Abstract
Esophageal squamous cell carcinoma (ESCC) is the most common type of esophageal cancer worldwide, with a high mortality due to advanced stage at diagnosis. Although most common in an area known as the Asian Esophageal Cancer Belt, which extends from the Caspian Sea to northern China, and in parts of Africa, high-risk populations also exist elsewhere in the world. Screening for ESCC has been practiced in a few geographic areas and high-risk populations, with varying levels of success. Esophageal squamous dysplasia is recognized as the precursor lesion for ESCC. Endoscopic screening for ESCC/esophageal squamous dysplasia is expensive and not sufficiently available in many high-risk regions. Recent advances in non-endoscopic screening enhanced by biomarker-based disease detection have raised the prospect of improved accuracy and availability of screening for esophageal squamous dysplasia and early stage ESCC. Development of a cost-effective, accurate, and well-tolerated screening test, if applied in endemic areas and high-risk populations, has the potential to reduce mortality from this deadly disease worldwide. In this review, we summarize recent developments in endoscopic and non-endoscopic screening modalities.
Introduction:
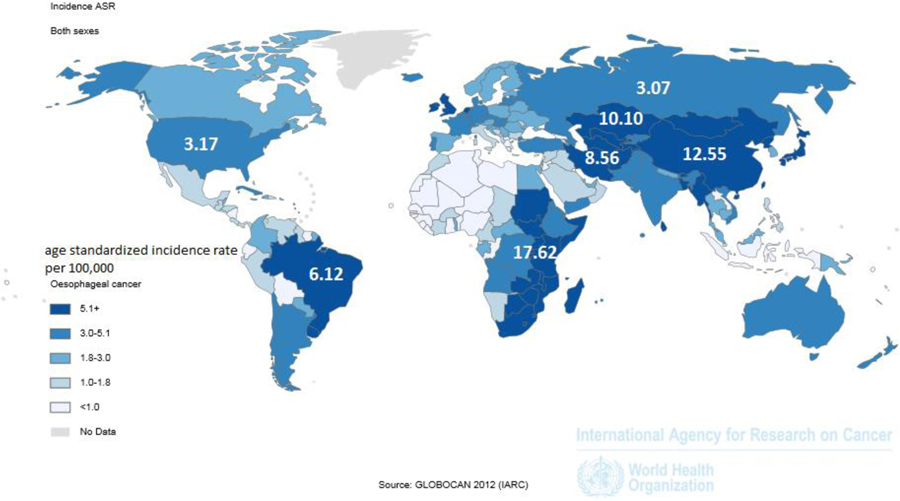
Esophageal cancer ranks as the 6th most common cause of cancer death worldwide, with 456,000 new cases and 400,000 deaths annually1, 2. Nearly 90% of esophageal cancer cases worldwide are esophageal squamous cell carcinoma (ESCC)2–4. The highest risk areas for ESCC are found in two geographic belts, the Asian Esophageal Cancer Belt across central Asia, from the Caspian Sea to northern China, and a belt on the eastern coast of Africa, from Ethiopia to South Africa (Figure 1).
Figure 1:
The Asian and African esophageal cancer belts (in dark blue), where over 90% of cases are esophageal squamous cell carcinoma.
(Reprinted with permission from Ferlay J SI, et al. GLOBOCAN 2012 v1.0, [Internet]. Lyon, France: International Agency for Research on Cancer, 2013.)
Unfortunately, most patients with ESCC present with advanced disease: 5-year survival rates are less than 20% in developed countries and less than 5% in many developing countries where most of the disease occurs3, 5, 6. Additionally, the outcomes of patients diagnosed after the onset of symptoms have not changed in the last several decades despite advances in therapy5. Early stage carcinoma, however, is associated with a substantially improved survival of 80–90% at 5 years when treated endoscopically or surgically3, 7. Therefore, early detection strategies are needed to improve outcomes of patients with ESCC.
Analogous to the association of Barrett’s esophagus (BE) and its related dysplasia to esophageal adenocarcinoma (EAC), esophageal squamous dysplasia has been shown to be a precursor to ESCC based on natural history studies conducted in China8, 9. These studies have shown that the risk of progression to cancer increases depending on the degree of dysplasia8. This has led to endoscopic treatment of high grade dysplasia (HGD). Given the considerable time needed in the transformation from dysplastic epithelium to malignancy10, a window exists in which detection and treatment of esophageal squamous dysplasia can reduce ESCC incidence.
In this review, we summarize and evaluate both conventional and emerging modalities to screen for this lethal disease and its precursor.
Rationale and Outcomes of Screening and Surveillance Programs
The main rationale for ESCC screening is to detect asymptomatic esophageal squamous dysplasia and early ESCC, to enable curative treatment. Several studies have demonstrated increases in early detection, mortality reduction, and cost effectiveness by endoscopic screening programs in high-risk populations.
In endemic areas in China, a community assignment trial with 10 years of follow up demonstrated a 33% reduction in the cumulative ESCC related mortality in intervention communities where 40–69 year-old adults were screened once by Lugol’s chromoendoscopy, compared to communities without screening.11 Similar results were found in other large retrospective studies of high-risk Chinese populations.12, 13 Modeling studies have shown that population-based screening of high risk populations in China can be cost effective.14, 15
In non-endemic areas, evidence supports endoscopic screening in patients with a history of head-and-neck cancers. In a case-control study, Su et al. found a higher prevalence of secondary ESCC in head-and-neck cancer patients receiving routine endoscopies compared to the non-screening group ( 4.5% vs. 3.0%, p=0.04), with earlier stage at diagnosis (p=0.03).16 Screening and early diagnosis correlated with improved survival, as Murakami et al. reported significantly superior 5-year survival in patients whose secondary ESCC was diagnosed on endoscopic screening versus symptomatically (60.4% versus 0.0%, p <0.01).17
For another high-risk population-tylosis, limited data from a small case series has shown that annual screening endoscopy detects early ESCC, with improved outcomes compared to those diagnosed after onset of symptoms.18
Given these findings, screening programs have been proposed for high-risk populations around the world. However, effective screening programs must be accurate, safe, cost-effective, and facilitate curative therapeutic intervention.19 With the advent of endoscopic treatment of esophageal squamous dysplasia and early stage ESCC, screening programs have gained additional impetus.
Current recommendations on ESCC Screening (Table 1)
Table 1:
Recommended Screening Guidelines for High Risk Populations
| Risk factor | Screening method and duration | Outcome | Level of evidence^20 |
|---|---|---|---|
| Head and neck cancer | Endoscopy with Lugol’s or NBI every 6 months to 1 year after completion of therapy for HNSCC, for 10 years | • Detects earlier stage disease • Improved survival • No evidence for cost-effectiveness, |
II-III (moderate) |
| Tylosis | 4 quadrant biopsies from proximal, middle, and distal esophagus starting at age 30; repeat every 1–3 years | • Effective for early diagnosis • Only beneficial for Type A (late onset) Tylosis |
III-IV (low) |
| Achalasia | Yearly EGD 10–15 years after disease onset +/− Lugol’s solution | • No evidence for cost effectiveness • Need to screen many patients to detect one cancer |
III (low) |
| Asian or African high-risk populations | One time Lugol’s chromoendoscopy beginning at the age of 40 | • Screened groups have lower ESCC incidence and mortality rates | II-III (moderate) |
| History of caustic esophageal injury | Endoscopy every 2–3 years 10–20 years following the injury | • No evidence for effectiveness | IV (low) |
Levels of evidence: Level I evidence: presence of at least one prospective, randomized, controlled trial, level II evidence: well-designed cohort or case-controlled studies; level III evidence: case series or flawed clinical trials; level IV evidence: opinions of respected authorities or expert committees; level V evidence: insufficient evidence to form any opinions
Given the low burden of disease in the general population of the United States, guidelines from the American Gastroenterology Association do not advocate population based screening for ESCC.20 However, screening may be worthwhile in endemic areas for individuals over a certain age, and screening may also be indicated in certain high-risk groups such as patients with head-and-neck cancers, tylosis, or history of caustic ingestions. Although smoking and alcohol abuse have a dose-dependent association with ESCC, screening for ESCC is not routinely recommended unless other risk factors are present. A recently published risk-prediction model from an endemic population in China incorporating more than 10 risk variables was able to predict severe squamous dysplasia with an AUC between 0.62–0.85, with age being the single most significant risk factor.21 Further refinement of similar risk-prediction models is crucial to identify the target individuals for screening in most populations. Currently proposed screening guidelines are summarized in Table 1.
