Abstract
Research indicates that lifestyle and genetic factors influence the course of cognitive impairment in aging, but their interactions have not been well-examined. This study examined the relationship between physical activity and genotypes related to brain-derived neurotrophic factor (BDNF) in predicting cognitive performance in a sample of older adults with up to 12 years of follow-up. Physical activity levels (sedentary, light, and moderate/vigorous) were determined for the sample of 3,591 participants (57% female) without dementia. The genotypes examined included BDNF gene single nucleotide polymorphisms (SNPs) (rs6265 and rs56164415) and receptor gene SNPs (NTRK2 rs2289656 and NGFR rs2072446). Cognition was assessed triennially using the Modified Mini-Mental State Exam. Unadjusted linear mixed models indicated that sedentary (β = −5.05) and light (β = −2.41) groups performed worse than moderate–vigorous (p < .001). Addition of interaction effects showed significant differences in rate of decline between activity levels, particularly among males (p = .006). A three-way interaction with sex, NGFR SNP rs2072446, and physical activity suggested that the C/C allele was associated with better cognitive performance among males engaging in light activity only (p = .004). Physical activity and sex, but not BDNF-related SNPs, predicted rate of cognitive decline in older adults, while NGFR rs2072446 may modify main effects.
Keywords: Cognitive decline, Genetics, Exercise
Reduced mortality trends across the globe are accompanied by increases in age-related chronic health conditions, including dementia and severe cognitive impairment (1,2). Severe cognitive impairment is one of the leading causes of institutionalization in the United States and increases exponentially with age (2,3). While less than 4% of those aged 70–74 have severe cognitive impairment, this condition affects 35% of those 90 and older (2). As reviewed below, modifiable lifestyle factors, including physical activity, are associated with cognitive outcomes in late-life.
Physical activity has been associated with cognitive benefits for older adults with (4) and without (5,6) dementia in both experimental and observational studies, though effectiveness may vary by type of physical activity and null findings have also been published (particularly in persons with dementia) (7,8). While meta-analyses of observational studies indicate that both moderate and high engagement in physical activity reduces risk for cognitive decline (6,9) and dementia (9), aerobic exercise (ie, that which noticeably accelerates the heart rate and breathing (10)) appears most effective, as suggested by randomized control trials (4,5,11,12). Specifically, Hindin and colleagues found that aerobic exercise interventions (mean [range] number of weeks = 23.8 [8–51]) across 17 randomized control trials resulted in better performance on several cognitive tests of reaction time, memory, and executive function in older adults without dementia (estimated effect size = 0.33) (5). In their meta-analysis of 18 randomized control trials, Groot and colleagues found similar results with participants who have dementia, such that combined (aerobic and nonaerobic) exercise (standardized mean difference = 0.59) and aerobic exercise-only (standardized mean difference = 0.41) interventions across mean (range) weeks of 15 (6–51) produced positive effects on cognition. Significant cognitive benefits were not obtained for participants who engaged in only nonaerobic exercise interventions (standardized mean difference = −0.10) (4). Delaying cognitive decline through physical activity may represent a viable route to lowering prevalence of severe cognitive impairment and dementia.
While the specific mechanisms underlying the beneficial effects of physical activity in cognitive function are still unclear, one possible mechanism is through the regulation of brain-derived neurotrophic factor (BDNF). BDNF, a protein belonging to the neurotrophin family of growth factors, is critical to neurogenesis, cell survival and differentiation, and synaptic plasticity in the nervous system of mammals (13). With its high expression in the hippocampus and promotion of plasticity of hippocampal neurons, BDNF is widely recognized to play an important role in cognitive health, particularly with regard to learning and memory (14). While the beneficial effects of mature BDNF are mediated through its specific receptor trkB (encoded by the NTRK2 gene), the common neurotrophin receptor p75NTR (encoded by the nerve growth factor receptor [NGFR] gene) mediates the proapoptotic and dendritic remodeling effects of neurotrophin precursors (15).
Physical activity is associated with neurotrophin production, neurogenesis, and reduction in the accumulation of harmful proteins such as β-amyloid in the brain (4). BDNF is widely recognized as a critical mechanism of exercise-induced cognitive enhancement both in humans (16) and in rodents (17,18), in that learning and memory are directly linked to exercise-related increases in BDNF expression. In humans, Erickson and colleagues demonstrated that an aerobic exercise intervention increased hippocampal volume (and improved spatial memory) in 60 adults aged 65 and older, reversing age-related cortical atrophy at 1- to 2-year follow-up compared to controls (N = 60). Increased hippocampal volume was associated with higher BDNF levels in serum (r = .36 and .37 for right and left hippocampi, respectively) in the aerobic exercise group (16).
Animal studies have provided strong evidence of the direct effects of exercise on the expression of neurotrophins and neurotrophin receptors. Vilela and colleagues showed that strength and aerobic exercises improve spatial memory in aging rats through increasing expression of BDNF and its receptors in the hippocampus (19). Inhibition of BDNF action via a BDNF antagonist results in the abolition of exercise-related cognitive gains (17,18). Maejima and colleagues found that long-term aerobic exercise improved cognitive function of senescence-accelerated mice, and, through epigenetic regulation of transcriptional activity upregulated the expression of mature BDNF, and downregulated p75, a receptor involved in detrimental effects of proneurotrophins (20).
A recent study demonstrated that one of the mechanisms through which physical exercise influences gene expression is through the release of endogenous molecules such as β-hydroxy butyrate (21), which inhibit histone deacetylases. β-Hydroxy butyrate acts on histone deacetylase 2 and histone deacetylase 3, affecting activity-dependent expression of BDNF by acting selectively on the BDNF gene promoters. As a result, BDNF expression is increased not only in the periphery (as revealed by serum levels) but also in the brain. Moreover, electrophysiological measurements in the brain indicate that β-hydroxy butyrate increases neurotransmitter release in the hippocampus, as a result of BDNF signaling through the TrkB receptor (product of the NTRK2 gene) (21).
Although physical exercise affects BDNF protein levels, they are ultimately regulated by genes involved in BDNF production and signaling. Several single nucleotide polymorphisms (SNPs) have been identified in the BDNF gene and in genes encoding BDNF receptors. Studies examining the relationship between BDNF-related SNPs and cognitive performance have revealed variable results, with most consistent findings occurring for BDNF rs6265 (or val66met) (15,22–24). One study found that having one or more copies of the minor rs6265 allele predicted worse performance on measures of processing speed, delayed recall, and general intelligence in a cross-sectional analysis of 722 older English adults (25). In this study, rs6265 best accounted for the variance in cognitive performance among 13 other BDNF-related SNPs (25). Another study found that BDNF rs6265 minor allele was associated with worse episodic memory, but not working memory, executive functioning, or language processes (26). However, this effect was only observed in carriers of the e4 allele of apolipoprotein E (26). In relation to Alzheimer’s disease (AD) risk, the heterozygous (AG or val-met) genotype was associated with higher risk for AD (odds ratio = 2.01) when compared to the homozygous variant, but only in the context of the heterozygous variants of two other BDNF SNPs (27). Interestingly, BDNF rs56164415 (or C270T), which is a SNP on promoter exon V, did not account for AD risk in this study (27), but has been associated with AD risk in other studies among the AlzGene database (28). There have also been null findings (odds ratios ranging from 0.38 to 3.31) for both rs6265 and rs56164415 in association with AD (27,28).
