Abstract
Background
Declining physical activity (PA) is a hallmark of aging. Wearable technology provides reliable measures of the frequency, duration, intensity, and timing of PA. Accelerometry-derived measures of PA are compared with established predictors of 5-year all-cause mortality in older adults in terms of individual, relative, and combined predictive performance.
Methods
Participants aged between 50 and 85 years from the 2003–2006 National Health and Nutritional Examination Survey (NHANES, n = 2,978) wore a hip-worn accelerometer in the free-living environment for up to 7 days. A total of 33 predictors of 5-year all-cause mortality (number of events = 297), including 20 measures of objective PA, were compared using univariate and multivariate logistic regression.
Results
In univariate logistic regression, the total activity count was the best predictor of 5-year mortality (Area under the Curve (AUC) = 0.771) followed by age (AUC = 0.758). Overall, 9 of the top 10 predictors were objective PA measures (AUC from 0.771 to 0.692). In multivariate regression, the 10-fold cross-validated AUC was 0.798 for the model without objective PA variables (9 predictors) and 0.838 for the forward selection model with objective PA variables (13 predictors). The Net Reclassification Index was substantially improved by adding objective PA variables (p < .001).
Conclusions
Objective accelerometry-derived PA measures outperform traditional predictors of 5-year mortality, including age. This highlights the importance of wearable technology for providing reproducible, unbiased, and prognostic biomarkers of health.
Keywords: Accelerometry, Physical activity, Physical performance, Exercise, Longevity
Physical activity (PA) is a major determinant of human health. Decline in PA reflects both age- and disease-related changes across multiple biological systems and physiological processes (1). PA has historically been assessed via questionnaires and has been subject to substantial recall bias. Even detailed PA questionnaires only provide coarse and imprecise information (2), as individuals have different definitions of “physical activity” and “intensity”. Moreover, self-reported PA can be affected by cognitive impairment, which is associated with disease, age, and psychosocial factors (2,3). Recognizing these limitations, scientific research has been refocusing on objective measurements of PA and sedentary time using accelerometers (1,4–13).
Objective PA measurements obtained from accelerometers have been increasingly used in aging research to predict changes in sleep, cognition, frailty, and mortality (14,15). Wanigatunga and colleagues (16) compared the daily objective PA patterns of older individuals in low-, intermediate-, and high-fatigability groups, whereas Huisingh-Scheetz and colleagues (17) showed that systematic within-day changes in PA are associated with frailty.
The 2003–2004 and 2005–2006 waves of the National Health and Nutrition Examination Survey (NHANES) collected objectively measured PA data using hip-worn accelerometers (18). The NHANES data are linked to US national mortality data, which allows the study of the association between accelerometer-based objective PA measurements and mortality. For example, Koster and colleagues (19) reported associations between sedentary behavior and mortality, independent of moderate-to-vigorous PA (MVPA), whereas Schmid and colleagues (20) reported an association between higher levels of sedentary time and lower levels of MVPA and mortality. Shorter sedentary time and longer periods of light, moderate, or vigorous activity was associated with reduced risk of mortality (21,22). At present, Raichlen and colleagues (23) associated fractal complexity of NHANES PA signals with mortality.
These studies have provided insights into the association between PA as measured by time spent in sedentary behaviors (24,25), light intensity PA (LiPA) (26) and MVPA and MVPA bouts (27–29) and mortality. In contrast, the focus here is on quantifying and comparing the individual, relative, and combined predictive performance of 5-year all-cause mortality predictors in the United States using the NHANES 2003–2006 data.
Methods
Study Population
The NHANES is a large study conducted by the Centers for Disease Control (CDC) to assess the health and nutritional status of the US population (30). These data include: (i) responses to demographic, socioeconomic, and health-related survey questions; (ii) medical, dental, physiological examination, and clinical laboratory tests; and (iii) PA information measured by accelerometers. Noninstitutionalized civilian residents of the United States were selected to participate in this study according to the CDC sample design specifications (31). Each study participant was assigned a survey weight equal to the number of people he or she represents in the US population. The NHANES 2003–2004 and 2005–2006 data were downloaded, processed, and combined with survey weights and mortality data (updated through 2015). Data are organized in the “R” package “rnhanesdata” (32).
The NHANES 2003–2004 and 2005–2006 have a total of 14,631 participants with accelerometry data. For this analysis, we excluded participants who (i) were younger than 50 years of age, or 85 and older at the time they wore the accelerometer (10,859 participants); (ii) had missing body mass index (BMI) or education predictor variables (41 participants); (iii) had fewer than 3 days of data with at least 10 hours of estimated wear time or were deemed by NHANES to have poor quality data (517 participants); non-wear periods were identified as intervals with at least 60 consecutive minutes of zero activity counts and at most 2 minutes with counts between 0 and 100 (18,33); (iv) had missing mortality information (21 participants); and (v) had missing systolic blood pressure, total or high-density lipoproteins (HDL) cholesterol measurements (293 participants). Among the remaining participants, 86 did not have alcohol consumption information and were retained in the data set by introducing the category “Missing Alcohol.” The final data set contained 2,978 participants with 297 deaths in the first 5 years after the accelerometer study.
