Abstract
Background
Despite the promise of immunotherapy for gastric adenocarcinoma, choices for the selection of effective antigenic targets are very limited. Previously published data and our own in-house computational analysis have suggested that ANTXR1 is a potential target, simultaneously expressed in malignant tumor cells and the endothelial cells of the tumors. However, the expression pattern of ANTXR1 protein in clinical samples of gastric adenocarcinoma has not been fully evaluated.
Methods
Using immunohistochemistry (IHC), we recorded the percentage of ANTXR1 positive cells separately in tumor cells and endothelial cells in the primary tumor, non-tumor gastric tissue adjacent to the primary tumor, and tumor in metastatic sites of 140 gastric adenocarcinoma patients. We also evaluated the association of ANTXR1 expression with the Lauren histological classification of the primary tumors, the patient’s history of neoadjuvant chemotherapy and/or radiotherapy, and the patient’s overall survival.
Results
ANTXR1 was expressed in a mean of 73.89 ± 30.12% of tumor cells and 13.55 ± 20.53% of endothelial cells in the primary tumors. Intestinal adenocarcinomas had lower ANTXR1 expression in the tumor cells and higher ANTXR1 expression in the endothelial cells of the tumor regions, and a history of neoadjuvant therapy was associated with increased ANTXR1 expression in the endothelial cells of the tumor regions. Finally, above median expression of ANTXR1 in the tumor cells of the tumor regions was associated with significantly lower overall patient survival.
Conclusions
Our findings suggest that ANTXR1 is a promising candidate for preclinical and clinical evaluation for gastric adenocarcinoma immunotherapy.
Electronic supplementary material
The online version of this article (10.1007/s00262-019-02392-y) contains supplementary material, which is available to authorized users.
Keywords: Chimeric antigen receptor T cell, Gastric adenocarcinoma, ANTXR1, TEM8, Antigenic targets, Immunotherapy
Introduction
Gastric cancer is the third most common cause of cancer death worldwide [1]. The initial symptoms of many gastric cancers are non-specific, leading to late-stage diagnosis, with high rates of regional and distant metastases [2]. In this setting, monomodality therapies, such as surgery, are often not curative, and overall survival is low [3]. To date, several key clinical trials have shown that multimodality therapies, including chemotherapy, radiotherapy, and more recently immunotherapy, can significantly improve the survival of patients with gastric cancer [4]. Among the targeted immunotherapies, trastuzumab [targeting ERBB2 (HER2)] [5], ramucirumab [targeting KDR (VEGFR2)] [6], and pembrolizumab [targeting PDCD1 (PD-1)] [7] have been FDA-approved for the treatment of locally advanced or metastatic gastric cancer. Despite the undeniable applicability of these targets, search for and validation of new antigenic targets for immunotherapy and evaluation of multidrug panels of immunotherapeutic agents still need to be actively pursued for several reasons. First, according to current algorithms, immunotherapy agents can only be prescribed if the targeted antigen is sufficiently expressed in the tumor bed [8], and second, resistance has been observed even in the face of continued therapy [9, 10]. Thus, monotherapy with one of these immune-targeted treatments is seldom completely curative, and eventually, relapse of the tumor nearly always occurs. Hence, the development and use of panels of different immunotherapy drugs, targeting different antigenic targets, are needed to diminish the risk of resistance and relapse.
ANTXR1 (TEM8) has been shown to be overexpressed in tumor-associated vs. normal endothelium in several cancer types [11, 12]. The subsequent use of an anti-TEM8 antibody–drug conjugate in a preclinical setting also revealed its potential to augment therapies in diverse cancer types [13]. In addition, at the beginning of 2018, Byrd et al. reported the first preclinical evaluation of ANTXR1-specific chimeric antigen receptor T cells (CAR T cells) for triple-negative breast cancer, showing obliteration of tumor neovasculature and regression of established patient-derived xenografts (PDXs) in local and metastatic murine models of this disease [14]. These findings, along with our earlier bioinformatics study which demonstrated the applicability of ANTXR1 (TEM8) for CAR T cell therapy of gastric adenocarcinoma [15], motivated us to assess the ANTXR1 (TEM8) protein expression pattern in clinical samples, to further evaluate its potential applicability as an antigenic target for immunotherapy of gastric adenocarcinoma.
