Abstract
Background:
Patients with unresectable locally advanced NSCLC who refuse or are not candidates for chemotherapy often receive radiation therapy (RT) alone. Hypofractionated RT (HFRT) regimens are becoming increasingly common. An analysis of the National Cancer Database (NCDB) was performed to evaluate the practice patterns and outcomes of HFRT vs. conventionally fractionated RT (CFRT) in patients with Stage III NSCLC undergoing definitive RT alone.
Materials and Methods:
The NCDB was queried for all patients with stage III NSCLC diagnosed between 2004 and 2014 who received RT alone. CFRT was defined as patients treated to a total dose of 60–80 Gy in 1.8–2 Gy daily fractions. HFRT was defined as patients treated to a total dose of 50–80 Gy in 2.25–4 Gy fractions. Logistic regression, univariable and multivariable analyses (MVA) for overall survival (OS), and propensity score matched analyses (PSMA) were performed.
Results:
A total of 6,490 patients were evaluated: 5,378 received CFRT and 1,112 received HFRT. Median CFRT dose was 66 Gy in 2 Gy fractions versus 58.5 Gy in 2.5 Gy fractions for HFRT. HFRT was associated with older age, lower biological effective dose (BED10), academic facility type, higher T-stage, and lower N-stage. On initial analysis, HFRT was associated with inferior OS (median 9.9 vs. 11.1 months, p<0.001), but after adjusting for the imbalance in covariates such as age, BED10, T-stage and N-stage using PSMA, the difference in survival was no longer significant (p=0.1).
Conclusion:
In the appropriate clinical context, HFRT can be an option for patients with locally advanced NSCLC who are not candidates for chemotherapy or surgical resection. HFRT needs to be further studied in prospective trials to evaluate toxicity and tumor control.
Keywords: Carcinoma, Non-Small-Cell Lung, Dose Hypofractionation, Dose Fractionation Radiation
Introduction:
Lung cancer is the most common cause of cancer-related deaths worldwide[1]. Patients with locally advanced, unresectable non-small cell lung cancer (NSCLC) typically undergo treatment with definitive concurrent chemoradiation over 6–7 weeks with daily conventionally fractionated radiation therapy (CFRT) in 1.8–2.0 Gy fractions. While concurrent chemoradiation improves overall survival compared to sequential chemoradiation or radiotherapy (RT) alone, there is an increased risk for esophagitis and other toxicities [2–4]. Many patients diagnosed with NSCLC are elderly, have medical comorbidities, poor functional status, or notable preexisting weight loss and may not be able to tolerate chemotherapy with radiation [5, 6]. Patients who refuse or are not candidates for chemotherapy often receive RT alone. RT alone, however, confers a 5-year overall survival rate of about 5–6%[4, 7, 8].
Hypofractionated RT (HFRT) regimens, in which radiation is administered using larger doses per fraction over a shorter period of time, are becoming increasingly common. Hypofractionated regimens range from stereotactic body radiation therapy (SBRT) treatments using high dose per fraction (ex: 8–18 Gy/fraction) in 3–5 fractions to moderately hypofractionated radiation regimens (ex: 2.25–4 Gy/fraction) in 15–25 fractions. HFRT offers the convenience of a shorter treatment duration and can also lower health-care costs and help with resource limitations as compared to CFRT. Depending on fractional dose and the number of fractions, a larger RT dose per fraction may also confer greater biological effectiveness and improve locoregional control [9].
HFRT is now a standard of care option for select breast and prostate cancer patients, as it has been shown to result in excellent local control with acceptable toxicity and is also known to be an effective treatment for early-stage NSCLC [10–14]. Stereotactic body radiation therapy (SBRT) and other HFRT regimens are well-established standard treatment regimens that have been shown to be safe and highly effective with excellent local control rates in early-stage NSCLC patients[14, 15]. As tumor target volume increases and targets become more centrally located, however, the risks of toxicities with SBRT and HFRT increase[15]. Therefore, there is some concern that HFRT may result in increased toxicities in patients with locally-advanced NSCLC, particularly in those with mediastinal lymph node involvement.
With advancements in radiation treatment techniques, image guidance, tumor localization and targeting, HFRT is being explored in locally advanced NSCLC. The HFRT regimen of 55 Gy in 20 fractions is now the most common fractionation schedule in the United Kingdom with or without chemotherapy[16]. CALGB 31102 (Alliance) conducted a phase I study to define the maximally tolerated dose of HFRT with concurrent chemotherapy in patients with Stage III NSCLC and found that only modest hypofractionation to 60 Gy in 24 fractions (2.5 Gy/fraction) was achievable as a result of long-term toxicities[17]. HFRT with concurrent chemotherapy in locally advanced NSCLC is also currently under investigation through the RTOG 1106 and PCG-LUN005 trials.
Data regarding HFRT regimens using RT alone in locally advanced NSCLC, however, are limited. Small, single-institution studies have found HFRT to be well-tolerated with acceptable rates of toxicity, disease control and overall survival [9, 18–20]. While these results are promising, these studies employed small patient numbers and further prognostic and outcome data are needed.
To further understand the role of HFRT in locally advanced NSCLC patients undergoing RT alone, a database analysis was performed to evaluate practice patterns and outcomes of HFRT vs. CFRT in patients with Stage III NSCLC undergoing definitive RT alone.
Methods and Materials:
The National Cancer Database (NCDB) is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society and is a nationwide hospital-based oncology outcomes database. NCDB captures the following patient and tumor characteristics: age, sex, race, insurance status, income, education, urban vs. rural dwelling, distance between patient’s residence and treatment facility, treatment facility type, treatment facility location, Charlson-Deyo comorbidity score, tumor location, tumor grade, tumor histology, tumor size, and AJCC staging (clinical and pathologic T, N, M and overall stage). Smoking status, toxicities, and tumor control status are not available in the database.