A recent study in the Cache County population-based cohort identified both BDNF rs56164415 and NGFR rs2072446, but not BDNF rs6265, as being independently associated with reduced risk for AD; however, the effects varied by sex (29). The NGFR gene is related to BDNF in that it encodes the proneurotrophin receptor p75, which has also been implicated in aging-related memory deficits and cognitive impairment in rodents (30–33). In the Cache County study, Matyi and colleagues found that female carriers of the minor T allele in BDNF rs56164415 and NGFR rs2072446 had 93% and 60% higher risk of developing AD, respectively, compared to males with the minor T allele (29). In a study of 795 individuals from 203 families as part of the Alzheimer’s Disease Gene Initiative conducted by the National Institute of Mental Health, NTRK2, the gene responsible for encoding the BDNF receptor TrkB, was associated with AD risk in the context of other BDNF-related haplotypes (34). Although the aforementioned studies investigated AD risk, BDNF rs56164415, BDNF rs6265, NGFR rs2072446, and NTRK2 may be relevant for cognitive decline as well.
The present study examined whether BDNF-related genes (BDNF rs6265, BDNF rs56164415, NTRK2 rs2289656, and NGFR rs2072446) modified the association between physical activity and cognitive decline in older adult participants of the Cache County Study on Memory in Aging.
Methods
Participants
The current study used extant data of the longitudinal, population-based Cache County Study on Memory in Aging. A detailed description of the study’s recruitment and participation has been documented elsewhere (35). Briefly, 5,092 residents aged 65 and older in Cache County, UT enrolled in Cache County Study on Memory in Aging in 1995 and were followed over three more triennial waves for a total of four waves across 12 years. Researchers conducted cognitive testing and dementia ascertainment procedures, and collected demographic, genetic, health, and behavioral data at each wave.
Procedure
The Institutional Review Boards of collaborating universities (Utah State University, Duke University, and the Johns Hopkins University) approved all study procedures. Participants completed a cognitive screening test, an adaptation of the Modified Mini-Mental State Exam (3MS) (36) at each wave. If participants were unable to complete testing, scored less than 60 points on the total or less than 15 points on orientation items, or were judged as unreliable by the interviewer, a proxy interview was completed using the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) (37). After adjusting for sensory impairments and level of education, participants with a 3MS score less than 87 or IQCODE greater than 3.27 were further screened over telephone with an interview with a knowledgeable informant using the Dementia Questionnaire. Those whose interview results were rated with significant cognitive impairment or possible dementia were invited to complete a clinical assessment. At the clinical assessment, the participant completed a physical and neurological exam and neuropsychological assessment. A nurse also interviewed a knowledgeable informant to obtain the participant’s clinical health and functional history including current neuropsychiatric symptoms and cognitive and functional limitations. A study geropsychiatrist and neuropsychologist reviewed the information gleaned from these procedures during case reviews to assign preliminary diagnoses of dementia using criteria of the Diagnostic Statistical Manual of Mental Disorders – 3rd Edition Revised (DSM-III-R) (38). The study’s multidisciplinary panel of dementia experts diagnosed type of dementia. Those who did not receive a diagnosis of dementia were followed in this study until they developed dementia, died, or refused further participation. In order to be eligible for the current study, participants had to be dementia-free at baseline and not missing data on key variables of interest. Persons with new/incident dementia were included in all analyses up to and including the wave at which their diagnosis of dementia was determined, but were not followed in subsequent waves (post-dementia onset).
Cognitive Decline
Cognitive status at each wave was assessed using an adapted version of the 3MS, which is an expansion of the Mini-Mental State Exam that provides increased sensitivity of global cognitive function (36,39). While the 3MS is often used for dementia screening, it has been widely used to detect changes in cognition this and other large-scale longitudinal studies (40). The 3MS has a scoring range of 0–100 and broadly measures the following cognitive constructs: psychomotor skills, memory, orientation, identification and association, and concentration and calculation (36,41). Psychometric properties include high internal consistency (α = .87) and test-retest reliability (r = .91–.93), as well as sensitivity (91%) and specificity (97%) for identifying dementia (36,39). Minor modifications were made to the 3MS for epidemiological fieldwork; for example, replacing an aural presentation of words with a visual presentation for hearing impaired individuals and information items that could be verified in the field (40). Because hearing loss was common among participants, further adaptations and score adjustments were made for sensory deficits as reported previously (35). Sensory/motor deficits included impairments in hearing, vision, and fine motor skills.
Physical Activity
At each wave with the exception of Wave 2, physical activity was assessed using a self-report questionnaire of participation in physical activities. The questionnaire was collected as a mail-in form for the first wave and then as part of in-person interviews during Waves 3 and 4. In Wave 1, the questionnaire included three items regarding general participation in light, moderate, or vigorous physical activity; however, the questionnaire was modified in Waves 3 and 4 to include more specific information about type, frequency, and duration of the physical activity over the previous year (see Supplementary Table 1 for specific items). Physical activity was coded into a categorical variable with levels of sedentary, light, moderate, or vigorous, based on considerations of intensity and cardiovascular value following guidelines of the American College of Sports Medicine (ACSM) and American Heart Association (AHA) (10). The ACSM/AHA uses the concept of metabolic equivalents (METs) to estimate energy expenditure and assigns an intensity value to a variety of common activities. Activities are allocated particular METs per minute (MET-min), depending on their intensity and categorization as light intensity (<3 MET-min; eg, walking at a pace < 4.8 km/h, housework, shopping), moderate intensity (3–6 MET-min; eg, bicycling at 16–19 km/h, walking at 4.8–6.4 km/h, golfing), or vigorous intensity (>6 MET-min; eg, jogging at 8–11.25 km/h, bicycling at 19–25.75 km/h, cross-country skiing). ACSM/AHA guidelines specify that persons are meeting recommended physical activity standards if they engage in 450–750 MET-min per week as long as this is achieved through engagement in moderate or vigorous activities. In order to be consistent with ACSM/AHA guidelines that specify a minimum threshold of 450 MET-min of moderate activity per week, we defined moderate and vigorous groups accordingly. Persons were categorized as “vigorous” if their MET-min per week exceeded 750 and at least one of their exercise activities met vigorous intensity standards. Persons were categorized as “moderate” if their MET-min per week was greater than or equal to 450, and they engaged in at least moderate intensity activities but they did not otherwise satisfy the vigorous criteria. Persons were categorized as “light” if they endorsed light physical activities and did not otherwise meet moderate or vigorous criteria (ie, MET-min/wk < 450 and did not engage in at least moderate activity). Persons were categorized as sedentary if they did not endorse engaging in light, moderate, or vigorous physical activity. Note, vigorous and moderate categories were ultimately collapsed into one group (see Exploratory Analyses section for rationale). This process was applied to each wave and physical activity was investigated as a time-varying predictor. Due to limitations in specificity of questions 2 and 3 at Wave 1, duration of activity was assigned as 30 minutes. Because physical activity was not assessed in Wave 2, Wave 1 data were imputed for Wave 2. Lastly, baseline physical activity questionnaires were not administered for participants who required a proxy interview due to 3MS score below 60.
