Abstract
R-spondin2 (RSPO2) is a member of the R-spondin family, which are secreted activators of the WNT/β-catenin (CTNNB1) signaling pathway. In the mouse postnatal ovary, WNT/CTNNB1 signaling is active in the oocyte and in the neighboring supporting cells, the granulosa cells. Although the role of Rspo2 has been previously studied using in vitro experiments, the results are conflicting and the in vivo ovarian function of Rspo2 remains unclear. In the present study, we found that RSPO2/Rspo2 expression is restricted to the oocyte of developing follicles in both human and mouse ovaries from the beginning of the follicular growth. In mice, genetic deletion of Rspo2 does not impair oocyte growth, but instead prevents cell cycle progression of neighboring granulosa cells, thus resulting in an arrest of follicular growth. We further show this cell cycle arrest to be independent of growth promoting GDF9 signaling, but rather associated with a downregulation of WNT/CTNNB1 signaling in granulosa cells. To confirm the contribution of WNT/CTNNB1 signaling in granulosa cell proliferation, we induced cell type specific deletion of Ctnnb1 postnatally. Strikingly, follicles lacking Ctnnb1 failed to develop beyond the primary stage. These results show that RSPO2 acts in a paracrine manner to sustain granulosa cell proliferation in early developing follicles. Taken together, our data demonstrate that the activation of WNT/CTNNB1 signaling by RSPO2 is essential for oocyte-granulosa cell interactions that drive maturation of the ovarian follicles and eventually female fertility.
Subject terms: Development, Gene regulation
Introduction
In the ovary, cell communication between the oocyte and the neighboring somatic cells, or granulosa cells, is essential for follicular growth that will eventually lead to the release of the oocyte during ovulation. In mice, the primordial follicles assemble perinatally and oocytes become individualized and surrounded by flattened granulosa cells [1, 2]. Once formed, most of the primordial follicles enter a resting phase until they are recruited to support an oocyte during the cyclic process of ovulation [3]. Their activation requires an interaction between the granulosa cells and the oocyte driven by the granulosa cell-derived factor KITL (Kit Ligand) and the KIT receptor expressed by the oocytes [4, 5]. Activated primordial follicles then undergo rapid growth including enlargement of the oocytes and proliferation of the granulosa cells [6, 7]. The formation of mature primary follicles begins with a change in the shape of the granulosa cells from flattened to cuboidal cells. This change is initiated by an increase of granulosa cell proliferation that promotes intercellular contacts between adjacent granulosa cells, followed by an extension of the intercellular adhesion to the oocyte surface [8]. With the increased proliferation, the granulosa cells become cuboidal and more packed on the oocyte surface. Furthermore, the increased packing density of the granulosa cells leads them to adopt a columnar shape with the nucleus becoming adjacent to the basal lamina [9]. Follicular growth continues with the formation of a second inner layer of granulosa cells leading to the formation of secondary follicles. Further divisions of granulosa cells give rise to tertiary, antral, and preovulatory follicles with more than 50,000 granulosa cells [10].
During the early phase of growth, the oocyte secretes three glycoproteins, ZP1-3, that contribute to the formation of an extracellular matrix, the zona pellucida, around the oocyte [10]. At that time, direct contact between the granulosa cells and the oocyte is mediated by transzonal projections. These specialized filopodia are elaborated by the granulosa cells upon the oocyte-secreted growth differentiation factor-9 (GDF9), cross the zona pellucida to reach the oocyte [11]. Accordingly, the lack of GDF9 has a dramatic consequence on follicle development; in its absence a second layer of granulosa cells fails to develop and primary follicles remain [12]. Strikingly, the oocyte continues to grow and reaches the size of an antral oocyte capable of resuming meiosis in Gdf9−/− ovaries [13].
R-spondins (RSPO) are secreted proteins that promote the activation of the canonical WNT/β-catenin (CTNNB1) signaling pathway [14, 15]. To be functional, RSPO bind their receptors LGR4/5/6 and RSPO2/3 and can also bind heparan sulfate proteoglycans. They next recruit the transmembrane E3-ubiquitin ligases ZNRF3 or RNF43 into an inhibition complex [16–20]. The inhibition of ZNRF3/RNF43 then leads to the stabilization of CTNNB1, which, in turn, interacts with the transcription factors LEF or TCF to induce the expression of various target genes including modulators of cell cycle progression [21]. WNT/CTNNB1 signaling notably promotes G1 phase progression via upregulation of Ccnd1 (CyclinD1) and downregulation of Cdkn2a (p21) [22–24]. R-spondins can stimulate cell proliferation by potentiating WNT/CTNNB1 signaling as previously shown in the intestine [25]. During development, Rspo1 is required for ovarian differentiation and is downregulated in the postnatal ovary [26–28]. In contrast, the expression of R-spondin2 (Rspo2) has been detected in mice oocytes at birth and Rspo2 heterozygous loss-of-function female mice gradually lose their fertility from 4 months of age [29–31]. In vitro, treatments of ovarian cultures with recombinant RSPO1, RSPO2, or WNT3A, three enhancers of WNT signaling or IWR1, a WNT inhibitor, promote granulosa cell expansion [31, 32]. Furthermore, treatments with WNT3A or LiCl also result in the formation of abnormal follicles [32]. In vivo, either forced activation of CTNNB1 or postnatal ectopic expression of RSPO1 induces precancerous lesions in ovaries that do not grow larger than the size of an antral follicle, contain few proliferative cells, and evolve into granulosa cell tumors [28, 33]. These data show divergent roles or even opposite effects of WNT signaling in ovaries. To date, the perinatal lethality associated with conditional loss-of-function mutations of Ctnnb1 (e.g., Sf1Cre; Ctnnb1fl/fl mice) has precluded to determine the precise contribution of WNT signaling to folliculogenesis [34]. To address the role of RSPO2 and WNT signaling in physiological conditions, we have performed a detailed analysis of the ovarian phenotype of the Rspo2 loss-of-function mouse model. Here we show that RSPO2 is a critical factor involved in the communication between the oocyte and the granulosa cells. Indeed, this oocyte-secreted factor induces the activation of WNT/CTNNB1 signaling in the granulosa cells allowing their proliferation and hence follicular growth. Consequently, lack of Rspo2 in the ovary or Ctnnb1 in granulosa cells leads to blockage of the follicles at the primary stage.
Results
Rspo2 expression is restricted to the oocyte in postnatal ovaries
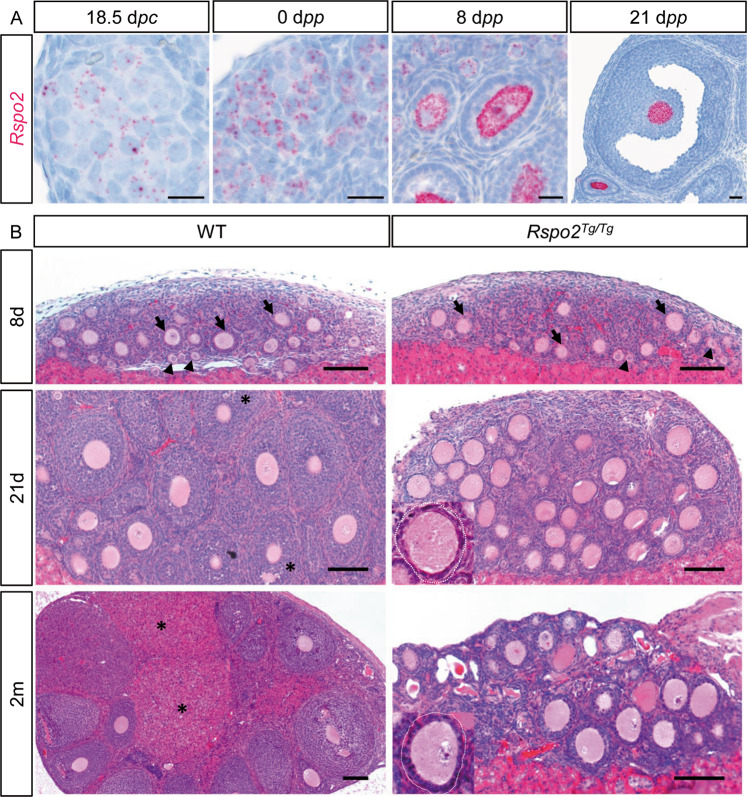
To gain insight into the role of Rspo2 in the ovary, we first characterized the expression pattern of Rspo2 in mouse ovaries. In situ hybridization experiments revealed that Rspo2 is weakly expressed in fetal ovaries. At birth (0 dpp—day postpartum), Rspo2 expression is restricted to the oocyte of primordial follicles (Fig. 1a). Rspo2 expression then increases in the oocyte of developing follicles at 8 and 21 dpp. Notably, granulosa cells are devoid of Rspo2 expression irrespective to the time-point considered. In a 12-month-old girl, immunolocalization of RSPO2 demonstrates that RSPO2 is also expressed within the oocyte of primordial, primary, and preantral follicles (Fig. S1a). This suggests that RSPO2/Rspo2 is expressed in the oocyte as soon as the primordial follicle stage both in humans and mice.