The NCDB was queried for patients with stage III NSCLC diagnosed between 2004 and 2014 who received external beam photon-based definitive radiation therapy (50 Gy-80 Gy) to the chest. Patients were excluded if they received chemotherapy, surgical resection or RT with palliative intent. See Figure 1 for the CONSORT diagram depicting patient selection.
Figure 1:
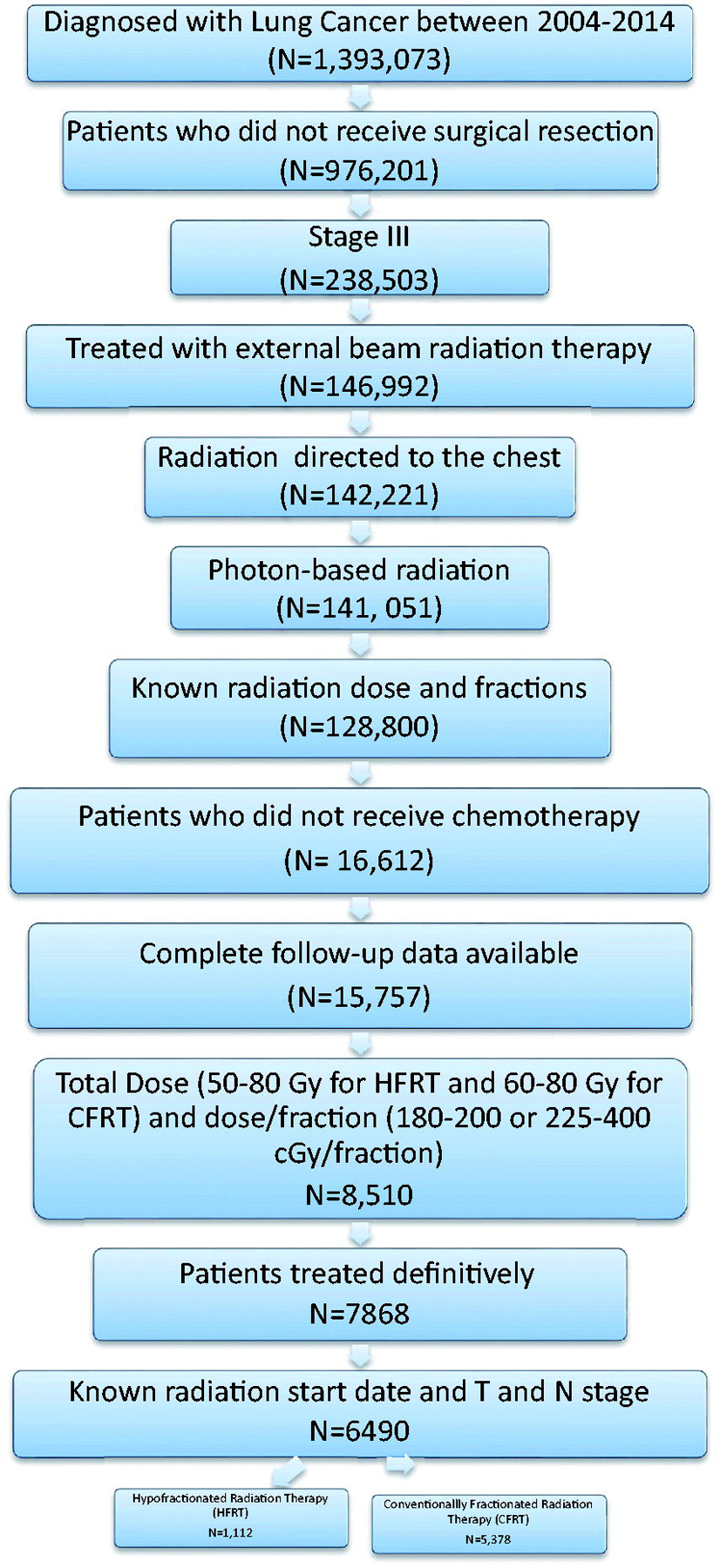
CONSORT Diagram depicting patient selection
The total radiation dose was the sum of the regional dose and boost dose, if applicable. The dose per fraction was calculated by dividing the total radiation dose by the number of fractions. Because Biological Effective Dose (BED10) is associated with improved local control and outcomes, particularly in patients receiving SBRT, the BED10 was calculated using the standard formula with 10 used as the alpha/beta ratio[21]. Patients were grouped into CFRT or HFRT. CFRT was defined as patients treated to a total dose of 60–80 Gy and a dose/fraction of 1.8–2 Gy/fraction. HFRT was defined as patients treated to a total dose of 50–80 Gy and a dose/fraction of 2.25–4 Gy/fraction.
We first performed logistic regression to evaluate factors associated with HFRT treatment assignment. The following baseline patient and tumor characteristics were analyzed using both univariable and multivariable logistic regression predicting HFRT vs CFRT treatment: Age, BED10, Miles from hospital (“Crowfly”), Sex, Race (white vs other), Insurance (Medicare vs others), Income (<$38K vs >=$38K), Education (No High School Diploma (HSD) vs others), Urban (population >1million vs other), Facility Location (East Coast, Central, West Coast), Facility type (Academic vs other), Charlson-Deyo Comorbidity Index (CDCC) Total, Year of Diagnosis (2004–2009 vs. 2010–2014), Tumor location (upper vs other), Tumor laterality (Right vs other), Histology (squamous, adenocarcinoma, other), Grade of differentiation (well, moderate, poor), T-stage (T3/T4 vs others), and N-stage (N2/N3 vs others). Next, Kaplan-Meier survival curves were used to evaluate overall survival based on the date of diagnosis until the date of death or last contact. Cox proportional-hazards regression models were used for univariable and multivariable analyses predicting overall survival. The multivariable analysis included variables that were statistically significant (p<0.05) on univariable analysis.