Technologies currently utilized and in development for ESCC/Squamous Dysplasia screening
Endoscopic screening methods (Table 2)
Table 2:
Pros and Cons of Various Endoscopic Screening Methods
| Endoscopic screening modalities | Pros | Cons | PIVI criteria for Squamous Dysplasia Reached? |
|---|---|---|---|
| All modalities | •Visualizes the mucosa | • Uncomfortable if not done with sedation (which is frequently not available in resource poor areas) • Requires operator training and experience • Expensive equipment |
• |
| Conventional white light endoscopy | • Readily available in developed areas | • Lacks sensitivity for precursor lesions | • No |
| Chromoendoscopy | • Inexpensive • Improves sensitivity for precursor lesions/dysplasia • Short learning curve • Clarity of lesion borders |
• Irritant/allergic reactions to iodine • Lower specificity for precursor lesions (before biopsy diagnosis) |
• Yes |
| Endoscopy with Narrow Band Imaging | • Improves sensitivity and specificity for precursor lesions/dysplasia • Does not require iodine |
• Increased cost of equipment • Longer learning curve • Requires more operator expertise |
• Yes |
| Transnasal endoscopy | • No need for sedation • Improves cost effectiveness |
• Currently not standard of care, not widely available • Lack of trained operators • Cannot be combined with Lugol’s or NBI to detect precursor lesions. • Not yet tested on precursor lesions • Smaller biopsies • No therapeutic capabilities • Increased risk for patients with ENT pathology |
• Yes (only if combined with FICE) |
| Endocytoscopy | • Cellular level resolution may obviate need for some or all biopsies | • A contact probe technology • Small field of view • Can only be used with other lesion localization technologies (Lugol’s or NBI) • Increased cost of equipment • Additional training needed • Not yet tested on precursor lesions |
• Yes |
| Microendoscopy | • Inexpensive and portable • Cellular level resolution may obviate need for some or all biopsies • Can be portable and added to standard endoscope • High sensitivity and specificity for dysplasia and early stage cancer. |
• A contact probe technology • Small field of view • Can only be used with other lesion localization technologies (Lugol’s or NBI) • Additional training needed • Variable cost-effectiveness when compared to standard endoscopic screening techniques |
• Yes |
Traditionally, endoscopy has been the modality of choice for ESCC screening. However, endoscopy encompasses a heterogeneous spectrum of technology: their applications in ESCC screening have been summarized in Table 2.
Lugol’s Chromoendoscopy
Although conventional white light endoscopy (WLE) can reliably detect invasive cancer of the esophagus, it lacks sensitivity for detecting esophageal squamous dysplasia, the precursor to ESCC and the main target of screening, which often appears similar to normal squamous mucosa.22, 23 While high-resolution WLE has been used in the detection of dysplasia in colonic polyps and Barrett’s esophagus, its role has not been formally evaluated in the detection of squamous dysplasia. Rather, Lugol’s chromoendoscopy is the current standard to highlight areas of abnormality, significantly increasing the ability to detect esophageal squamous dysplasia.24–27
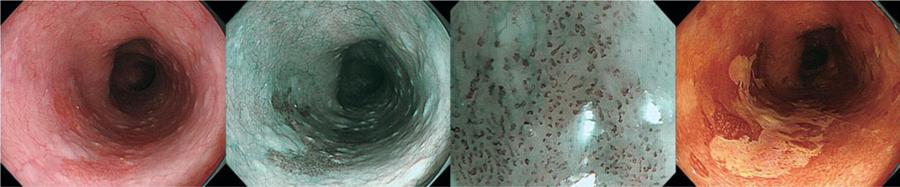
In Lugol’s chromoendoscopy, iodine binds reversibly to glycogen, which is less abundant in immature and rapidly dividing cells in the squamous epithelium, such as those found in esophageal squamous dysplasia or esophagitis. Dysplastic and inflammatory esophageal mucosa remain unstained compared to normal epithelium, allowing for targeted biopsies.28 Figure 3 shows a dysplastic lesion on WLE, NBI, and Lugol’s chromoendoscopy.
Figure 3:
Endoscopic images of the same lesion of esophageal squamous dysplasia, with WLE, NBI, Magnifying NBI, and Lugol’s chromoendoscopy.
a. | Endoscopic finding with conventional white light imaging. A flat reddish lesion was seen in the upper thoracic esophagus (between the arrows). At the edge of the lesion, the vascular pattern in the surrounding mucosa was interrupted.
b. | Endoscopic finding with narrow band imaging of the lesion from panel a. The lesion was contrasted as darker area (arrow) and the boundary of the lesion is clearer than in white light images.
c. | Endoscopic finding with magnifying narrow-band imaging. Dilated irregular microvascular pattern was clearly visualized in the lesion. A distinct demarcation line between the background mucosa and the lesion is visible (arrow). Note the color of the epithelium in the lesion is browner than the surrounding mucosa. Accordingly, endoscopic diagnosis of intramucosal squamous cell carcinoma or high-grade dysplasia can be made with high confidence.
d. | Endoscopic finding using Lugol’s chromoendoscopy for the lesion depicted in panels a and b. The lesion is clearly visualized as a lighter (yellow) unstained area within the brown stained area. The boundary of the lesion can be clearly delineated with chromoendoscopy. (Reprinted with permission from Veitch AM, et al. Nat Rev Gastroenterol & Hepatol. 2015)
In a study of patients with head and neck cancer undergoing WLE followed by Lugol’s, the detection of advanced cancer was similar between WLE and Lugol’s, but WLE was only able to detect 55% of esophageal squamous dysplasia identified with chromoendoscopy.25 Chromoendoscopy has been found to have a sensitivity ranging from 92%–100% and specificity of 37%–82% for detecting esophageal squamous dysplasia.27, 29–32 The main reason for the wide range in specificity is that esophagitis can appear as an unstained lesion, but this is readily discernable on histology.
Lugol’s chromoendoscopy is inexpensive and relatively easy to perform and interpret. Lugol’s solution contains iodine, potassium iodide and water; it can be made on site in developing countries. Therefore, Lugol’s chromoendoscopy is currently the standard of care for ESCC screening. Adverse effects of Lugol’s solution are infrequent but include irritant esophagitis/gastritis, hypersensitivity reactions, aspiration pneumonia, and thyrotoxicosis in patients with endemic goiters.33, 34 Furthermore, the interpretation of abnormal findings depends on the clinician administering the test, and interobserver variability has not been adequately described for ESCC screening.
Optical enhancement techniques: Narrow Band Imaging
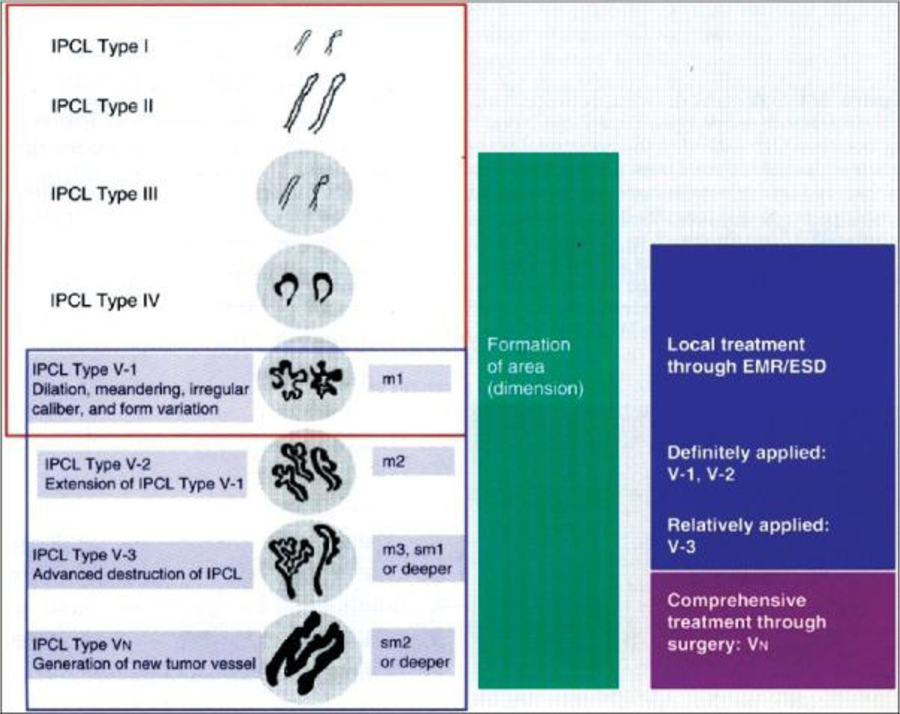
By utilizing specific “narrow band” wavelengths (415 nm and 540 nm) that match the absorption peaks of hemoglobin, narrow band imaging (NBI) allows for clearer depiction of capillary networks and superficial mucosal patterns.35 esophageal squamous dysplasia or early ESCC is characterized by a “brownish” appearance with abnormal vascular patterns of intracapillary papillary loops (IPCLs) (Figure 2). Detailed classifications of these abnormalities have been proposed and validated in the identification and staging of dysplasia and early carcinoma.36
Figure 2:
Original intrapapillary capillary loop (IPCL) pattern classification.