Variables and Measures
Traditional mortality predictions
We integrated the NHANES data with the US national mortality registries and started with the sociodemographic factors age, sex, race/ethnicity, and educational attainment. In NHANES, race/ethnicity was coded as Non-Hispanic White (White), Mexican American (Mexican), Non-Hispanic Black (Black), Other Hispanic and Other. Educational attainment was coded as less than high school, high school equivalent, and greater than high school. We further included smoking status (never, former, current), alcohol consumption (nondrinker, moderate drinker, heavy drinker, missing alcohol), BMI (kg/m2), mobility difficulty (yes/no), diabetes, coronary heart disease, congestive heart failure, stroke, cancer, systolic blood pressure, total cholesterol (mg/dL), and HDL cholesterol (mg/dL). Mobility difficulty was defined as a positive response to any of the following questions (1): difficulty walking a quarter-mile (2); difficulty climbing 10 stairs; or (3) use of any special equipment to walk.
Accelerometry-Derived Predictors
In NHANES, the minute-by-minute activity data were recorded using a hip-worn ActiGraph AM-7164 accelerometer. Each participant was instructed to wear the device for a period of 7 consecutive days from the NHANES examination and remove it during sleep and water-related activity, such as swimming and bathing. The device was returned to the CDC by mail. Not every study participant wore the device for the full 7-day period.
Because minute-level accelerometer-derived PA data are large, the current practice is to take summary measures. Popular PA summaries based on actigraphy include: (i) total activity count (TAC); (ii) total log(1+activity count), or total log activity count (TLAC); and (iii) total minutes of MVPA, where MVPA is defined as more than 2,020 counts per minute. Although informative, these summaries may not reflect the full complexity of daily PA patterns. Therefore, we also considered the 2-hour summary variables (TLAC 12–2 am, TLAC 2—4 am, …, TLAC 10 pm–12 am), where each variable is TLAC, but calculated in the corresponding time interval. We also use two measures of activity fragmentation: transition probabilities from sedentary to active (SATPsl/nw) and active to sedentary (ASTPsl/nw) (29). Finally, we used principal component analysis to derive the surrogate for the standard deviation (SD) of the sixth principal component (PC). In the analysis, we refer to this measure as SD on PC 6 (surrogate). A detailed description of all these measures is available in Supplementary Materials. To ensure reproducibility, we also provide an accompanying R vignette in the “rnhanesdata” package (32).
Statistical Analysis
The demographic and clinical characteristics of the participants are presented in Table 1. They are separated by mortality status 5 years after the accelerometry study. For continuous variables, the mean is reported along with the SD (in parentheses). For binary or categorical variables, the number of study participants in each category is reported along with the percent number of participants (in parentheses) out of the total number in the corresponding alive or dead category. Variables are ranked in decreasing order of their predictive performance as measured by the receiver operating characteristic curve (AUC) in single predictor logistic regression with the 5-year all-cause mortality as outcome. The TAC is the top-ranked 5-year mortality predictor (AUC = 0.771) whereas age is a close second (AUC = 0.758).
Table 1.
Demographic and Clinical Characteristics Separated by Alive and Deceased Status 5 Years After Participation in the Accelerometry Study, National Health and Nutritional Examination Survey Pooled Cohorts Study, United States, 2003–2006
| Alive | Deceased | AUCb | ||
|---|---|---|---|---|
| Rank | Characteristics | Mean(SD)/N(%)a | ||
| 1 | TAC | 217,926 (111,868.8) | 136,307.9 (94,316.3) | 0.771 |
| 2 | Age | 65 (9.3) | 73.4 (8.9) | 0.758 |
| 3 | MVPA | 14.7 (17.3) | 6.5 (12.1) | 0.745 |
| 4 | ASTPsl/nw | 0.29 (0.08) | 0.37 (0.11) | 0.733 |
| 5 | Sedentary time | 1,102.4 (105.1) | 1,184 (110.5) | 0.728 |