Propensity score matched analysis was subsequently performed to adjust the imbalance in patient and clinical characteristics between HFRT and CFRT groups. This is a method for analyzing observational data by aiming to reduce the bias due to confounding variables, which is especially important in a retrospective study. Propensity scores were computed as the conditional probability of HFRT vs CFRT using a logistic regression model which included 19 demographic and clinical variables (listed above). Propensity-score-matched pairs were identified without replacement using a 1:1 nearest neighbor matching algorithm with caliper width equal to 0.132 (determined by 0.2 of the standard deviation of the logit of the propensity score recommended by Austin et al.[22]). Criteria of balance was satisfied for each of the variables included in the propensity score matching, given by absolute standardized mean difference (ASMD) between the two groups <0.1. p-values and 95% confidence intervals were two sided, and a p-value less than 0.05 was considered statistically significant. STATA/IC v13 software (College Station, TX) and R 3.5.2 (www.r-project.org) were used for statistical analysis.
Results:
Patient Characteristics
A total of 6,490 patients were evaluated: 5,378 received CFRT and 1,112 received HFRT. Patient, tumor and treatment characteristics are listed in Table 1.
Table 1 -.
Patient, tumor and treatment characteristics:
| Variable | All Patients | CFRT | HFRT |
|---|---|---|---|
| N = 6490 (100%) | N = 5378 (83%) | N = 1112 (17%) | |
| Age (Mean (min, max)) | 75 (40, 90) | 74 (40, 90) | 76 (41, 90) |
| BED (Mean (min, max)) | 77.0 (61.4, 112.0) | 77.6 (70.9, 96.0) | 74.3 (61.4, 112.0) |
| Miles from hosp (Mean (min, max)) | 18 (0, 2588) | 17 (0, 2420) | 20 (0, 2588) |
| Sex (Female vs Male) | 2877 (44%) | 2369 (44%) | 508 (46%) |
| Race (White vs Other) | 5566 (86%) | 4618 (86%) | 948 (85%) |
| Insurance (Medicare vs Other) | 4934 (76%) | 4069 (76%) | 865 (78%) |
| Urban (Pop. >1 million) | 2802 (43%) | 2277 (42%) | 525 (47%) |
| Facility Location | |||
| 1. East Coast | 2570 (40%) | 2089 (39%) | 481 (43%) |
| 2. Central | 3105 (48%) | 2631 (49%) | 474 (43%) |
| 3. West Coast | 815 (13%) | 658 (12%) | 157 (14%) |
| CDCC_TOTAL | |||
| 0 | 3817 (59%) | 3178 (59%) | 639 (57%) |
| 1 | 1709 (26%) | 1411 (26%) | 298 (27%) |
| 2 | 686 (11%) | 558 (10%) | 128 (12%) |
| 3 | 278 (4.3%) | 231 (4.3%) | 47 (4.2%) |
| Year of Diagnosis >2009 | 2828 (44%) | 2312 (43%) | 516 (46%) |
| Tumor location (Upper vs Lower) | 4098 (63%) | 3391 (63%) | 707 (64%) |
| Tumor laterality (Right vs Left) | 3825 (59%) | 3162 (59%) | 663 (60%) |
| Histology | |||
| 1. Squamous | 3081 (47%) | 2554 (47%) | 527 (47%) |
| 2. Adeno | 1754 (27%) | 1461 (27%) | 293 (26%) |
| 3. Other | 1655 (26%) | 1363 (25%) | 292 (26%) |
| Grade | |||
| 1. Well/mod | 1323 (20%) | 1075 (20%) | 248 (22%) |
| 2. Poor | 1940 (30%) | 1624 (30%) | 316 (28%) |
| 3. Not determined | 3227 (50%) | 2679 (50%) | 548 (49%) |
| T-stage 3/4 | 3337 (51%) | 2684 (50%) | 653 (59%) |
| N-stage 2/3 | 5051 (78%) | 4248 (79%) | 803 (72%) |
| Median Income <$38K | 1532 (24%) | 1264 (24%) | 268 (24%) |
| Variable | All Patients | CFRT | HFRT |
Continuous variables presented as Mean (Minimum, Maximum). Categorical variables presented as n (%).
HFRT = Hypofractionated Radiotherapy, CFRT = Conventional Radiotherapy, BED = Biologic Effective Dose, HSD = High School Diploma, CDCC = Charlson Deyo Comorbidity Index
For the CFRT versus HFRT groups, median RT dose was 66 Gy (range: 60–80 Gy, Interquartile Range (IQR): 61.2–68) vs. 58.5 Gy (range: 50–80 Gy, IQR: 51.0 – 64.0), median RT dose per fraction was 2 Gy (range: 1.8–2 Gy, IQR: 1.8–2) vs. 2.50 Gy (range: 2.25–4 Gy, IQR: 2.5–2.75), median number of fractions was 34 (range: 30–43, IQR: 32–36) vs. 22 (range: 13–35, IQR: 20–25), and median BED10 was 78.6 (range: 70.9–96, IQR: 72.22–80.71) vs. 74.1(range: 61.36–112, IQR: 66.15–81.25).
Logistic regression results for factors associated with HFRT vs CFRT assignment are presented (Table 2). HFRT was associated with older age (odds ratio [OR] 1.01, p<0.001), lower BED10 (OR: 0.91, p<0.001), Academic Facility Type (OR 1.32, p<0.001), higher T-stage (T3/4 OR: 1.26, p=0.004), lower N-stage (N2/3 OR: 0.77, p=0.003), Central Location vs. East Coast Location (OR: 0.80, p=0.003) and poorly differentiated grade vs. well/moderately differentiated grade (OR: 0.79, p=0.018).