Fig. 1. RSPO2 is required for follicular growth.
a Rspo2 in situ hybridization analyses at 18.5 dpc and 0, 8, and 21 dpp show Rspo2 expression in oocytes after birth in mouse ovaries. Scale bar, 20 µm. b Histological analyses (HE staining) of wild-type (WT) and Rspo2Tg/Tg ovaries at 8 and 21 post-transplantation days (8d, 21d) and 2 months (2m) of 18.5 dpc ovaries. WT and Rspo2Tg/Tg ovaries contain primordial (arrowheads) and primary (arrows) follicles at 8d. At 21d, Rspo2Tg/Tg ovaries lack secondary, tertiary, and antral (*) follicles, whereas WT ovaries contain these different types of follicles. After 2 months, corpora lutea (*) are observed in WT ovaries. In contrast, follicles remain blocked at the primary–secondary transition in Rspo2Tg/Tg ovaries. Follicles are outlined with a white dotted line. Scale bars, 100 µm.
Rspo2 loss-of-function ovaries lack secondary follicles
To investigate the function of Rspo2 in postnatal ovaries, we used a loss-of-function Rspo2 allele created by a transgene insertional mutation (Rspo2Tg/Tg) [35]. Since the Rspo2Tg/Tg pups die at birth due to respiratory distress, we transplanted Rspo2Tg/Tg and wild-type fetal ovaries (18.5 dpc—day post coitum) under the kidney capsule of athymic female mice. At day 8 post-transplantation (8d), histological analyses revealed that both wild-type and Rspo2Tg/Tg ovaries contain primordial and primary follicles (Figs. 1b and S1c). This indicates a developmental delay due to transplantation, since non-transplanted wild-type ovaries already harbor secondary follicles at 8 dpp (Fig. 1a) [2]. At 12d, wild-type transplanted ovaries contained 45% of secondary follicles (Fig. S1c). At 21d, 36% of the wild-type follicles were tertiary follicles, as evidenced by multiple granulosa cell layers (Fig. S1c). In addition, some follicles had reached the antral stage with the formation of a fluid-filled antrum (Fig. 1b). In contrast, in Rspo2Tg/Tg ovaries, 62% of the follicles were still at the primary stage with only one layer of nucleus-centered cuboidal granulosa cells (Figs. 1b and S1c). Moreover, some oocytes were surrounded by flattened somatic cells, suggesting that they remained as primordial follicles or were developmentally blocked at the transition from primordial to primary follicle. Others exhibited an asymmetric appearance, with one layer of cells on one side and a disorganized multilayer of granulosa cells on the other side (Fig. S1b). These abnormalities were not observed in wild-type ovaries. Corpora lutea resulting from ovulation were apparent in 2-month-old wild-type transplanted ovaries, whereas Rspo2Tg/Tg follicles remained blocked as primary follicles (Fig. 1b). In conclusion, our data show that most primary follicles cannot develop beyond early secondary follicles in the absence of Rspo2.
Granulosa cell identity is altered in the absence of RSPO2
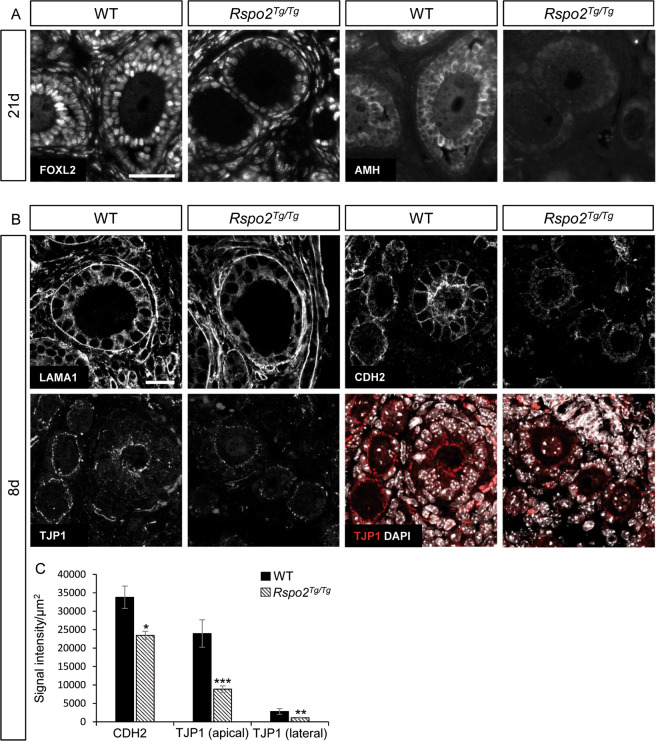
Previous data indicate that the transcription factor Foxl2 is expressed in granulosa and theca cells and is required for the transition from flattened to cuboidal granulosa cells [36, 37]. In Rspo2Tg/Tg ovaries, FOXL2 immunolocalizations confirmed the identity of the granulosa cells. A subset of these cells also expresses AMH, a marker of granulosa cells in primary to antral follicles [38], but the expression of AMH is sporadic with some cells expressing high levels and others low levels of AMH in Rspo2Tg/Tg follicles at 12d (Fig. S2a). At 21d, most of the Rspo2Tg/Tg granulosa cells fail to express AMH but remain FOXL2-positive (Fig. 2a). This indicates that Rspo2Tg/Tg follicles do not exhibit some hallmarks of primary follicles like AMH expression. FOXL2 immunostainings also revealed some positive stromal cells [39] in Rspo2Tg/Tg ovaries. These cells were also positive for SF1/NR5A1 indicating that Rspo2Tg/Tg ovaries contain theca cells (Fig. S2a).
Fig. 2. Granulosa cell intercellular junctions are less abundant in Rspo2Tg/Tg follicles.
a Immunodetection analyses of FOXL2 and AMH (granulosa cells) at 21d in wild-type (WT) and Rspo2Tg/Tg transplanted ovaries. In Rspo2Tg/Tg ovaries, FOXL2 is still expressed in granulosa cells, but AMH expression is impaired compared to wild-type. b Immunodetection of LAMA1 (basal lamina), CDH2 (adherens junctions), and TJP1 (tight junctions) at 8d in WT and Rspo2Tg/Tg transplanted ovaries. Follicles are surrounded by a continuous basal lamina in both genotypes as evidenced by LAMA1 immunostaining. CDH2 and TJP1 signals are decreased, notably at the interface between the oocyte and granulosa cells, indicating a deficit of cohesion in Rspo2Tg/Tg follicles. Scale bar, 20 µm. c This result is corroborated by quantitative analysis of CDH2 and TJP1 signal intensity on confocal sections. Data are presented as mean ± SEM. n = 5 WT and n = 9 Rspo2Tg/Tg follicles from one transplanted ovary of each genotype. Student’s t test, unpaired two sided (*p < 0.05; **p < 0.01; ***p < 0.001).
Intercellular junctions are impaired in Rspo2Tg/Tg follicles
Given that granulosa cells were disorganized in Rspo2Tg/Tg primordial/primary follicles (Fig. S1b), we investigated the establishment of the normal granulosa cell polarity. We first analyzed the localization of laminin (LAMA1), a major component of the basal lamina. Immunostainings for LAMA1 did not reveal differences between the wild type and the mutant follicles, indicating a normal deposition of the basal lamina (Fig. 2b). CDH2 (N-Cadherin), present in the adherens junctions of granulosa cells, is robustly expressed from primary follicles onwards [40]. In Rspo2Tg/Tg follicles, CDH2 was less abundant especially at the interface between oocyte and granulosa cells (Fig. 2b, c). In addition, the tight junction protein TJP1 (ZO1) was found enriched at the interface between oocyte and granulosa cells (apical TJP1), but also present at a lower level between granulosa cells (lateral TJP1) of wild-type ovaries. Immunostainings and quantification analyses highlighted that TJP1 is less expressed in Rspo2Tg/Tg follicles (Fig. 2b, c). Altogether this shows a defect of the establishment of adherens junctions between the follicular cells and tight junctions at the apical pole of granulosa cells.
