Abstract
Background
Fatigability is a construct that measures whole-body tiredness anchored to activities of a fixed intensity and duration; little is known about its epidemiology and heritability.
Methods
Two generations of family members enriched for exceptional longevity and their spouses were enrolled (2006–2009) in the Long Life Family Study (LLFS). At Visit 2 (2014–2017, N = 2,355) perceived physical fatigability was measured using the 10-item self-administered Pittsburgh Fatigability Scale (PFS), along with demographic, medical, behavioral, physical, and cognitive risk factors.
Results
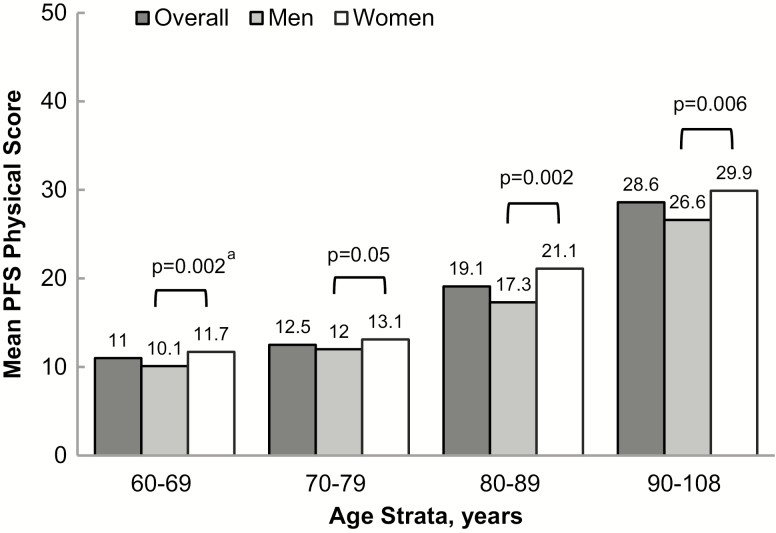
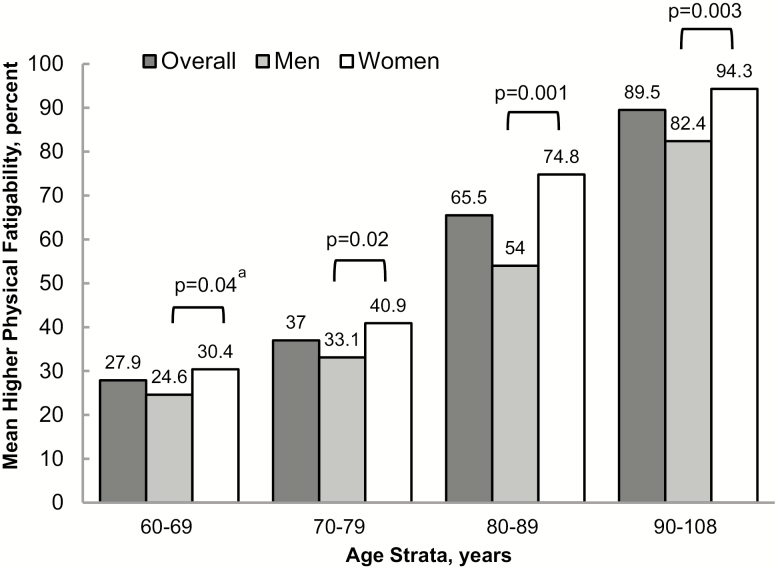
Residual genetic heritability of fatigability was 0.263 (p = 6.6 × 10–9) after adjustment for age, sex, and field center. PFS physical scores (mean ± SD) and higher physical fatigability prevalence (% PFS ≥ 15) were greater with each age strata: 60–69 (n = 1,009, 11.0 ± 7.6, 28%), 70–79 (n = 847, 12.5 ± 8.1, 37%), 80–89 (n = 253, 19.3 ± 9.9, 65.2%), and 90–108 (n = 266, 28.6 ± 9.8, 89.5%), p < .0001, adjusted for sex, field center, and family relatedness. Women had a higher prevalence of perceived physical fatigability compared to men, with the largest difference in the 80–89 age strata, 74.8% versus 53.5%, p < .0001. Those with greater body mass index, worse physical and cognitive function, and lower physical activity had significantly higher perceived physical fatigability.
Conclusions
Perceived physical fatigability is highly prevalent in older adults and strongly associated with age. The family design of LLFS allowed us to estimate the genetic heritability of perceived physical fatigability. Identifying risk factors associated with higher perceived physical fatigability can inform the development of targeted interventions for those most at risk, including older women, older adults with depression, and those who are less physically active.