Table 2 –
Logistic Regression predicting HFRT vs CFRT assignment:
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| N = 6490 | OR | 95% CI | p-value | OR | 95% CI | p-value |
| Increasing Age | 1.02 | 1.01, 1.02 | <0.001 | 1.01 | 1.01, 1.02 | <0.001 |
| Increasing BED | 0.91 | 0.90, 0.92 | <0.001 | 0.91 | 0.90, 0.92 | <0.001 |
| Miles from hosp. | 1.00 | 1.00, 1.00 | 0.2 | 1.00 | 1.00, 1.00 | 0.3 |
| Sex (Female vs Male) | 1.07 | 0.94, 1.22 | 0.3 | 1.03 | 0.90, 1.18 | 0.7 |
| Race (White vs Other) | 0.95 | 0.79, 1.14 | 0.6 | 1.03 | 0.84, 1.26 | 0.8 |
| Insurance (Medicare vs Other) | 1.13 | 0.97, 1.32 | 0.13 | 0.99 | 0.83, 1.19 | >0.9 |
| Urban (Pop. >1 million) | 1.22 | 1.07, 1.39 | 0.003 | 1.08 | 0.94, 1.24 | 0.3 |
| Facility Type (Academic vs Other) | 1.34 | 1.16, 1.54 | <0.001 | 1.32 | 1.14, 1.54 | <0.001 |
| CDCC_TOTAL | ||||||
| 0 | Ref. | Ref. | ||||
| 1 | 1.05 | 0.90, 1.22 | 0.5 | 1.04 | 0.89, 1.21 | 0.6 |
| 2 | 1.14 | 0.92, 1.40 | 0.2 | 1.17 | 0.94, 1.45 | 0.2 |
| 3 | 1.01 | 0.72, 1.39 | >0.9 | 1.04 | 0.74, 1.45 | 0.8 |
| Year of Diagnosis >2009 | 1.15 | 1.01, 1.31 | 0.037 | 1.12 | 0.97, 1.28 | 0.12 |
| Tumor location (Upper vs Lower) | 1.02 | 0.89, 1.17 | 0.7 | 1.05 | 0.92, 1.21 | 0.5 |
| Histology | ||||||
| Squamous | Ref. | Ref. | ||||
| Adeno | 0.97 | 0.83, 1.14 | 0.7 | 1.02 | 0.86, 1.20 | 0.8 |
| Other | 1.04 | 0.89, 1.21 | 0.6 | 1.18 | 0.99, 1.40 | 0.061 |
| T-stage 3/4 | 1.43 | 1.25, 1.63 | <0.001 | 1.26 | 1.08, 1.47 | 0.004 |
| N-stage 2/3 | 0.69 | 0.60, 0.80 | <0.001 | 0.77 | 0.65, 0.91 | 0.003 |
| Location | ||||||
| East Coast | Ref. | Ref. | ||||
| Central | 0.78 | 0.68, 0.90 | <0.001 | 0.80 | 0.69, 0.93 | 0.003 |
| West Coast | 1.04 | 0.85, 1.26 | 0.7 | 1.04 | 0.84, 1.28 | 0.7 |
| Tumor laterality (Right vs Left) | 1.03 | 0.91, 1.18 | 0.6 | 1.05 | 0.92, 1.20 | 0.5 |
| Grade | ||||||
| Well/mod | Ref. | Ref. | ||||
| Poor | 0.84 | 0.70, 1.01 | 0.069 | 0.79 | 0.65, 0.96 | 0.018 |
| Not determined | 0.89 | 0.75, 1.05 | 0.2 | 0.82 | 0.69, 0.98 | 0.029 |
| Median Income <$38K | 1.03 | 0.89, 1.20 | 0.7 | 1.15 | 0.95, 1.38 | 0.14 |
| Education (No HSD vs other) | 1.05 | 0.89, 1.24 | 0.5 | 1.09 | 0.90, 1.33 | 0.4 |
Univariable (UVA) Odds Ratio (OR), 95% Confidence Interval (CI), and p-value calculated by univariable logistic regression predicting HFRT assignment. Multivariable (MVA) OR, 95% CI and p-value calculated by using multivariable logistic regression predicting HFRT assignment, adjusting for all variables in the model.
HFRT = Hypofractionated Radiotherapy, CFRT = Conventional Radiotherapy, BED = Biologic Effective Dose, HSD = High School Diploma, CDCC = Charlson Deyo Comorbidity Index
Overall Survival
The median, 1-year, 2-year and 5-year rates of overall survival were 11.1 months, 47.3%, 23.4% and 6.5%, respectively, for patients who received CFRT versus 9.9 months, 42.9%, 20.7% and 5.1%, respectively, for patients who received HFRT, and survival time between the groups was statistically significant by log-rank test (p<0.001) (Figure 2A).