Granulosa cells exhibit cell cycle progression defects in Rspo2Tg/Tg follicles
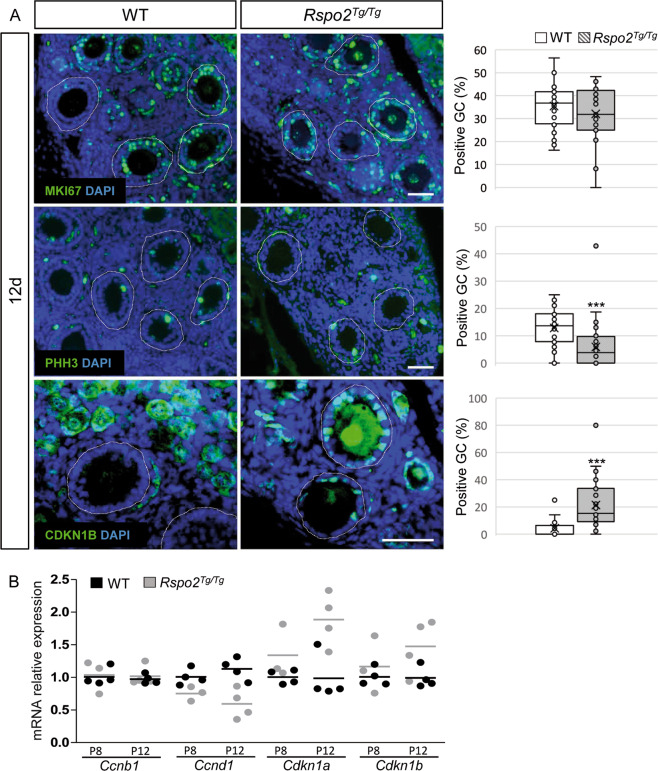
The lack of secondary follicles suggested a defect in proliferation of the granulosa cells in Rspo2Tg/Tg females. We examined cell cycle progression using MKI67 that is expressed in all active phases of the cell cycle (G1, S, G2, M) at 12d (Fig. 3a). The number of MKI67-positive granulosa cells was similar in wild-type and mutant ovaries, indicating that these cells are proliferating. To evaluate the rate of cell cycle progression, we analyzed the G2/M transition by the quantification of the expression of Ccnb1 (CyclinB1) and phosphohistone H3 (PHH3) using qRT-PCR and immunolocalization, respectively (Fig. 3a, b). No difference was observed in the level of Ccnb1 transcripts, but the percentage of PHH3-positive granulosa cells was decreased in Rspo2Tg/Tg ovaries in comparison with wild types. These observations indicate that fewer granulosa cells attain the G2/M transition phase of the cell cycle in Rspo2Tg/Tg females. Ccnd1, a cyclin involved in G1 phase progression, was downregulated in the Rspo2Tg/Tg ovary. Cdkn1a and Cdkn1b (cyclin-dependent kinase inhibitor 1a-b also denoted p21 and p27, respectively), two proteins that mediate cell cycle arrest, were trending to be upregulated at 8d and were confirmed to be upregulated by 12d (Fig. 3b). In addition, immunolocalization of CDKN1B showed an increase of CDKN1B-positive granulosa cells in Rspo2Tg/Tg females at this stage (Fig. 3a). Altogether, our data indicate that the progression of the granulosa cell cycle is impaired, giving an explanation for the blockage of follicular growth observed in Rspo2Tg/Tg ovaries. Thus, oocyte-specific RSPO2 appears to be an enhancer of cell cycle progression of granulosa cells during follicular growth.
Fig. 3. Granulosa cell cycle progression is impaired in the absence of RSPO2.
a Immunodetection and quantitative analyses of MKI67, PHH3, and CDKN1B (p27) to assess the proliferation status of granulosa cells at 12d in WT and Rspo2Tg/Tg transplanted ovaries. Left panel: follicular cells are engaged in cell cycle as indicated by MKI67 immunostaining but exhibit an interphase delay illustrated by a decrease of PHH3-positive and an increase of CDKN1B-positive granulosa cells in mutant ovaries. Follicles are outlined with a white dotted line. Right panel: quantification of the % of positive granulosa cells for MKI67, PHH3, and CDKN1B. n = 1070, 1107, and 280 WT and n = 889, 678, and 583 Rspo2Tg/Tg granulosa cells (identified with DAPI) from 31, 30, and 10 and 28, 42, and 23 follicles, respectively, from at least two individual transplanted ovaries of each genotype. Data are presented in a box and whisker plot representation to illustrate positive granulosa cell dispersion according to the follicle considered. Student’s t test, unpaired two sided (*p < 0.05; **p < 0.01; ***p < 0.001). Scale bar, 50 µm. b QRT-PCR analysis of Ccnb1 (CyclinB1), CcnD1 (CyclinD1), Cdkn1a (p21), and Cdkn1b (p27). Both inhibitors of CDK are upregulated, while Ccnd1 expression is decreased, confirming a reduction of the cell cycle progression in Rspo2Tg/Tg granulosa cells. Data are presented as individual data points. n = 3 (8d) and 4 (12d) individual ovaries per genotype. Mean values are indicated as black (WT) and gray (Rspo2Tg/Tg) bars.
Oocyte growth is independent of RSPO2 signaling
Whereas proliferation of the granulosa cells is impaired, the oocytes appear to have a similar size in wild-type and Rspo2Tg/Tg ovaries at 21d (Figs. 1a and S1b). Before carrying out quantifications, we evaluated the impact of transplantation on follicular growth by comparing the oocyte and the follicular diameters in non-transplanted and transplanted wild-type ovaries at 21 dpp and 21d (Fig. S3). This shows that the diameter of the oocyte increases regularly during follicle growth in both conditions (a threefold increase from 20 to 60 µm in tertiary/antral follicles). There is, however, a noticeable difference in the size of antral follicles displaying an average diameter of 250 µm for non-transplanted ovaries and 170 µm for transplanted ovaries. This reflects that the fluid-filled cavity enlargement of antral follicles is less advanced in transplanted ovaries (Fig. 1b). However, our results demonstrate that transplantation does not hamper oocyte and follicle growth.
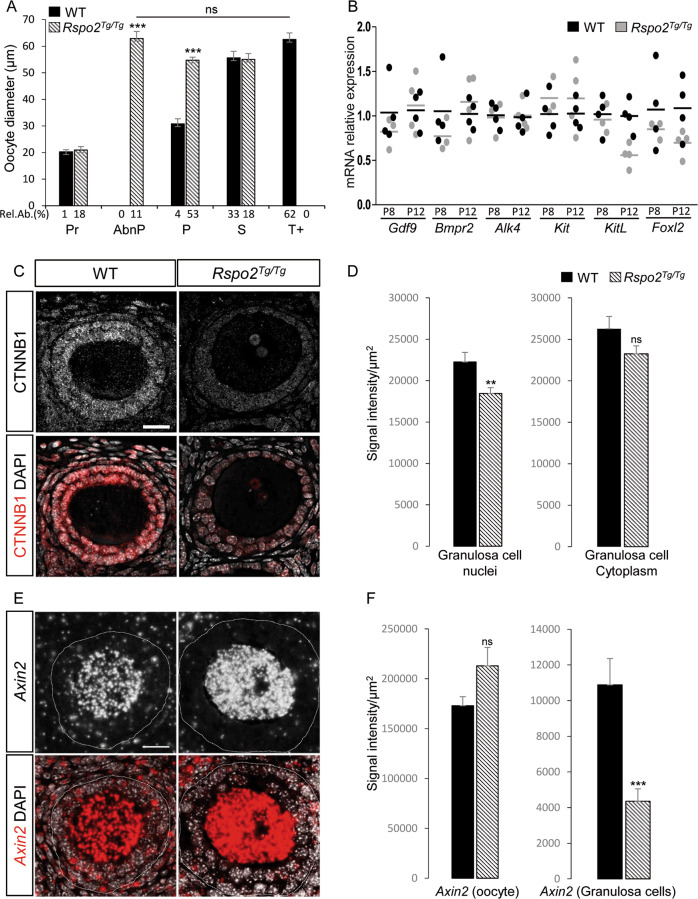
Next, we compared the size of oocytes in wild-type and Rspo2Tg/Tg follicles. At 21d, primordial, primary, secondary, and tertiary/antral follicles constitute 1%, 4%, 33%, and 62% of all wild-type follicles, respectively (Fig. 4a). On average, the oocyte diameter increases from 20 µm in primordial to 30 µm in primary and 56 µm in secondary in wild-type follicles. In Rspo2Tg/Tg ovaries, primary follicles contain oocytes of 55 µm in diameter, corresponding to the diameter of oocytes of secondary wild-type follicles (Fig. 4a). In addition, 11% of the follicles are abnormal primordial follicles surrounded by a layer of flattened granulosa cells, with oocytes of 63 µm in diameter, corresponding to the size of the oocyte in tertiary/antral wild-type follicles. At 21d, 82% of the follicles are primordial and primary follicles in Rspo2Tg/Tg ovaries. Our data demonstrate that Rspo2 ablation does not impair oocyte growth despite the growth arrest of the primary follicles.
Fig. 4. RSPO2 signaling specifically targets granulosa cells.
a Quantitative analysis of the oocyte diameter size according to follicular stage in WT (n = 55; black histogram) and Rspo2Tg/Tg (n = 90; striped histogram) transplanted ovaries at 21d (at least three ovaries of each genotype). Pr primordial, AbnP abnormal primordial Rspo2Tg/Tg specific follicles, P primary, S secondary, T+ tertiary and antral follicles. Data show that the oocyte growth is not impacted by the absence of Rspo2 (mean ± SEM). Relative abundance (Rel.Ab.) of each follicular stage is indicated as percentage of total follicles. Student’s t test, unpaired two sided (***p < 0.001). b QRT-PCR analysis of Gdf9, Bmpr2, and Alk4 (GDF9 receptors); Kit receptor (Kit); Kit ligand (KitL); and Foxl2 expression. Data are presented as individual data points. n = 3 (8d) and 4 (12d) individual ovaries per genotype. Mean values are indicated as black (WT) and gray (Rspo2Tg/Tg) bars. c Immunodetection and d corresponding quantitative analysis of nuclear versus cytoplasmic CTNNB1 in granulosa cells to assess the activation of WNT/CTNNB1 signaling at 12d in wild-type (WT) and Rspo2Tg/Tg transplanted ovaries (n = 20 WT and n = 23 Rspo2Tg/Tg follicles of three ovaries per genotype). In Rspo2Tg/Tg follicles, CTNNB1 expression in granulosa cell nuclei is significantly decreased. Data are presented as mean ± SEM. Student’s t test, unpaired two sided (**p < 0.01). Scale bar, 20 µm. e In situ hybridization analysis of Axin2 expression and f signal quantification in WT and Rspo2Tg/Tg follicles at 12d (n = 18 WT and n = 14 Rspo2Tg/Tg follicles of two ovaries per genotype). In Rspo2Tg/Tg ovaries, Axin2 expression is unchanged within the oocyte but is decreased in granulosa cells when compared with WT. Data are presented as mean ± SEM. Student’s t test, unpaired two sided (***p < 0.001). Scale bar, 20 µm.