Genotyping
At baseline, participants provided a buccal-swab sample from which DNA was extracted (35). DNA was also extracted from blood draws completed in Waves 3 and 4. Apolipoprotein E genotype, included as a covariate, was determined from DNA from buccal cells and processed using polymerase chain reaction (42). Genotyping for BDNF and related genes SNPs rs6265, rs2289656, rs2072446, and rs56164415 was completed using standard TaqMan Assays (Life Technologies) via DNA from blood samples (or if missing, buccal samples) (29).
Additional Information
Demographic variables such as age, sex, and level of education were collected during baseline interviews. Subjective health status was obtained at each wave by asking the participant to rate his/her health on the day of the interview as “excellent,” “good,” “fair,” or “poor”. Subjective health served as a brief and simple way of controlling for general medical comorbidities that may affect an individual’s level of physical activity. Body mass index (BMI) was determined by self-reported height and weight from participant’s baseline interview, and categorized into the following standard groups: underweight (BMI < 18.5), normal weight (BMI = 18.5–24.9), overweight (BMI = 25–29.9), and obese (BMI ≥ 30). Depression was assessed using an adaptation of the Diagnostic Interview Schedule (DIS) for the Diagnostic Statistical Manual of Mental Disorders – 3rd Edition Revised (DSM-III-R) (38,43) and evaluated as a binary, time-varying covariate in which both major depressive and dysthymic episodes at or between waves were considered positive for depression. A diagnosis of major depression was assigned if the participant endorsed at least five of the nine depressive symptom criteria, including depressed mood and/or anhedonia, that persisted for a period of 2 weeks or more. Dysthymia was assigned if the participant did not meet the criteria for major depressive disorder but endorsed persistent depressed mood and at least two other symptoms for 2 years or more.
Statistical Analysis
The sample was characterized as a whole using descriptive statistics. Inferential statistics, with t tests for continuous variables and Pearson’s chi-square tests of independence for categorical variables, were used to examine demographic differences in comparing those who were missing or nonmissing on variables of interest. The stability of genotype frequencies was verified with the Hardy–Weinberg equilibrium chi-square goodness-of-fit method, using the p < .001 criterion as in other large-scale association studies (29).
Linear mixed effects models were used to examine the association between physical activity (time-varying) and cognitive decline. All linear mixed models included random slopes and intercepts. Base models included the independent variable, time, and time2, the latter to assess nonlinear change in 3MS (44). Next, an interaction of the independent variable with time and time2 was examined to test for effects on rate of change in 3MS. Subsequently, covariates were tested and finally, SNPs related to BDNF processing were included with a term testing an interaction between each SNP and the independent variable. Covariates tested here included baseline age, sex, level of education, apolipoprotein E e4 allele, BMI, and time-varying subjective health status and depression. Based on prior results in the Cache County population regarding possible sex differences in BDNF functioning (29), interactions between each SNP and sex were tested. Exploratory interactions between sex and physical activity were also evaluated. Model-building proceeded in this sequential manner, and model fit was determined using the chi-square test of independence in comparing negative 2-log likelihood values (−2LL) of nested models. Although results are shown for p < .05, statistical significance was determined based on model fit and alpha was set to p = .0125, adjusting for multiple comparisons using the Bonferroni procedure to account for each SNP. The maximum likelihood method of estimation was used in determining model fit, but final models employed the restricted maximum likelihood method of estimation. All statistical models were run using SPSS software version 25.
Results
Exploratory Analyses
Table 1 includes baseline sample characteristics of persons with and without complete physical activity data at Wave 1. Those excluded from analyses due to incomplete physical activity data were at baseline: significantly older, completed fewer years of education, had lower 3MS scores, and were significantly different regarding subjective health and depression status. Note that participants were only excluded from linear mixed effects models at the specific wave of missing data, and were included at all visits for which physical activity data were complete; therefore, descriptive analyses convey incidental differences at baseline only. One hundred and fifty-six persons with prevalent dementia (N = 105) or uncertain diagnoses (N = 51) were additionally excluded from all analyses. Initial linear mixed models adjusting for covariates showed that persons included in moderate and vigorous categories of physical activity were virtually indistinguishable in terms of cognitive outcomes over time as evidenced by lack of statistically significant differences for both main (p = .690) and interaction effects (with time2, p = .415) as well as similar parameter estimates in main effects when using sedentary as the reference (vigorous β = 4.18 and moderate β = 4.09). Thus, moderate and vigorous categories were collapsed into one group for final analyses. Only BDNF SNP rs56164415 showed a significant deviation from Hardy–Weinberg equilibrium (χ 2 = 13.01, p < .001). See Table 1 for allelic frequencies in our cohort.
Table 1.
Baseline Characteristics† of CCSMA Participants With and Without Complete Physical Activity Data
| Characteristic | Complete (N = 3,337) | Incomplete (N = 961) | χ 2 | t | p Value |
|---|---|---|---|---|---|
| Female, N (%) | 1,868 (55.98) | 548 (57.02) | 0.33 | .565 | |
| Physical activity, N (%) | N/A | ||||
| Sedentary | 37 (1.11) | — | |||
| Light | 267 (8.00) | — | |||
| Moderate–vigorous | 3,033 (90.89) | — | |||
| Presence of APOE e4, N (%) | 1,037 (31.08) | 273 (28.41) | 2.51 | .113 | |
| Subjective health status, N (%)* | 30.54 | <.001 | |||
| Excellent | 949 (28.44) | 197 (20.50) | |||
| Good | 1,906 (57.12) | 578 (60.15) | |||
| Fair | 427 (12.80) | 163 (16.96) | |||
| Poor | 55 (1.65) | 23 (2.39) | |||
| BDNF rs6265, N (%) | 0.04 | .979 | |||
| G/G | 2,108 (63.17) | 597 (62.12) | |||
| G/A | 973 (29.16) | 273 (28.41) | |||
| A/A | 139 (4.17) | 38 (3.95) | |||
| Missing | 117 (3.51) | 53 (5.52) | |||
| NGFR rs2072446, N (%) | 3.15 | .207 | |||
| C/C | 2,917 (87.41) | 828 (86.16) | |||
| C/T | 260 (7.79) | 68 (7.08) | |||
| T/T | 9 (0.27) | 6 (0.62) | |||
| Missing | 151 (4.53) | 59 (6.14) | |||
| NTRK2 rs2289656, N (%) | 2.02 | .364 | |||
| C/C | 2,154 (64.55) | 579 (60.25) | |||
| T/C | 922 (27.63) | 274 (28.51) | |||
| T/T | 112 (3.36) | 36 (3.75) | |||
| Missing | 149 (4.47) | 72 (7.49) | |||
| BDNF rs56164415, N (%) | 0.13 | .723 | |||
| C/C | 2,794 (83.73) | 800 (83.25) | |||
| T/C | 368 (11.03) | 101 (10.51) | |||
| Missing | 175 (5.24) | 60 (6.24) | |||
| BMI, N (%) | 1.83 | .609 | |||
| Underweight | 65 (1.95) | 25 (2.60) | |||
| Normal weight | 1,366 (40.93) | 390 (40.58) | |||
| Overweight | 1,314 (39.38) | 370 (38.50) | |||
| Obese | 592 (17.74) | 176 (18.31) | |||
| Depressive episode, N (%)* | 150 (4.50) | 71 (7.39) | 12.80 | <.001 | |
| Age, M (SD)* | 74.40 (6.56) | 76.38 (7.30) | −7.58 | <.001 | |
| Education, M (SD)* | 13.39 (2.84) | 12.99 (3.01) | 3.81 | <.001 | |
| 3MS, M (SD)* | 91.59 (6.00) | 88.86 (8.36) | 9.43 | <.001 | |
| MET-min, Median (min–max) | 4,894.63 (0–18,345.60) | — | N/A | ||
| Sedentary | 0 (0.00–0.00) | — | |||
| Light | 2,608.20 (7.69–13,910.40) | — | |||
| Moderate–vigorous | 5,304.60 (547.63–18,345.60) | — |
Notes: 3MS = Modified Mini-Mental State Exam; APOE = apolipoprotein E; BDNF = brain-derived neurotrophic factor; BMI = body mass index; CCSMA = Cache County Study on Memory in Aging.
