Abstract
Background
Some studies have investigated the prognostic value exhibited by the Prognostic Nutritional Index (PNI) in patients suffering diffuse large B-cell lymphoma (DLBCL), but varying results were obtained. In order to determine the specific prognostic value more accurately, a meta-analysis was conducted in this study.
Methods
Literatures were searched from the China National Knowledge Infrastructure (CNKI), Wanfang, PubMed, Embase, the Cochrane Library, and Web of Science. Pooled hazard ratio (HR) and the 95% confidence interval (CI) were calculated to assess the association between PNI and the overall survival (OS) and the progression-free survival (PFS) of patients with DLBCL.
Results
Based on seven studies with a total number of 1311 patients, our meta-analysis revealed that low PNI may meant poor OS (HR = 2.14, 95% CI 1.66–2.75, p < 0.001) and poor PFS (HR = 1.75, 95% CI 1.36–2.25, p = 0.438). Subgroup analysis showed that, in Asians, low PNI was correlated to poor OS (pooled HR = 2.06 95% CI 1.59–2.66) and poor PFS (pooled HR = 1.66, 95% CI 1.28–2.15). Similar results were obtained from one European study, which is the only study performed outside of Asia from our literature search.
Conclusion
For patients with DLBCL, low PNI may be interpreted as adverse prognosis. More data from European patients are required in this study to avoid analysis bias.
Keywords: Meta-analysis, Prognostic nutritional index, Diffuse large B-cell lymphoma, Prognosis
Introduction
As the most commonly diagnosed tumor in adults, diffuse large B-cell lymphoma (DLBCL) constitutes about 20% of newly diagnosed lymphoid neoplasms [1]. In Western countries, DLBCL accounts for 31% of all non-Hodgkin’s lymphomas (NHL) [2]. Due to the biological and clinical heterogeneity of the tumor, DLBCL patients are typically treated strategically with different drugs, such as rituximab, cyclophosphamide, vincristine, doxorubicin, and prednisone; R-CHOP [3]. Although about 60%-70% of patients suffering DLBCL are curable by different regimens, chemotherapy is insensitive for some patients who sometimes exhibit a poor long-term survival outcome [4]. Gene expression profiling (GEP), International Prognostic Index (IPI) and other indexes are useful for identifying high-risk patients [5, 6], however they are not easily available in daily clinical practice and are incapable of predicting prognosis accurately. Therefore, there is an urgent call for the development of simple and easily accessible prognostic biomarkers at a low cost.
A number of studies in recent years have shown that malnutrition, which is a frequently-encountered issue in patients with DLBCL, is associated with the poor overall survival (OS) [7–9]. Lymphoma patients with poor nutrition supply have a higher risk of developing febrile neutropenia that can lead to delays in chemotherapy treatment due to decreased drug usage. Recent studies have found that PNI, an indicator that reflects the nutritional and immune status of patients, can be used to predict the clinical outcomes of patients with various malignant tumors, regardless of the tumor location and origin [10–14]. Some studies have focused on exploring the prognostic value of PNI for DLBCL, however the results were inconsistent and contradictory [3, 15–20], possibly due to small sample sizes and patient heterogeneity in individual studies. In order to achieve a comprehensive evaluation of PNI for DLBCL, we aggregated the data from related studies and performed a meta-analysis to investigate how PNI is used in predicting the OS and the progression-free survival (PFS) of patients.
Materials and methods
Search strategy
Literatures published since inception till April 2020 from PubMed, Embase, Cochrane Library, Web of Science, CNKI (Chinese), and Wanfang were searched using search terms (“Prognostic Nutritional Index”) AND (Lymphoma), and evaluated by two investigators (N.W. and CY.L) independently. A consensus was reached to resolve conflicting opinions during the searching process. Relevant studies referenced in the literatures were also examined.