Oocyte-secreted RSPO2 activates WNT/CTNNB1 signaling in granulosa cells
The phenotype of Rspo2Tg/Tg ovaries shows similarities with Gdf9−/− ovaries regarding the lack of granulosa cell proliferation and the maintenance of the oocyte growth [12, 13]. This prompted us to investigate the expression level of key genes involved in GDF9 signaling like Gdf9 and its receptors Bmpr2 and Alk4 [41] by qRT-PCR analyses. None of these transcripts were downregulated, suggesting that RSPO2 signaling acts independently of the GDF9 pathway (Fig. 4b). In addition, we analyzed the expression of Kit and KitL, two factors involved in primordial follicle activation [42]. Kit expression was similar in wild-type and mutant ovaries, indicating the maintenance of this signaling pathway from the oocyte toward granulosa cells. The expression of KitL was lowered in Rspo2Tg/Tg ovaries at 12d, but the expression of the granulosa cell marker Foxl2 was also reduced, suggesting that KitL reduction is associated with the diminution of the granulosa cell numbers.
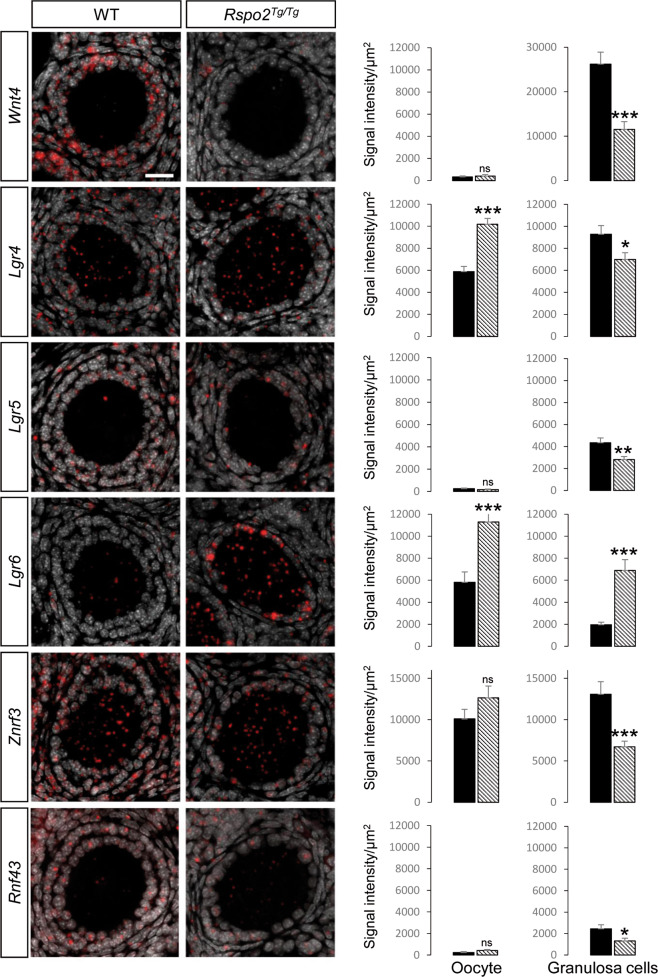
RSPO2 is an activator of WNT/CTNNB1 signaling that is mediated by nuclear translocation of CTNNB1. Immunolocalization analyses of CTNNB1 using an antibody that recognizes nuclear and cytoplasmic, including membrane forms, of CTNNB1 revealed an overall downregulation of CTNNB1 in granulosa cells (Fig. 4c). Quantification analyses using DAPI to measure CTNNB1 expression restricted to nuclei revealed higher levels of CTNNB1 in the nuclei of granulosa cells in wild types when compared with Rspo2Tg/Tg follicles (Fig. 4d). Our data suggest that RSPO2 promotes nuclear translocation of CTNNB1. To verify the activation of this signaling pathway, we then studied the level of expression of different genes including Axin2, a universal readout of the activation of this signaling pathway [43]. In situhybridization revealed that Axin2 is highly expressed in the oocyte and weaker in granulosa cells in wild-type ovaries at 12d (Fig. 4e). In Rspo2Tg/Tg follicles, the expression of Axin2 is restricted to the oocytes. Quantification of the signal intensity confirmed the downregulation of Axin2 in granulosa cells as evidenced by a robust decrease of Axin2 mRNA in these cells (Fig. 4f). Next, we performed expression analyses for the main actors of the RSPO/WNT/CTNNB1 signaling pathway, e.g., the receptors Lgr4, Lgr5, and Lgr6 and the two E3-ubiquitin ligases Znrf3 and Rnf43. Among them, Lgr5, Rnf43, and Znrf3 have been described to be targets of WNT/CTNNB1 signaling [17, 44]. In wild-types, Lgr5 and Rnf43 expression is restricted to the granulosa cells, whereas Lgr4, Lgr6, and Znrf3 are expressed in oocytes and granulosa cells (Fig. 5). In Rspo2Tg/Tg granulosa cells, Lgr5, Znrf3, and Rnf43 expression is downregulated, confirming that RSPO2 is involved in the activation of WNT/CTNNB1 signaling in early developing follicles.
Fig. 5. RSPO2 activates WNT/CTNNB1 signaling in granulosa cells.
In situ hybridization analysis of six main actors of the RSPO/WNT/CTNNB1 signaling pathway, e.g., Wnt4, Lgr4, Lgr5, Lgr6, Znrf3, and Rnf43 and expression signal quantification in wild-type (WT) and Rspo2Tg/Tg follicles at 12d. The number of counted follicles (of three ovaries per genotype) is n = 20, 11, 14, 17, 15, and 6 for WT and n = 26, 15, 14, 23, 16, and 9 for Rspo2Tg/Tg ovaries in Wnt4, Lgr4, Lgr5, Lgr6, Znrf3, and Rnf43 staining, respectively. In WT follicles, Lgr5, Rnf43, and Wnt4 expression is restricted to the granulosa cells, whereas Lgr4, Lgr6, and Znrf3 are expressed in oocytes and granulosa cells. In Rspo2Tg/Tg granulosa cells, the expression of all these markers, except Lgr6, is downregulated compared with WT. Data are presented as mean ± SEM. Student’s t test, unpaired two sided (*p < 0.05; **p < 0.01; ***p < 0.001).
It is noteworthy that WNT4 can signal via the WNT/CTNNB1 pathway in granulosa cells and promote late follicular development [45]. In the Rspo2Tg/Tg ovaries, we observed a downregulation of Wnt4 that likely contributes to the proliferation defects of the granulosa cells in primary follicles (Fig. 5). Altogether, our data suggest that RSPO2 activates WNT/CTNNB1 signaling in granulosa cells.
WNT/CTNNB1 signaling is required for granulosa cell proliferation
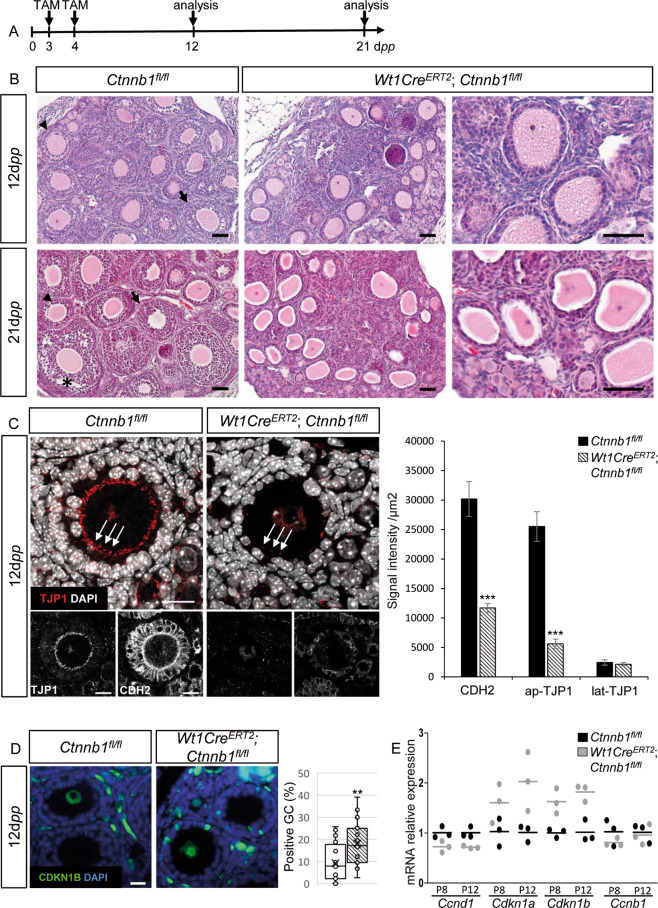
To assess the contribution of WNT/CTNNB1 signaling in granulosa cell proliferation, conditional deletion of Ctnnb1 was performed by using Wt1CreERT2; Ctnnb1fl/fl mice. Wt1CreERT2 is driven by the endogenous Wt1 promoter and is expressed in granulosa cells [46]. Genetic deletion was induced by tamoxifen administration at 3 and 4 dpp and analyses were performed at 12 and 21 dpp (Fig. 6a). At both stages, mutant ovaries lack secondary follicles (Fig. 6b), as previously observed in Rspo2Tg/Tg transplanted ovaries (Fig. 1b). FOXL2 and AMH immunodetection analyses revealed that granulosa cells express low levels of AMH in Wt1CreERT2; Ctnnb1fl/fl ovaries at 12 dpp, as observed in Rspo2Tg/Tg ovaries (Fig. 2a).