The IPCL pattern classification includes two sets of diagnostic criteria. IPCL pattern classification from IPCL type I to type V-1 is used for the tissue characterization of flat lesions (red outline). IPCL pattern classification from IPCL type V-1 to type VN reflects cancer infiltration depth (blue outline). IPCL type III corresponds to borderline lesions which potentially include esophagitis or low-grade intraepithelial neoplasia. IPCL type III should be considered for endoscopic follow up. In IPCL type IV, high-grade intraepithelial neoplasia appears, and then further treatment with endoscopic mucosal resection (EMR) / endoscopic submucosal dissection is recommended. EMR/esophageal squamous dysplasia for IPCL types V-1 and V-2 should be also considered as they are definite M1 or M2 lesion with no risk of lymph node metastasis. IPCL type V3 corresponds to an M3 lesion and diagnostic EMR/esophageal squamous dysplasia should be applied as a “complete biopsy” to decide on a final treatment strategy. IPCL type VN corresponds to a “new tumor vessel” often associated with sm2 invasion with significantly increased risk of lymph node metastasis. Surgical treatment should be recommended.
(Reprinted with permission from Inoue H, et al. Ann Gastroenterol. 2015 Jan-Mar; 28(1): 41–48.)
In a prospective study with 202 subjects with high-risk factors for ESCC, where each subject sequentially underwent WLE, NBI, and chromoendoscopy, Nagami, et al., found that the accuracy, sensitivity, and specificity of NBI for detecting invasive ESCC and high grade intraepithelial neoplasia were 77.0, 88.3, and 75.2 %, respectively; compared to chromoendoscopy with 68.0, 94.2, and 64.0%, respectively.31 NBI was not statistically inferior to chromoendoscopy in sensitivity (p=0.67); but significantly superior in specificity and overall accuracy (p=0.01).31 A recent meta-analysis of 18 studies including more than 1,900 patients also found that NBI had a significantly improved specificity compared to chromoendoscopy, with no significant difference in sensitivity.32
Fujifilm Blue Laser Imaging (BLI) (LASEREO; Fujifilm Co., Tokyo, Japan) utilizes two lasers in addition to white light to sharpen image quality.37 A small retrospective study showed that endoscopists preferred BLI for detection of ESCC38 but no objective evidence exists in its application as a screening tool for ESCC. Furthermore, the interobserver variability of narrow band imaging for esophageal pathology is not clear, but promising results have been reported for upper aerodigestive tract lesions.39
Post Processing Imaging technology (i-SCAN, FICE)
Several software driven advances have allowed for increased mucosal sharpness, clarity, and image processing in real-time. The i-SCAN (Pentax, Tokyo, Japan) technology utilizes surface, contrast, and tone enhancement.40 FICE (Fuji Intelligent Chromoendoscopy) (Fujinon, Fujifilm Medical Co, [Saitama, Japan]) enhances image quality through the use of spectral estimation technology.41
Using Lugol’s and FICE, Li et al 44 examined 257 patients with suspicious esophageal lesions (27 ESCC, 22 HGIN, 40 LGIN, and 12 normal esophagus). Compared to Lugol’s, which detected ESCC, HGIN, LGIN with a sensitivity of 88.9%, 77.3%, and 77.5% respectively, FICE’s sensitivity was 92.6%, 86.4%, 87.5%, respectively. However, these improvements were not statistically significant (p=0.64).
When combined with transnasal endoscopy (TNE) in a study of 99 patients with history of head and neck cancer, FICE was found to be more sensitive than TNE with WLE alone for detection of superficial ESCC (100% vs. 25%, respectively), with comparable specificities (96.8% vs. 97.8%).42
Transnasal endoscopy
Ultrathin transnasal endoscopy (TNE) allows for visualization of the esophagus without sedation, abrogating the risks of sedation and reducing costs. Huang, et al., found TNE to be well-tolerated in over 98% of patients.43 Wang, et al., demonstrated a reasonable safety profile with only 2 of 441 patients undergoing TNE having complications treated conservatively.44 When combined with FICE, Arantes, et al., observed sensitivities and specificities of over 90% for detecting ESCC.45 Further study with this modality in the identification of esophageal squamous dysplasia is required, but without the risks and costs of sedation, TNE could become highly utilized in endemic areas.
Despite extensive data on patient acceptability, TNE is not widely used. This may reflect lack of physician training or experience, and perhaps lack of patient and physician preference for unsedated transnasal procedures.
Endocytoscopy
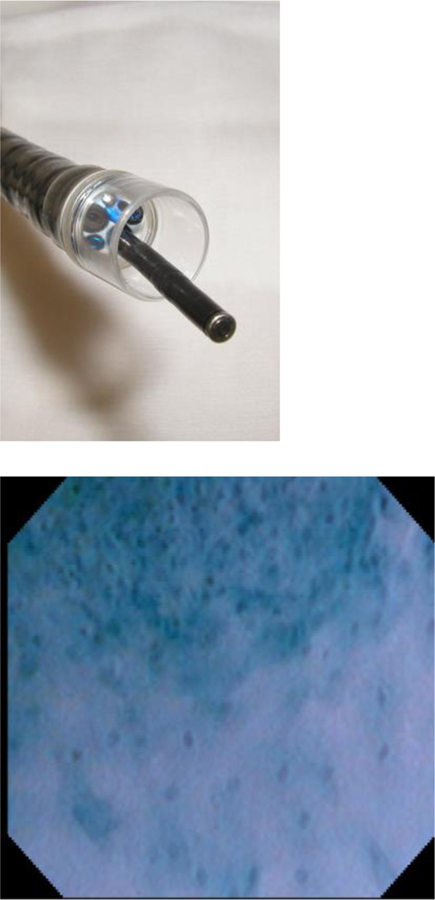
The ability to combine a microscope with a flexible endoscope was introduced in the early 2000s.46 Endocytoscopy can diagnose dysplastic lesions without biopsy, reducing the wait for pathology and the costs for another procedure. The endocytoscope (Olympus GIF-1T240, Olympus Medical Systems Corp. Tokyo, Japan) can be passed through the instrument channel of a standard endoscope, where a soft hood at the end of the endoscope allows for continuous contact with the mucosa (Figure 4).41 Methylene or toluene blue spraying is required for staining to enhance visualization of dysplastic cellular elements.
Figure 4:
Microendoscopic imaging of squamous dysplasia.
a. | Endocytoscope probe which can be passed through the instrument channel of an endoscope.
b. | The border between esophageal squamous cancer (upper) and normal squamous epithelium (lower). The density and nucleus:cytoplasm ratio is much higher in cancer compared to normal epithelium.
(Reprinted with permission from Kumagai Y, et al. Endoscopy 2004;36:590–594.)
An early study of 75 patients by Inoue, et al., found a diagnostic accuracy of 82% for distinguishing between malignant and non-malignant lesions of the esophagus.47 Kumagai, et al., found that the need for histologic biopsy could be obviated in up to 93.5% of cases.48 More recently, Kumagai reported a sensitivity and specificity of 100% and 80%, respectively, for the detection of ESCC using endocytoscopy.49 Further research in this field includes the application of higher magnification endoscopes, in which preliminary results appear promising.50, 51
However, while endocytoscopy may obviate the need for biopsies, this approach is more operator dependent, requiring experience to interpret the imaging and initiate appropriate treatment. No studies on ESCC screening have reported the interobserver variability of this technique. Furthermore, in order to highlight abnormalities, Lugol’s staining or NBI may be required, again increasing the costs. Further study is required to elucidate the role of endocystoscopy in the detection of esophageal squamous dysplasia.