Selection criteria
Literatures with the following features were included in our meta-analysis: (1) DLBCL patients must be diagnosed by histology; (2) Must contain prognostic value of PNI for OS and/or PFS, or with sufficient data for relevant calculation; (3) Hazard Ratio (HR) must be reported as the prognostic index (4) The PNI must be calculated before the first chemotherapy cycle. Meanwhile, articles in the form of comments, reviews, case reports, or thesis were excluded from our study. Latest articles with the largest sample size were chosen in our analysis.
Data extraction and quality assessment
The data were extracted independently by two investigators (N.W and CY.L). A third investigator (BA.C) participated in discussions to resolve discrepancies. Date of eligible studies including author, country, publication year, sample size, patient age, treatment plans, DLLBCL state, cut-off values exhibited by PNI, follow-up time, and survival outcomes were extracted. The quality of the included studies was assessed based on the Newcastle–Ottawa Scale (NOS) [21], where studies with a score of ≥ 6 out of 9 were regarded as high quality research.
Statistical analysis
HR as well as the 95% confidence interval (CI) values were pooled using Stata version 15.0 (STATA, College Station, TX) to evaluate the association of different level of PNI and OS and PFS. All HR and 95%CI were extracted directly from the included articles. Heterogeneity of the included studies was evaluated by Q and I2 statistics. Random effects model was used when the data were considered highly inconsistent with I2 > 50% or P < 0.05; otherwise, fixed effect model was used instead. For sensitivity analysis, the result credibility of each study was examined by sequential omission. Publication bias was evaluated using Egger’s test by STATA. A two-tailed p-value of < 0.05 was considered statistically significant.
Results
Search results
Initial literature search included a total of 45 studies, but only 19 studies remained following the removal of duplicating studies. Another 8 studies were excluded after title/abstract screening. Due to the type of lymphoma not being DLBCL, 4 full-text articles were further removed. As a result, a total of 7 relevant studies were included in the current meta-analysis [15−20, 22] (Fig. 1).
Fig. 1.
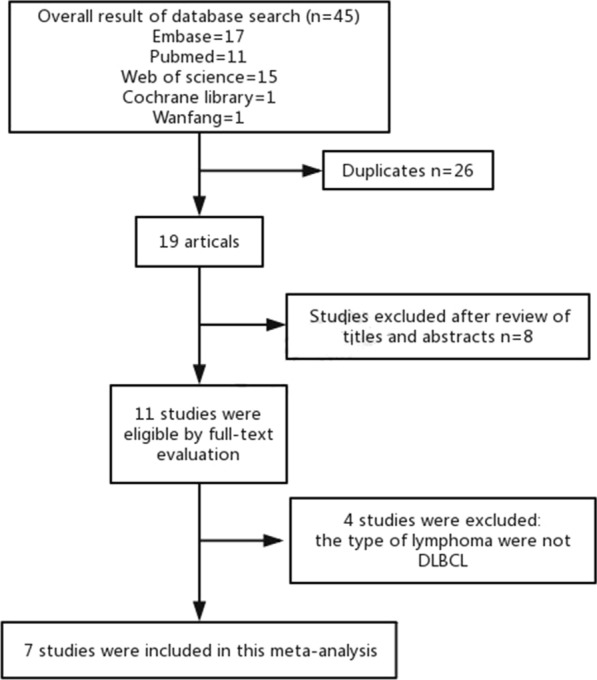
Flow chart of the screening process in choosing eligible studies
Features of included studies
Out of the 7 chosen studies that were published from 2016 to 2020, 4 were conducted in China [15−17, 19], while the remaining 3 were each carried out in Korea [18], Japan [20] and Croatia [22], respectively. In terms of language, 6 studies were published in English [15, 16, 18–20, 22] and 1 in Chinese [17]. The total sample size was 1311, and the cut-off values of PNI were between 40 and 45. Whilst R-CHOP or R-CHOP like regimen was employed in 3 studies [18, 19, 22]; R-CHOP/CHOP or CHOP like regimen was used in another 3 studies [15–17]; whereas rituximab-containing chemotherapy regimens (R-CHOP /R-CVP/rituximab alone) and palliative therapy were used in the remaining study [20]. The prognostic values of PNI on OS [15−20, 22] were reported in all 7 studies; while the association between PNI and PFS [15, 16, 18, 22] was shown in 4 studies. All included studies had a NOS score of ≥ 6 (Table 1).