Fig. 6. Genetic deletion of Ctnnb1 in granulosa cells induces similar phenotypes as Rspo2Tg/Tg ovaries.
a Timeline of tamoxifen administration (TAM) at 3 and 4 dpp and ovary analyses of control (Ctnnb1fl/fl) and mutant (Wt1CreERT2; Ctnnb1fl/fl) mice at 12 and 21 dpp. b Histological section analyses (HE stainings) of Ctnnb1fl/fl and Wt1CreERT2; Ctnnb1fl/fl ovaries at 12 and 21 dpp. Mutant ovaries lack secondary (arrowheads), tertiary (arrows), and antral (*) follicles in contrast to Ctnnb1fl/fl ovaries. Scale bars, 50 µm. c Immunodetection and quantitative analyses of TJP1 (tight junctions) and CDH2 (adherens junctions) in Ctnnb1fl/fl and Wt1CreERT2; Ctnnb1fl/fl ovaries at 12 dpp. CDH2 and TJP1 signals are decreased in Wt1CreERT2; Ctnnb1fl/fl follicles, notably at the interface between the oocyte and granulosa cells (indicated by white arrows). Data are presented as mean ± SEM. n = 6 Ctnnb1fl/fl and n = 7 Wt1CreERT2; Ctnnb1fl/fl follicles of each genotype. Student’s t test, unpaired two sided (***p < 0.001). Scale bars, 20 µm. d Immunodetection and quantitative analyses of CDKN1B (p27) to assess the proliferation status of granulosa cells in Ctnnb1fl/fl and Wt1CreERT2; Ctnnb1fl/fl ovaries at 12 dpp (n = 700 and 499 granulosa cells from 18 and 16 follicles, respectively, of one transplanted ovary per genotype). Data are presented in a box and whisker plot representation to illustrate positive granulosa cell dispersion according to the follicle considered. Student’s t test, unpaired two sided (*p < 0.05; **p < 0.01). Scale bar, 20 µm. E QRT-PCR analysis of, Ccnd1 (CyclinD1), Cdkn1a (p21), Cdkn1b (p27), and Ccnb1 (CyclinB1). Data are presented as individual data points. n = 3 individual ovary per genotype. Mean values are indicated as black (Ctnnb1fl/fl) and gray (Wt1CreERT2; Ctnnb1fl/fl) bars.
Next, the analysis of the actors of the WNT/CTNNB1 signaling pathway showed that the active nuclear form of CTNNB1 appears to be less abundant in Wt1CreERT2; Ctnnb1fl/fl ovaries in comparison with wild types (Fig. S4a, b). In addition, in situ hybridization experiments at 21 dpp showed that Axin2 expression is reduced in Wt1CreERT2; Ctnnb1fl/fl granulosa cells in comparison with Ctnnb1fl/fl ovaries (Fig. S4c, d). This indicates that WNT/CTNNB1 signaling is activated in granulosa cells in early developing follicles and RSPO2 is a/the main factor of this activation. Furthermore, the adherens and tight junction markers CDH2 and TJP1, respectively, are less abundant in Wt1CreERT2; Ctnnb1fl/fl follicles than in Ctnnb1fl/fl follicles at 12 dpp (Fig. 6c). This suggests that granulosa cells are not packed enough to allow the formation of adherens and tight junctions in ovaries lacking Ctnnb1.
We then characterized the proliferative status of the granulosa cells. QRT-PCR experiments revealed that the direct target of CTNNB1, Ccnd1, is downregulated in mutant ovaries, whereas the two cell cycle inhibitors Cdkn1a and Cdkn1b are upregulated (Fig. 6e). Moreover, immunolocalization of CDKN1B showed an upregulation of this marker in granulosa cells in mutant ovaries (Fig. 6d), highlighting an arrest in the G1 phase of the cell cycle, as evidenced in Rspo2Tg/Tg ovaries (Fig. 3b). QRT-PCR analysis indicated an absence of significant modification of the expression of Gdf9 and its receptors Bmpr2 and Alk4 (Fig. S4e). In contrast, KitL expression was decreased at 8 and 12 dpp, whereas oocyte-specific Kit expression was increased in the Wt1CreERT2; Ctnnb1fl/fl ovaries (Fig. S4e). In summary, conditional deletion of Ctnnb1 within the granulosa cells of primordial follicles impaired their proliferation and prevented follicular growth beyond the primary follicular stage in a GDF9 signaling independent manner. Given that this phenotype recapitulates the phenotype induced by Rspo2 ablation, our data confirm that RSPO2 is an enhancer of WNT/CTNNB1 signaling in granulosa cells. Remarkably, our data show that RSPO2/CTNNB1 is mediated by paracrine signaling with the oocyte-secreted RSPO2 promoting CTNNB1 activation in the neighboring granulosa cells.
Discussion
In the ovary, the regulation of Rspo2 expression is attributed to the oocyte-specific homeobox gene, Nobox as evidenced by microarray analysis of Nobox loss-of-function ovaries showing a downregulation of Rspo2 [47] and by in silico analysis of the Rspo2 promoter revealing the presence of two NOBOX binding elements [48]. However, the ablation of Nobox triggers rapid degeneration of perinatal oocytes, thus impacting the expression of many oocyte-specific genes like Rspo2 [49]. The expression of RSPO2 has also been shown to be regulated by the transcription factor FOXL2 in goat ovaries [50]. In mouse, FOXL2 cannot directly regulate Rspo2 given that Rspo2 is expressed in the oocyte and Foxl2 in granulosa cells. Thus, regulation of Rspo2 expression in the postnatal oocyte remains to be investigated.
Our study exemplifies the importance of the communication between the oocyte and the neighboring granulosa cells. Here we show that the oocyte factor RSPO2 induces the activation of WNT/CTNNB1 signaling, also called canonical WNT signaling, in granulosa cells, thereby promoting cell cycle progression and eventually follicular growth (Fig. 7). Consequently, ablation of Rspo2 leads to a lack of growing numbers of granulosa cells that are required to develop secondary follicles and follicles remain at the primary stage. This arrest is associated with changes of the granulosa cells when they age as evidenced by downregulation of AMH. This does not trigger follicular degeneration, since primary follicles are still observed in 2-month-old Rspo2Tg/Tg ovaries. Thus, these follicles unlikely gain the competency to become atretic as previously reported in Gdf9−/− ovaries [12]. Overall, our results demonstrate that RSPO2 is an oocyte-secreted mitogen required for granulosa cell proliferation and eventually differentiation.
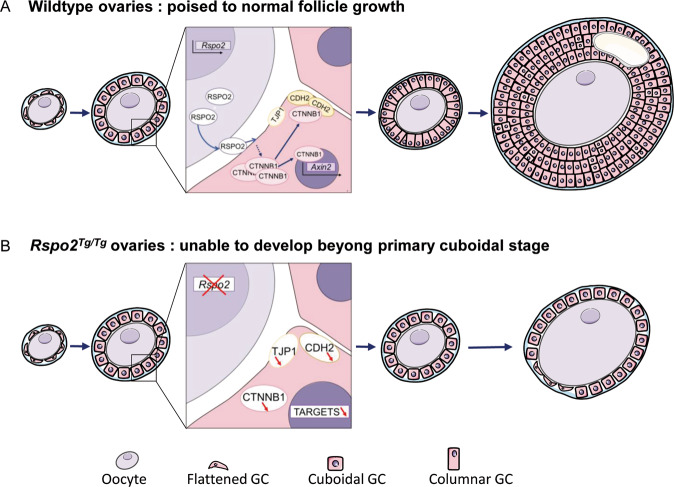
Fig. 7. Proposed model for RSPO2 signaling in mouse ovaries.
a Follicular growth requires the oocyte-secreted RSPO2 protein. RSPO2 promotes the stabilization of CTNNB1 in the neighboring granulosa cells and, in turn, their proliferation and cell adhesion. This leads to follicular growth and development of antral follicles. b In the absence of RSPO2, activation of CTNNB1 is decreased in granulosa cells, thus leading to defects in proliferation and adhesion. Although oocyte growth occurs as in control follicles, the Rspo2Tg/Tg follicles remain as primary follicles containing flattened and cuboidal granulosa cells.