Keywords: Fatigue, Physical function, Cognitive function, Heritability
Fatigue is a highly prevalent symptom in the aging population and is an important risk factor for loss of function, disability, future disease, and mortality (1–4). However, the use of traditional fatigue scales is limited due to their broad, subjective, qualitative nature, which makes them prone to self-pacing bias (5,6). Fatigue, as a concept decontextualized from daily activities and lifestyle, may not be accurately capturing symptoms and impact. This is particularly true for older adult populations who often report lower physical activity levels than their younger counterparts. Physical (whole-body) fatigability measures an individual’s tiredness anchored to standardized tasks or activities (ie, demand) of a specific intensity and duration, which may be able to overcome the limitations of traditional fatigue scales and better pinpoint older adults at risk for loss of function and mobility decline (5,7). Measuring fatigability accounts for self-pacing (ie, activity adjustment) and may lead to comparisons that are more meaningful between participants and across studies as well as explain the inconsistent relationship with fatigue measures across age (5,8–14).
To date, we know little about the epidemiology of perceived physical fatigability and its associations with demographic and lifestyle characteristics, physical and cognitive function measures, and health and comorbidities. Emerging work indicates that slower gait speed, a measure of physical function and an established predictor of aging outcomes, is associated with higher physical fatigability in older adults (15). Higher physical and mental fatigability also predicts meaningful functional decline in older adults, and higher body mass index (BMI) and lower physical activity levels are associated with higher physical fatigability (9,10,16–18). Expanding fatigability research across the older adult age span is crucial for our understanding of its role along the disablement pathway, and identifying risk factors in older adults that will aid in the future development of targeted interventions and improve comparisons across cohorts.
We chose to examine the relationship between age and fatigability in the Long Life Family Study (LLFS), a novel family cohort enriched for longevity (19,20). Although this cohort overall has better health and higher physical function compared to the general population, the LLFS was able to provide a broad range of ages as well as a comprehensive set of measurements to carefully examine risk factors for fatigability. Therefore, the aims of this paper were twofold: (i) examine the prevalence of higher perceived physical fatigability by age and sex and (ii) evaluate potential risk factors related to higher perceived physical fatigability across age strata. We hypothesize that the prevalence of perceived physical fatigability will be greater across age and higher for women than for men.
Method
Study Population
LLFS is a longitudinal cohort study conducted at three U.S. field centers (University of Pittsburgh, Boston University School of Medicine, Columbia University Medical Center) and one in Denmark (University of Southern Denmark) in families determined to have “exceptional survival” by the Family Longevity Selection Score (19). From 2006 to 2009 probands, their siblings and spouse controls (n = 1,727) and offspring and spouse controls (n = 3,226), were enrolled for a total cohort size of 4,953 participants. The details of participant eligibility and screening have been described elsewhere (20). Once enrolled, we followed up participants on an annual basis by telephone interview to track physical function, medical history, and vital status. A second round of in-person examinations was conducted from 2014 to 2017. For both examinations, if an in-person visit was not feasible, we conducted telephone interviews and a blood or saliva sample was obtained by an outside physician’s office or laboratory (20).
Assessment of Perceived Physical Fatigability
Perceived physical fatigability was measured using the validated self-administered 10-item Pittsburgh Fatigability Scale (PFS) at Visit 2 (8). The PFS evaluates both physical and mental fatigability in separate subscales. Participants were asked to rate on a scale from 0 (no fatigue) to 5 (extreme fatigue) the level of tiredness/exhaustion they expected or imagined they would feel after completing 10 different activities ranging in type and intensity such as watching TV for 2 h to brisk or fast walking for 1 h (8). Responses were summed to derive a PFS physical score ranging from 0 to 50, with higher scores indicating greater perceived physical fatigability (8). A cut point for higher (PFS ≥15) and lower (PFS <15) perceived physical fatigability was established during the initial validation in the Baltimore Longitudinal Study of Aging based on discrimination of objective physical performance and function outcomes, and is further detailed elsewhere (7,16). Participants from Denmark completed the Danish translated version of the PFS. Validation of the psychometric properties of the Danish PFS included forward and backward translation, cross-cultural adaptation as well as test–retest reliability. The detailed validation of the Danish PFS and the examination of the epidemiology of perceived mental fatigability are beyond the scope of this paper.
Ascertainment and Measurement of Risk Factors
Age was validated using date of birth from an official document or source such as a driver’s license or birth certificate at the baseline visit (21). At this visit, we also collected self-reported information on sex, race, ethnicity, and education levels (20). Self-reported marital status was collected at in-person Visit 1 and updated at Visit 2.
Smoking status was assessed by self-reported current cigarette or cigar smoking at Visit 2. Physical activity levels were measured using total Metabolic Equivalent of Task (MET) hours/day for a typical day over the past year using the Framingham Physical Activity Index at Visit 2 (22). Standing height was measured to the nearest 0.1 cm at Visit 2 with a Handi-stat set square (Perspective Enterprises, Portage, MI); an average of two to four measurements was taken. At Visit 2, weight was measured to the nearest 0.1 kg with an electronic digital scale (SECA 841, Hanover, MD). BMI was calculated from these measurements as weight (kg)/height (m2) (21). Average abdominal circumference was measured twice to the nearest 0.1 cm, and if the difference between those two measurements was greater than 1.0 cm, two additional measurements were taken. The average of these waist circumference measurements was calculated and used in this analysis. Waist-to-height ratio, a predictor of intra-abdominal fat, was derived by dividing waist circumference by average standing height (23).