Table 1.
Features of the studies included
| Author | Country | Year | Sample size (high/low PNI) | Cut-off value of PNI | Median (range) of PNI | Adjusted factors | Follow-up time (month) | Age (year)(range) | NOS score | Treatment | Stage | Survival outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xiaoxiao Hao | China | 2017 | 125/127 | 45 | – | IPI, GPS, NLR, PNI, PI | – | 49 (16–82) | 6 |
R-CHOP; CHOP/CHOP-like |
I–IV | OS, PFS |
| Wenjuan Yu | China | 2019 | 114/195 | 45 | 48.4 (23.9–86.2) |
BMI, hemoglobin, NCCI-IPI |
– | – | 7 | R-CHOP | I–IV | OS |
| Se-Il Go | Korea | 2019 | 69/159 | 40 | – | Sarcopenia, albumin, ALC, BMI, IPI, B-symptoms | – | 64 (21–88) | 7 | R-CHOP | I–IV | OS, PFS |
| Vlatka Periˇsa | Croatia | 2017 | 75/28 | 44.55 | 50.26 (22.91–65.3) |
Age, gender, IPI ECOG-PS, LDH, Ann Arbor stage, B-symptoms |
Median: 27 (range: 1–105) | 63 (22–87) | 6 |
R-CHOP/ R-CHOP-like |
I–IV | OS, PFS |
| Qinjun Zhou | China | 2016 | 129/124 | 44.675 | - |
B-symptoms, LDH, Ann Arbor stage, ECOG-PS, extra-nodal, IPI |
– | 49 (19–81) | 6 | R‑CHOP | I–IV | OS, PFS |
| Teng Song | China | 2019 | 44/38 | 44.15 | – | ECOG-PS, Ann Arbor stage, LDH,IPI, ALC | – | 59(23–79) | 6 |
CHOP; R-CHOP |
I–IV | OS |
|
Erina Hamada |
Japan | 2020 | 38/46 | 41.3 | – |
Albumin, ALC, IPI, extra-nodal ECOG-PS, LDH, gender, Ann Arbor stage, B-symptoms |
Median: 39 | 84 (80–94) | 6 |
R-CHOP; R-CVP; R alone; palliative |
I–IV | OS |
IPI International Prognostic Index, PNI Platelet Lymphocyte Ratio, PNI Prognostic Nutritional Index, GPS Glasgow prognostic score, PI Prognostic Index, NLR Neutrophil Lymphocyte Ratio, R-CHOP rituximab plus cyclophosphamide doxorubicin vincristine and prednisone, BMI Body Mass Index, ECOG PS Eastern Cooperative Oncology Group performance status, LDH lactate dehydrogenase, PFS progression-free survival, OS overall survival, ALC Absolute lymphocyte count, NCCN National Comprehensive Cancer Network
Results of meta-analysis
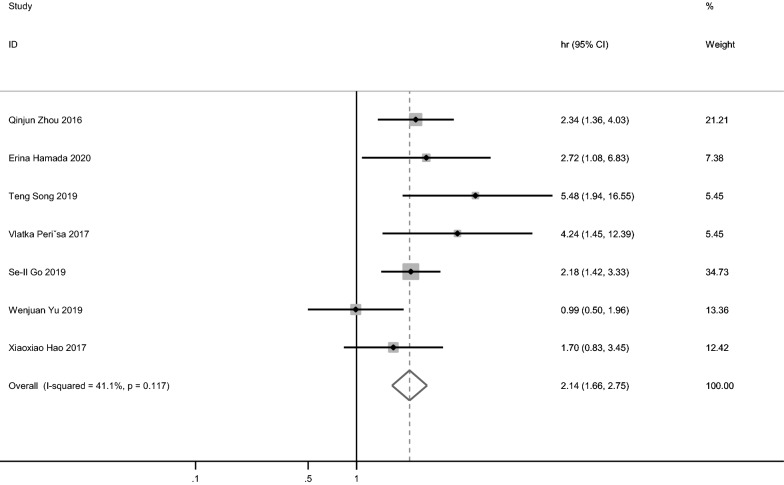
All 7 studies [15−20, 22] reported the correlation between PNI and OS. Fixed-effect model was used with P = 0.117 and I2 = 41.1%. Our meta-analysis showed that a low PNI was significantly correlated to worse OS (HR = 2.14, 95% CI 1.66–2.75; Fig. 2, Table 2). Due to the lack of obvious heterogeneity, only ethnic (Asian or not) subgroup analysis was conducted to study the impact of PNI on OS. The combined results of six studies indicated that PNI was still a significant marker in Asian (Pooled HR = 2.06. 95% CI 1.59–2.66); while the only study with non-Asian showed that PNI also had a significant predictive value (HR = 4.24, 95% CI 1.451–12.392).