| 6 | TLAC | 2,811.7 (705.6) | 2,278.7 (744.9) | 0.721 |
| 7 | TLAC 12–2 pm | 410.2 (113.9) |
333.4 (125.3) | 0.697 |
| 8 | TLAC 4–6 pm | 381 (112.2) |
309.3 (116.4) | 0.697 |
| 9 | TLAC 2–4 pm | 398.1 (116.7) |
323 (121.5) | 0.693 |
| 10 | TLAC 6–8 pm | 320.5 (118.4) |
250.7 (108.7) | 0.692 |
| 11 | TLAC 10 am–12 pm | 410.9 (127.4) |
335.3 (132.3) | 0.682 |
| 12 | Mobility problem | 768 (28.6%) | 172 (57.9%) | 0.672 |
| 13 | SD on PC 6 (surrogate) | 0.7 (0.27) | 0.57 (0.25) | 0.661 |
| 14 | SATPsl/nw | 0.08 (0.02) | 0.07 (0.02) | 0.658 |
| 15 | TLAC 8–10 am | 344.7 (153.3) | 282.4 (149.3) | 0.629 |
| 16 | Education | 0.612 | ||
| Less than high school | 822 (30.7%) | 123 (41.4%) | ||
| High school | 659 (24.6%) | 80 (26.9%) | ||
| More than high school | 1,200 (44.8%) | 94 (31.6%) | ||
| 17 | TLAC 8–10 pm | 208.8 (122.6) | 165.4 (104.1) | 0.603 |
| 18 | Alcohol consumption | 0.602 | ||
| Moderate drinker | 1,346 (50.2%) | 99 (33.3%) | ||
| Nondrinker | 1,106 (41.3%) | 160 (53.9%) | ||
| Heavy drinker | 153 (5.7%) | 28 (9.4%) | ||
| Missing alcohol | 76 (2.8%) | 10 (3.4%) | ||
| 19 | TLAC 6–8 am | 171.1 (153.5) | 127 (121.9) | 0.59 |
| 20 | Smoking status | |||
| Never | 1,233 (46%) | 93 (31.3%) | 0.586 | |
| Former | 1,010 (37.7%) | 137 (46.1%) | ||
| Current | 438 (16.3%) | 67 (22.6%) | ||
| 21 | CHF | 119 (4.4%) | 49 (16.5%) | 0.57 |
| 22 | Gender | 0.559 | ||
| Male | 1,331 (49.6%) | 192 (64.6%) | ||
| Female | 1,350 (50.4%) | 105 (35.4%) | ||
| 23 | Diabetes | 444 (16.6%) | 74 (24.9%) | 0.558 |
| 24 | Cancer | 382 (14.2%) | 73 (24.6%) | 0.558 |
| 25 | BMI | 0.553 | ||
| Normal | 663 (24.7%) | 97 (32.7%) | ||
| Underweight | 22 (0.8%) | 7 (2.4%) | ||
| Overweight | 1,048 (39.1%) | 102 (34.3%) | ||
| Obese | 948 (35.4%) |
91 (30.6%) | ||
| 26 | CHD | 196 (7.3%) | 48 (16.2%) | 0.552 |
| 27 | Stroke | 132 (4.9%) | 42 (14.1%) | 0.546 |
| 28 | Race | 0.525 | ||
| White | 1,556 (58%) | 200 (67.3%) | ||
| Mexican American | 504 (18.8%) | 34 (11.4%) | ||
| Other Hispanic | 53 (2%) | 3 (1%) | ||
| Black | 481 (17.9%) | 53 (17.8%) | ||
| Other | 87 (3.2%) | 7 (2.4%) | ||
| 29 | TLAC 12–2 am | 25.1 (61.4) | 24.7 (47.7) | 0.511 |
| 30 | TLAC 10 pm–12 | 86.3 (100.5) | 75.3 (80.9) | 0.506 |
| 31 | TLAC 4–6 am | 39.6 (85.1) | 34.1 (64.1) | 0.504 |
| 32 | TLAC 2–4 am | 15.4 (52.7) | 18.3 (44.9) | 0.5 |
| 33 | Wear time | 877.1 (134.4) | 891.7 (170.8) | 0.454 |
BMI = body mass index; CHD = coronary heart disease; CHF = congestive heart failure; MVPA = moderate-to-vigorous physical activity; SATP = transition probabilities from sedentary to active; SD = standard deviation; TAC = total activity count; TLAC = total log activity count.
aFor continuous variables, the mean is reported with the standard deviation shown in parentheses. For binary or categorical variables, the number of study participants in that category is reported with the alive/deceased specific prevalence of each category in parentheses.
bVariables are ranked by their predictive ability as measured by the AUC in single predictor logistic regressions with 5-year all-cause mortality as the outcome.
Mortality Prediction Models
Our main goals are to (i) rank predictors in terms of their 5-year mortality predictive performance; and (ii) identify the best subset of 5-year mortality predictors. To ensure that results are generalizable to the US population weights were calculated for the selected subset of participants using the function “reweight_accel()” in the “rnhanesdata” package. After reweighting, we used survey-weighted logistic regression using the function “svyglm()” in the R package “survey.” Variables are ranked according to the 10-fold complex survey-weighted cross-validated AUC in univariate models, where one predictor at a time is used to predict 5-year mortality. To select the best 5-year mortality predictors, we use forward selection survey-weighted logistic regression with the weighted cross-validated AUC as optimization criterion. The number of variables in the final model was selected to maximize the cross-validated AUC, though we also report the Akaike’s information criterion(34) and the efficient parsimony information criterion (35).
