrilli P, Gavasso S, Rosing J and Castoldi E. Residual platelet factor V ensures thrombin generation in patients with severe congenital factor V deficiency and mild bleeding symptoms. Blood. 2010;115:879–886. [DOI] [PubMed] [Google Scholar]
- 20.Ren M, Li R, Chen N, Pang N, Li Y, Deng X, Wang L, Luo M, Liu Y, Hao H, et al. Platelet-Derived Factor V Is a Critical Mediator of Arterial Thrombosis. J Am Heart Assoc. 2017;6:e006345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu YJ, Schmidt AF, Dudbridge F, Holmes MV, Brophy JM, Tragante V, Li Z, Liao P, Quyyumi AA, McCubrey RO, et al. Impact of Selection Bias on Estimation of Subsequent Event Risk. Circ Cardiovasc Genet. 2017;10:e001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchetti M, Pistorio A and Barosi G. Extended anticoagulation for prevention of recurrent venous thromboembolism in carriers of factor V Leiden--cost-effectiveness analysis. Thromb Haemost. 2000;84:752–757. [PubMed] [Google Scholar]
- 23.Makris M. Thrombophilia: grading the risk. Blood. 2009;113:5038–5039. [DOI] [PubMed] [Google Scholar]
- 24.Mahmoodi BK, Brouwer JL, Veeger NJ and van der Meer J. Hereditary deficiency of protein C or protein S confers increased risk of arterial thromboembolic events at a young age: results from a large family cohort study. Circulation. 2008;118:1659–1667. [DOI] [PubMed] [Google Scholar]
- 25.Anand SS and Yusuf S. Oral anticoagulant therapy in patients with coronary artery disease: a meta-analysis. JAMA. 1999;282:2058–2067. [DOI] [PubMed] [Google Scholar]
- 26.Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura T, Maeng M, Merkely B, Zeymer U, Gropper S, et al. Dual Antithrombotic Therapy with Dabigatran after PCI in Atrial Fibrillation. N Engl J Med. 2017;377:1513–1524. [DOI] [PubMed] [Google Scholar]
- 27.Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, Birmingham M, Ianus J, Burton P, van Eickels M, et al. Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI. N Engl J Med. 2016;375:2423–2434. [DOI] [PubMed] [Google Scholar]
- 28.Raitoharju E, Seppala I, Levula M, Kuukasjarvi P, Laurikka J, Nikus K, Huovila AP, Oksala N, Klopp N, Illig T, et al. Common variation in the ADAM8 gene affects serum sADAM8 concentrations and the risk of myocardial infarction in two independent cohorts. Atherosclerosis. 2011;218:127–133. [DOI] [PubMed] [Google Scholar]
- 29.Barnet CS, Liu X, Body SC, Collard CD, Shernan SK, Muehlschlegel JD, Jarolim P and Fox AA. Plasma corin decreases after coronary artery bypass graft surgery and is associated with postoperative heart failure: a pilot study. J Cardiothorac Vasc Anesth. 2015;29:374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ellis KL, Pilbrow AP, Frampton CM, Doughty RN, Whalley GA, Ellis CJ, Palmer BR, Skelton L, Yandle TG, Palmer SC, et al. A common variant at chromosome 9P21.3 is associated with age of onset of coronary disease but not subsequent mortality. Circ Cardiovasc Genet. 2010;3:286–293. [DOI] [PubMed] [Google Scholar]
- 31.Hoefer IE, Sels JW, Jukema JW, Bergheanu S, Biessen E, McClellan E, Daemen M, Doevendans P, de Groot P, Hillaert M, et al. Circulating cells as predictors of secondary manifestations of cardiovascular disease: design of the CIRCULATING CELLS study. Clin Res Cardiol. 2013;102:847–856. [DOI] [PubMed] [Google Scholar]
- 32.Mehta SR, Yusuf S and Clopidogrel in Unstable angina to prevent Recurrent Events Study I. The Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) trial programme; rationale, design and baseline characteristics including a meta-analysis of the effects of thienopyridines in vascular disease. Eur Heart J. 2000;21:2033–2041. [DOI] [PubMed] [Google Scholar]
- 33.Cambou JP, Simon T, Mulak G, Bataille V and Danchin N. The French registry of Acute ST elevation or non-ST-elevation Myocardial Infarction (FAST-MI): study design and baseline characteristics. Arch Mal Coeur Vaiss. 2007;100:524–534. [PubMed] [Google Scholar]