High Resolution Microendoscopy
High resolution microendoscopy (HRME) utilizes a contact probe containing a fiber optic microendoscope to visualize the esophageal epithelium (Figure 5). Before insertion of the probe through the instrument channel of an endoscope, the mucosal area of interest (usually identified by Lugol’s or NBI) is sprayed with proflavine dye, which enables visualization of the superficial mucosal nuclei.52 Image analysis software is then used to interpret the images and identify dysplastic cellular features. This approach has been shown to be feasible for detection of ESCC, with sensitivities ranging from 84% to 93% and specificities ranging from 92% to 97%.53, 54
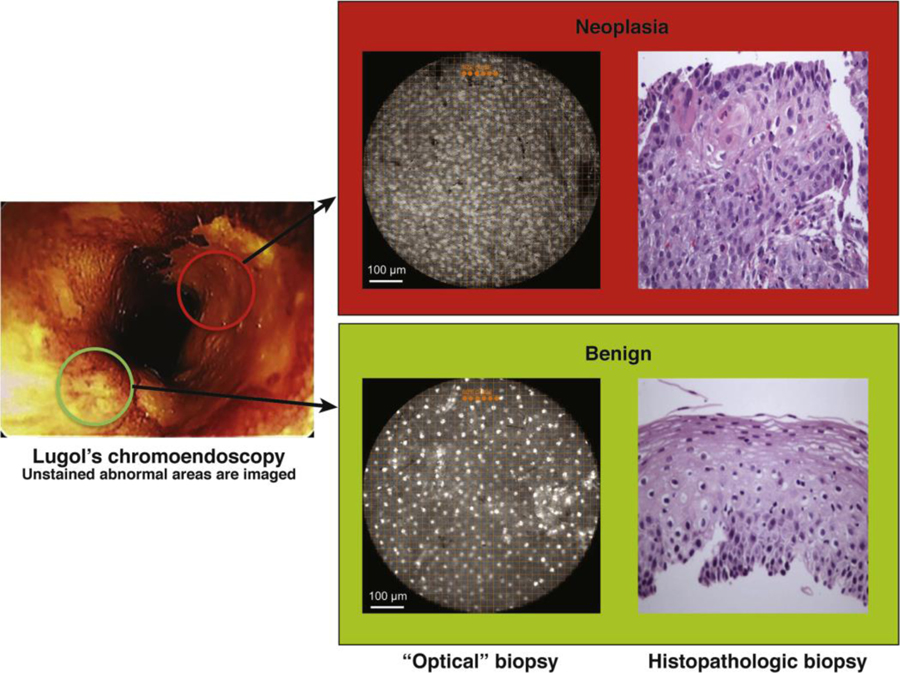
Figure 5:
High-resolution microendoscope92
Lugol’s unstained lesions (left) are imaged with HRME (“optical” biopsy) and compared with corresponding tissue biopsy (histopathologic biopsy) (original magnification, 100x). Only 1 of the 2 Lugol’s abnormal areas was neoplastic (upper panel) as determined by the imaging software, based on mean nuclear area, nuclear-to cytoplasmic ratio, nearest inter-nuclear distance, nuclear eccentricity, nuclear solidity, and the major axis of the ellipse best approximating each nucleus.
(Reprinted with permission from Protano et al. Gastroenterology 2015; 149: 321–329.)
HRME systems cost considerably less than other advanced screening platforms, making this modality more attractive for developing nations. A screening trial of 147 patients in the United States and China showed that the addition of HRME to Lugol’s significantly improved the specificity (88% vs 48%, p<0.001) and positive predictive value (45% vs 22%, p<0.0001) for detecting severe esophageal squamous dysplasia and ESCC, compared to Lugol’s alone. Fifty-five patients would have been spared from biopsy with HRME.51 Markov models incorporating both average and high risk populations in China have shown that HRME is more cost-effective compared to no screening, but results were mixed when compared to traditional screening methods.15
Image analysis advances that highlight abnormal nuclei or automatically calculate a score for the degree of dysplasia present based on an algorithm of microscopic features may improve accuracy by decreasing interobserver variation.55 Furthermore, portable tablet systems have now been developed, potentially allowing for the use of HRME in remote regions.56
Video Capsule Endoscopy
Video capsule endoscopy has emerged as a non-invasive method to image the gastrointestinal tract. Accordingly, there has been interest in using this as a screening method for ESCC. However, an initial study comparing the use of video capsule endoscopy to Lugol’s chromoendoscopy in a high-risk population of patients with head and neck cancer found poor sensitivity and specificity in detecting neoplastic lesions in the esophagus.57
Non-endoscopic Screening Methods
The community-assignment study by Wei et al shows that endoscopic screening with appropriate treatment of esophageal squamous dysplasia and early ESCC can reduce ESCC mortality in a very high-risk population in China.11 However, widespread endoscopic screening is not possible or cost-effective in most high-risk areas in Asia and Africa. Table 3 shows the esophageal cancer incidence rates in 2012 from high-quality population-based cancer registries in ESCC endemic areas along with proportions of esophageal squamous dysplasia and early ESCC diagnosed on screening endoscopies of asymptomatic adults in three of these sites. The Wei study was done in Cixian, China, where ESCC incidence was ~150cases/100,000 population/year, and found HGD in 11 of every 100 endoscopies.58 In such a site, primary endoscopic screening of the population may well be indicated and cost-effective. But in most high-risk populations, with ESCC incidence rates of ~20–40/100,000/year, only 1%–3% prevalence of HGD, and less developed health infrastructures, primary endoscopic screening of the population will not be possible or cost-effective. To reduce ESCC mortality in these populations, simple, inexpensive, and minimally-invasive tests need to be developed that are acceptable to unsedated asymptomatic adults and can specifically triage those with moderate to HGD to endoscopy for definitive diagnosis and treatment. Table 4 and Table 5 summarize the characteristics and current status of non-endoscopic screening methods for ESCC.
Table 3:
Esophageal Cancer Incidence and the Prevalence of High-Grade Dysplasia in Endoscopic Screening Studies from Selected Sites in Asia and Africa11, 28, 64, 93
| EC incidence | HGD Prevalence | ||
|---|---|---|---|
| Males | Females | ||
| Cixian, China | 193 | 109 | 11% |
| Yanting, China | 101 | 68 | |
| Golestan, Iran | 23 | 19 | 1% |
| Nairobi, Kenya | 21 | 15 | 3% |
| Blantyre, Malawi | 38 | 23 | |
| Eastern Cape Province, RSA | 32 | 20 | |
Table 4:
Non-endoscopic screening methods and characteristics
| Screening Method | Author (year) | Study Size (N) | Dysplasia and ESCC (N) | Assay method | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| Direct Sampling Techniques | ||||||
| Inflatable Balloon | Roth (1997)63 | 439 | 123 (28%) esophageal squamous dysplasia 16 (4%) ESCC |
Cytology only | 47% esophageal squamous dysplasia+ | 81% esophageal squamous dysplasia+ |
| Inflatable Balloon | Pan (2008) 62 | 725 | 232 (32%) esophageal squamous dysplasia + | Cytology only | 46% esophageal squamous dysplasia+ | 84% esophageal squamous dysplasia+ |
| Mechanical Balloon | Pan (2008)62 | 725 | 232 (32%) esophageal squamous dysplasia+ | Cytology only | 39% esophageal squamous dysplasia+ | 85% esophageal squamous dysplasia+ |
| Sponge | Roth (1997)63 | 439 | 123 (28%) esophageal squamous dysplasia 16 (4%) ESCC |
Cytology only | 24% esophageal squamous dysplasia+ | 92% esophageal squamous dysplasia+ |
| Inflatable Balloon | Adams (2008)58 | 147 | 72 esophageal squamous dysplasia (25 mild, 26 moderate, 21 severe) | Cytology + Panel of Methylated DNA markers* | 50% HGD+ | 28% HGD+ |
| Sponge | Roshandel (2014)64 | 301 | 18 esophageal squamous dysplasia, (4 with HGD) | Cytology + p53 | 22% esophageal squamous dysplasia+_ 100% HGD+ |
97% HGD+ |
| Breath Markers | ||||||
| Volatile Organic Compounds | Zou (2016)68 | 86 | 29 esophageal cancers (not specified) | 7 ions analyzed on a home-made breath mass spectrometer—PTR-MS apparatus (Ion Sniffer 2020Q). | 86.2% for Esophageal cancer | 89.5% for Esophageal cancer |
| Blood Markers | ||||||
| Autoantibodies | Zhou (2014)94 | 567 | 65 esophageal squamous dysplasia 88 ESCC |
6 marker panel^ | 64% (for ESCC) 38% for esophageal squamous dysplasia+ |
94% (for ESCC) |
| Autoantibodies | Xu (2014)71 | 513 | 237 ESCC | 6 marker panel~ | 45% for ESCC | 95% for ESCC |
| miRNA | Zhang 201095 | 430 | 290 ESCC | 7 miRNA panel# | 80% for ESCC | 90% for ESCC |
| Circulating tumor cells and mRNA | Cao (2009)96 | 182 | 108 ESCC | Survivin mRNA expressing circulating tumor cells | 47.2% for ESCC | 100% for ESCC |
AHRR, p16INK4a, MT1G, and CLDN3
P53, IMP1, P16, cyclin B1, P62, and C-myc
p53, NY-ESO-1, MMP-7, Hsp70, Prx VI, and BMI-1
miR-10a, miR-22, miR-100, miR-148b, miR-223, miR-133a, and miR-127–3p
Table 5:
Pros and Cons of Non-endoscopic Screening Methods
| Pros | Cons | |
|---|---|---|
| Esophageal mucosal sampling devices (brushes, balloons, sponges) | • Minimally invasive direct tissue sampling •Can be combined with any molecular test • Low operating cost • High specificity |
• Cytology-only yields low sensitivity • Devices are hard to swallow and may lead to poor compliance |
| Auto-antibodies (blood) | • Non-invasive (serum) • Stable assay |
• Low sensitivity |
| Circulating tumor cells (blood) | • Non-invasive • High specificity • Prognostic value |
• Low sensitivity • Not found in early disease |
| Circulating miRNA (blood | • Non-invasive (detected from plasma or serum) • Stable and consistently expressed • High sensitivity and specificity |
•Limited number of studies |
| Methylated DNA (cell sample or blood) | • Can be non-invasively detected in plasma • Stable and consistently expressed |
• Limited number of studies |
| Volatile Organic Compounds (breath) | • Non-invasive | • Limited number of studies |
Esophageal cytology samples
Non-endoscopic esophageal sampling devices have been studied extensively as inexpensive, tools for ESCC screening. The unsedated patient swallows a brush, a deflated balloon, or a sponge in a gelatin capsule attached to a cannula or string that is tethered outside the mouth. After it reaches the stomach, the balloon is inflated or the gelatin capsule dissolves and the sponge expands, and then the cell collection device is pulled up the esophagus and out, collecting superficial squamous cells from the esophagus. Traditionally, the recovered material is smeared on slides for Papanicolaou-stained cytologic examination. Figure 6 illustrates some of the common balloons and sponges used in China and Japan.