In this study, we evaluated ANTXR1 protein expression in tumor cells and endothelial cells in three anatomical sites, the primary tumor, non-tumor gastric tissue adjacent to the primary tumor, and tumor in metastatic sites. We also evaluated the homogeneity of ANTXR1 expression in each anatomical site. Finally, we evaluated the association of ANTXR1 expression with the Lauren histological classification of the primary tumors, the patient’s history of neoadjuvant chemotherapy and/or radiotherapy, and the patient’s overall survival.
Materials and methods
We evaluated 140 patients who underwent surgery for gastric adenocarcinoma between March 2011 and February 2018 at Shariati and Atieh hospitals. Immunohistochemistry (IHC) for ANTXR1 was carried out on tissue microarray (TMA) blocks, which were serially sectioned to ensure comparability of the tissue components on each slide (Fig. 1). For each patient, two 1 mm cores were randomly collected from each of three anatomical locations, the primary tumor (PT), non-tumor gastric tissue adjacent to the primary tumor (the non-tumor region, NT), and tumor in a metastatic site (lymph node or distant metastasis; metastatic tumor, MT), and all of the cores from each patient were embedded in the same TMA block. The ANTXR1 expression was evaluated separately in the tumor cells (in the PT and MT cores) and in the endothelial cells (in the NT, PT, and MT cores).
Fig. 1.
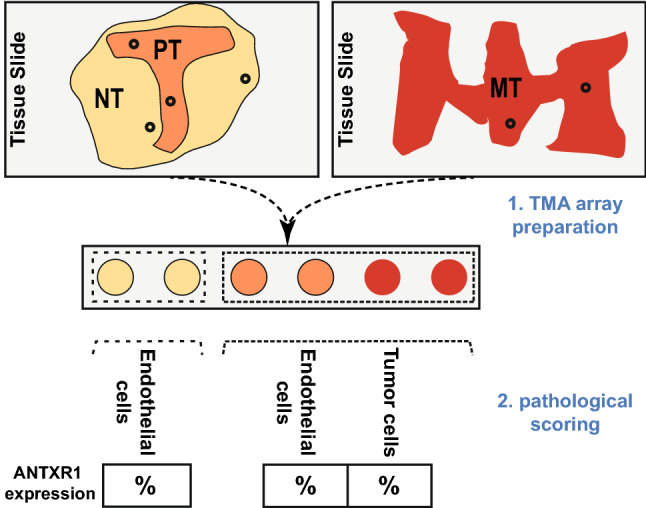
Steps from tissue microarray (TMA) preparation to data acquisition. Paraffin blocks of patients with gastric adenocarcinoma were cored according to earlier inspection of their tissue slides with H and E staining. Two cores were collected from each anatomical region, i.e., the non-tumor region (NT), the primary tumor (PT) and the metastatic tumor (MT). Subsequently, after ANTXR1 immunostaining, the proportion of positively stained endothelial and tumor cells among all endothelial and tumor cells in each core was reported as a percentage
Immunohistochemistry
All steps in the IHC of the paraffin-embedded tissues were adapted from the appropriate Abcam guidelines [16]. In brief, antigen retrieval was performed with a microwave oven (850-watt power, 5 min; 340-watt power, 11 min) in Tris–EDTA Buffer pH: 9. Primary monoclonal mouse anti-human TEM8 (Abcam, Cat No: ab13798) was used at 1/50 dilution. UnoVue Mouse/Rabbit HRP Detection Reagent (MRUHRP100) was used for final visualization of the IHC staining, and then the TMA slides were counterstained with hematoxylin. In parallel, we evaluated a mouse IgG1 kappa monoclonal isotype control (ab170190) with the same protocol to check the validity of the ANTXR1 primary antibody staining.
Each core in the TMA was scored separately. In the tumor and metastatic regions, the number of positively stained tumor cells divided by the number of all counted tumor cells was reported on a scale from 0 to 100%. In all three anatomical regions, the same approach was followed to determine the percentage of positive endothelial cells. In both the tumor and endothelial cell evaluations, all cells were scored as either positive or negative; cells with any specific IHC staining were recorded as positive, and the intensity of staining was not taken into account.