Fig. 2.
Pooled results of the association between PNI and overall survival (OS)
Table 2.
Results of subgroup meta-analysis
| Group | No. of studies | HR (95% CI) | Heterogeneity | |
|---|---|---|---|---|
| I2 (%) | P | |||
| OS | 7 | 2.14(1.66–2.75) | 41.1 | 0.117 |
| Ethnicity | ||||
| Asian | 6 | 2.06(1.59–2.66) | 41.4 | 0.129 |
| Non-Asian | 1 | 4.24(1.451–12.392) | – | – |
| Other treatment | 4 | 2.43(1.68–3.51) | 8.2 | 0.352 |
| PFS | 4 | 1.7 (1.36–2.25) | 39.2 | 0.117 |
| Ethnicity | ||||
| Asian | 3 | 1.66(1.28–2.15) | 4.7 | 0.350 |
| Non-Asian | 1 | 4.007(1.48–10.852) | – | – |
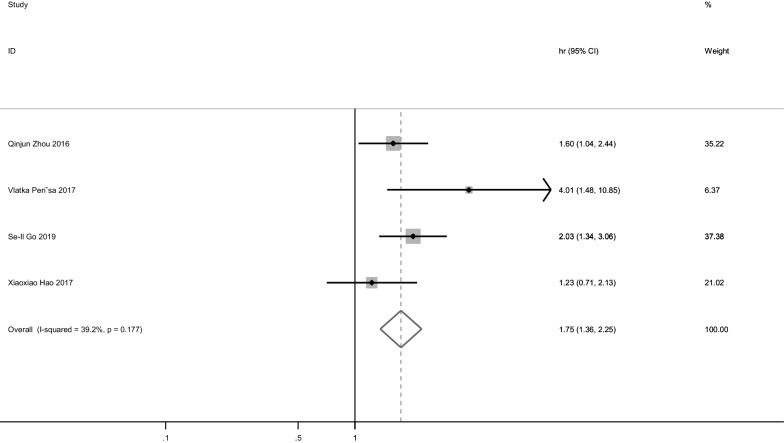
The association of PNI and PFS was reported in four studies [15, 16, 18, 22], which included 836 patients. Due to the low heterogeneity, a fixed-effect model was applied (P = 0.177, I2 = 39.2%). Our analysis showed that the pooled HR was 1.75 with a 95% CI of 1.36–2.25 (Table 2, Fig. 3), indicating that lower PNI and poorer PFS are closely associated. Subgroup analysis revealed that PNI was correlated to PFS of Asian (pooled HR = 1.66, 95% CI 1.28–2.15). Meanwhile, the only European study suggested that PNI can predict PFS (HR = 4.007 95% CI 1.48–10.852).
Fig. 3.