Figure 6:
Balloons and sponges used in prior studies for screening of esophageal squamous cell carcinoma and/or dysplasia.
(Reprinted with permission from Pan QJ, et al. Acta Cytol 2008;52:14–23.)
These methods are attractive as they can be performed without endoscopy or sedation, and can be applied at scale in limited resource settings.59–61 Unfortunately, the results have been less than optimal. A study of more than 700 patients comparing balloon cytology to endoscopic biopsy reported sensitivities close to 40%.62 Similarly, cytology obtained with sponges from a study of 439 Chinese patients also produced a disappointing sensitivity of 24% for esophageal squamous dysplasia and ESCC.63
Esophageal cytology specimens combined with biomarkers
Combining molecular biomarkers to esophageal cytology obtained non-endoscopically may improve the sensitivity of the test. In a study of 147 Chinese patients with esophageal biopsies ranging from normal to severe esophageal squamous dysplasia,89 a 4-marker panel of differentially methylated promoter regions in AHRR, p16INK4a, MT1G, and CLDN3, assayed from cells collected with an inflatable balloon, was able to detect HGD with a sensitivity and specificity of 50% and 68%, respectively.
In another study, 301 Iranian adults were examined by a swallowed sponge (Cytosponge, Medtronic, [Minneapolis, USA]), followed by Lugol’s chromoendoscopy. Combined analyses of cytology and p53 staining showed a sensitivity of 100% at 97% specificity for detecting four patients with HGD, although sensitivity was much lower for any grade of dysplasia (64% by morphology, 11% by p53).64 Despite the small number of cases, this study shows promise for the Cytosponge as a safe and feasible tool for diagnosing esophageal squamous dysplasia, and should be evaluated with other biomarkers in larger studies.
Breath markers
Volatile organic compounds
The measurement of volatile organic compounds (VOCs) from breath is completely non-invasive. Human breath is a complex biological sample containing more than 250 VOCs.65 The potential role of VOCs in detecting cancer was creatively demonstrated by Sonoda et al., who showed that canine olfaction of VOCs found in exhaled breath and stool samples was accurate in discriminating patients with colorectal cancer from normal controls (sensitivity 0.91 and specificity 0.99).66 Since this study, many breath mass spectrometry devices have been developed to replace the canine nose.67 Although no studies specific to ESCC have been conducted, a pilot study from China on 29 patients with esophageal cancer and 57 healthy relatives reported an AUC of 0.943 with 7 ions analyzed on a home-made breath mass spectrometer—Ion Sniffer 2020Q (Hefei, China).68 Since the study population was from China, where ESCC comprises over 90% of esophageal cancer cases, one could assume that most of the esophageal cancer cases were ESCC. However, these results should be interpreted with caution given the small sample size and lack of blinding.
Blood Markers
Autoantibodies
Auto-antibodies to tumor associated antigens (TAAs) have been proposed as biomarkers for cancer due to their stability in serum. The most comprehensively studied TAA is P53, in which a mutation results in a non-functioning protein that has a longer half-life than the native protein, and the subsequent anti-P53 can be detected non-invasively in serum. However, a meta-analysis of 15 studies reported a disappointing sensitivity range of 15%–60% at specificity 91–100% for anti-P53 as a single marker.69 A 6-marker panel of auto-antibodies in 237 late-stage ESCCs versus 134 normal did not do much better, with a sensitivity of 45% at a specificity of 95%.70 Evidence is even more limited for esophageal squamous dysplasia. Therefore, autoantibodies for screening purposes of esophageal cancer are still far from clinical application.
Circulating tumor cells
Circulating tumor cells (CTCs) assayed from blood have been investigated for their prognostic use in esophageal cancer. The presence of CTCs has been found to correlate strongly with nodal metastases, and poor progression-free survival in patients with esophageal cancer.71–73 Since the presence of CTCs portends advanced disease, CTCs would not be an appropriate screening test for early cancer.
Circulating miRNA
MicroRNAs (miRNAs) are small noncoding RNAs that bind to target messenger RNAs (mRNAs) resulting in RNA degradation and/or post-translational inhibition. MiRNAs are stable, abundantly expressed, and can be consistently detected in blood. In a meta-analysis of 27 studies, the overall sensitivity and specificity of miRNAs for detecting ESCC were 79.9% (95% CI: 76.2%–83.1%) and81.3% (95% CI: 75.7–85.9), respectively, with an AUC of 0.87 (95% CI: 0.84–0.90).74 However, only four of the 19 miRNAs were studied in more than one of the publications. Other meta-analyses were limited by small numbers of studies and lack of validation.75 Nevertheless, the concept of miRNAs does hold potential as a non-invasive screening tool for ESCC, and further studies are underway.76–78
Methylated DNA markers
Oncogenesis by way of modifications in DNA methylation occurs via two fundamental changes: (1) hypermethylation of CpG islands in gene promoters, which can silence tumor suppressor genes; and (2) hypomethylation of repetitive genetic elements, which may lead to genomic instability or oncogene activation.79 Methylated DNA markers (MDMs) have been shown to be broadly informative markers of neoplasia82 and are a critical component of a multi-target stool DNA test, FDA-approved for average risk colorectal cancer screening.83
MDMs have been utilized to develop minimally invasive diagnostic tools for BE and BE related dysplasia. The identification, validation and pilot testing of MDMs for BE diagnosis has been recently described. Following agnostic discovery by reduced representation bisulfite sampling (RRBS) and validation in independent biopsy and whole esophageal brushing cohorts84, the utility of these markers was confirmed in a pilot randomized trial. In 19 BE cases and 20 controls, esophageal cytology specimens obtained via a 25 mm capsule sponge device (Sponge On a String (SOS) (EsophaCap) (Capnostics, New Jersey, USA), coupled with a 2 marker MDM panel detected all 19 cases of BE (100% sensitivity at 100% specificity).85 In a subsequent ongoing validation study (SOS 2 trial) including 90 BE and 58 controls, a two-marker MDM panel assayed from SOS samples accurately distinguished BE cases from controls with an AUC of 0.97 (95% CI 0.94–0.99).86 A three marker panel of MDMs for the detection of BE related dysplasia has also been previously described.87 MDMs have also been applied to the Cytosponge as mentioned previously, with AUCs as high as 0.877 (95% CI 0.84–0.91) in a pilot study with 20 BE cases and 10 normal controls, and a validation cohort with 149 BE cases and 129 normal controls.88 In samples from non-endoscopic balloons from 86 individuals, tests of CCNA1 and VIM DNA methylation detected BE metaplasia with 90.3% sensitivity and 91.7% specificity in another recently published study.84
Along these same lines, MDMs for ESCC and squamous dysplasia, partnered with a device such as SOS, can lead to the development of a cost-effective, minimally-invasive, and highly accurate way to screen for this deadly disease. In a prior discovery study, we reported MDMs with high discrimination for the detection of ESCC.89 The 15 best-performing MDMs were then chosen for a multi-national validation study with tissue from 86 ESCC cases from the US, 114 from Iran, and 133 from China, with similar numbers of country-specific normal controls. Although the subjects came from three distinct populations with varying risks of ESCC, our candidate MDMs performed well at discriminating ESCC tissue from normal esophageal tissue across all sites. Using a marker panel of 2–4 MDMs, the AUCs were 0.997 (95% CI 0.99–1.0), 0.99 (95% CI 0.98–1.0), and 0.97 (95% CI 0.93–0.99), for US, Iran, and China respectively, corresponding to sensitivities and specificities >95%.90 These markers need to be tested on esophageal cytology specimens obtained via a capsule sponge sampling device. A report from China applied MDMs to esophageal balloon cytology samples from 147 patients with endoscopic biopsy diagnoses ranging from normal to severe squamous dysplasia in an endemic area, and a 4-marker panel (AHRR, p16INK4a, MT1G, and CLDN3) was able to detect severe squamous dysplasia with a sensitivity and specificity of 50% and 68%.58
Not only can ESCC-specific MDMs be detected in esophageal tissue, we have also demonstrated its feasibility as a plasma-based biomarker for esophageal cancer in a recent pilot study (ref DDW abstract).91 Using MDMs discovered through the aforementioned whole methylome discovery effort, 12 candidate MDMs were chosen for a Phase I study with 85 cases (76 EAC and 9 ESCC) and 98 controls. At a specificity of 91%, a five-marker panel assayed from plasma detected 74% of esophageal cancer overall (74% of EAC, and 78% of ESCC), with an overall AUC of 0.93 (95% CI 0.89–0.96). The test was more sensitive for higher-stage disease, but was still able to detect Stage I and Stage II disease at sensitivities of 56% and 83%, respectively. Although only 9 cases of ESCC were included in this study, this preliminary research has established a potential for using MDMs for detection of ESCC in an entirely non-invasive way. With ongoing larger-studies and optimized assays, active research in this area may transform the future of screening for ESCC especially in resource-poor endemic areas.