Statistical analysis
For each of the five cell-type/anatomical region categories (tumor cells in PT and MT; endothelial cells in PT, NT and MT), the ANTXR1 IHC staining percentage for each core (the core %) was recorded. Then, for patients with two cores from one or more anatomical regions, the total positive cells in both cores were divided by the total eligible cells in the two cores, to get the ANTXR1 IHC staining percentage for that cell-type/anatomical region category in that patient (the patient %). Then, to get mean values for groups (e.g., the average tumor cell expression of ANTXR1 in the tumor region of all patients, or the average endothelial expression of ANTXR1 in the non-tumor regions of patients who received neoadjuvant therapy), the appropriate patient percentages were averaged. Independent t tests or ANOVA with subsequent scheffe post hoc tests were used, as appropriate, to evaluate the expression differences between groups. Missing data, due to missing cores (cores not collected, or core sections lost during staining) or missing patient data (e.g., data on neoadjuvant therapy or survival), resulted in different numbers of patients evaluated in each analysis group.
The homogeneity of staining between different areas of each anatomical region was evaluated by calculating the percentage of cases in which the core percentages of individual cases differed by > 20%.
Overall patient survival was plotted on Kaplan–Meier curves, and the difference in the curves was tested for significance by log-rank tests. In addition, Cox proportional hazards regression models, with age as the time scale, with and without adjustment for age, sex, and disease stage, were used to estimate hazard ratios (HR) and 95% confidence intervals (95% CI) for mortality within the follow-up period.
STATA software, version 14, was used for all statistical comparisons. When significant, P values were reported at two levels, P < 0.05(*) and P < 0.01(**).
Results
The clinicopathological characteristics of the 140 patients in this study are summarized in Table 1. Figure 2a shows a representative tumor core of gastric adenocarcinoma, immunostained by the anti-ANTXR1 antibody. Immunostaining of some extra cores is also provided in Supplementary Fig. 1.
Table 1.
Clinicopathological characteristics of the evaluated patients
| Characteristic | Number of patients/tumors (%) |
|---|---|
| Age | |
| Mean ± SD | 59.94 ± 13.29 |
| Median age | 61 |
| Range | 28–93 |
| Sex | |
| Male | 97 (69.3%) |
| Female | 43 (30.7%) |
| Total | N = 140 |
| Tumor stage (pTNM) | |
| Stage I (IA+IB) | 29 (21.6%) |
| Stage II (IIA+IIB) | 44 (32.8%) |
| Stage III (IIIA+IIIB+IIIC) | 33 (24.6%) |
| Stage IV | 28 (20.9%) |
| Lauren tumor classification | |
| Diffuse type | 51 (38.6%) |
| Intestinal type | 81 (61.4%) |
| History of neoadjuvant therapy | |
| Yes | 32 (22.86%) |
| No | 108 (77.14%) |
pTNM pathologic tumor-node-metastasis stage
Fig. 2.