We have also compared the best multivariate model with and without accelerometry-derived PA measurements with the model obtained from this model by removing the accelerometry-derived PA measurements. The reclassification improvement of these two nested models (with and without PA measures) was evaluated using the categorical (36) and continuous (37) Net Reclassification Index, as used in the “R” package “PredictABEL.”
Results
Participant characteristics by mortality status are provided in Table 1. The mean age of the study sample was 65.9 (± 9.6, range 50.0–84.9) years. The proportions of men (51%) and women (49%) were similar with a larger proportion of men (65%) dying within 5 years of the follow-up. The participants who died within 5 years were on average 8.4 years older and had less time in MVPA, higher active to sedentary/sleep/non-wear transition probability (ASTPsl/nw), TAC, and TLAC, lower sedentary/sleep/non-wear to active transition probability (SATPsl/nw), and more sedentary/sleep/non-wear time. There was a larger proportion of nondrinkers and smaller proportion of moderate drinkers among the individuals who died compared were the group who did not. The proportion of smokers and former smokers was higher among the individuals who died. There was a larger proportion of individuals with less than high school education and a smaller proportion of individuals with more than high school education who died versus those who survived. The proportion of participants with CHF, coronary heart disease, and diabetes was higher among those who died within 5 years. There was a slightly larger proportion of deceased participants with underweight BMI, whereas the proportion of deceased and alive participants with normal and overweight BMI was similar. Finally, the proportion of alive individuals was slightly higher among Mexican Americans, whereas the proportion of alive and deceased participants was similar in other racial categories.
Table 1 shows the predictors ranking according to AUC in univariate logistic regression models, where each mortality prediction model was fit with one covariate at a time. TAC is the strongest predictor of 5-year mortality (AUC = 0.771) with age (AUC = 0.758) and MVPA (AUC = 0.745) being close second and third predictors, respectively. The transition probability from active to sedentary/sleep/non-wear (ASTPsl/nw, AUC = 0.733) and total sedentary time (sedentary/sleep/non-wear time, AUC = 0.728) round up the list of the top five predictors of 5-year all-cause mortality. The next eight most predictive covariates (excluding mobility difficulty) are all derived from accelerometry with AUCs from 0.721 to 0.658. These results indicate that accelerometry-derived variables are strong predictors of mortality that outperform traditional risk factors including smoking, total cholesterol, gender, cancer, stroke, diabetes, and coronary heart disease.
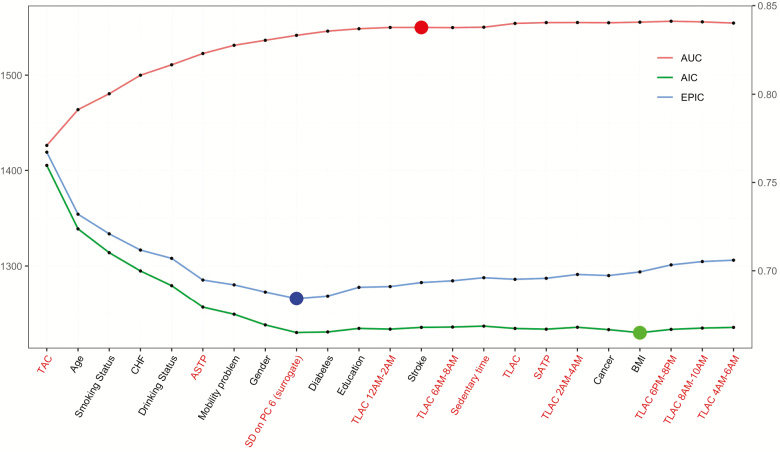
We also consider multipredictor models and use forward selection that adds one variable at a time by maximizing the cross-validated AUC. Figure 1 displays the Akaike’s information criterion, efficient parsimony information criterion, and AUC at each stage of the forward selection process. The scale for Akaike’s information criterion and efficient parsimony information criterion is shown on the left y-axis, whereas the scale for AUC is shown on the right y-axis. The final 5-year mortality prediction model selected based on the cross-validated AUC criterion contains 13 predictors (arranged in the order of their selection): TAC, age, smoking status, CHF, drinking status, ASTPsl/nw, mobility problem, gender, the surrogate for the SD on the sixth PC (SD on PC 6 surrogate), diabetes, education, TLAC 12–2 am, and stroke.
Figure 1.
Model selection criteria plotted as a function of the forward selection procedure. Accelerometry predictors are shown in red on the horizontal axis. National Health and Nutritional Examination Survey Pooled Cohorts Study, United States, 2003–2006.