Figure 2:
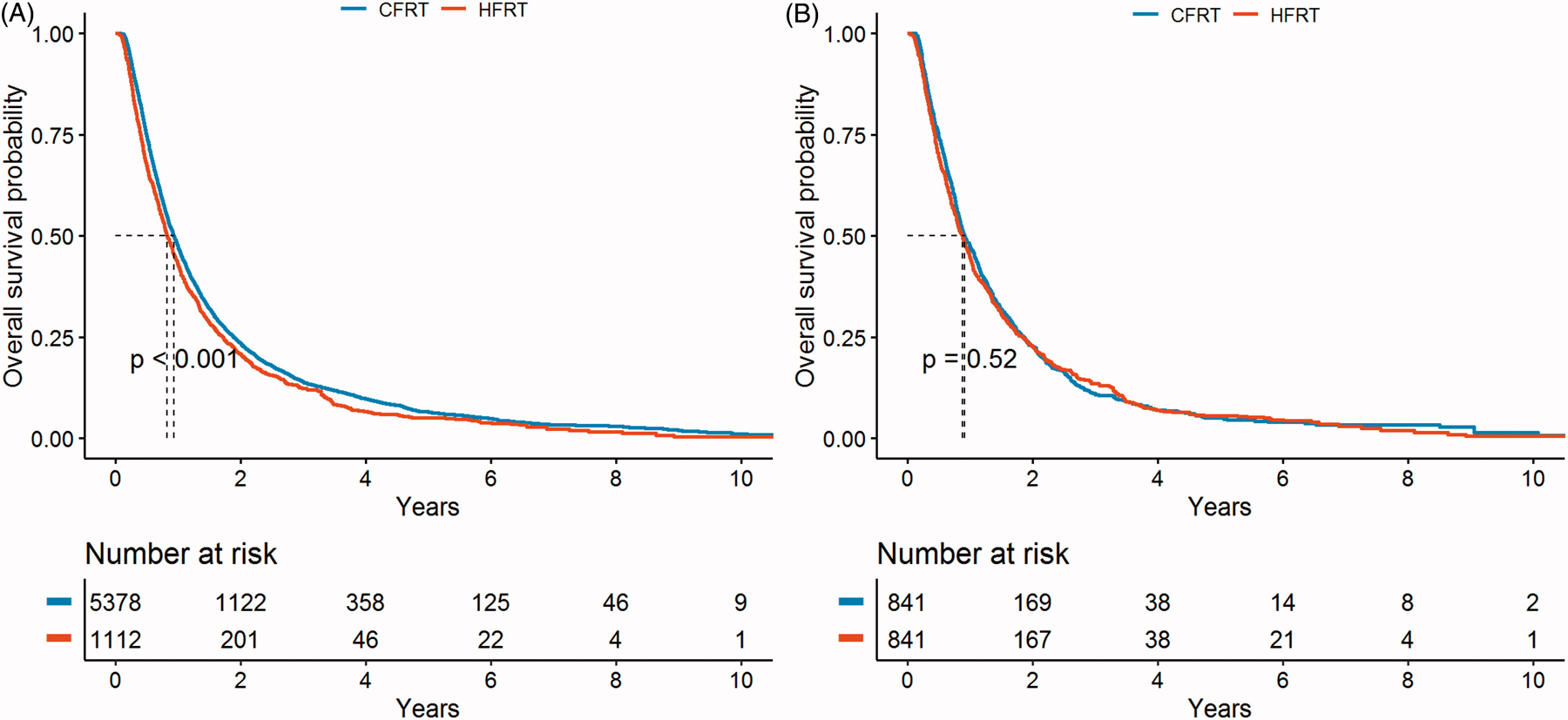
Overall Survival Rates Comparing HFRT vs. CFRT
Due to the overall poor prognosis of this patient population, there is a steep drop off in survival early on and the few survivors beyond 5 years. To minimize the bias from patients treated with palliative intent, a secondary analysis was performed on patients with a minimum survival of 3 months. In this analysis, HFRT was no longer significantly associated with poorer survival (median survival: 12.2 months with CFRT vs. 11.8 months with HFRT, p=0.052 (log-rank test)).
Univariable and Multivariable Cox Regression Analyses
The results from univariable and multivariable analyses predicting overall survival are demonstrated in Table 3. According to multivariable Cox regression analysis predicting overall survival, HFRT (compared to CFRT), increasing age, Medicare insurance, increasing Charlson-Deyo Comorbidity Score, higher T-stage, and higher N-stage were associated with inferior overall survival. In contrast, increasing BED10, female gender, urban dwelling, treatment at an academic center, more recent diagnosis (after 2009), upper lobe location and adenocarcinoma histology were associated with better overall survival.
Table 3-.