In Foxl2 loss-of-function mice, granulosa cells hardly proliferate and this is associated with their inability to become cuboidal cells [36]. In agreement with this, previous results showed that the change in shape from flattened to cuboidal granulosa cells is associated with an increase in proliferation [51, 52]. Indeed, the flat granulosa cells occasionally start to proliferate in response to unknown signals in primordial follicles [8]. The two adjacent daughter granulosa cells exhibit the first substantial region of adhesion that extends perpendicular to the oocyte surface in primordial follicles. The dividing granulosa cells become more packed on the oocyte surface and then cuboidal due to the space constraints. In Rspo2Tg/Tg follicles, KIT signaling is not impaired at an earlier stage like 8d, suggesting that the activation of the primordial follicles is maintained as previously described by [4]. The granulosa cells proliferate less than in wild types and exhibit less adherens and tight junctions. Some granulosa cells develop until the cuboidal stage but rarely evolve into dense polarized columnar cells in comparison with wild types. It is likely that the lower number of granulosa cells prevents them from forming contacts between them and between them and the oocyte and this might hinder the formation of junctions in Rspo2Tg/Tg follicles and their cuboidalization. Interestingly, it has been shown that the elimination of protein geranylgeranylation, involved in posttranslational modifications of proteins, impairs cell adhesion between the oocyte and the granulosa cells and promotes the arrest at the primary–secondary follicle transition [53]. Thus, the decrease of tight and adherens junctions in Rspo2Tg/Tg and Wt1CreERT2; Ctnnb1fl/fl ovaries might contribute to the blockage of growth of primary follicles.
The oocyte factor GDF9 plays a central role in the signaling from the oocyte to the somatic cells [6]. Strikingly, GDF9 and RSPO2 signaling are both involved in granulosa cell proliferation. RSPO2 activates WNT/CTNNB1 signaling and GDF9 signaling acts through the BMPR2/ALK4 heterodimer receptor and SMAD2/3 pathway in the granulosa cells [41]. Recently, GDF9 was also reported to promote the formation of the transzonal projections from the neighboring granulosa cells to the oocyte through the zona pellucida [11]. The mechanism of action of RSPO is still not very well understood, but RSPOs might use filopodia to reach their target cells as has been shown for WNT ligands [54]. The lack of filopodia in Gdf9−/− follicles may prevent the delivery of RSPO2 to the granulosa cells once the zona pellucida is formed, thus preventing the follicular growth. GDF9 might therefore promote RSPO2/WNT signaling in the ovarian follicle.
Considering the fertility defect in heterozygous Rspo2 mutant mice, the severe follicular growth defect in Rspo2 homozygous mutant ovaries and the conservation of RSPO2 expression in ovaries in a little girl, we can assume that RSPO2 is a candidate gene for premature ovarian insufficiency (POI) in women. Bouilly et al. [48] previously screened 100 patients suffering from POI and did not associate RSPO2 as a potential cause. This might be due to the heterozygous condition. Indeed, homozygous mutations of RSPO2 have been described in human patients with a rare malformation disorder, the tetra-amelia and lung hypo/aplasia syndrome characterized by the absence of the forelimbs and pulmonary anomalies [55, 56]. The patients die at birth due to respiratory distress but in most cases, ultrasonography evaluation leads to an early termination of the pregnancy. Few cases have been described and the pathological examination of one female fetus at the 22nd gestational weeks excluded genitourinary anomalies, but there is no detail on folliculogenesis [57]. At this age, primary follicles are present, but secondary follicles are still rare, thus preventing to know whether loss of RSPO2 could induce a follicular growth defect in these patients. Given the severity of the phenotype described here, the different actors involved in RSPO2/WNT signaling in the ovary, once identified, will likely become new candidates for the diagnosis of POI.
Material and methods
Mouse strains and genotyping
The experiments were carried out in compliance with the relevant institutional and French animal welfare laws, guidelines, and policies and have been approved by the French ethics committees (CIEPAL, accreditation APAFIS#4035–2016012712367693). The mice were kept on a mixed 129/C57Bl6/J genetic background. Homozygous mutant Rspo2Tg/Tg embryos were identified by limb malformations and hypoplasia and confirmed by genotyping as described in [29, 35]. Homozygous females carrying the Ctnnb1fl allele were mated with Wt1CreERT2 males to generate Wt1CreERT2; Ctnnb1fl/fl mice [58, 59]. Activation of the Cre recombinase was performed by intraperitoneal injections of 0.4 mg of tamoxifen (Sigma-Aldrich) dissolved in corn oil (Sigma-Aldrich) per pup. All the pups of the litters were treated in the same way, and genotypes were determined when samples were collected. Hence, the controls (Ctnnb1fl/fl) got the same treatments as Wt1CreERT2; Ctnnb1fl/fl mutants.
Transplantation of fetal mouse ovaries
Wild-type and Rspo2Tg/Tg embryos were collected from 18.5 dpc fetuses. Ovaries were transplanted under the kidney capsule of 6-week-old female athymic mice (Envigo, France). Transplants were dissected from host animals 8, 12, 21 days or 2 months posttransplantation and processed as described below.
Histological analysis
Kidneys including the transplants or ovaries were dissected, fixed in Bouin’s solution overnight embedded in paraffin, sectioned at 5 µm thickness, and stained with hematoxylin and eosin (HE). Pictures were taken with an Axioscope 2 (Zeiss) or MZ9.5 (Leica) microscope coupled with an Axiocam MRc5 (Zeiss) or DHC490 (Leica) camera and Axiovision 4.8 (Zeiss) or application suite V3.3.0 (Leica) software, and processed with Adobe Photoshop for mosaics and Fiji [60].
Morphometric analyses of ovarian follicles
The developmental stages of ovarian follicles (primordial, primary, secondary, tertiary, and antral) were determined on HE stained Bouin’s fixed sections, on the basis of the standards established by [61]. Follicles were counted in ten randomly selected sections of three wild-type and mutant grafted ovaries and expressed as a percentage of the total number of follicles counted. The average oocyte and follicle diameters were evaluated as performed by [42]. Only the follicles sectioned through the nucleus of the oocyte were scored.
In situ hybridization analyses
Samples were fixed in 4% paraformaldehyde (PFA) overnight at room temperature. 5 µm sections were processed for RNA in situ detection using the RNAscope 2.0 High Definition-RED Kit according to the manufacturer’s instructions (ACDBio, [62]). Axin2, Lgr4, Lgr5, Lgr6, Rnf43, Rspo2, Wnt4, and Znrf3 probes were designed by ACDBio. Slides were counterstained with DAPI diluted in the mounting medium at 10 µg/ml (Vectashield, Vector laboratories) to detect nuclei. RNAscope results were examined under a LSM 780 NLO inverted Axio Observer.Z1 confocal microscope (Carl Zeiss Microscopy GmbH, Jena, Germany) using a C-Apo 40X water 1.2 NA objective. The lasers used were diodes laser (405, 488, 532, and 635 nm). The microscope was equipped with a galvanometric stage in order to do z acquisitions. For Rspo2 detection, slides were counterstained with 50% Hematoxylin for 10 s and incubated in 0.1% sodium bicarbonate for 1 min. Slides were then mounted in VectaMount AQ medium (Vector Lab), scanned with Vectra Polaris Automated Imaging System, and examined with Phenochart Software (PerkinElmer).
Immunolabeling analyses
Mouse samples were fixed in 4% PFA overnight at 4 °C and then processed for paraffin embedding. Microtome sections of 6 μm thickness were processed for immunostaining, and antigens were retrieved in Envision Flex Target Retrieval solution (pH9) on the PTlink Pre Treatment Module (DAKO, Agilent). The following dilutions of primary antibodies were used: AMH (C-20, sc6886, Santa Cruz) 1:200, CDH2 (cat 33–3900, Invitrogen) 1:200, CTNNB1 (cat 2206, Sigma) 1:100, FOXL2 (NB100-1277, Novus Bio) 1:400, LAMA1 (cat L9393, Sigma) 1:150, MKi67 (clone SP6, cat 9106, Thermo-Scientific) 1:200, p27 (sc-528, Santa Cruz) 1:200, PHH3 (ab14955, Abcam) 1:300, SF1 (kindly provided by Prof. Morohashi) 1:1000, TJP1 (cat 40–2300, Invitrogen) 1:200. Slides were counterstained with DAPI diluted in the mounting medium (Vectashield, Vector laboratories). Imaging was performed with a motorized Axio ImagerZ1 microscope (Zeiss) coupled with an Axiocam Mrm camera (Zeiss) and processed with Axiovision LE (Zeiss) or on a LSM 780 NLO inverted Axio Observer.Z1 confocal microscope (Carl Zeiss Microscopy GmbH, Jena, Germany) using a C-Apo 40X water 1.2 NA objective when quantification was needed. Images were acquired in mono-photon mode using a LASER diode 405 nm and/or Argon LASER (458, 488, 514 nm) nm and/or DPSS 561 nm and/or HeNe 633 nm. Fluorescence emission was detected on a descanned spectral GaAsp PMT 32 channels. The microscope z-drive was used for z acquisitions.