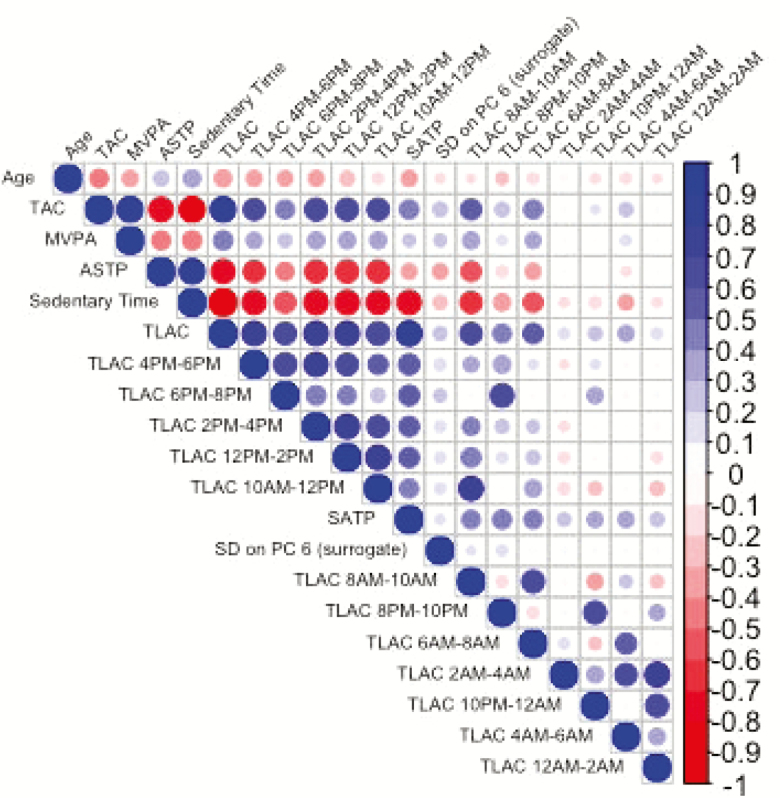
Figure 2 displays the correlation plot between age and all activity-derived variables. Age has high negative correlations with TAC, TLAC, and positive correlation with sedentary time. TAC is highly correlated with most activity-derived measures, including MVPA, SATPsl/nw, sedentary time, TLAC, and TLAC 4–6 pm, 6–8 pm, 2–4 pm, 12 –2 pm, 10–12 pm, 8–10 am, 6–8 am. ASTPsl/nw, sedentary time, TLAC, and SATPsl/nw are the most highly correlated with multiple other variables. The surrogate for the SD on the sixth PC has low correlation with other activity-derived variables, which may explain why it was selected in the joint model in addition to the other covariates.
Figure 2.
Correlation plot between age and accelerometry derived measures. National Health and Nutritional Examination Survey Pooled Cohorts Study, United States, 2003–2006.
Table 2 provides the results (point estimates odds ratio [OR], and confidence intervals [CIs], p-value) for the 13-variable model obtained via forward selection using the cross validated AUC. The mortality risk increases significantly with age (OR = 1.087, CI: (1.063, 1.112); p < .001) and history of coronary heart failure (OR = 2.175, CI: (1.177, 3.930); p = .013). Females have a lower probability of death (OR = 0.523, CI: (0.332, 0.817); p = .007), whereas former (OR = 1.394, CI: (0.835, 2.345); p = .176) and current smokers (OR = 2.219, CI: (1.412, 3.478); p = .002) have a higher mortality risk than nonsmokers. Nondrinkers (OR = 1.759, CI: (1.165, 2.677); p = .010), heavy drinkers (OR = 2.620, CI: (1.148, 5.673); p = .018) have higher 5-year mortality risk compared with individuals who consume alcohol moderately. When adjusted for age and other risk factors, including accelerometry-derived variables, the mortality risk was not statistically associated with a higher total activity (OR = 1.007, CI: (0.508, 1.832); p = .982) but was positively associated with the active to sedentary transition probability (ASTPsl/nw; OR = 1.465, CI: (1.078, 1.993); p = .016). Finally, higher values of the surrogate for the SD on the sixth PC (SD on PC6 surrogate; OR = 0.748, CI: (0.629, 0.885); p = .002) are associated with a lower probability of 5-year all-cause mortality. Although TAC is the most predictive variable of the 5-year mortality in single-regression models, its importance is substantially reduced after forward selection. This likely happens because many accelerometry-derived variables are highly correlated among themselves and with age (Figure 2).
Table 2.