Pooled results of the association between PNI and progression-free survival (PFS)
Sensitivity analysis and publication bias evaluation
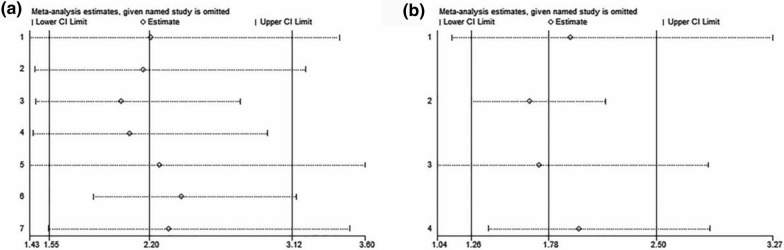
Sensitivity analysis showed that changes of the pooled HRs of OS or PFS remain insignificant following omission any individual study (Fig. 4), indicating that the results were reliable.
Fig. 4.
Sensitivity analysis of the pooled hazard ratios (HRs) to evaluate the association between PNI and OS (a) and PFS (b)
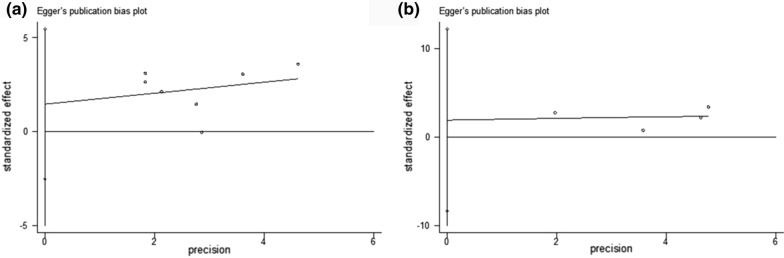
No publication bias was found in the this meta-analysis (Egger’s test: OS, p = 0.391; PFS, p = 0.509) (Fig. 5).
Fig. 5.
Publication bias analysis using Egger’s test for OS (a) and PFS (b)
Discussion
Tumor progression has been shown to be remarkably affected by inflammation and nutrition [23]. Recent studies have identified a simple prognostic score based on nutritional status and PNI as biomarkers that can be used to independently predict the prognosis of DLBCL patients in terms of OS and PFS. PNI was initially used for assessing patients receiving digestive tract surgery due to immunological and nutritional complications [24-27]. Later, it was found that PNI could simply be used to powerfully predict the prognosis of various diseases, including solid tumors and hematological diseases. Previous studies have revealed that PNI exhibit a prognostic value for DLBCL patients [15−20, 22]. However, whilst most studies [15, 17, 18, 20, 22] have demonstrated PNI as a significant prognostic factor for DLBCL patients; two studies have reported the opposite results [16, 19].
To our knowledge, our meta-analysis was the first study that focused on the prognostic value exhibited by PNI in DLBCL patients. In this study, data aggregation was performed from 7 studies that covered 1311 patients in total. Our results showed that, regardless of ethnicity, low PNI was a significant prognostic marker for poorer OS (pooled HR = 2.14, 95% CI 1.66–2.75) and poorer PFS (pooled HR = 1.7 95% CI 1.36–2.25).
Although the accurate mechanism of how low PNI is associated with poor prognosis remains unclear, there are a number of possible explanations: 1) hypoalbuminemia may be due to malnutrition, and malnourished patients may show a worse response to treatments as well as a weaker treatment tolerance compared to that of well-nourished patients; (2) the decreased concentrations of serum albumin and lymphopenia were possibly due to cytokine release by the tumors, such as tumor necrosis factor-alpha and interleukin 6, indicating that the disease is strongly aggressive; (3) low ALC caused by the pre-existing immunosuppression, indicating that the antitumor immunological reaction of the host was insufficient; (4) low ALC that was possibly caused by lympholytic cytokines arising from lymphoma cells, and this kind of lymphomas could exhibit an intrinsic treatment resistance [28-32].
Limitation
There were several limitations identified in our study. Firstly, our analysis involved a relatively small sample size, including only 7 studies where most of the data were obtained from Asian countries. Accordingly, the predictive value of PNI in European countries requires further discussion. In addition, our analysis only involved studies published in Chinese and English, excluding those that were reported in other languages.
Conclusion
Low PNI may represent adverse prognosis in patients with DLBCL. However, since our analysis mainly focused on Asian studies, our findings should be interpreted with caution in European patients.