Formalin-fixed paraffin-embedded ovaries from girls aged 12 postnatal months dying suddenly and unexpectedly were accrued under the French autopsy law that allows the use of such tissues for in-depth anatomopathological examination (Law 94–654 published on July 29, 1994). Autopsy was performed with informed consent of the parents in all cases. These methods were carried out in accordance with relevant guidelines and regulations. All the experiments and experimental protocols on human subjects were approved by the institutional committee of the French agency for biomedical research (Agence de la Biomédecine, Saint-Denis la Plaine, France). Immunohistochemistry was performed as previously described (François et al., Scientific report 2017). Slides were incubated overnight with primary antibodies for RSPO2 (Dilution 1/100; Sigma, HPA024764). They were counterstained in hematoxylin, dehydrated and mounted in Eukitt (Sigma).
Image posttreatments and quantitative analyses
Images were assembled using the open source software platform OMERO (https://www.openmicroscopy.org/omero/). Posttreatment image acquisition and analysis were performed on Fiji [60], using a homemade semiautomated macro when needed.
For CTNNB1 expression quantification, z-stack images were processed through sum slices projection. Nuclear CTNNB1 signal intensity was determined as the signal colocalized with DAPI staining and cytoplasmic CTNNB1 signal intensity was determined as the signal localized outside the DAPI staining but within granulosa cell crown (drawn manually). CTNNB1 signal intensity was quantified as raw integrated density after threshold adjustment normalized on nuclei or cytoplasmic area (µm2). Axin2, TJP1, and CDH2 expression was quantified on confocal z-stack images according to the different steps described in Fig. S5. For all the three Lgr, Rnf43, Wnt4, and Znrf3, signal intensity was quantified on z-stack images processed through sum slices projection. Oocyte surface was obtained by manual drawing of the external contour of the oocyte and granulosa cell layer region of interest (ROI) was determined as the whole follicle area (drawn manually) minus the oocyte surface on DAPI channel. Signal intensity within the oocyte and the granulosa cell ROI was quantified as raw integrated density after threshold adjustment normalized on oocyte and granulosa cell surface (µm2), respectively.
Cell proliferation was assessed by counting MKI67, PHH3, and CDKN1B-positive cells among total DAPI-positive granulosa cells on at least ten different follicles per genotype and per marker.
Quantitative PCR analysis
RNA from snap frozen ovaries was extracted using the RNeasy Micro Kit (Qiagen) and reverse transcribed using the RNA RT-PCR kit (Promega). Primers (Supplementary Table 1) were designed by Roche Assay Design Center (http://qpcr.probefinder.com/organism.jsp). All real-time PCR assays were carried out using LightCycler 480 SYBR Green Master kit (Roche). QRT-PCR were performed on cDNA from one gonad and compared with a standard curve. Relative expression levels of each gene were determined in the same run and normalized on the levels of endogenous Sdha cDNA. QPCR were repeated in duplicate on cDNA issued from three ovaries of each genotype.
Statistical analysis
When appropriate, values are depicted as mean ± SEM. Data were analyzed by unpaired two-sided Student’s t test using Microsoft Excel. Asterisks highlight the pertinent comparisons and indicate levels of significance: *p < 0.05, **p < 0.01, and ***p < 0.001. Cell proliferation counting is shown as box and whisker plot representation to illustrate positive granulosa cell dispersion according to the follicle considered. QRT-PCR analyses are presented as individual data points. The mean value between individual points is shown as a horizontal bar.
Supplementary information
Acknowledgements
We are grateful to Isabelle Gillot and Aitana Perea-Gomez for helpful discussions. We thank the services of the iBV, especially the animal facility members for their help in breeding mice and Cécile Passot and Mireille Cutajar-Bossert for the management of funding. We also thank Samah Rekima and the Experimental Histopathology Platform for assistance and histological sample processing. The microscopy analyses were carried out at the PRISM iBV-imaging platform. This work was supported by the Agence Nationale de la Recherche (ANR-11-LABX-0028-01; ANR-18-CE14-0012; ANR-19-CE14-0022) and Ligue Nationale Contre le cancer (MLR fellowship).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Edited by E. Baehrecke
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version of this article (10.1038/s41418-020-0547-7) contains supplementary material, which is available to authorized users.
References
- 1.Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30:624–712. doi: 10.1210/er.2009-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pelosi E, Forabosco A, Schlessinger D. Genetics of the ovarian reserve. Front Genet. 2015;6:308. doi: 10.3389/fgene.2015.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lei L, Spradling AC. Female mice lack adult germ-line stem cells but sustain oogenesis using stable primordial follicles. Proc Natl Acad Sci USA. 2013;110:8585–90. doi: 10.1073/pnas.1306189110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang H, Risal S, Gorre N, Busayavalasa K, Li X, Shen Y, et al. Somatic cells initiate primordial follicle activation and govern the development of dormant oocytes in mice. Curr Biol. 2014;24:2501–8. doi: 10.1016/j.cub.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 5.Jones RL, Pepling ME. KIT signaling regulates primordial follicle formation in the neonatal mouse ovary. Dev Biol. 2013;382:186–97. doi: 10.1016/j.ydbio.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 6.Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296:2178–80. doi: 10.1126/science.1071965. [DOI] [PubMed] [Google Scholar]
- 7.Hirshfield AN. Granulosa cell proliferation in very small follicles of cycling rats studied by long-term continuous tritiated-thymidine infusion. Biol Reprod. 1989;41:309–16. doi: 10.1095/biolreprod41.2.309. [DOI] [PubMed] [Google Scholar]
- 8.Hardy K, Mora JM, Dunlop C, Carzaniga R, Franks S, Fenwick MA. Nuclear exclusion of SMAD2/3 in granulosa cells is associated with primordial follicle activation in the mouse ovary. J Cell Sci. 2018;131:jcs218123. doi: 10.1242/jcs.218123. [DOI] [PubMed] [Google Scholar]
- 9.Da Silva-Buttkus P, Jayasooriya GS, Mora JM, Mobberley M, Ryder TA, Baithun M, et al. Effect of cell shape and packing density on granulosa cell proliferation and formation of multiple layers during early follicle development in the ovary. J Cell Sci. 2008;121:3890–900. doi: 10.1242/jcs.036400. [DOI] [PubMed] [Google Scholar]
- 10.Wassarman PM, Litscher ES. The mouse egg’s zona pellucida. Curr Top Dev Biol. 2018;130:331–56. doi: 10.1016/bs.ctdb.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 11.El-Hayek S, Yang Q, Abbassi L, FitzHarris G, Clarke HJ. Mammalian oocytes locally remodel follicular architecture to provide the foundation for germline-soma communication. Curr Biol. 2018;28:1124–31. doi: 10.1016/j.cub.2018.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–5. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- 13.Carabatsos MJ, Elvin J, Matzuk MM, Albertini DF. Characterization of oocyte and follicle development in growth differentiation factor-9-deficient mice. Dev Biol. 1998;204:373–84. doi: 10.1006/dbio.1998.9087. [DOI] [PubMed] [Google Scholar]
- 14.Kazanskaya O, Glinka A, del Barco Barrantes I, Stannek P, Niehrs C, Wu W. R-Spondin2 is a secreted activator of Wnt/beta-catenin signaling and is required for Xenopus myogenesis. Dev Cell. 2004;7:525–34. doi: 10.1016/j.devcel.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 15.Nam JS, Turcotte TJ, Smith PF, Choi S, Yoon JK. Mouse cristin/R-spondin family proteins are novel ligands for the Frizzled 8 and LRP6 receptors and activate beta-catenin-dependent gene expression. J Biol Chem. 2006;281:13247–57. doi: 10.1074/jbc.M508324200. [DOI] [PubMed] [Google Scholar]
- 16.Koo BK, Spit M, Jordens I, Low TY, Stange DE, van de Wetering M, et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature. 2012;488:665–9. doi: 10.1038/nature11308. [DOI] [PubMed] [Google Scholar]
- 17.Hao HX, Xie Y, Zhang Y, Charlat O, Oster E, Avello M, et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature. 2012;485:195–200. doi: 10.1038/nature11019. [DOI] [PubMed] [Google Scholar]