†Baseline characteristics of participants with complete data for physical activity and covariates, compared to excluded participants with incomplete data. Significant differences were evaluated using Pearson’s chi-square tests of independence and t-test analyses for categorical and continuous variables, respectively.
*p < .05 in t test and Pearson’s chi-square tests of independence.
Physical Activity
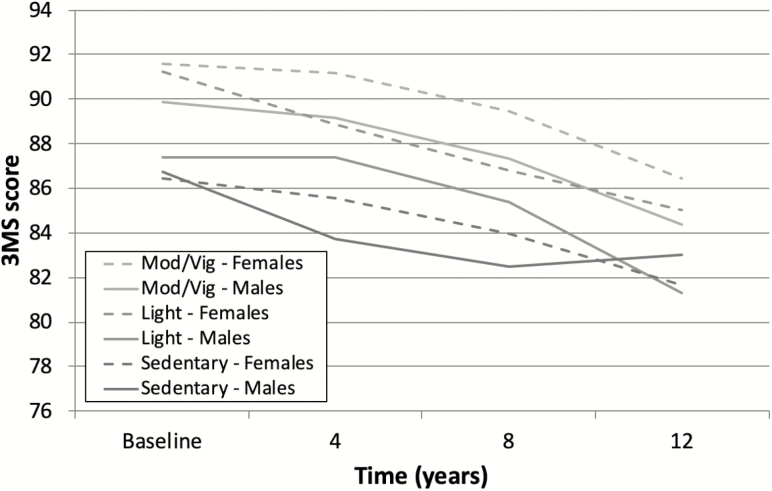
In unadjusted analyses examining physical activity and cognitive performance, persons who were sedentary or engaged in only light activity had significantly lower cognitive performance (β = −5.05 and −2.41, respectively, p < .001) compared to persons engaged in moderate–vigorous activity. In the final model adjusting for education, baseline age, sex, apolipoprotein E status, subjective health, and BMI, a three-way interaction term between physical activity, sex, and time showed that lower levels of physical activity predicted faster rate of cognitive decline, particularly among males (p = .006). Note, inclusion of the interaction did not significantly improve model fit with the Bonferroni adjustment (χ 2 = 17.68, df = 8, p = .024), but we provide the model information with this caveat. Table 2 shows parameter estimates of fixed effects in unadjusted and adjusted linear mixed models. Figure 1 illustrates the effect of physical activity in predicting rate of cognitive decline by sex, as determined by the fully adjusted model. See Table 3 for accompanying estimated marginal means.
Table 2.
Estimates of Fixed Effects for Physical Activity and Cognitive Decline as Modified by Sex
| 95% Confidence Interval | ||||||
|---|---|---|---|---|---|---|
| β | SE | p | Lower | Upper | Type III Test p | |
| Intercept | 118.48 | 1.12 | <.001 | 116.28 | 120.68 | <.001 |
| Time (y) | 0.06 | 0.06 | .291 | −0.06 | 0.18 | .029 |
| Time2 | −0.04 | 0.01 | <.001 | −0.05 | −0.03 | .088 |
| Physical activity (ref = mod–vig) | <.001 | |||||
| Sedentary | −5.14 | 1.25 | <.001 | −7.59 | −2.69 | |
| Light | −0.36 | 0.39 | .362 | −1.13 | 0.41 | |
| Physical activity × Time | .072 | |||||
| Sedentary × Time | −0.18 | 0.38 | .624 | −0.92 | 0.55 | |
| Light × Time | −0.69 | 0.16 | <.001 | −1.01 | −0.38 | |
| Physical activity × Time2 | .099 | |||||
| Sedentary × Time2 | 0.02 | 0.03 | .565 | −0.04 | 0.08 | |
| Light × Time2 | 0.05 | 0.02 | .001 | 0.02 | 0.08 | |
| Less than HS educ | −2.65 | 0.18 | <.001 | −3.00 | −2.30 | <.001 |
| Baseline age | −0.36 | 0.01 | <.001 | −0.39 | −0.34 | <.001 |
| Male | −1.69 | 0.20 | <.001 | −2.08 | −1.30 | .005 |
| No APOE e4 allele | 0.51 | 0.19 | .008 | 0.13 | 0.88 | .008 |
| Subjective health (ref = poor) | <.001 | |||||
| Excellent | 2.56 | 0.40 | <.001 | 1.78 | 3.34 | |
| Good | 2.28 | 0.38 | <.001 | 1.53 | 3.03 | |
| Fair | 1.72 | 0.39 | <.001 | 0.96 | 2.49 | |
| BMI (ref = obese) | .001 | |||||
| Underweight | −2.61 | 0.69 | <.001 | −3.96 | −1.26 | |
| Normal weight | −0.00 | 0.25 | .997 | −0.50 | 0.50 | |
| Overweight | −0.20 | 0.25 | .430 | −0.69 | 0.30 | |
| Physical activity × Sex | .005 | |||||
| Sedentary × Male | 1.97 | 1.74 | .257 | −1.44 | 5.37 | |
| Light × Male | −2.15 | 0.71 | .002 | −3.54 | −0.77 | |
| Male × Time | −0.10 | 0.09 | .279 | −0.27 | 0.08 | .929 |
| Male × Time2 | 0.01 | 0.01 | .529 | −0.01 | 0.02 | .861 |
| Physical activity × Sex × Time | .001 | |||||
| Sedentary × Male × Time | −0.76 | 0.60 | .204 | −1.92 | 0.41 | |
| Light × Male × Time | 0.99 | 0.29 | .001 | 0.42 | 1.56 | |
| Physical activity × Sex × Time2 | .006 | |||||
| Sedentary × Male × Time2 | 0.07 | 0.05 | .172 | −0.03 | 0.18 | |
| Light × Male × Time2 | −0.08 | 0.03 | .004 | −0.13 | −0.03 | |
Notes: BMI = body mass index; APOE = apolipoprotein E; HS = high school. Model parameters excluded persons missing on any primary predictors and covariates. Nested model comparisons used the same model parameters.
Figure 1.
Physical activity and sex interact to predict cognitive decline. Figure derived from estimated marginal means of each group while adjusting for mean baseline age and marginal means across education, apolipoprotein E (APOE) e4, subjective health, and body mass index (BMI).
Table 3.