Univariable and Multivariable Cox Regression Analyses Predicting OS:
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| N = 6490 | HR | 95% CI | p-value | HR | 95% CI | p-value |
| HFRT vs CFRT | 1.14 | 1.06, 1.22 | <0.001 | 1.08 | 1.01, 1.16 | 0.027 |
| Increasing Age | 1.01 | 1.01, 1.01 | <0.001 | 1.01 | 1.01, 1.01 | <0.001 |
| Increasing BED | 0.98 | 0.98, 0.99 | <0.001 | 0.98 | 0.98, 0.99 | <0.001 |
| Miles from hosp. | 1.00 | 1.00, 1.00 | 0.2 | |||
| Sex (Female vs Male) | 0.83 | 0.79, 0.87 | <0.001 | 0.83 | 0.79, 0.88 | <0.001 |
| Race (White vs Other) | 1.09 | 1.02, 1.18 | 0.018 | 1.03 | 0.95, 1.11 | 0.5 |
| Insurance (Medicare vs Other) | 1.24 | 1.17, 1.32 | <0.001 | 1.14 | 1.07, 1.23 | <0.001 |
| Urban (Pop. >1 million) | 0.91 | 0.86, 0.96 | <0.001 | 0.91 | 0.86, 0.96 | <0.001 |
| Facility Type (Academic vs Other) | 0.89 | 0.84, 0.95 | <0.001 | 0.92 | 0.86, 0.97 | 0.004 |
| CDCC_TOTAL | ||||||
| 0 | Ref. | Ref. | ||||
| 1 | 1.10 | 1.03, 1.16 | 0.003 | 1.09 | 1.02, 1.15 | 0.007 |
| 2 | 1.20 | 1.10, 1.31 | <0.001 | 1.19 | 1.09, 1.30 | <0.001 |
| 3 | 1.32 | 1.16, 1.50 | <0.001 | 1.33 | 1.17, 1.51 | <0.001 |
| Year of Diagnosis >2009 | 0.91 | 0.86, 0.96 | <0.001 | 0.88 | 0.84, 0.93 | <0.001 |
| Tumor location (Upper vs Lower) | 0.84 | 0.79, 0.88 | <0.001 | 0.84 | 0.80, 0.89 | <0.001 |
| Histology | ||||||
| Squamous | Ref. | Ref. | ||||
| Adeno | 0.83 | 0.78, 0.88 | <0.001 | 0.89 | 0.84, 0.95 | <0.001 |
| Other | 0.97 | 0.91, 1.03 | 0.3 | 0.99 | 0.93, 1.06 | 0.8 |
| T-stage 3/4 | 1.17 | 1.11, 1.23 | <0.001 | 1.34 | 1.26, 1.42 | <0.001 |
| N-stage 2/3 | 1.10 | 1.03, 1.17 | 0.003 | 1.33 | 1.24, 1.43 | <0.001 |
| Location | ||||||
| East Coast | Ref. | |||||
| Central | 1.03 | 0.98, 1.09 | 0.2 | |||
| West Coast | 0.97 | 0.89, 1.05 | 0.4 | |||
| Tumor laterality (Right vs Left) | 0.98 | 0.93, 1.03 | 0.4 | |||
| Grade | ||||||
| Well/mod | Ref. | |||||
| Poor | 1.03 | 0.96, 1.11 | 0.4 | |||
| Not determined | 0.97 | 0.91, 1.04 | 0.4 | |||
| Median Income <$38K | 0.98 | 0.92, 1.04 | 0.5 | |||
| Education (No HSD vs other) | 0.94 | 0.88, 1.01 | 0.084 | |||
Univariable (UVA) Hazard Ratio (HR), 95% Confidence Interval (CI), and p-value calculated by univariable Cox proportional hazards regression with an endpoint of overall survival. Multivariate (MVA) OR, 95% CI and p-value calculated by using multivariable Cox proportional hazards regression with an endpoint of overall survival, adjusting for all variables that were significant in the UVA model. HFRT = Hypofractionated Radiotherapy, CFRT = Conventional Radiotherapy, BED = Biologic Effective Dose, HSD = High School Diploma, CDCC = Charlson Deyo Comorbidity Index
Propensity score matching analysis
To minimize selection bias, we performed propensity score matching analysis using the 19 demographic and clinical covariates. After matching to adjust for the imbalanced demographic and clinical characteristics between CFRT and HFRT groups (including age, BED10, T-stage and N-stage), there were 841 matched pairs for both treatments. Using a Cox regression model stratified by propensity score matched pairs, HFRT compared to CFRT was no longer a significant predictor of OS with a HR (95% CI) of 1.13 (0.98–1.3) and p-value =0.095. In this matched cohort, the median, 1-year, 2-year and 5-year rates of overall survival were 10.9 months, 47.2 %, 22.7 % and 5.0 %, respectively, for patients who received CFRT versus 10.5 months, 44.6 %, 22.7 % and 5.6 %, respectively, for patients who received HFRT (Figure 2B).
Discussion:
This large analysis presents data from 6,490 patients with Stage III NSCLC treated with radiation therapy alone administered as either HFRT or CFRT. Because these patients did not receive surgical resection or chemotherapy, they are presumably patients with poor prognosis due to older age, medical comorbidities and/or extent of disease.
The data presented reflect a trend for prioritizing convenience as older patients and patients treated at academic centers were more likely to receive HFRT on logistic regression. HFRT was also associated with higher T-stage and lower N-stage on logistic regression. This is not surprising as practitioners may prefer a higher dose per fraction in larger tumors (T-stage) to improve local control. Additionally, practitioners are likely more comfortable using HFRT in patients with limited mediastinal nodal disease (N-stage) because toxicity is often related to the degree of target overlap with central structures.
Although hypofractionation is used to deliver an increased BED10 to patients, particularly for those who have tumors with high propensities for local failures like NSCLC, this study demonstrates that HFRT administered clinically was associated with a lower BED10. Although patients were selected for treatment with definitive intent, clinically, there are often cases treated with “aggressive palliation,” that are difficult to identify and exclude in a database study. Because patients in the HFRT group were older with larger tumors and treated with a lower BED10, the HFRT group likely included more patients treated with some degree of palliative intent and practitioners are likely selecting out worse-prognosis patients for HFRT. Unfortunately, NCDB does not record baseline performance status, which is an important predictor for outcome in this patient population. Due to the concern for confounding, MVA, PSMA and secondary analysis omitting patients surviving less than 3 months were performed. After adjusting for the confounders of age, BED10, T-stage and N-stage through PSMA, the difference in overall survival with HFRT was no longer statistically significant. Presuming that patients in the HFRT group had worse baseline performance status, if it were possible to control for baseline performance status, we anticipate that the difference in overall survival would be further diminished.
It should be noted that, despite statistically significant differences in the initial analysis, the absolute differences in overall survival rates were small: 2.7% difference at 2 years, 1.4% at 5 years and 1.2 months at median. This small difference in overall survival needs to be considered in the appropriate clinical context as convenience may need to be prioritized in a patient population that is not fit for chemotherapy or surgery. The difference in median number of fractions of RT between CFRT and HFRT was 12, which indicates that the patients who received CFRT spent on average an additional >0.5 months of their lives receiving radiation, compared to the HFRT patients. Therefore, despite the small OS detriment seen on initial analysis with HFRT, HFRT can be considered in the appropriate clinical context.