Acknowledgements
None.
Authors’ contributions
Conceptualization: CL, BC, NW. Data analysis:NW, CL, FW. Draft writing: CL, FW, NW. Review and editing: BC, FW, NW, CL. All authors read and approved the final manuscript.
Funding
This work was supported by the Natural Science Foundation of Jiangsu Province for Youth (number: BK20180372) and Jiangsu Provincial Medical Youth Talent (number: QNRC2016812).
Availability of data and materials
The databases analyzed during the current study are available.
Ethics approval and consent to participate
No ethical approval are required for this meta-analysis.
Consent for publication
Not applicable.
Competing interests
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chunyan Luan and Fei Wang contributed equally to this work
Contributor Information
Ning Wei, Email: 18795883826@163.com.
Baoan Chen, Email: cba8888@hotmail.com.
References
- 1.Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107(1):265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher SG, Fisher RI. The epidemiology of non-Hodgkin's lymphoma. Oncogene. 2004;23(38):6524–6534. doi: 10.1038/sj.onc.1207843. [DOI] [PubMed] [Google Scholar]
- 3.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. The New England journal of medicine. 2002;346(4):235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 4.Sehn LH. Early detection of patients with poor risk diffuse large B-cell lymphoma. Leuk Lymphoma. 2009;50(11):1744–1747. doi: 10.3109/10428190903308064. [DOI] [PubMed] [Google Scholar]
- 5.Ziepert M, Hasenclever D, Kuhnt E, et al. Standard International prognostic index remains a valid predictor of outcome for patients with aggressive CD20+ B-cell lymphoma in the rituximab era. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(14):2373–2380. doi: 10.1200/JCO.2009.26.2493. [DOI] [PubMed] [Google Scholar]
- 6.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 7.Park S, Han B, Cho JW, et al. Effect of nutritional status on survival outcome of diffuse large B-cell lymphoma patients treated with rituximab-CHOP. Nutr Cancer. 2014;66(2):225–233. doi: 10.1080/01635581.2014.867065. [DOI] [PubMed] [Google Scholar]
- 8.Raffetti E, Donato F, Castelnuovo F, et al. The prognostic role of systemic inflammatory markers on HIV-infected patients with non-Hodgkin lymphoma, a multicenter cohort study. Journal of translational medicine. 2015;13:89. doi: 10.1186/s12967-015-0446-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun HL, Pan YQ, He BS, et al. Prognostic performance of lymphocyte-to-monocyte ratio in diffuse large B-cell lymphoma: an updated meta-analysis of eleven reports. OncoTargets and therapy. 2016;9:3017–3023. doi: 10.2147/OTT.S96910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun K, Chen S, Xu J, et al. The prognostic significance of the prognostic nutritional index in cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2014;140(9):1537–1549. doi: 10.1007/s00432-014-1714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimizu K, Okita R, Saisho S, et al. Prognostic nutritional index before adjuvant chemotherapy predicts chemotherapy compliance and survival among patients with non-small-cell lung cancer. Ther Clin Risk Manag. 2015;11:1555–1561. doi: 10.2147/TCRM.S92961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sasahara M, Kanda M, Ito S, et al. The Preoperative Prognostic Nutritional Index Predicts Short-Term and Long-Term Outcomes of Patients with Stage II/III Gastric Cancer: Analysis of a Multi-Institution Dataset. Digestive surgery. 2020;37(2):135–144. doi: 10.1159/000497454. [DOI] [PubMed] [Google Scholar]