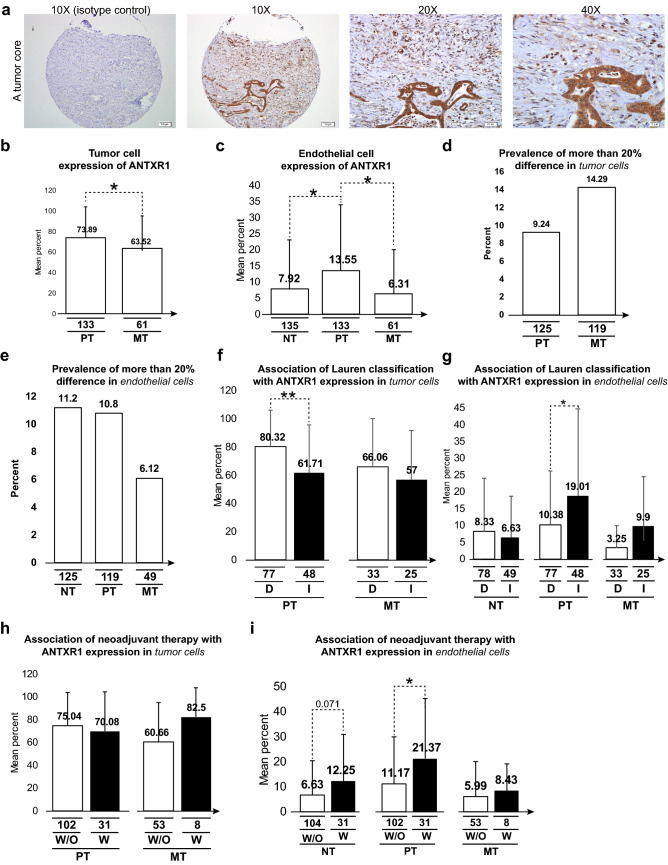
ANTXR1 protein expression in different conditions. a Representative core stained for ANTXR1; 100% of the tumor cells and 40% of the endothelial cells were read as positive for the target. b, c Expression of ANTXR1 is compared in tumor cells (b) or endothelial cells (c) in the three anatomical regions. d, e Graphs show the percentage of patients with more than a 20% difference in the expression of ANTXR1 in tumor cells (d) or endothelial cells (e) in two duplicate cores from the same anatomical region. f, g Association of the Lauren histological subtypes of the primary tumors and ANTXR1 expression in tumor cells (f) or endothelial cells (g) in the three anatomical regions. h, i Association of a history of neoadjuvant therapy with ANTXR1 expression in tumor cells (h) or endothelial cells (i) in the three anatomical regions. The first line in the subtitle of each subfigure represents the number of analyzed cases. Abbreviations of the subtitles in order of presence include NT non-tumor, PT primary tumor, MT metastatic tumor, ANTXR1 ANTXR cell adhesion molecule 1, D diffuse type, I intestinal type, W/O without a history of neoadjuvant therapy, W with a history of neoadjuvant therapy
The cores of the primary tumors had a higher percentage of ANTXR1 positive tumor cells than the cores of the metastatic tumors (73.89 ± 30.12 vs. 63.52 ± 34.19, P < 0.05) (Fig. 2b). The cores of the primary tumors also had a higher percentage of positively staining endothelial cells (13.55 ± 20.53) than the cores of the adjacent non-tumor sites (7.92 ± 15.26) or the metastatic tumor sites (6.31 ± 13.78) (P < 0.05 for both comparisons) (Fig. 2c).
ANTXR1 staining was similar in duplicate cores from the same anatomical regions. Fewer than 15% of the duplicate cores had a > 20% difference in ANTXR1 positive tumor or endothelial cells (Fig. 2d, e).
In the primary tumors, fewer tumor cells (61.71 ± 34.09 vs. 80.32 ± 25.84, P < 0.01) and more endothelial cells (19.01 ± 25.92 vs. 10.38 ± 15.84, P < 0.05) expressed ANTXR1 protein in intestinal-type tumors than in diffuse-type tumors (Fig. 2f, g).
Neoadjuvant chemotherapy did not affect ANTXR1 expression in the tumor cells of the primary or metastatic tumor tissues (Fig. 2h), but it was associated with a significantly higher expression of this protein in the endothelial cells of the primary tumors (21.37 ± 23.93 vs. 11.17 ± 18.87 of cells staining positively with ANTXR1, P < 0.05) (Fig. 2i).
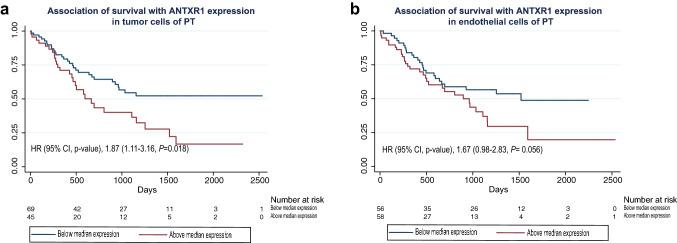
During a median follow-up of 1.82 years (range 0.01–6.93 years), 21 patients were lost to follow-up. Of the remaining 119 patients, 60 died and 59 were alive at the end of the study (23 November 2018), for a total of 269.76 person-years of follow-up time. Higher than median expression of ANTXR1 in the tumor cells of the primary tumor region was associated with significantly lower survival (Fig. 3a, Table 2) and an increased risk of death during the follow-up period [unadjusted HR (95% CI) = 1.87 (1.11–3.16), P = 0.018; adjusted HR (95% CI) = 1.67 (0.98–2.85), P = 0.060] (Table 2). Staining of the endothelial cells in the tumor region had a similar inverse association with survival (Fig. 3b) and a similar increase in the risk of death during the follow-up period (Table 2). Analysis of metastatic tumors showed similar results, with higher ANTXR1 staining being non-significantly associated with lower survival and a higher probability of death within the follow-up period (Supplementary Fig. 2, Table 2).