The overall survival rates (2-year: HFRT 20.7%- CFRT 23.4%; 5 year: HFRT 5.1% -CFRT 6.5%) are consistent with the published literature on locally advanced NSCLC patients treated with radiation therapy alone[4, 7]. To our knowledge, there are no randomized trials directly comparing CFRT vs. HFRT. Small, single-institution, single-arm studies have found RT alone with HFRT to be relatively well-tolerated with acceptable toxicity rates, disease control and overall survival [9, 18, 19]. The two systematic literature reviews examining HFRT in locally advanced NSCLC found that in patients treated with RT alone, the 2-year overall survival rates ranged from 18–68.7% and 5-year overall survival rates ranged from 0–7.4%. Acute grade ≥3 esophagitis occurred in 0–15%, while late esophageal toxicity was 0–16%. Acute pneumonitis (all grades) occurred in 0–44%, whereas late pneumonitis (all grades) occurred in 0–47%, most commonly grade ≤3[23, 24].
The strengths of our study include the large patient population and the novelty of being the first to compare CFRT vs. HFRT for locally advanced NSCLC on a large scale. Our study is limited by inherent weaknesses of the NCDB, including the absence of information regarding tumor control and cancer-specific survival and the lack of recording of other potential confounding variables, such as baseline performance status, toxicity rates and more aggressive biology (histologic subtype, PET scan avidity, etc.).
Prospective data and data comparing CFRT vs. HFRT are necessary to further understand the role of HFRT in locally advanced NSCLC patients undergoing RT alone. With the recent publication of the PACIFIC trial demonstrating improved overall survival with immunotherapy in patients with locally advanced NSCLC undergoing definitive concurrent chemoradiation,[25] the role of fractionation in the setting of immunotherapy needs to be explored. The recently activated NRG-LU004 (NCI number: NCT03801902) trial will evaluate these two questions. This study is evaluating the role of the addition of durvalumab to CFRT or HFRT without chemotherapy in patients with locally advanced NSCLC. We are also investigating the role of immunotherapy and CFRT in patients who cannot undergo concurrent chemotherapy through a phase II study, which will determine the safety and clinical outcomes with such a regimen (DART study, NCI number: NCT03999710).
Conclusions:
In conclusion, HFRT is associated with older age, lower BED10, academic facility type, higher T-stage, and lower N-stage. The slightly inferior overall survival rates seen in patients who received HFRT on initial analysis was no longer statistically significant after adjusting for the confounders of age, BED10, T-stage and N-stage through PSMA. HFRT can be considered in the appropriate clinical context, such as in older patients with a higher comorbidity score and more limited life expectancy. Further data comparing CFRT vs. HFRT are necessary to better understand the role of HFRT in locally advanced NSCLC patients undergoing RT alone and in conjunction with adjuvant or concurrent immunotherapy
Acknowledgments:
The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Grants: MSKCC Cancer Center Support Grant, Principal Investigator (Thompson), Agency: NIH/NCI, 5 P30 CA008748-50, Period: 1/1/14 – 12/31/18
Footnotes
Disclosures:
Consulting: Varian Medical Systems
Disclosures:
Research grants from CivaTech Oncology
Consulting for AstraZeneca
Honoraria (travel grant) from AlphaTau Medical
Disclosures:
Consulting: AstraZeneca, Varian Medical Systems, Merck, Cybrex, MoreHealth
Research Grants: AstraZeneca, Varian Medical Systems, Boehringer Ingelheim, Pfizer
Travel Reimbursement: Philips/Elekta
Disclosures:
Travel Reimbursement and Consulting: ASCO
Contributor Information
Michelle Iocolano, Stony Brook University School of Medicine, Stony Brook, NY.
Aaron T. Wild, Department of Radiation Oncology, Memorial Sloan Kettering Cancer Center, NYC, NY.
Margaret Hannum, Department of Epidemiology and Biostatistics, Memorial Sloan Kettering Cancer Center, NYC, NY.
Zhigang Zhang, Department of Epidemiology and Biostatistics, Memorial Sloan Kettering Cancer Center, NYC, NY.
Charles B. Simone, II, Department of Radiation Oncology, University of Maryland, School of Medicine.
Daphna Gelblum, Department of Radiation Oncology, Memorial Sloan Kettering Cancer Center, NYC, NY.
Abraham J. Wu, Department of Radiation Oncology, Memorial Sloan Kettering Cancer Center, NYC, NY.
Andreas Rimner, Department of Radiation Oncology, Memorial Sloan Kettering Cancer Center, NYC, NY.
Annemarie F. Shepherd, Department of Radiation Oncology, Memorial Sloan Kettering Cancer Center, NYC, NY.