Future Directions
ESCC is a lethal disease largely due to its usually advanced stage at diagnosis. Screening programs targeting high-risk populations are needed to detect curable dysplasia and early stage ESCC. Critical advances have been made in high quality endoscopic screening modalities. However, the majority of patients afflicted by ESCC are found in resource-poor regions. For screening to occur successfully at a population level in these regions, minimally-invasive tools that are accurate and cost effective need to be developed.
The use of molecular biomarkers in esophageal cell samples acquired non-endoscopically (via a sampling device such as a sponge) or in blood holds great potential in the future for screening in populations at high risk for ESCC. However, the performance of such biomarkers is yet to be optimized, and results need to be validated in large studies. Additionally, further risk-stratification of individuals needs to be achieved in areas with lower incidence of ESCC and esophageal squamous dysplasia. Such risk-stratification models combined with a minimally-invasive test could transform screening and reduce mortality from ESCC.
Acknowledgments
Funding: None
Acronyms:
- ESCC
esophageal squamous cell carcinoma
- HGD
high grade dysplasia
- EAC
esophageal adenocarcinoma
- BE
Barrett’s esophagus
- NBI
narrow band imaging
- TNE
transnasal endoscopy
- WLE
white light endoscopy
- FICE
Fuji Intelligent Chromoendoscopy
- BLI
Blue Laser Imaging
- HRME
High Resolution Microendoscopy
- HGIN
High Grade Intraepithelial Neoplasia
- LGIN
Low Grade Intraepithelial Neoplasia
Footnotes
Conflicts of Interest:
Prasad G. Iyer: Research funding from Exact Sciences, C2 Therapeutics, Medtronic, Nine Point Medical
John B. Kisiel and David A. Ahlquist: Listed as co-inventors in an intellectual property development agreement with Exact Sciences and could receive future royalties.
References:
- 1.Ferlay JSI, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer, 2013. [Google Scholar]
- 2.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: A Cancer Journal for Clinicians 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 4.Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015;64:381–7. [DOI] [PubMed] [Google Scholar]
- 5.Torre LA, Siegel RL, Ward EM, et al. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev 2016;25:16–27. [DOI] [PubMed] [Google Scholar]
- 6.Aghcheli K, Marjani HA, Nasrollahzadeh D, et al. Prognostic factors for esophageal squamous cell carcinoma--a population-based study in Golestan Province, Iran, a high incidence area. PLoS One 2011;6:e22152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang GQ, Jiao GG, Chang FB, et al. Long-term results of operation for 420 patients with early squamous cell esophageal carcinoma discovered by screening. Ann Thorac Surg 2004;77:1740–4. [DOI] [PubMed] [Google Scholar]
- 8.Taylor PR, Abnet CC, Dawsey SM. Squamous dysplasia – the precursor lesion for esophageal squamous cell carcinoma. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2013;22:540–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawsey SMFDE Screening for Esophageal Squamous Cell Carcinoma and Its Precursor Lesions. Clinical Gastrointestinal Endoscopy. Second ed. St. Louis, Missouri: Saunders, 2012:385–399. [Google Scholar]
- 10.Wang J-W, Guan C-T, Wang L-L, et al. Natural History Analysis of 101 Severe Dysplasia and Esophageal Carcinoma Cases by Endoscopy. Gastroenterology Research and Practice 2017;2017:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei WQ, Chen ZF, He YT, et al. Long-Term Follow-Up of a Community Assignment, One-Time Endoscopic Screening Study of Esophageal Cancer in China. J Clin Oncol 2015;33:1951–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng X, Mao X, Xu K, et al. Massive Endoscopic Screening for Esophageal and Gastric Cancers in a High-Risk Area of China. PLoS One 2015;10:e0145097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Q, Yu L, Hao C, et al. Effectiveness evaluation of organized screening for esophageal cancer: a case-control study in Linzhou city, China. Scientific Reports 2016;6:35707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, Wei WQ, Niu J, et al. Cost-benefit analysis of esophageal cancer endoscopic screening in high-risk areas of China. World J Gastroenterol 2012;18:2493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinh P, Anaparthy R, Young PE, et al. Clinical outcomes in patients with a diagnosis of “indefinite for dysplasia” in Barrett’s esophagus: a multicenter cohort study. Endoscopy 2015;47:669–74. [DOI] [PubMed] [Google Scholar]
- 16.Su YY, Chen WC, Chuang HC, et al. Effect of routine esophageal screening in patients with head and neck cancer. JAMA Otolaryngology - Head and Neck Surgery 2013;139:350–354. [DOI] [PubMed] [Google Scholar]
- 17.Murakami S, Hashimoto T, Noguchi T, et al. The utility of endoscopic screening for patients with esophageal or head and neck cancer. Diseases of the Esophagus 1999;12:186–190. [DOI] [PubMed] [Google Scholar]
- 18.Risk JM, Mills HS, Garde J, et al. The tylosis esophageal cancer (TOC) locus: more than just a familial cancer gene. Diseases of the Esophagus 1999;12:173–176. [DOI] [PubMed] [Google Scholar]
- 19.Obuchowski NA, Graham RJ, Baker ME, et al. Ten criteria for effective screening: their application to multislice CT screening for pulmonary and colorectal cancers. AJR Am J Roentgenol 2001;176:1357–62. [DOI] [PubMed] [Google Scholar]
- 20.Wang KK, Wongkeesong M, Buttar NS. American gastroenterological association technical review on the role of the gastroenterologist in the management of esophageal carcinoma. Gastroenterology 2005;128:1471–1505. [DOI] [PubMed] [Google Scholar]
- 21.Liu M, Liu Z, Cai H, et al. A Model To Identify Individuals at High Risk for Esophageal Squamous Cell Carcinoma and Precancerous Lesions in Regions of High Prevalence in China. Clin Gastroenterol Hepatol 2017;15:1538–1546.e7. [DOI] [PubMed] [Google Scholar]
- 22.Petit T, Georges C, Jung GM, et al. Systematic esophageal endoscopy screening in patients previously treated for head and neck squamous-cell carcinoma. Ann Oncol 2001;12:643–6. [DOI] [PubMed] [Google Scholar]
- 23.Roshandel G, Nourouzi A, Pourshams A, et al. Endoscopic screening for esophageal squamous cell carcinoma. Arch Iran Med 2013;16:351–7. [PubMed] [Google Scholar]
- 24.Muto M, Hironaka S, Nakane M, et al. Association of multiple Lugol-voiding lesions with synchronous and metachronous esophageal squamous cell carcinoma in patients with head and neck cancer. Gastrointest Endosc 2002;56:517–21. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto CL, Iriya K, Baba ER, et al. Lugol’s Dye Spray Chromoendoscopy Establishes Early Diagnosis of Esophageal Cancer in Patients with Primary Head and Neck Cancer. American Journal of Gastroenterology 2005;100:275–282. [DOI] [PubMed] [Google Scholar]
- 26.Sugimachi K, Kitamura K, Baba K, et al. Endoscopic diagnosis of early carcinoma of the esophagus using Lugol’s solution. Gastrointestinal Endoscopy 1992;38:657–661. [DOI] [PubMed] [Google Scholar]
- 27.Dawsey SM, Fleischer DE, Wang GQ, et al. Mucosal iodine staining improves endoscopic visualization of squamous dysplasia and squamous cell carcinoma of the esophagus in Linxian, China. Cancer 1998;83:220–31. [PubMed] [Google Scholar]
- 28.Mwachiro MM, Burgert SL, Lando J, et al. Esophageal Squamous Dysplasia is Common in Asymptomatic Kenyans: A Prospective, Community-Based, Cross-Sectional Study. The American Journal Of Gastroenterology 2016;111:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carvalho R, Areia M, Brito D, et al. Diagnostic accuracy of lugol chromoendoscopy in the oesophagus in patients with head and neck cancer. Revista Espanola de Enfermedades Digestivas 2013;105:79–83. [DOI] [PubMed] [Google Scholar]