Estimated Final Model Coefficients Odds Ratio (OR) with Corresponding Standard Errors and Significance Values in the Final Complex Survey Design Model, National Health and Nutritional Examination Survey Pooled Cohorts Study, United States, 2003–2006
| Estimate | p-value | Confidence interval (95%) | |
|---|---|---|---|
| Intercept | 0.000 | <.001 | (0.000, 0.001) |
| Total activity count | 1.007 | .982 | (0.508, 1.832) |
| Age | 1.087 | <.001 | (1.063, 1.112) |
| Former smoker | 1.394 | .176 | (0.835, 2.345) |
| Current smoker | 2.219 | .002 | (1.412, 3.478) |
| Coronary heart failure: yes | 2.175 | .013 | (1.177, 3.930) |
| Nondrinker | 1.759 | .010 | (1.165, 2.677) |
| Heavy drinker | 2.620 | .018 | (1.148, 5.673) |
| Missing alcohol | 2.111 | .106 | (0.752, 5.193) |
| ASTP | 1.465 | .016 | (1.078, 1.993) |
| Mobility problem | 1.726 | .028 | (1.057, 2.816) |
| Gender: female | 0.523 | .007 | (0.332, 0.817) |
| SD on PC 6 (surrogate) | 0.748 | .002 | (0.629, 0.885) |
| Diabetes: yes | 1.241 | .310 | (0.780, 1.937) |
| High school education | 0.992 | .973 | (0.582, 1.694) |
| More than high school education | 0.794 | .309 | (0.489, 1.294) |
| TLAC 12–2 am | 1.137 | .099 | (0.958, 1.322) |
| Stroke: yes | 1.213 | .505 | (0.636, 2.227) |
ASTP = active to sedentary transition probability; SD = standard deviation; TLAC = total log activity count.
To study the added prediction performance of accelerometry-derived PA variables, we started with the optimal model using forward selection with PA and non-PA variables. This model had 13 variables, a cross-validated AUC = 0.838 and is summarized in Table 2. From this model, we constructed a model without PA variables by removing TAC, ASTPsl/nw, the surrogate for the SD on the sixth PC (SD on PC 6 surrogate), and TLAC 12–2 am. The resulting model had a cross-validated AUC = 0.798 with the following non-PA variables: age, smoking status, CHF, drinking status, mobility problem, gender, diabetes, education, and stroke. The improvement in the continuous Net Reclassification Index (37), when comparing the 9-predictor (without PA covariates) and 13 predictor (with additional PA covariates) models was strongly statistically significant (p < .001).
Discussion
Table 1 illustrates the strong predictive performance of objective PA measures derived from measurements collected by a hip-placed accelerometer. These predictors substantially outperform established predictors of mortality in single predictor regression models.
There are important limitations to the results in Table 1. Indeed, they are based on single variable regressions, which provide ranking of predictors of 5-year all-cause mortality if one can measure only one variable at a time. This is useful, but one is often interested in building risk scores based on combinations of variables. Indeed, one could argue that accelerometer-derived PA measurements may be so predictive because they are highly correlated with age. The practical implication would be that objective PA measurements might not be modifiable. For this reason, we have conducted forward selection with a rich pool of potential mortality predictors including the standard demographic, behavioral, and comorbidities.
Another limitation is the exclusion of interaction terms from the analysis. Unreported results indicate that most predictive interactions were between age and objective PA predictors. Although some of the interactions were significant, they did not fundamentally change the results. Thus, to preserve simplicity, the focus here is on main effects prediction.
The association between PA and time to death using Cox models in the NHANES population has been investigated in several publications (21,29). Here, we focused on the 5-year mortality instead of time-to-death because (i) the model and the results are easy to communicate; (ii) potential problems with Cox model assumptions are avoided; and (iii) it is one of the standard horizons for prediction modeling (38,39). We further considered excluding all participants who died within 1 and 2 years from the time when the survey was conducted to avoid potential reverse causation. When doing that, results remained qualitatively consistent, identifying the same core predictors of mortality. However, as the focus is on mortality prediction (and not causal associations), results are presented without removing mortality data for the first years.
To study the robustness of finding, we have also conducted the same analyses in two age subgroups: (i) participants aged 50–70 (1,828 alive and 98 deceased within 5 years); and (ii) participants aged 70 and older (853 alive and 199 deceased within 5 years). In both age subgroups, 8 and 9 of the top 10 mortality predictors were accelerometry-derived PA summaries, respectively.
The selection of the exact collection of PA summaries derived from accelerometry that predict the 5-year all-cause mortality outcome can vary when data sets are slightly modified. This is likely due to the strong correlation among the objective PA summaries as well as to their correlation to other, established, risk factors. A better understanding of these relationships may further strengthen our understanding of the mutual effects of activity and other risk factors and their joint effect on mortality risk. However, these results indicate that (i) there is a strong association between PA summaries derived from accelerometry and mortality; (ii) these effects are encapsulated in different dimensions of PA measures; and (iii) the combined effects of these summaries are independent of other, well-known, mortality risk factors.
Given the large body of research on possible predictors of mortality, we conclude that PA summaries derived from accelerometry should become one of the top standard predictors of mortality risk. These measurements are becoming increasingly routine, are cheap and nonintrusive. Once they are normalized across cohorts and can be quantified in terms of easy to understand activities, duration, and timing, this could lead to more targeted PA intervention research.
Funding
This work was supported by the National Institutes of Health (RO1 HL123407 and RO1 NS060910 to Ciprian Crainiceanu) and National Institute on Aging Training Grant (T 32 AG000247).
Conflict of Interest
None reported.