References:
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018. September 12. [DOI] [PubMed] [Google Scholar]
- 2.O’Rourke N, Roque IFM, Farre Bernado N, et al. Concurrent chemoradiotherapy in non-small cell lung cancer. The Cochrane database of systematic reviews. 2010. June 16(6):Cd002140 Epub 2010/06/18. eng. [DOI] [PubMed] [Google Scholar]
- 3.Curran WJ Jr., Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011. October 5;103(19):1452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dillman RO, Herndon J, Seagren SL, et al. Improved survival in stage III non-small-cell lung cancer: seven-year follow-up of cancer and leukemia group B (CALGB) 8433 trial. J Natl Cancer Inst. 1996. September 04;88(17):1210–5. [DOI] [PubMed] [Google Scholar]
- 5.Bayman N, Alam N, Faivre-Finn C. Radiotherapy for lung cancer in the elderly. Lung cancer (Amsterdam, Netherlands). 2010. May;68(2):129–36. Epub 2010/01/19. eng. [DOI] [PubMed] [Google Scholar]
- 6.Amini A, Lin SH, Wei C, et al. Accelerated hypofractionated radiation therapy compared to conventionally fractionated radiation therapy for the treatment of inoperable non-small cell lung cancer. Radiat Oncol. 2012. March 15;7:33 Epub 2012/03/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson DH, Einhorn LH, Bartolucci A, et al. Thoracic radiotherapy does not prolong survival in patients with locally advanced, unresectable non-small cell lung cancer. Annals of internal medicine. 1990. July 1;113(1):33–8. Epub 1990/07/01. eng. [DOI] [PubMed] [Google Scholar]
- 8.Sonnick MA, Oro F, Yan B, et al. Identifying the Optimal Radiation Dose in Locally Advanced Non-Small-cell Lung Cancer Treated With Definitive Radiotherapy Without Concurrent Chemotherapy. Clin Lung Cancer. 2018. January;19(1):e131–e40. Epub 2017/08/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osti MF, Agolli L, Valeriani M, et al. Image guided hypofractionated 3-dimensional radiation therapy in patients with inoperable advanced stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013. March 1;85(3):e157–63. Epub 2012/11/28. eng. [DOI] [PubMed] [Google Scholar]
- 10.Ray KJ, Sibson NR, Kiltie AE. Treatment of Breast and Prostate Cancer by Hypofractionated Radiotherapy: Potential Risks and Benefits. Clinical oncology (Royal College of Radiologists (Great Britain)). 2015. July;27(7):420–6. Epub 2015/03/11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. The New England journal of medicine. 2010. February 11;362(6):513–20. Epub 2010/02/12. eng. [DOI] [PubMed] [Google Scholar]
- 12.Datta NR, Stutz E, Rogers S, et al. Conventional Versus Hypofractionated Radiation Therapy for Localized or Locally Advanced Prostate Cancer: A Systematic Review and Meta-analysis along with Therapeutic Implications. Int J Radiat Oncol Biol Phys. 2017. November 1;99(3):573–89. Epub 2017/12/28. eng. [DOI] [PubMed] [Google Scholar]
- 13.Hegemann NS, Guckenberger M, Belka C, et al. Hypofractionated radiotherapy for prostate cancer. Radiat Oncol. 2014. December 6;9:275 Epub 2014/12/07. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung P, Faria S, Ahmed S, et al. Phase II study of accelerated hypofractionated three-dimensional conformal radiotherapy for stage T1–3 N0 M0 non-small cell lung cancer: NCIC CTG BR.25. J Natl Cancer Inst. 2014. August;106(8). Epub 2014/07/31. eng. [DOI] [PubMed] [Google Scholar]
- 15.Videtic GMM, Donington J, Giuliani M, et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive Summary of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol. 2017. Sep-Oct;7(5):295–301. [DOI] [PubMed] [Google Scholar]
- 16.Prewett SL, Aslam S, Williams MV, et al. The management of lung cancer: a UK survey of oncologists. Clinical oncology (Royal College of Radiologists (Great Britain)). 2012. August;24(6):402–9. Epub 2012/04/21. eng. [DOI] [PubMed] [Google Scholar]
- 17.Urbanic JJ, Wang X, Bogart JA, et al. Phase 1 Study of Accelerated Hypofractionated Radiation Therapy With Concurrent Chemotherapy for Stage III Non-Small Cell Lung Cancer: CALGB 31102 (Alliance). Int J Radiat Oncol Biol Phys. 2018. May 1;101(1):177–85. Epub 2018/03/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris JP, Chang-Halpenny CN, Maxim PG, et al. Outcomes of Modestly Hypofractionated Radiation for Lung Tumors: Pre- and Mid-Treatment Positron Emission Tomography-Computed Tomography Metrics as Prognostic Factors. Clin Lung Cancer. 2015. November;16(6):475–85. Epub 2015/03/17. eng. [DOI] [PubMed] [Google Scholar]
- 19.Pollom EL, Qian Y, Durkee BY, et al. Hypofractionated Intensity-Modulated Radiotherapy for Patients With Non-Small-Cell Lung Cancer. Clin Lung Cancer. 2016. November;17(6):588–94. Epub 2016/10/23. eng. [DOI] [PubMed] [Google Scholar]
- 20.Westover KD, Loo BW Jr., Gerber DE, et al. Precision Hypofractionated Radiation Therapy in Poor Performing Patients With Non-Small Cell Lung Cancer: Phase 1 Dose Escalation Trial. Int J Radiat Oncol Biol Phys. 2015. September 1;93(1):72–81. [DOI] [PubMed] [Google Scholar]
- 21.Falkson CB, Vella ET, Yu E, et al. Radiotherapy With Curative Intent in Patients With Early-stage, Medically Inoperable, Non-Small-cell Lung Cancer: A Systematic Review. Clin Lung Cancer. 2017. March;18(2):105–21 e5. [DOI] [PubMed] [Google Scholar]
- 22.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011. May;46(3):399–424. Epub 2011/08/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaster TS, Yaremko B, Palma DA, et al. Radical-intent hypofractionated radiotherapy for locally advanced non-small-cell lung cancer: a systematic review of the literature. Clin Lung Cancer. 2015. March;16(2):71–9. [DOI] [PubMed] [Google Scholar]
- 24.Parisi G, Mazzola R, Ciammella P, et al. Hypofractionated radiation therapy in the management of locally advanced NSCLC: a narrative review of the literature on behalf of the Italian Association of Radiation Oncology (AIRO)-Lung Working Group. La Radiologia medica. 2018. October 27 Epub 2018/10/29. eng. [DOI] [PubMed] [Google Scholar]
- 25.Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. The New England journal of medicine. 2018. September 25. [DOI] [PubMed] [Google Scholar]