- 30.Dubuc J, Legoux JL, Winnock M, et al. Endoscopic screening for esophageal squamous-cell carcinoma in high-risk patients: a prospective study conducted in 62 french endoscopy centers. Endoscopy 2006;38:690–695. [DOI] [PubMed] [Google Scholar]
- 31.Nagami Y, Tominaga K, Machida H, et al. Usefulness of Non-Magnifying Narrow-Band Imaging in Screening of Early Esophageal Squamous Cell Carcinoma: A Prospective Comparative Study Using Propensity Score Matching. American Journal of Gastroenterology 2014;109:845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morita FHA, Bernardo WM, Ide E, et al. Narrow band imaging versus lugol chromoendoscopy to diagnose squamous cell carcinoma of the esophagus: a systematic review and meta-analysis. BMC Cancer 2017;17:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thuler FPBM, de Paulo GA, Ferrari AP. Chemical esophagitis after chromoendoscopy with Lugol’s solution for esophageal cancer: case report. Gastrointestinal Endoscopy 2004;59:925–926. [DOI] [PubMed] [Google Scholar]
- 34.Leustean L, Preda C, Ungureanu MC, et al. Jod-Basedow effect due to prolonged use of lugol solution-case report. Rev Med Chir Soc Med Nat Iasi 2014;118:1013–7. [PubMed] [Google Scholar]
- 35.Gono K Narrow Band Imaging: Technology Basis and Research and Development History. Clinical Endoscopy 2015;48:476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inoue H, Kaga M, Ikeda H, et al. Magnification endoscopy in esophageal squamous cell carcinoma: a review of the intrapapillary capillary loop classification. Annals of Gastroenterology : Quarterly Publication of the Hellenic Society of Gastroenterology 2015;28:41–48. [PMC free article] [PubMed] [Google Scholar]
- 37.Kaneko K, Oono Y, Yano T, et al. Effect of novel bright image enhanced endoscopy using blue laser imaging (BLI). Endoscopy International Open 2014;2:E212–E219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomie A, Dohi O, Yagi N, et al. Blue Laser Imaging-Bright Improves Endoscopic Recognition of Superficial Esophageal Squamous Cell Carcinoma. Gastroenterol Res Pract 2016;2016:6140854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zwakenberg MA, Dikkers FG, Wedman J, et al. Narrow band imaging improves observer reliability in evaluation of upper aerodigestive tract lesions. Laryngoscope 2016;126:2276–81. [DOI] [PubMed] [Google Scholar]
- 40.Hancock S, Bowman E, Prabakaran J, et al. Use of i-scan Endoscopic Image Enhancement Technology in Clinical Practice to Assist in Diagnostic and Therapeutic Endoscopy: A Case Series and Review of the Literature. Diagn Ther Endosc 2012;2012:193570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krystallis C, Koulaouzidis A, Douglas S, et al. Chromoendoscopy in small bowel capsule endoscopy: Blue mode or Fuji Intelligent Colour Enhancement? Dig Liver Dis 2011;43:953–7. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka T, Niwa Y, Tajika M, et al. Prospective evaluation of a transnasal endoscopy utilizing flexible spectral imaging color enhancement (FICE) with the Valsalva maneuver for detecting pharyngeal and esophageal cancer. Hepato-gastroenterology 2014;61:1627–1634. [PubMed] [Google Scholar]
- 43.Huang Y-C, Lee Y-C, Tseng P-H, et al. Regular screening of esophageal cancer for 248 newly diagnosed hypopharyngeal squamous cell carcinoma by unsedated transnasal esophagogastroduodenoscopy. Oral Oncology 2016;55:55–60. [DOI] [PubMed] [Google Scholar]
- 44.Wang CH, Lee YC, Wang CP, et al. Use of transnasal endoscopy for screening of esophageal squamous cell carcinoma in high-risk patients: yield rate, completion rate, and safety. Dig Endosc 2014;26:24–31. [DOI] [PubMed] [Google Scholar]
- 45.Arantes V, Albuquerque W, Salles JM, et al. Effectiveness of unsedated transnasal endoscopy with white-light, flexible spectral imaging color enhancement, and lugol staining for esophageal cancer screening in high-risk patients. J Clin Gastroenterol 2013;47:314–21. [DOI] [PubMed] [Google Scholar]
- 46.Kumagai Y, Monma K, Kawada K. Magnifying Chromoendoscopy of the Esophagus: In-Vivo Pathological Diagnosis Using an Endocytoscopy System. Endoscopy 2004;36:590–594. [DOI] [PubMed] [Google Scholar]
- 47.Inoue H, Sasajima K, Kaga M, et al. Endoscopic in vivo evaluation of tissue atypia in the esophagus using a newly designed integrated endocytoscope: a pilot trial. Endoscopy 2006;38:891–5. [DOI] [PubMed] [Google Scholar]
- 48.Kumagai Y, Kawada K, Yamazaki S, et al. Endocytoscopic observation for esophageal squamous cell carcinoma: can biopsy histology be omitted? Dis Esophagus 2009;22:505–12. [DOI] [PubMed] [Google Scholar]
- 49.Kumagai Y, Kawada K, Yamazaki S, et al. Current status and limitations of the newly developed endocytoscope GIF-Y0002 with reference to its diagnostic performance for common esophageal lesions. J Dig Dis 2012;13:393–400. [DOI] [PubMed] [Google Scholar]
- 50.Kumagai Y, Kawada K, Higashi M, et al. Endocytoscopic observation of various esophageal lesions at x600: can nuclear abnormality be recognized? Dis Esophagus 2015;28:269–75. [DOI] [PubMed] [Google Scholar]
- 51.Protano MA, Xu H, Wang G, et al. Low-Cost High-Resolution Microendoscopy for the Detection of Esophageal Squamous Cell Neoplasia: An International Trial. Gastroenterology 2015;149:321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pierce M, Yu D, Richards-Kortum R. High-resolution Fiber-optic Microendoscopy for in situ Cellular Imaging. Journal of Visualized Experiments : JoVE 2011:2306. [DOI] [PMC free article] [PubMed]
- 53.Patsias A, Giraldez-Rodriguez L, Polydorides AD, et al. Feasibility of transoral robotic-assisted high-resolution microendoscopic imaging of oropharyngeal squamous cell carcinoma. Head Neck 2015;37:E99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shin D, Protano MA, Polydorides AD, et al. Quantitative analysis of high-resolution microendoscopic images for diagnosis of esophageal squamous cell carcinoma. Clin Gastroenterol Hepatol 2015;13:272–279.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ishijima A, Schwarz RA, Shin D, et al. Automated frame selection process for high-resolution microendoscopy. Journal of Biomedical Optics 2015;20:046014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quang T, Schwarz RA, Dawsey SM, et al. A tablet-interfaced high-resolution microendoscope with automated image interpretation for real-time evaluation of esophageal squamous cell neoplasia. Gastrointestinal Endoscopy 2016;84:834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heresbach D, Leray E, d’Halluin PN, et al. Diagnostic accuracy of esophageal capsule endoscopy versus conventional upper digestive endoscopy for suspected esophageal squamous cell carcinoma. Endoscopy 2010;42:93–7. [DOI] [PubMed] [Google Scholar]
- 58.Adams L, Roth MJ, Abnet CC, et al. Promoter methylation in cytology specimens as an early detection marker for esophageal squamous dysplasia and early esophageal squamous cell carcinoma. Cancer Prevention Research 2008;1:357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dowlatshahi K, Skinner DB, DeMeester TR, et al. Evaluation of brush cytology as an independent technique for detection of esophageal carcinoma. J Thorac Cardiovasc Surg 1985;89:848–51. [PubMed] [Google Scholar]
- 60.Shroff CP, Nanivadekar SA. Endoscopic brushing cytology and biopsy in the diagnosis of upper gastrointestinal tract lesions. A study of 350 cases. Acta Cytol 1988;32:455–60. [PubMed] [Google Scholar]