Fig. 3.
Associations between ANTXR1 expression in the tumor cells and the endothelial cells of the primary tumors and overall survival. a Kaplan–Meier curves of overall survival among patients with below median expression (blue line) vs. above median expression (red line) of ANTXR1 expression in tumor cells of the primary tumor. b Kaplan–Meier curves of overall survival among patients with below median expression (blue line) vs. above median expression (red line) of ANTXR1 expression in endothelial cells of the primary tumor. Abbreviations in order of appearance. ANTXR1 ANTXR cell adhesion molecule 1, PT primary tumor
Table 2.
Association of ANTXR1 expression and risk of death within the follow-up period
| Cell-type/anatomical region category | ANTXR1 expression level | Unadjusted HR (95% CI) | P value | Adjusted HRa (95% CI) | P value |
|---|---|---|---|---|---|
| Tumor cells in PT | Below median | 1.00 (Ref) | 1.00 (ref) | ||
| Above median | 1.87 (1.11–3.16) | 0.018 | 1.67(0.98–2.85) | 0.060 | |
| Tumor cells in MT | Below median | 1.00 (Ref) | 1.00 (ref) | ||
| Above median | 1.28 (0.65–2.50) | 0.46 | 1.18 (0.58–2.39) | 0.63 | |
| Endothelial cells in NT | Below median | 1.00 (Ref) | 1.00 (ref) | ||
| Above median | 0.67 (0.39–1.14) | 0.14 | 0.83 (0.48–1.44) | 0.51 | |
| Endothelial cells in PT | Below median | 1.00 (Ref) | 1.00 (ref) | ||
| Above median | 1.67 (0.98–2.83) | 0.056 | 1.29 (0.75–2.23) | 0.75 | |
| Endothelial cells in MT | Below median | 1.00 (Ref) | 1.00 (ref) | ||
| above median | 1.61 (0.82–3.17) | 0.16 | 1.59 (0.77–3.31) | 0.20 |
HR hazard ratio, 95% CI 95% confidence interval, PT primary tumor, MT metastatic tumor, NT non-tumor gastric tissue
aAdjusted for age, sex, and pTNM tumor stage
Discussion
In this study, we found that ANTXR1 (TEM8) protein was expressed in over 70% of the tumor cells in 133 gastric adenocarcinomas and in over 60% of the tumor cells in metastases of 61 of these tumors, and this expression was similar in duplicate cores of individual tumors or individual metastatic sites. Although there is no consensus for the required expression level of a suitable immunotherapeutic target, antigen expression in > 50% of tumor cells has been shown to increase objective clinical response in clinical trials of some agents [17].
ANTXR1 protein was also expressed in 13% of the endothelial cells of the primary tumors, 6% of the endothelial cells of the metastatic sites, and 8% of the endothelial cells of non-tumor gastric tissue adjacent to the primary tumors, and this expression was also similar in duplicate cores from these different anatomical sites. We detected ANTXR1 expression in endothelial cells lining the vasculature of all tumor sites; an observation which was previously reported for other cancer types [18, 19]. The observation of ANTXR1 expression in endothelial cells of non-tumor tissue adjacent to ANTXR1 expressing tumors has been previously reported, and has been attributed to the induction of its expression by the nearby tumor [20, 21]. Despite this ectopic expression, studies have shown no toxicity while using ANTXR1 antibodies [18, 20] or CAR T cells [14] in preclinical (mouse model) evaluations. While the possibility of anti-ANTXR1 toxicity in humans has not yet been tested, the more than 98% homology of the mouse and human ANTXR1 antigens [22] and the lack of toxicity in mouse studies [14], in spite of CAR T cells’ capability of detecting low copy antigens [23], collectively make it unlikely that significant human toxicity will be found.