Estimated Marginal Means for Rate of Cognitive Decline by Physical Activity and Sex
| Follow-up | |||||
|---|---|---|---|---|---|
| Group | Baseline | 4 y | 8 y | 12 y | |
| Sedentary | Males | 86.71 | 83.70 | 82.47 | 83.01 |
| Females | 86.43 | 85.58 | 83.99 | 81.65 | |
| Light | Males | 87.37 | 87.41 | 85.38 | 81.27 |
| Females | 91.22 | 88.86 | 86.80 | 85.05 | |
| Mod–vigorous | Males | 89.89 | 89.19 | 87.36 | 84.40 |
| Females | 91.58 | 91.18 | 89.48 | 86.47 | |
Notes: Model parameters excluded persons missing on any primary predictors and covariates. Estimated marginal means of each group adjust for mean baseline age and marginal means across education, apolipoprotein E (APOE) e4, subjective health, and body mass index (BMI).
BDNF-Related Genes and Physical Activity Predict Cognitive Performance
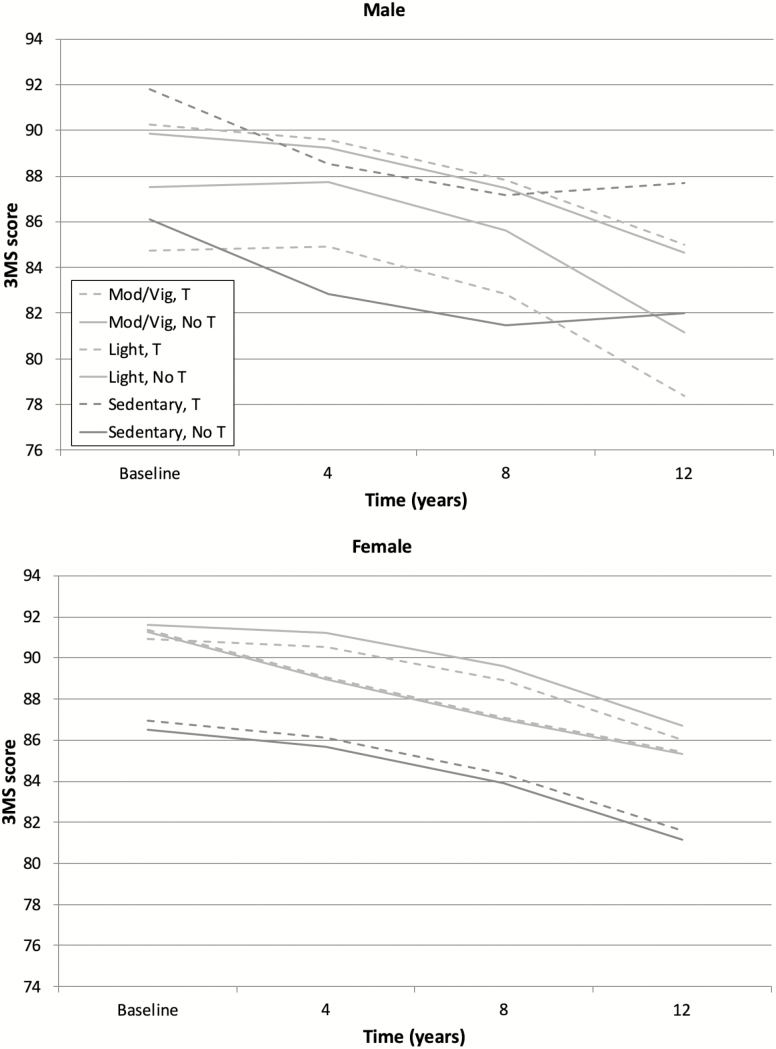
Models testing the association of BDNF-related SNPs and their interactions with physical activity indicated that NGFR rs2072446 was significantly associated with cognitive performance, but only when interacted with physical activity and sex (p = .004). These effects were significant in the presence of terms included in the previously adjusted model (see Supplementary Table 2 for parameter estimates of fixed effects). Among those in the light activity group, males who were homozygous for the dominant C allele had better cognitive performance compared to males with a minor T allele. Genotype differences for NGFR rs2072446 were not evident in other physical activity groups. See an illustration of this three-way interaction in Figure 2. None of the other BDNF-related SNPs, nor their interactions with physical activity or sex, were significantly associated with cognitive performance on the 3MS (p > .110).
Figure 2.
Physical activity as modified by single nucleotide polymorphism (SNP) rs2072446 predicts cognitive performance by sex. An interaction between brain-derived neurotrophic factor (BDNF)-related NGFR SNP rs2072446, physical activity, and sex indicated that there was a significant discrepancy in cognitive performance depending on rs2072446 genotype, but this effect was seen in males only. Within males engaging in light levels of physical activity, those homozygous for the dominant allele had better Modified Mini-Mental State Exam (3MS) scores compared to those with at least one minor allele. Note that differences at the sedentary level among males portrayed here were not statistically significant. Figure derived from estimated marginal means of each group while adjusting for mean baseline age and marginal means across education, apolipoprotein E (APOE) e4, subjective health, and body mass index (BMI).
Discussion
This study demonstrated that physical activity was associated with cognitive performance, though effects varied by sex and BDNF-related NGFR SNP rs2072446. Not only were lower levels of physical activity associated with worse cognitive performance, but level of physical activity predicted rate of cognitive decline differently among males and females. For example, males engaged in only light activity had a nonlinear, inverted U-shaped cognitive trajectory such that their discrepancy with females was most prominent at baseline and 12-year follow-up (approximately 4 points). Although there appeared to be a trend for differential rate of decline between sedentary males and females, this was not statistically significant. Lastly, the BDNF-related NGFR SNP rs2072446 modified the relationship between physical activity and sex in predicting cognitive performance, with no other significant interactions with BDNF-related genes. Overall, the results support that moderate–vigorous levels physical activity may help to preserve cognitive function in late-life with little overall contribution of BDNF-related SNPs.
Results of previous studies have hinted that sex differences may exist in the relationship of physical activity and cognitive decline (45); however, our study is the first to demonstrate an effect modification by sex. For example, a prior meta-analysis of aerobic intervention studies in older adults without dementia found that the relationship between exercise and cognitive performance was stronger in samples with a higher proportion of females (45). Additionally, a large prospective study of a community-based Canadian sample found that higher levels of physical activity significantly reduced risk for cognitive impairment, no dementia, AD, and all-cause dementia; however, when analyses were stratified by sex, the effects were only significant in females (46). While physical activity has been associated with better cognitive performance and reduced risk for dementia in both males and females, the benefits may be more pronounced in females (47) due to possible differential neuroprotective effects of sex hormones produced during exercise (48). Males experience a gradual decrease of sex hormones across mid- to late-life, while females experience a marked drop in reproductive hormones and, therefore, may be at increased risk for cognitive impairment. Exercise leads to increased production of sex hormones, particularly testosterone. Because older females have lower baseline levels of these hormones, they may benefit more than males from an influx of testosterone production associated with exercise (47). Further, level of physical activity may be associated with other health indicators differently for males and females due to possible baseline differences in physical activity. For example, in our sample, a greater proportion of males were engaged in moderate–vigorous activity (93.4% compared to 88.9% females at baseline) and a greater proportion of females were observed in the light group (10.2% compared to 5.2% males at baseline). Although we controlled for other health indicators (eg, subjective health status and BMI), it is possible that lower physical activity is generally more predictive of more severe medical comorbidities in males than females. Lastly, low power due to a small number of participants in the sedentary group may account for the lack of statistically significant differences observed between males and females at this activity level.