Supplementary Material
Acknowledgments
Author’s role: Study concept and design: E.S., A.L., V.Z., C.C. Acquisition of the data: A.L., J.U., V.Z., C.C. Analysis and interpretation of the data: E.S., A.L., Q.C., L.T., V.Z., C.C., J.U. Preparation of manuscript: E.S., A.L., Q.C., L.T., V.Z., C.C., J.U. The funding agencies that supported the research had no role in the development of these analyses or the preparation of the manuscript.
References
- 1. Varma VR, Dey D, Leroux A, et al. Re-evaluating the effect of age on physical activity over the lifespan. Prev Med.. 2017;101:102–108. doi: 10.1016/j.ypmed.2017.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport.. 2000;71(sup2):1–14. doi: 10.1080/02701367.2000.11082780 [DOI] [PubMed] [Google Scholar]
- 3. Washburn RA. Assessment of physical activity in older adults. Res Q Exerc Sport.. 2000;71(sup2):79–87. doi: 10.1080/02701367.2000.11082790 [DOI] [PubMed] [Google Scholar]
- 4. Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports.. 2006;16(S1):3–63. doi: 10.1111/sms.12581 [DOI] [PubMed] [Google Scholar]
- 5. Lavie CJ, Arena R, Swift DL, et al. Exercise and the cardiovascular system: clinical science and cardiovascular outcomes. Circ Res.. 2015;117:207–219. doi: 10.1161/CIRCRESAHA.117.305205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stewart RAH, Held C, Hadziosmanovic N, et al. ; STABILITY Investigators Physical activity and mortality in patients with stable coronary heart disease. J Am Coll Cardiol.. 2017;70:1689–1700. doi: 10.1016/j.jacc.2017.08.017 [DOI] [PubMed] [Google Scholar]
- 7. van der Berg JD, Stehouwer CD, Bosma H, et al. Associations of total amount and patterns of sedentary behaviour with type 2 diabetes and the metabolic syndrome: the Maastricht Study. Diabetologia.. 2016;59:709–718. doi: 10.1007/s00125-015-3861-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamilton MT, Hamilton DG, Zderic TW. Sedentary behavior as a mediator of type 2 diabetes. Med Sport Sci.. 2014;60:11–26. doi: 10.1159/000357332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thune I, Furberg AS. Physical activity and cancer risk: dose-response and cancer, all sites and site-specific. Med Sci Sports Exerc.. 2001;33(6 Suppl):S530–550; discussion S609. [DOI] [PubMed] [Google Scholar]
- 10. Wen CP, Wai JP, Tsai MK, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378:1244–1253. doi: 10.1016/S0140-6736(11)60749-6 [DOI] [PubMed] [Google Scholar]
- 11. Keadle SK, Conroy DE, Buman MP, Dunstan DW, Matthews CE. Targeting reductions in sitting time to increase physical activity and improve health. Med Sci Sports Exerc.. 2017;49:1572–1582. doi: 10.1249/MSS.0000000000001257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matthews CE, Kozey SK, Moore SC, et al. Measurement of active and sedentary behavior in context of large epidemiologic studies. Med Sci Sports Exerc.. 2018;50:266–276. doi: 10.1249/MSS.0000000000001428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Troiano RP, McClain JJ, Brychta RJ, Chen KY. Evolution of accelerometer methods for physical activity research. Br J Sports Med.. 2014;48:1019–1023. doi: 10.1136/bjsports-2014-093546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shiroma EJ, Schrack JA, Harris TB. Accelerating accelerometer research in aging. J Gerontol A Biol Sci Med Sci.. 2018;73:619–621. doi: 10.1093/gerona/gly033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zeitzer JM, Blackwell T, Hoffman AR, Cummings S, Ancoli-Israel S, Stone K, Osteoporotic Fractures in Men (MrOS) Study Research Group. daily patterns of accelerometer activity predict changes in sleep, cognition, and mortality in older men. J Gerontol: Ser A. 2017;73:682–687. doi: 10.1093/gerona/glw250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wanigatunga AA, Simonsick EM, Zipunnikov V, et al. Perceived fatigability and objective physical activity in mid-to late-life. J Gerontol: Ser A. 2017;73:630–635. doi: 10.1093/gerona/glx181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huisingh-Scheetz M, Wroblewski K, Kocherginsky M, et al. The relationship between physical activity and frailty among U.S. older adults based on hourly accelerometry data. J Gerontol A Biol Sci Med Sci.. 2018;73:622–629. doi: 10.1093/gerona/glx208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc.. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3 [DOI] [PubMed] [Google Scholar]