One interesting finding in this study was that ANTXR1 expression varied by the Lauren histologic classification of the primary tumor. In both the primary tumors and the metastatic sites, fewer tumor cells and more endothelial cells expressed ANTXR1 in the intestinal-type tumors than in the diffuse-type tumors. Clinical trials will be needed to determine which (if either) histological subtype will benefit more from ANTXR1 therapy.
Many immunotherapies, such as ramucirumab, a VEGFR2 antibody, are prescribed along with chemoradiotherapies or as second-line treatments [6, 24]. Hence, the previous neoadjuvant therapy might change the pattern of expression for many antigenic targets. In our study, a history of neoadjuvant therapy did not affect the expression of ANTXR1 in tumor cells, but it increased the number of endothelial cells expressing ANTXR1 in the primary tumor site. As with the Lauren histological subtyping analysis, the clinical effects of the previous neoadjuvant therapies will need to be evaluated further in future clinical studies.
In this study, a higher than median expression of ANTXR1 in either the tumor or endothelial cells of the primary or metastatic tumor sites was associated with significantly lower overall survival and a higher risk of death during the follow-up period. This suggests that ANTXR1 expression is associated with more aggressive tumors.
In conclusion, our study found expression of ANTXR1 protein in over 70% of tumor cells in primary gastric adenocarcinomas and in over 60% of tumor cells in the metastases of these tumors. It also found ANTXR1 expression in endothelial cells in both primary and metastatic tumors, and it found that greater expression of ANTXR1 in either the tumor or endothelial cells in tumor tissue was associated with decreased overall patient survival. These findings suggest that targeting ANTXR1 protein as an antigen for immunotherapy would target two important compartments of tumors (the tumor cells and the endothelial cells). In addition, unlike ramucirumab (anti-VEGFR2), ANTXR1 could be used in more intense treatments such as CAR T-cell immunotherapy [14], whereas the use of anti-VEGFR2 has more toxicities and subsequent constraints due to the role of the antigen in normal adult physiology [25]. Collectively, these features make ANTXR1 (TEM8) protein a promising candidate for future preclinical and clinical studies to evaluate its utility in gastric adenocarcinoma immunotherapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (PDF 108337 kb)
Abbreviations
- ANTXR1
ANTXR cell adhesion molecule 1
- CAR T cell
Chimeric antigen receptor T cells
- ERBB2
Erb-b2 receptor tyrosine kinase 2
- KDR
Kinase insert domain receptor
- MT
Metastatic tumor
- NT
Non-tumor region
- PDCD1
Programmed cell death 1
- PDX
Patient-derived xenograft
- PT
Primary tumor
- TMA
Tissue microarray
Author contribution
MS, NA, and MN designed the study; MS and BS performed the IHC evaluations; RS performed the statistical data analyses; and MN and SMD prepared the manuscript.
Funding
This project was funded by Tehran University of Medical Sciences, Digestive Disease Research Institute grant number 38435-37-02-97.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This study was performed after the approval of Institutional Review Board of Tehran University of Medical Sciences (approval ID: IR.TUMS.DDRI.REC.1397.007).