- 13.Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI) Br J Cancer. 2012;106(8):1439–1445. doi: 10.1038/bjc.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Z, Zhang B, Hou L, et al. Pre-operative prognostic nutritional index predicts the outcomes for triple-negative breast cancer. Tumour Biol. 2014;35(12):12165–12171. doi: 10.1007/s13277-014-2524-6. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Q, Wei Y, Huang F, et al. Low prognostic nutritional index predicts poor outcome in diffuse large B-cell lymphoma treated with R-CHOP. Int J Hematol. 2016;104(4):485–490. doi: 10.1007/s12185-016-2052-9. [DOI] [PubMed] [Google Scholar]
- 16.Hao XX, Wei YQ, Wei XL, et al. Glasgow prognostic score is superior to other inflammation-based scores in predicting survival of diffuse large B-cell lymphoma. Oncotarget. 2017;8(44):76740–76748. doi: 10.18632/oncotarget.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song T, Zhang Y, Zhao K, et al. The prognostic value of prognostic nutritional index in patients with diffuse large Bcell lymphoma. Chin J Clin Oncol. 2019;46(17):903–908. [Google Scholar]
- 18.Go SI, Park S, Kang MH, et al. Clinical impact of prognostic nutritional index in diffuse large B cell lymphoma. Ann Hematol. 2019;98(2):401–411. doi: 10.1007/s00277-018-3540-1. [DOI] [PubMed] [Google Scholar]
- 19.Yu W, Guo Q, Wang Z, et al. Clinical Significance of Prognostic Nutritional Index for Patients with Diffuse Large B-cell Lymphoma. Nutr Cancer. 2019;71(4):569–574. doi: 10.1080/01635581.2018.1540718. [DOI] [PubMed] [Google Scholar]
- 20.Hamada E, Shinji O, Nishiyama-Fujita Y, et al. The clinical significance of the prognostic nutritional index in very elderly patients over 80 years of age with diffuse large B-cell lymphoma. Ann Hematol. 2020;99(5):1153–1155. doi: 10.1007/s00277-020-04012-7. [DOI] [PubMed] [Google Scholar]
- 21.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 22.Perisa V, Zibar L, Knezovic A, et al. Prognostic nutritional index as a predictor of prognosis in patients with diffuse large B cell lymphoma. Wien Klin Wochenschr. 2017;129(11–12):411–419. doi: 10.1007/s00508-016-1077-7. [DOI] [PubMed] [Google Scholar]
- 23.Zitvogel L, Pietrocola F, Kroemer G. Nutrition, inflammation and cancer. Nat Immunol. 2017;18:843–850. doi: 10.1038/ni.3754. [DOI] [PubMed] [Google Scholar]
- 24.Gp B, Jl M, Dc M, et al. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. 1980;139(1):160–167. doi: 10.1016/0002-9610(80)90246-9. [DOI] [PubMed] [Google Scholar]
- 25.Kanda M, Fujii T, Kodera Y, et al. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011;98(2):268–274. doi: 10.1002/bjs.7305. [DOI] [PubMed] [Google Scholar]
- 26.Nozoe T, Kimura Y, Ishida M, et al. Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for oesophageal carcinoma. Eur J Surg Oncol. 2002;28(4):396–400. doi: 10.1053/ejso.2002.1257. [DOI] [PubMed] [Google Scholar]
- 27.Nozoe T, Ninomiya M, Maeda T, et al. Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40(5):440–443. doi: 10.1007/s00595-009-4065-y. [DOI] [PubMed] [Google Scholar]
- 28.Oki Y, Yamamoto K, Kato H, et al. Low absolute lymphocyte count is a poor prognostic marker in patients with diffuse large B-cell lymphoma and suggests patients' survival benefit from rituximab. Eur J Haematol. 2008;81(6):448–453. doi: 10.1111/j.1600-0609.2008.01129.x. [DOI] [PubMed] [Google Scholar]
- 29.Aviles A, Yanez J, Lopez T, et al. Malnutrition as an adverse prognostic factor in patients with diffuse large cell lymphoma. Arch Med Res. 1995;26(1):31–34. [PubMed] [Google Scholar]
- 30.Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69. doi: 10.1186/1475-2891-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ray-Coquard I, Cropet C, Van Glabbeke M, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69(13):5383–5391. doi: 10.1158/0008-5472.CAN-08-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McMillan DC, Watson WS, O'Gorman P, et al. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer. 2001;39(2):210–213. doi: 10.1207/S15327914nc392_8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The databases analyzed during the current study are available.