- 19. Koster A, Caserotti P, Patel KV, et al. Association of sedentary time with mortality independent of moderate to vigorous physical activity. PLoS One.. 2012;7:e37696. doi: 10.1371/journal.pone.0037696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schmid D, Ricci C, Leitzmann MF. Associations of objectively assessed physical activity and sedentary time with all-cause mortality in US adults: the NHANES study. PLoS One.. 2015;10:e0119591. doi: 10.1371/journal.pone.0119591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fishman EI, Steeves JA, Zipunnikov V, et al. Association between objectively measured physical activity and mortality in NHANES. Med Sci Sports Exerc.. 2016;48:1303–1311. doi: 10.1249/MSS.0000000000000885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schmid D, Ricci C, Baumeister SE, Leitzmann MF. Replacing sedentary time with physical activity in relation to mortality. Med Sci Sports Exerc.. 2016;48:1312–1319. doi: 10.1249/MSS.0000000000000913 [DOI] [PubMed] [Google Scholar]
- 23. Raichlen DA, Klimentidis YC, Hsu C-H, Alexander GE. Fractal complexity of daily physical activity patterns differs with age over the life span and is associated with mortality in older adults J Gerontol: Ser A. 2018;74:1461–1467. doi: 10.1093/gerona/gly247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Theou O, Blodgett JM, Godin J, Rockwood K. Association between sedentary time and mortality across levels of frailty. CMAJ. 2017;189:E1056–E1064. doi: 10.1503/cmaj.161034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Diaz KM, Howard VJ, Hutto B, et al. Patterns of sedentary behavior and mortality in U.S. middle-aged and older adults. Ann Intern Med. [Internet]. 2017;167:465–475. Available from: http://annals.org/article.aspx?doi=10.7326/M17-0212. doi: 10.7326/M17-0212 [DOI] [PMC free article] [PubMed]
- 26. Jefferis BJ, Parsons TJ, Sartini C, et al. Objectively measured physical activity, sedentary behaviour and all-cause mortality in older men: does volume of activity matter more than pattern of accumulation? Br J Sports Med. Published Online First: 12 February 2019;53:1013–1020. doi: 10.1136/bjsports-2017-098733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saint-Maurice PF, Troiano RP, Berrigan D, et al. Volume of light versus moderate‐to‐vigorous physical activity: similar benefits for all‐cause mortality?. J Am Heart Assoc. 2018;7:e008815. doi: 10.1161/JAHA.118.008815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saint-Maurice PF, Troiano RP, Matthews CE, et al. Moderate‐to‐vigorous physical activity and all‐cause mortality: do bouts matter?. J Am Heart Assoc. 2018;7:e007678. doi: 10.1161/JAHA.117.007678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Di J, Leroux A, Urbanek J, et al. Patterns of sedentary and active time accumulation are associated with mortality in US adults: the NHANES study. bioRxiv. 2017;182337. doi: 10.1101/182337 [Google Scholar]
- 30. Centers for Disease Control and Prevention: About the national health and nutrition examination survey 2016. http://www.cdc.gov/nchs/nhanes/about_nhanes.htm. Accessed June 1, 2018.
- 31. Mirel LB, Mohadjer LK, Dohrmann SM, et al. National health and nutrition examination survey: estimation procedures, 2007–2010. Vital Health Stat. Ser 2, Data Eval Methods Res. 2013;159:1–17. [PubMed] [Google Scholar]
- 32. Leroux A, Crainiceanu C, Smirnova E, Cao Q. rnhanesdata: NHANES Accelerometry Data Pipeline https://github.com/andrew-leroux/rnhanesdata. R package version 1.0. Accessed July 1, 2019.
- 33. Atienza AA, Moser RP, Perna F, et al. Self-reported and objectively measured activity related to biomarkers using NHANES. Med Sci Sports Exerc.. 2011;43:815–821. doi: 10.1249/MSS.0b013e3181fdfc32 [DOI] [PubMed] [Google Scholar]
- 34. Lumney T, Scott A. AIC and BIC for modeling with complex survey data. J Surv Stat Methodol. 2015;3:1–18. doi: 10.1093/jssam/smu021 [Google Scholar]
- 35. Shinohara RT, Crainiceanu CM, Caffo BS, et al. Longitudinal analysis of spatiotemporal processes: a case study of dynamic contrast-enhanced magnetic resonance imaging in multiple sclerosis. Neuroimage. 2011;57:1430–46. doi: 10.1016/j.neuroimage.2011.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med.. 2008;27:157–72; discussion 207. doi: 10.1002/sim.2929 [DOI] [PubMed] [Google Scholar]
- 37. Pencina MJ, D’Agostino RB Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med.. 2011;30:11–21. doi: 10.1002/sim.4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lloyd-Jones DM. Cardiovascular risk prediction: basic concepts, current status, and future directions. Circulation. 2010;121:1768–1777. doi: 10.1161/CIRCULATIONAHA.109.849166 [DOI] [PubMed] [Google Scholar]
- 39. Choudhury PP, Wilcox A, Brook M, et al. Comparative validation of breast cancer risk prediction models and projections for future risk stratification. Journal of the National Cancer Institute. 2019;djz113. doi: doi.org/10.1093/jnci/djz113 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.