- 34.Nieminen T, Lehtinen R, Viik J, Lehtimaki T, Niemela K, Nikus K, Niemi M, Kallio J, Koobi T, Turjanmaa V, et al. The Finnish Cardiovascular Study (FINCAVAS): characterising patients with high risk of cardiovascular morbidity and mortality. BMC Cardiovasc Disord. 2006;6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doney AS, Fischer B, Lee SP, Morris AD, Leese G and Palmer CN. Association of common variation in the PPARA gene with incident myocardial infarction in individuals with type 2 diabetes: a Go-DARTS study. Nucl Recept. 2005;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evans JM, Wang J and Morris AD. Comparison of cardiovascular risk between patients with type 2 diabetes and those who had had a myocardial infarction: cross sectional and cohort studies. BMJ. 2002;324:939–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gong Y, Beitelshees AL, Cooper-DeHoff RM, Lobmeyer MT, Langaee TY, Wu J, Cresci S, Province MA, Spertus JA, Pepine CJ, et al. Chromosome 9p21 haplotypes and prognosis in white and black patients with coronary artery disease. Circ Cardiovasc Genet. 2011;4:169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, Sigamani A, Xavier D, Chan MTV, Srinathan SK, Walsh M, Abraham V, Pearse R, et al. Association of Postoperative High-Sensitivity Troponin Levels With Myocardial Injury and 30-Day Mortality Among Patients Undergoing Noncardiac Surgery. JAMA. 2017;317:1642–1651. [DOI] [PubMed] [Google Scholar]
- 40.Beutner F, Teupser D, Gielen S, Holdt LM, Scholz M, Boudriot E, Schuler G and Thiery J. Rationale and design of the Leipzig (LIFE) Heart Study: phenotyping and cardiovascular characteristics of patients with coronary artery disease. PLoS ONE. 2011;6:e29070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winkelmann BR, Marz W, Boehm BO, Zotz R, Hager J, Hellstern P, Senges J and Group LS. Rationale and design of the LURIC study--a resource for functional genomics, pharmacogenomics and long-term prognosis of cardiovascular disease. Pharmacogenomics. 2001;2:S1–73. [DOI] [PubMed] [Google Scholar]
- 42.James S, Akerblom A, Cannon CP, Emanuelsson H, Husted S, Katus H, Skene A, Steg PG, Storey RF, Harrington R, et al. Comparison of ticagrelor, the first reversible oral P2Y(12) receptor antagonist, with clopidogrel in patients with acute coronary syndromes: Rationale, design, and baseline characteristics of the PLATelet inhibition and patient Outcomes (PLATO) trial. Am Heart J. 2009;157:599–605. [DOI] [PubMed] [Google Scholar]
- 43.Richards AM, Nicholls MG, Espiner EA, Lainchbury JG, Troughton RW, Elliott J, Frampton C, Turner J, Crozier IG and Yandle TG. B-type natriuretic peptides and ejection fraction for prognosis after myocardial infarction. Circulation. 2003;107:2786–2792. [DOI] [PubMed] [Google Scholar]
- 44.Breet NJ, van Werkum JW, Bouman HJ, Kelder JC, Ruven HJ, Bal ET, Deneer VH, Harmsze AM, van der Heyden JA, Rensing BJ, et al. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA. 2010;303:754–762. [DOI] [PubMed] [Google Scholar]
- 45.Simons PC, Algra A, Bots ML, Grobbee DE and van der Graaf Y. Common carotid intima-media thickness and arterial stiffness: indicators of cardiovascular risk in high-risk patients. The SMART Study (Second Manifestations of ARTerial disease). Circulation. 1999;100:951–957. [DOI] [PubMed] [Google Scholar]
- 46.STABILITY Investigators, White HD, Held C, Stewart R, Tarka E, Brown R, Davies RY, Budaj A, Harrington RA, Steg PG, et al. Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med. 2014;370:1702–1711. [DOI] [PubMed] [Google Scholar]
- 47.Arnold SV, Chan PS, Jones PG, Decker C, Buchanan DM, Krumholz HM, Ho PM, Spertus JA and Cardiovascular Outcomes Research C. Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status (TRIUMPH): design and rationale of a prospective multicenter registry. Circ Cardiovasc Qual Outcomes. 2011;4:467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gijsberts CM, den Ruijter HM, de Kleijn DP, Huisman A, ten Berg MJ, van Wijk RH, Asselbergs FW, Voskuil M, Pasterkamp G, van Solinge WW, et al. Hematological Parameters Improve Prediction of Mortality and Secondary Adverse Events in Coronary Angiography Patients: A Longitudinal Cohort Study. Medicine (Baltimore). 2015;94:e1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kroger E, Van Marum R, Souverein P, Carmichael PH and Egberts T. Treatment with rivastigmine or galantamine and risk of urinary incontinence: results from a Dutch database study. Pharmacoepidemiology and drug safety. 2015;24:276–285. [DOI] [PubMed] [Google Scholar]
- 50.Rutten-Jacobs LCA, Tozer DJ, Duering M, Malik R, Dichgans M, Markus HS and Traylor M. Genetic Study of White Matter Integrity in UK Biobank (N=8448) and the Overlap With Stroke, Depression, and Dementia. Stroke. 2018;49:1340–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Samani NJ, Burton P, Mangino M, Ball SG, Balmforth AJ, Barrett J, Bishop T, Hall A and Group BHFFHSR. A genomewide linkage study of 1,933 families affected by premature coronary artery disease: The British Heart Foundation (BHF) Family Heart Study. Am J Hum Genet. 2005;77:1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