- 61.Patel AA, Strome M, Blitzer A. Directed balloon cytology of the esophagus: A novel device for obtaining circumferential cytologic sampling. Laryngoscope 2017. [DOI] [PubMed]
- 62.Pan QJ, Roth MJ, Guo HQ, et al. Cytologic detection of esophageal squamous cell carcinoma and its precursor lesions using balloon samplers and liquid-based cytology in asymptomatic adults in Llinxian, China. Acta Cytol 2008;52:14–23. [DOI] [PubMed] [Google Scholar]
- 63.Roth MJ, Liu SF, Dawsey SM, et al. Cytologic detection of esophageal squamous cell carcinoma and precursor lesions using balloon and sponge samplers in asymptomatic adults in Linxian, China. Cancer 1997;80:2047–59. [DOI] [PubMed] [Google Scholar]
- 64.Roshandel G, Merat S, Sotoudeh M, et al. Pilot study of cytological testing for oesophageal squamous cell dysplasia in a high-risk area in Northern Iran. Br J Cancer 2014;111:2235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pauling L, Robinson AB, Teranishi R, et al. Quantitative analysis of urine vapor and breath by gas-liquid partition chromatography. Proc Natl Acad Sci U S A 1971;68:2374–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sonoda H, Kohnoe S, Yamazato T, et al. Colorectal cancer screening with odour material by canine scent detection. Gut 2011. [DOI] [PMC free article] [PubMed]
- 67.Chan DK, Zakko L, Visrodia KH, et al. Breath Testing for Barrett’s Esophagus Using Exhaled Volatile Organic Compound Profiling With an Electronic Nose Device. Gastroenterology 2017;152:24–26. [DOI] [PubMed] [Google Scholar]
- 68.Zou X, Zhou W, Lu Y, et al. Exhaled gases online measurements for esophageal cancer patients and healthy people by proton transfer reaction mass spectrometry. J Gastroenterol Hepatol 2016;31:1837–1843. [DOI] [PubMed] [Google Scholar]
- 69.Zhang J, Xu Z, Yu L, et al. Assessment of the Potential Diagnostic Value of Serum p53 Antibody for Cancer: A Meta-Analysis. PLoS ONE 2014;9:e99255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu YW, Peng YH, Chen B, et al. Autoantibodies as potential biomarkers for the early detection of esophageal squamous cell carcinoma. Am J Gastroenterol 2014;109:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qiao GL, Qi WX, Jiang WH, et al. Prognostic significance of circulating tumor cells in esophageal carcinoma: a meta-analysis. Onco Targets Ther 2016;9:1889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang S, Du H, Li G. Significant prognostic value of circulating tumor cells in esophageal cancer patients: A meta-analysis. Oncotarget 2017;8:15815–15826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu H-T, Miao J, Liu J-W, et al. Prognostic value of circulating tumor cells in esophageal cancer. World Journal of Gastroenterology 2017;23:1310–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu F, Tian T, Xia LL, et al. Circulating miRNAs as novel potential biomarkers for esophageal squamous cell carcinoma diagnosis: a meta-analysis update. Dis Esophagus 2017;30:1–9. [DOI] [PubMed] [Google Scholar]
- 75.Wang Y, Wang Q, Zhang N, et al. Identification of microRNAs as novel biomarkers for detecting esophageal squamous cell carcinoma in Asians: a meta-analysis. Tumour Biol 2014;35:11595–604. [DOI] [PubMed] [Google Scholar]
- 76.Chiam K, Wang T, Watson DI, et al. Circulating Serum Exosomal miRNAs As Potential Biomarkers for Esophageal Adenocarcinoma. J Gastrointest Surg 2015;19:1208–15. [DOI] [PubMed] [Google Scholar]
- 77.Lindner K, Haier J, Wang Z, et al. Circulating microRNAs: emerging biomarkers for diagnosis and prognosis in patients with gastrointestinal cancers. Clin Sci (Lond) 2015;128:1–15. [DOI] [PubMed] [Google Scholar]
- 78.Fu W, Pang L, Chen Y, et al. The microRNAs as prognostic biomarkers for survival in esophageal cancer: a meta-analysis. ScientificWorldJournal 2014;2014:523979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dickinson BT, Kisiel J, Ahlquist DA, et al. Molecular markers for colorectal cancer screening. Gut 2015;64:1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barault L, Amatu A, Siravegna G, et al. Discovery of methylated circulating DNA biomarkers for comprehensive non-invasive monitoring of treatment response in metastatic colorectal cancer. Gut 2017. [DOI] [PMC free article] [PubMed]
- 81.Tang Q, Cheng J, Cao X, et al. Blood-based DNA methylation as biomarker for breast cancer: a systematic review. Clin Epigenetics 2016;8:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zou H, Allawi H, Cao X, et al. Quantification of methylated markers with a multiplex methylation-specific technology. Clin Chem 2012;58:375–83. [DOI] [PubMed] [Google Scholar]
- 83.Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget Stool DNA Testing for Colorectal-Cancer Screening. New England Journal of Medicine 2014;370:1287–1297. [DOI] [PubMed] [Google Scholar]
- 84.Moinova HR, LaFramboise T, Lutterbaugh JD, et al. Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett’s esophagus. Science Translational Medicine 2018;10(424): pii: eaao5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iyer P, Taylor WR, Johnson ML, et al. (in press). Highly Discriminant Methylated DNA Markers for Non-Endoscopic Detection of Barrett’s Esophagus. Am J Gastroenterol 2018. [DOI] [PubMed]
- 86.Iyer PG, Ramona L; Johnson ML,et al. (in press). Accurate Non-endoscopic Detection of Barrett’s Esophagus in a Multicenter Prospective Validation Cohort: The SOS 2 Trial. Gastroenterology 2018; Supplement 1.
- 87.Iyer PG, Taylor WR, Yab TC, et al. 55 Detection of Barrett’s Dysplasia by Assay of Methylated DNA Markers on Whole Esophageal Brushings: A Prospective Feasibility Study. Gastroenterology 2015;148:S-16. [Google Scholar]
- 88.Chettouh H, Mowforth O, Galeano-Dalmau N, et al. Methylation panel is a diagnostic biomarker for Barrett’s oesophagus in endoscopic biopsies and non-endoscopic cytology specimens. Gut 2017;pii:gutjnl-2017-314026. [DOI] [PMC free article] [PubMed]
- 89.Taylor WR, Kisiel JB, Yab TC, et al. Novel Epigenetic Markers for Detection of Esophageal Cancer: Selection by Whole Methylome Sequencing and Tissue Validation. Gastroenterology 2015;148:S-550. [Google Scholar]
- 90.Iyer PG, Buglioni A, Cao XM, et al. (in press). Validation of novel methylated DNA markers for the detection of esophageal squamous cell carcinoma and dysplasia: multi-national tissue study. Gastroenterology 2018; Supplement 1. [Google Scholar]
- 91.Qin Y, Wu CW, Taylor WR, et al. (in press). Detection of Esophageal Cancer by Assay of Novel Methylated DNA Markers in Plasma. Gastroenterology 2018; Supplement 1.
- 92.Pech O, Rabenstein T, Manner H, et al. Confocal laser endomicroscopy for in vivo diagnosis of early squamous cell carcinoma in the esophagus. Clin Gastroenterol Hepatol 2008;6:89–94. [DOI] [PubMed] [Google Scholar]
- 93.Bray FCM, Mery L, Piñeros M, Znaor A, Zanetti R and Ferlay J, editors. Cancer Incidence in Five Continents, Vol. XI (electronic version). Lyon: International Agency for Research on Cancer., 2017. [Google Scholar]
- 94.Zhou SL, Yue WB, Fan ZM, et al. Autoantibody detection to tumor-associated antigens of P53, IMP1, P16, cyclin B1, P62, C-myc, Survivn, and Koc for the screening of high-risk subjects and early detection of esophageal squamous cell carcinoma. Dis Esophagus 2014;27:790–7. [DOI] [PubMed] [Google Scholar]
- 95.Zhang C, Wang C, Chen X, et al. Expression profile of microRNAs in serum: a fingerprint for esophageal squamous cell carcinoma. Clin Chem 2010;56:1871–9. [DOI] [PubMed] [Google Scholar]
- 96.Cao M, Yie SM, Wu SM, et al. Detection of survivin-expressing circulating cancer cells in the peripheral blood of patients with esophageal squamous cell carcinoma and its clinical significance. Clin Exp Metastasis 2009;26:751–8. [DOI] [PubMed] [Google Scholar]