To our knowledge, this is the first study to investigate NTRK2 or NGFR polymorphisms in relation to the effects of exercise on cognition. Here, we demonstrated a relationship between cognitive performance and NGFR functional polymorphism rs2072446 (Ser205Leu) in older adults. The NGFR gene encodes the proneurotrophin receptor p75, previously shown to influence learning and memory in rodents. Barrett and colleagues showed that the p75 knockout mouse exhibits enhanced spatial memory and hippocampal long-term potentiation (30). Blocking the p75 receptor improves spatial learning and memory in aged mice (31) and a reduction in p75 expression ameliorates cognitive dysfunction in a mouse model of AD (32). LM11A-31, a small molecule ligand for p75 reduces pathological phosphorylation and misfolding of tau, inflammatory changes, cholinergic degeneration, and cognitive deficits in AβPP(L/S) transgenic mice (33). Consistent with these findings, neurotrophin processes at the synapse level appear to also support the beneficial effects of physical activity on memory. In amnestic rats, exercise was shown to improve the function of the septo-hippocampal cholinergic system (49) and spatial memory; however, sequestering of the nerve growth factor (NGF) abolished the exercise-induced recovery of spatial working memory and acetylcholine efflux in the hippocampus. Furthermore, the reemergence of the cholinergic neuronal phenotype within the medial septum/diagonal band following exercise was also selectively dependent on the actions of NGF (50). This research on the exercise-dependent enhancement of the septo-hippocampal cholinergic system through NGF has broad implications for recovery of memory function across a variety of neurological disorders. Our results add to the existing literature implicating proneurotrophin receptor p75 function in neurodegenerative disease (ie, AD) to demonstrate that it may also be relevant for cognitive performance in older adults without dementia.
Our study is also the first to investigate interactive effects of BDNF-related genotypes and physical activity. In this sample, the effects of NGFR rs2072446 varied by level of physical activity and sex, such that the effects of NGFR rs2072446 were significant among males engaged in only light activity. Among this light activity group, males who were homozygous for the dominant allele (C/C) of NGFR rs2072446 performed better by approximately 3 points on a measure of global cognitive function, compared to males with at least one minor T allele. Previous research indicates that a difference of 5 or more points on the 3MS represents clinically meaningful change (51), suggesting that the effects of NGFR rs2072446 may translate to minimal cognitive advantages in older males without dementia. In prior analyses using the Cache County sample, we reported sex differences in the risk of AD by NGFR rs2072446 such that female carriers of the minor T allele had significantly increased hazard of developing AD compared to male noncarriers (29). Alternatively, male carriers of the minor T allele had reduced risk of AD compared to male noncarriers at a trend-level significance. The former analyses did not examine physical activity, which may account for some of the seemingly discrepant results; however, the prior work was examining AD and not all cause-dementia, whereas the current study is examining more broadly, cognitive decline from any cause. Note that this study may not have been able to detect true differences in the effect of rs2072446 among sedentary males due to low sample and corresponding power at this level of physical activity. Replication in other populations with greater number of sedentary persons is recommended. Also of interest would be to examine interactions between a modifiable lifestyle factor, physical activity, and associations between rs2072446 and risk for AD.
Previous research has implicated BDNF rs6265 and rs56164415 in increased risk for AD, though the overall odds ratios have been modest (ie, 1.09 and 1.02, respectively) (28). The present study did not find evidence for rs6265 or rs56164415 in predicting cognitive decline in late-life. Prior research within the Cache County cohort also did not find evidence for rs6265 in predicting AD risk, but did find increased risk for female carriers of the minor T allele of rs56164415 (29). Differences between prior results and our current work on cognitive decline may reflect the exclusion of other causes of dementia and cognitive impairment (eg, vascular, mixed, or all-cause dementia) in the prior analyses and population differences with regard to ethnicity. Further, our findings do not account for possible gene–gene interactions found in other investigations of BDNF genes (26,52).
The present study’s strengths included a large population-based sample, high participation rates, and extensive longitudinal follow-up extending to 12 years. Our large sample size allowed us to examine sex differences in the associations between physical activity and cognitive decline, as well as those for BDNF-related SNPs. Additionally, we note that some studies have evaluated physical activity by characterizations of simple “yes/no” or grouping by low–moderate intensity together (6); however, we evaluated levels of physical activity: sedentary, light, and moderate–vigorous. Notably, we found no differences between moderate and vigorous activity groups prior to collapsing these two categories, which provides additional evidence in support of existing literature suggesting diminishing returns above moderate activity on cognitive outcomes (9). This contrasts with the dose–response relationship between physical activity and general health in adults aged 18–65 (10).
Nonetheless, the implications of our study are limited by our measurements of physical activity and cognition. For example, physical activity data were not collected at Wave 2, requiring imputation from Wave 1. This study also relied on self-report for participant engagement in physical activity, which may have led to response bias or inaccurate recall. The self-reported level of physical activity in this sample is high (eg, only 1% sedentary) but consistent with other indicators of health in older adult Utahns. For example, a 2013 report showed that the prevalence rate for diabetes in Cache County is among the lowest in the country at just 5.6% (53). Additionally, a 2014 report showed that 55.8% of Utahns met the aerobic exercise guidelines of the AHA, although this is broadly consistent with the western United States (54). Additionally, study results were consistent with other studies on physical activity (6,9) and robust such that moderate to vigorous levels of physical activity were associated with lower rates of cognitive decline, indicating the importance and strength of effects. In terms of cognition, our use of a global cognitive screening tool did not allow for an examination of specific cognitive domains that may be affected by physical activity with age. Literature on cognitive changes in normal aging suggests that fluid intelligence (eg, processing speed, complex attention) reduces with age while crystallized knowledge is preserved (55). While the hypothesis tested here implicates BDNF in exercise-related gains, especially for learning and memory (14), existing research suggests that aerobic exercise also improves processing speed and executive functioning in adults without dementia (5). Future research may benefit from use of specific neuropsychological tests of several domains. Nonetheless, the 3MS is a measure of global cognitive abilities, and our use of it here allows for meaningful contribution to existing literature in examining physical activity, BDNF, and cognitive decline in older adults.
In the present sample, there was a significant deviation from Hardy–Weinberg equilibrium with BDNF rs56164415; however, disequilibrium in other SNPs is uncommon in the broader Cache County sample (56). Lastly, our study examines an ethnically and religiously homogenous sample of rural Utahns, which potentially limits the generalizability of findings. However, previous research with this sample has been largely consistent with other large population-based studies and extensive genetic characterizations of the Utah population show genetic stability and similar genetic diversity to other ethnically homogenous groups (57,58).
In conclusion, this study offers evidence that physical activity may be protective against cognitive decline in older adults. Notably, sex differences were suggested for the effects of physical activity and may provide insight into potential interventions differentially tailored to males versus females. While associations between cognition and most BDNF-related SNPs investigated here were negligible, BDNF-related NGFR SNP rs2072446 appeared to modify cognitive performance in late-life among males only. Future research may benefit from evaluating the mechanisms behind these risk factors for cognitive impairment in older adults.