Informed consent
Informed consent was obtained from all patients at the time of surgery for the use of their paraffin-embedded tissue blocks in research after finalizing their diagnostic procedure.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Maconi G, Manes G, Porro GB. Role of symptoms in diagnosis and outcome of gastric cancer. World J Gastroenterol. 2008;14(8):1149–1155. doi: 10.3748/wjg.14.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Batran S-E, Lorenzen S. Management of locally advanced gastroesophageal cancer: still a multidisciplinary global challenge? Hematol Oncol Clin. 2017;31:441–452. doi: 10.1016/j.hoc.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Charalampakis N, Economopoulou P, Kotsantis I, Tolia M, Schizas D, Liakakos T, Elimova E, Ajani JA, Psyrri A. Medical management of gastric cancer: a 2017 update. Cancer Med. 2018;7:123–133. doi: 10.1002/cam4.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bang Y-J, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4:e180013-e. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network (2018) NCCN clinical practice guidelines in oncology, gastric cancer, (Version 2.2018). https://www.nccn.org/professionals/physician_gls/default.aspx#gastric. Accessed 22 May 2018
- 9.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raimondi A, Nichetti F, Peverelli G, Bartolomeo MD, Braud FD, Pietrantonio F. Genomic markers of resistance to targeted treatments in gastric cancer: potential new treatment strategies. Pharmacogenomics. 2018;19:1047–1068. doi: 10.2217/pgs-2018-0077. [DOI] [PubMed] [Google Scholar]
- 11.Croix BS, Rago C, Velculescu V, et al. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 12.Nanda A, Carson-Walter EB, Seaman S, Barber TD, Stampfl J, Singh S, Vogelstein B, Kinzler KW, Croix BSJCR. TEM8 interacts with the cleaved C5 domain of collagen α3 (VI) Can Res. 2004;64:817–820. doi: 10.1158/0008-5472.CAN-03-2408. [DOI] [PubMed] [Google Scholar]
- 13.Szot C, Saha S, Zhang XM, et al. Tumor stroma–targeted antibody-drug conjugate triggers localized anticancer drug release. J Clin Investig. 2018;128(7):2927–2943. doi: 10.1172/JCI120481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byrd TT, Fousek K, Pignata A, et al. TEM8/ANTXR1-specific CAR T cells as a targeted therapy for triple-negative breast cancer. Can Res. 2018;78:489–500. doi: 10.1158/0008-5472.CAN-16-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sotoudeh M, Shirvani SI, Merat S, Ahmadbeigi N, Naderi M. MSLN (Mesothelin), ANTXR1 (TEM8), and MUC3A are the potent antigenic targets for CAR T cell therapy of gastric adenocarcinoma. J Cell Biochem. 2018;120(4):5010–5017. doi: 10.1002/jcb.27776. [DOI] [PubMed] [Google Scholar]
- 16.Abcam IHC-PARAFFIN PROTOCOL (IHC-P). https://www.abcam.com/ps/pdf/protocols/ihc_p.pdf. Accessed 13 Nov 2017
- 17.Klebanoff CA, Rosenberg SA, Restifo NP. Prospects for gene-engineered T cell immunotherapy for solid cancers. Nat Med. 2016;22(1):26. doi: 10.1038/nm.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaudhary A, Hilton MB, Seaman S, et al. TEM8/ANTXR1 blockade inhibits pathological angiogenesis and potentiates tumoricidal responses against multiple cancer types. Cancer Cell. 2012;21:212–226. doi: 10.1016/j.ccr.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byrd T, Fousek K, Pignata A, et al. TEM8/ANTXR1 specific T cells co-target tumor stem cells and tumor vasculature in triple-negative breast cancer. Cancer Res. 2016 doi: 10.1158/1538-7445.AM2016-2312. [DOI] [Google Scholar]
- 20.Szot C, Saha S, Zhang XM, et al. Tumor stroma–targeted antibody-drug conjugate triggers localized anticancer drug release. J Clin Invest. 2018;128(7):2927–2943. doi: 10.1172/JCI120481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutwein LG, Al-Quran SZ, Fernando S, Fletcher BS, Copeland EM, Grobmyer SR. Tumor endothelial marker 8 expression in triple-negative breast cancer. Anticancer Res. 2011;31:3417–3422. [PubMed] [Google Scholar]
- 22.Carson-Walter EB, Watkins DN, Nanda A, Vogelstein B, Kinzler KW, Croix BS. Cell surface tumor endothelial markers are conserved in mice and humans. Cancer Res. 2001;61:6649–6655. [PubMed] [Google Scholar]
- 23.Posey AD, Jr, Clausen H, June CHJI. Distinguishing truncated and normal MUC1 glycoform targeting from Tn-MUC1-specific CAR T cells: specificity is the key to safety. Immunity. 2016;45(5):947–948. doi: 10.1016/j.immuni.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 25.Verheul HM, Pinedo HMJNRC. Possible molecular mechanisms involved in the toxicity of angiogenesis inhibition. Nat Rev Cancer. 2007;7(6):475. doi: 10.1038/nrc2152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (PDF 108337 kb)