- 18.Zebisch M, Xu Y, Krastev C, MacDonald BT, Chen M, Gilbert RJ, et al. Structural and molecular basis of ZNRF3/RNF43 transmembrane ubiquitin ligase inhibition by the Wnt agonist R-spondin. Nat Commun. 2013;4:2787. doi: 10.1038/ncomms3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohkawara B, Glinka A, Niehrs C. Rspo3 binds syndecan 4 and induces Wnt/PCP signaling via clathrin-mediated endocytosis to promote morphogenesis. Dev Cell. 2011;20:303–14. doi: 10.1016/j.devcel.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Lebensohn AM, Rohatgi R. R-spondins can potentiate WNT signaling without LGRs. Elife. 2018;7:e33126. doi: 10.7554/eLife.33126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niehrs C, Acebron SP. Mitotic and mitogenic Wnt signalling. Embo J. 2012;31:2705–13. doi: 10.1038/emboj.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–6. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 23.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D’Amico M, Pestell R, et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA. 1999;96:5522–7. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 25.Kim KA, Kakitani M, Zhao J, Oshima T, Tang T, Binnerts M, et al. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005;309:1256–9. doi: 10.1126/science.1112521. [DOI] [PubMed] [Google Scholar]
- 26.Parma P, Radi O, Vidal V, Chaboissier MC, Dellambra E, Valentini S, et al. R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat Genet. 2006;38:1304–9. doi: 10.1038/ng1907. [DOI] [PubMed] [Google Scholar]
- 27.Chassot AA, Ranc F, Gregoire EP, Roepers-Gajadien HL, Taketo MM, Camerino G, et al. Activation of beta-catenin signaling by Rspo1 controls differentiation of the mammalian ovary. Hum Mol Genet. 2008;17:1264–77. doi: 10.1093/hmg/ddn016. [DOI] [PubMed] [Google Scholar]
- 28.De Cian MC, Pauper E, Bandiera R, Vidal VP, Sacco S, Gregoire EP, et al. Amplification of R-spondin1 signaling induces granulosa cell fate defects and cancers in mouse adult ovary. Oncogene. 2017;36:208–18. doi: 10.1038/onc.2016.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell SM, Schreiner CM, Hess KA, Anderson KP, Scott WJ. Asymmetric limb malformations in a new transgene insertional mutant, footless. Mech Dev. 2003;120:597–605. doi: 10.1016/s0925-4773(03)00021-2. [DOI] [PubMed] [Google Scholar]
- 30.Nam JS, Park E, Turcotte TJ, Palencia S, Zhan X, Lee J, et al. Mouse R-spondin2 is required for apical ectodermal ridge maintenance in the hindlimb. Dev Biol. 2007;311:124–35. doi: 10.1016/j.ydbio.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng Y, Kawamura K, Takae S, Deguchi M, Yang Q, Kuo C, et al. Oocyte-derived R-spondin2 promotes ovarian follicle development. FASEB J. 2013;27:2175–84. doi: 10.1096/fj.12-223412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, Ji SY, Yang JL, Li XX, Zhang J, Zhang Y, et al. Wnt/beta-catenin signaling regulates follicular development by modulating the expression of Foxo3a signaling components. Mol Cell Endocrinol. 2014;382:915–25. doi: 10.1016/j.mce.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Boerboom D, White LD, Dalle S, Courty J, Richards JS. Dominant-stable beta-catenin expression causes cell fate alterations and Wnt signaling antagonist expression in a murine granulosa cell tumor model. Cancer Res. 2006;66:1964–73. doi: 10.1158/0008-5472.CAN-05-3493. [DOI] [PubMed] [Google Scholar]
- 34.Liu CF, Bingham N, Parker K, Yao HH. Sex-specific roles of beta-catenin in mouse gonadal development. Hum Mol Genet. 2009;18:405–17. doi: 10.1093/hmg/ddn362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bell SM, Schreiner CM, Wert SE, Mucenski ML, Scott WJ, Whitsett JA. R-spondin 2 is required for normal laryngeal-tracheal, lung and limb morphogenesis. Development. 2008;135:1049–58. doi: 10.1242/dev.013359. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier AC, et al. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development. 2004;131:933–42. doi: 10.1242/dev.00969. [DOI] [PubMed] [Google Scholar]
- 37.Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. 2009;139:1130–42. doi: 10.1016/j.cell.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 38.Munsterberg A, Lovell-Badge R. Expression of the mouse anti-mullerian hormone gene suggests a role in both male and female sexual differentiation. Development. 1991;113:613–24. doi: 10.1242/dev.113.2.613. [DOI] [PubMed] [Google Scholar]
- 39.Ikeda Y, Lala DS, Luo X, Kim E, Moisan MP, Parker KL. Characterization of the mouse FTZ-F1 gene, which encodes a key regulator of steroid hydroxylase gene expression. Mol Endocrinol. 1993;7:852–60. doi: 10.1210/mend.7.7.8413309. [DOI] [PubMed] [Google Scholar]
- 40.Mora JM, Fenwick MA, Castle L, Baithun M, Ryder TA, Mobberley M, et al. Characterization and significance of adhesion and junction-related proteins in mouse ovarian follicles. Biol Reprod. 2012;86:153. doi: 10.1095/biolreprod.111.096156. [DOI] [PubMed] [Google Scholar]
- 41.Peng J, Li Q, Wigglesworth K, Rangarajan A, Kattamuri C, Peterson RT, et al. Growth differentiation factor 9:bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc Natl Acad Sci USA. 2013;110:E776–85. doi: 10.1073/pnas.1218020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saatcioglu HD, Cuevas I, Castrillon DH. Control of oocyte reawakening by Kit. PLoS Genet. 2016;12:e1006215. doi: 10.1371/journal.pgen.1006215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–93. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van der Flier LG, Sabates-Bellver J, Oving I, Haegebarth A, De Palo M, Anti M, et al. The intestinal Wnt/TCF signature. Gastroenterology. 2007;132:628–32. doi: 10.1053/j.gastro.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 45.Boyer A, Lapointe E, Zheng X, Cowan RG, Li H, Quirk SM, et al. WNT4 is required for normal ovarian follicle development and female fertility. FASEB J. 2010;24:3010–25. doi: 10.1096/fj.09-145789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pelletier J, Schalling M, Buckler AJ, Rogers A, Haber DA, Housman D. Expression of the Wilms’ tumor gene WT1 in the murine urogenital system. Genes Dev. 1991;5:1345–56. doi: 10.1101/gad.5.8.1345. [DOI] [PubMed] [Google Scholar]
- 47.Choi Y, Qin Y, Berger MF, Ballow DJ, Bulyk ML, Rajkovic A. Microarray analyses of newborn mouse ovaries lacking Nobox. Biol Reprod. 2007;77:312–9. doi: 10.1095/biolreprod.107.060459. [DOI] [PubMed] [Google Scholar]
- 48.Bouilly J, Beau I, Barraud S, Bernard V, Delemer B, Young J, et al. R-spondin2, a novel target of NOBOX: identification of variants in a cohort of women with primary ovarian insufficiency. J Ovarian Res. 2017;10:51. doi: 10.1186/s13048-017-0345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science. 2004;305:1157–9. doi: 10.1126/science.1099755. [DOI] [PubMed] [Google Scholar]
- 50.Kocer A, Pinheiro I, Pannetier M, Renault L, Parma P, Radi O, et al. R-spondin1 and FOXL2 act into two distinct cellular types during goat ovarian differentiation. BMC Dev Biol. 2008;8:36. doi: 10.1186/1471-213X-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meredith S, Dudenhoeffer G, Jackson K. Classification of small type B/C follicles as primordial follicles in mature rats. J Reprod Fertil. 2000;119:43–8. doi: 10.1530/jrf.0.1190043. [DOI] [PubMed] [Google Scholar]
- 52.Wandji SA, Srsen V, Nathanielsz PW, Eppig JJ, Fortune JE. Initiation of growth of baboon primordial follicles in vitro. Hum Reprod. 1997;12:1993–2001. doi: 10.1093/humrep/12.9.1993. [DOI] [PubMed] [Google Scholar]
- 53.Jiang C, Diao F, Sang YJ, Xu N, Zhu RL, Wang XX, et al. GGPP-mediated protein geranylgeranylation in oocyte is essential for the establishment of oocyte-granulosa cell communication and primary-secondary follicle transition in mouse ovary. PLoS Genet. 2017;13:e1006535. doi: 10.1371/journal.pgen.1006535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stanganello E, Hagemann AI, Mattes B, Sinner C, Meyen D, Weber S, et al. Filopodia-based Wnt transport during vertebrate tissue patterning. Nat Commun. 2015;6:5846. doi: 10.1038/ncomms6846. [DOI] [PubMed] [Google Scholar]
- 55.Basaran S, Yuksel A, Ermis H, Kuseyri F, Agan M, Yuksel-Apak M. Tetra-amelia, lung hypo-/aplasia, cleft lip-palate, and heart defect: a new syndrome? Am J Med Genet. 1994;51:77–80. doi: 10.1002/ajmg.1320510116. [DOI] [PubMed] [Google Scholar]
- 56.Szenker-Ravi E, Altunoglu U, Leushacke M, Bosso-Lefevre C, Khatoo M, Thi Tran H, et al. RSPO2 inhibition of RNF43 and ZNRF3 governs limb development independently of LGR4/5/6. Nature. 2018;557:564–9. doi: 10.1038/s41586-018-0118-y. [DOI] [PubMed] [Google Scholar]
- 57.Sousa SB, Pina R, Ramos L, Pereira N, Krahn M, Borozdin W, et al. Tetra-amelia and lung hypo/aplasia syndrome: new case report and review. Am J Med Genet A. 2008;146A:2799–803. doi: 10.1002/ajmg.a.32489. [DOI] [PubMed] [Google Scholar]
- 58.Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, et al. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–64. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 59.Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–13. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pedersen T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil. 1968;17:555–7. doi: 10.1530/jrf.0.0170555. [DOI] [PubMed] [Google Scholar]
- 62.Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14:22–9. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.