Supplementary Material
Supplementary data are available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Supplementary Table 1. Physical Activity Questionnaires by Wave.
Supplementary Table 2. Physical Activity as Modified by BDNF-Related Gene and Sex Predicts Cognitive Performance.
Funding
This work was supported by National Institutes of Health (NIH) grants R01AG11380, R01AG018712, RF1AG054052, and R21AG038767 to M.B., and Utah State University Research Catalyst Grant to M.B.
Acknowledgments
The authors would like to express gratitude and appreciation to the original Cache County Study investigators and the dedicated study participants that continue to inspire our work. The co-authors included in this study contributed to the development of the hypotheses tested here, literature review, statistical analyses, and preparation of this manuscript.
Conflict of Interest
Dr. J.S.K.K. is on the Scientific Advisory Board for ADx Neurosciences and is the Co-founder and on the board of Halia Therapeutics.
References
- 1. Alzheimer’s Association. 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016;12(4):459–509. doi:10.1016/j.jalz.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 2. Suthers K, Kim JK, Crimmins E. Life expectancy with cognitive impairment in the older population of the United States. J Gerontol B Psychol Sci Soc Sci. 2003;58(3):S179–S186. doi:10.1093/geronb/58.3.S179 [DOI] [PubMed] [Google Scholar]
- 3. Luppa M, Luck T, Weyerer S, König H-H, Brähler E, Riedel-Heller SG. Prediction of institutionalization in the elderly. A systematic review. Age Ageing. 2010;39(1):31–38. doi:10.1093/ageing/afp202 [DOI] [PubMed] [Google Scholar]
- 4. Groot C, Hooghiemstra AM, Raijmakers PG, et al. The effect of physical activity on cognitive function in patients with dementia: a meta-analysis of randomized control trials. Ageing Res Rev. 2016;25:13–23. doi:10.1016/j.arr.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 5. Hindin SB, Zelinski EM. Extended practice and aerobic exercise interventions benefit untrained cognitive outcomes in older adults: a meta-analysis. J Am Geriatr Soc. 2012;60(1):136–141. doi:10.1111/j.1532-5415.2011.03761.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sofi F, Valecchi D, Bacci D, et al. Physical activity and risk of cognitive decline: a meta-analysis of prospective studies. J Intern Med. 2011;269(1):107–117. doi:10.1111/j.1365-2796.2010.02281.x [DOI] [PubMed] [Google Scholar]
- 7. Lamb SE, Sheehan B, Atherton N, et al. Dementia And Physical Activity (DAPA) trial of moderate to high intensity exercise training for people with dementia: randomised controlled trial. BMJ. 2018;361:k1675. doi:10.1136/bmj.k1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Forbes D, Forbes SC, Blake CM, Thiessen EJ, Forbes S. Exercise programs for people with dementia. Cochrane Database Syst Rev. 2015;( 4):Cd006489. doi:10.1002/14651858.CD006489.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blondell SJ, Hammersley-Mather R, Veerman JL. Does physical activity prevent cognitive decline and dementia?: a systematic review and meta-analysis of longitudinal studies. BMC Public Health. 2014;14:510. doi:10.1186/1471-2458-14-510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–1434. doi:10.1249/mss.0b013e3180616b27 [DOI] [PubMed] [Google Scholar]
- 11. Bossers WJ, van der Woude LH, Boersma F, Hortobagyi T, Scherder EJ, van Heuvelen MJ. A 9-week aerobic and strength training program improves cognitive and motor function in patients with dementia: a randomized, controlled trial. Am J Geriatr Psychiatry. 2015;23(11):1106–1116. doi:10.1016/j.jagp.2014.12.191 [DOI] [PubMed] [Google Scholar]
- 12. Vreugdenhil A, Cannell J, Davies A, Razay G. A community-based exercise programme to improve functional ability in people with Alzheimer’s disease: a randomized controlled trial. Scand J Caring Sci. 2012;26(1):12–19. doi:10.1111/j.1471-6712.2011.00895.x [DOI] [PubMed] [Google Scholar]
- 13. Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors (Chur, Switzerland). 2004;22(3):123–131. doi:10.1080/08977190410001723308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yamada K, Nabeshima T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J Pharmacol Sci. 2003;91(4):267–270. doi:10.1254/jphs.91.267 [DOI] [PubMed] [Google Scholar]
- 15. Lu B, Nagappan G, Lu Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. In: Lewin GR, Carter BD, eds. Neurotrophic Factors. Berlin, Heidelberg: Springer Berlin Heidelberg; 2014:223–250. [DOI] [PubMed] [Google Scholar]
- 16. Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108(7):3017–3022. doi:10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gomez-Pinilla F, Vaynman S, Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur J Neurosci. 2008;28(11):2278–2287. doi:10.1111/j.1460-9568.2008.06524.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20(10):2580–2590. doi:10.1111/j.1460-9568.2004.03720.x [DOI] [PubMed] [Google Scholar]
- 19. Vilela TC, Muller AP, Damiani AP, et al. Strength and aerobic exercises improve spatial memory in aging rats through stimulating distinct neuroplasticity mechanisms. Mol Neurobiol. 2017;54(10):7928–7937. doi:10.1007/s12035-016-0272-x [DOI] [PubMed] [Google Scholar]
- 20. Maejima H, Kanemura N, Kokubun T, Murata K, Takayanagi K. Exercise enhances cognitive function and neurotrophin expression in the hippocampus accompanied by changes in epigenetic programming in senescence-accelerated mice. Neurosci Lett. 2018;665:67–73. doi:10.1016/j.neulet.2017.11.023 [DOI] [PubMed] [Google Scholar]
- 21. Sleiman SF, Henry J, Al-Haddad R, et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. ELife. 2016;5:e15092. doi:10.7554/eLife.15092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kennedy KM, Reese ED, Horn MM, et al. BDNF val66met polymorphism affects aging of multiple types of memory. Brain Res. 2015;1612:104–117. doi:10.1016/j.brainres.2014.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Toh YL, Ng T, Tan M, Tan A, Chan A. Impact of brain-derived neurotrophic factor genetic polymorphism on cognition: a systematic review. Brain Behav. 2018;8(7):e01009. doi:10.1002/brb3.1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adachi N, Numakawa T, Richards M, Nakajima S, Kunugi H. New insight in expression, transport, and secretion of brain-derived neurotrophic factor: implications in brain-related diseases. World J Bioll Chem. 2014;5(4):409–428. doi:10.4331/wjbc.v5.i4.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miyajima F, Ollier W, Mayes A, et al. Brain-derived neurotrophic factor polymorphism Val66Met influences cognitive abilities in the elderly. Genes Brain Behav. 2008;7(4):411–417. doi:10.1111/j.1601-183X.2007.00363.x [DOI] [PubMed] [Google Scholar]
- 26. Ward DD, Summers MJ, Saunders NL, Janssen P, Stuart KE, Vickers JC. APOE and BDNF Val66Met polymorphisms combine to influence episodic memory function in older adults. Behav Brain Res. 2014;271:309–315. doi:10.1016/j.bbr.2014.06.022 [DOI] [PubMed] [Google Scholar]
- 27. Huang R, Huang J, Cathcart H, Smith S, Poduslo SE. Genetic variants in brain-derived neurotrophic factor associated with Alzheimer’s disease. J Med Genet. 2007;44(2):e66. doi:10.1136/jmg.2006.044883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39(1):17–23. doi:10.1038/ng1934 [DOI] [PubMed] [Google Scholar]
- 29. Matyi J, Tschanz JT, Rattinger GB, et al. Sex differences in risk for Alzheimer’s disease related to neurotrophin gene polymorphisms: the Cache County Memory Study. J Gerontol A Biol Sci Med Sci. 2017;72(12):1607–1613. doi:10.1093/gerona/glx092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barrett GL, Reid CA, Tsafoulis C, et al. Enhanced spatial memory and hippocampal long-term potentiation in p75 neurotrophin receptor knockout mice. Hippocampus. 2010;20(1):145–152. doi:10.1002/hipo.20598 [DOI] [PubMed] [Google Scholar]
- 31. Buhusi M, Etheredge C, Granholm AC, Buhusi CV. Increased hippocampal proBDNF contributes to memory impairments in aged mice. Front Aging Neurosci. 2017;9:284. doi:10.3389/fnagi.2017.00284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murphy M, Wilson YM, Vargas E, et al. Reduction of p75 neurotrophin receptor ameliorates the cognitive deficits in a model of Alzheimer’s disease. Neurobiol Aging. 2015;36(2):740–752. doi:10.1016/j.neurobiolaging.2014.09.014 [DOI] [PubMed] [Google Scholar]
- 33. Simmons DA, Knowles JK, Belichenko NP, et al. A small molecule p75NTR ligand, LM11A-31, reverses cholinergic neurite dystrophy in Alzheimer’s disease mouse models with mid- to late-stage disease progression. PLoS One. 2014;9(8):e102136. doi:10.1371/journal.pone.0102136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen Z, Simmons MS, Perry RT, Wiener HW, Harrell LE, Go RC. Genetic association of neurotrophic tyrosine kinase receptor type 2 (NTRK2) With Alzheimer’s disease. Am J Med Genet B Neuropsychiatr Genet. 2008;147(3):363–369. doi:10.1002/ajmg.b.30607 [DOI] [PubMed] [Google Scholar]
- 35. Breitner JC, Wyse BW, Anthony JC, et al. APOE-epsilon4 count predicts age when prevalence of AD increases, then declines: the Cache County Study. Neurology. 1999;53(2):321–331. doi:10.1212/wnl.53.2.321 [DOI] [PubMed] [Google Scholar]
- 36. Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 37. Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24(1):145–153. doi:10.1017/S003329170002691X [DOI] [PubMed] [Google Scholar]
- 38. Association AP. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. Revised. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 39. McDowell I, Kristjansson B, Hill GB, Hebert R. Community screening for dementia: the Mini Mental State Exam (MMSE) and Modified Mini-Mental State Exam (3MS) compared. J Clin Epidemiol. 1997;50(4):377–383. doi:10.1016/S0895-4356(97)00060-7 [DOI] [PubMed] [Google Scholar]
- 40. Tschanz JT, Welsh-Bohmer KA, Plassman BL, Norton MC, Wyse BW, Breitner JC. An adaptation of the modified mini-mental state examination: analysis of demographic influences and normative data: the cache county study. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15(1):28–38. [PubMed] [Google Scholar]
- 41. Abraham IL, Manning CA, Boyd MR, et al. Cognitive screening of nursing home residents: factor structure of the modified mini-mental state (3MS) examination. Int J Geriatr Psychiatry. 1993;8(2):133–138. doi:10.1002/gps.930080205 [Google Scholar]
- 42. Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele ϵ4 with late‐onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43(8):1467. doi:10.1212/wnl.43.8.1467 [DOI] [PubMed] [Google Scholar]
- 43. Robins LN, Helzer JE, Ratcliff KS, Seyfried W. Validity of the diagnostic interview schedule, version II: DSM-III diagnoses. Psychol Med. 1982;12:855–870. doi:10.1017/S0033291700049151 [DOI] [PubMed] [Google Scholar]
- 44. Wengreen HJ, Munger RG, Corcoran CD, et al. Antioxidant intake and cognitive function of elderly men and women: the Cache County Study. J Nutr Health Aging. 2007;11(3):230–237. [PubMed] [Google Scholar]
- 45. Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14(2):125–130. doi:10.1111/1467–9280.t01-1-01430 [DOI] [PubMed] [Google Scholar]
- 46. Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58(3):498–504. doi:10.1001/archneur.58.3.498 [DOI] [PubMed] [Google Scholar]
- 47. Brown BM, Peiffer JJ, Martins RN. Multiple effects of physical activity on molecular and cognitive signs of brain aging: can exercise slow neurodegeneration and delay Alzheimer’s disease? Mol Psychiatry. 2013;18(8):864–874. doi:10.1038/mp.2012.162 [DOI] [PubMed] [Google Scholar]
- 48. Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer’s disease. Front Neuroendocrinol. 2009;30(2):239–258. doi:10.1016/j.yfrne.2009.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hall JM, Savage LM. Exercise leads to the re-emergence of the cholinergic/nestin neuronal phenotype within the medial septum/diagonal band and subsequent rescue of both hippocampal ACh efflux and spatial behavior. Exp Neurol. 2016;278:62–75. doi:10.1016/j.expneurol.2016.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hall JM, Gomez-Pinilla F, Savage LM. Nerve growth factor is responsible for exercise-induced recovery of septohippocampal cholinergic structure and function. Front Neurosci. 2018;12:773. doi:10.3389/fnins.2018.00773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Andrew MK, Rockwood K. A five-point change in Modified Mini-Mental State Examination was clinically meaningful in community-dwelling elderly people. J Clin Epidemiol. 2008;61(8):827–831. doi:10.1016/j.jclinepi.2007.10.022 [DOI] [PubMed] [Google Scholar]
- 52. Hwang JP, Tsai SJ, Hong CJ, Yang CH, Lirng JF, Yang YM. The Val66Met polymorphism of the brain-derived neurotrophic-factor gene is associated with geriatric depression. Neurobiol Aging. 2006;27(12):1834–1837. doi:10.1016/j.neurobiolaging.2005.10.013 [DOI] [PubMed] [Google Scholar]
- 53. Prevention CfDCa. Diagnosed Diabetes Percentage 2013. https://www.cdc.gov/diabetes/atlas/countydata/atlas.html. Accessed April 24, 2018.
- 54. Centers for Disease Control and Prevention. State Indicator Report on Physical Activity, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Harada CN, Natelson Love MC, Triebel KL. Normal cognitive aging. Clin Geriatr Med. 2013;29(4):737–752. doi:10.1016/j.cger.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sharp AR, Ridge PG, Bailey MH, et al. Population substructure in Cache County, Utah: the Cache County study. BMC Bioinformatics. 2014;15 (suppl 7):S8. doi:10.1186/1471-2105-15-S7-S8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jorde LB, Morgan K. Genetic structure of the Utah Mormons: isonymy analysis. Am J Phys Anthropol. 1987;72(3):403–412. doi:10.1002/ajpa.1330720313 [DOI] [PubMed] [Google Scholar]
- 58. Skolnick M. The Utah geneological database: a resource for genetic epidemiology. In: Banbury Report No. 4: Cancer Incidence in Defined Populations. New York, NY: Cold Spring Harbor Laboratory Press; 1980:285–297. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.