Physical function at Visit 2 was assessed using questions about ease of activities of daily living (ADLs) and instrumental activities of daily living (IADLs), performance tests of grip strength, Short Physical Performance Battery (SPPB) scores, and usual gait speed. ADL difficulty was defined as self-reported difficulty with at least one of the following three items: bathing/showering, walking up 10 steps, or getting in/out of a bed or chair. IADL dependence was defined as self-reported inability to perform any of the following tasks independently: using a telephone, shopping, preparing food, doing laundry, administering medications, going places, housekeeping, or personal finances. The SPPB, an objective assessment of lower extremity function in older adults, consisted of three balance tests, a 4 m walking test, and five timed chair stands and was scored 0–12 with higher scores indicating better physical function (24). Grip strength was assessed by taking the maximum of three trials on the dominant hand, using an isometric dynamometer in a seated position to the nearest 2 kg (21). Gait speed (m/s) was obtained from the fastest of two timed 4 m (or 3 m when space was not available in the home) walk tests administered within the SPPB.
At Visit 2, cognition was assessed using the 30-item Mini Mental State Exam (MMSE) and the Digit Symbol Substitution Test (DSST), two validated tests conducted as part of a larger neuropsychological battery (25,26). These two tests represent widely used measures, general cognition (MMSE) and psychomotor speed (DSST).
Self-reported diagnosis of cancer (excluding skin) was collected at Visit 1 and then updated during the annual telephone follow-ups and again at Visit 2. Centrally trained research assistants measured sitting blood pressure at Visit 2 using the average of three readings from an automated blood pressure machine. Hypertension was defined as systolic blood pressure ≥130 mmHg and diastolic blood press ≥80 mmHg; diabetes was derived according to the 2018 criteria from the American Diabetes Association that uses hemoglobin A1c as gold standard (≥6.5) (27). Blood samples were taken at Visit 2 after a minimum of 6 h fast, and the central laboratory at the University of Minnesota measured blood glucose (21). Depressive symptoms at Visit 2 were assessed using the 10-item (30 point) Center for Epidemiologic Studies—Depression Scale (CES-D) (28). Participants from the Denmark field center completed the entire LLFS battery in Danish, including using validated country specific versions of the MSSE, DSST, and CES-D.
Statistical Analysis
Of the original cohort, 2,746 participants were alive and agreed to participate in the second in-person visit, plus an additional 160 new family members were enrolled for a total of 2,906.
Our final analytic sample excluded participants with insufficient specific information on age (n = 3) and those <60 years of age (n = 306) as our fatigability outcome measure was validated for adults ≥60 years old. Of the N = 2,597 Visit 2 participants aged ≥60 years, n = 379 had no or incomplete PFS physical score data. To handle incomplete PFS data, if ≤3 items were missing, but the related question on whether the activity had been done in the past month was answered, a value for the missing response was imputed (16). Thus, the final analytic sample was N = 2,355 (n = 603 Pittsburgh, n = 652 Boston, n = 468 Columbia, and n = 632 Denmark). Scores were imputed for 137 participants.
We examined the residual genetic heritability of perceived physical fatigability using the variance component framework implemented in the Sequential Oligogenic Linkage Analysis Routines program, which estimates the effect of additive genetics adjusted for family relatedness (29,30). Perceived physical fatigability was log-transformed for normality prior to Sequential Oligogenic Linkage Analysis Routines analysis. Genetic heritability was also adjusted for age, sex, and field center.
First, our sample was classified into four age strata (60–69, 70–79, 80–89 and 90–108 years) to examine the prevalence of potential risk factors by age. Next, we used generalized linear modeling with family relatedness as repeated subject to examine differences in PFS physical scores and higher perceived physical fatigability across age strata and by sex, adjusted for field center. Then, to examine potential risk factors for higher perceived physical fatigability across age strata, we used age-stratified generalized estimating equations with an exchangeable covariance matrix with p-values for differences accounting for family relatedness and adjusted for field center, sex, and education (cognitive measures only). Statistical significance was considered at p < .05 with Bonferroni correction for multiple comparisons at p = .0024.
Finally, a generalized linear model with backward selection (p = .15 to stay), adjusted for field center and accounting for family relatedness, was used to assess the strongest independent risk factors associated with higher perceived physical fatigability. Multicollinearity was evaluated, and weight-to-height ratio, waist circumference, and grip strength were removed due to high levels of collinearity with other variables. We repeated this analysis by replacing our fatigability outcome measure with a commonly used single-item global fatigue measure, “I felt everything I did was an effort” from the CES-D to assess the relationship between age and fatigue after adjustment for other risk factors. For the CES-D measure, higher fatigue was classified as those answering “some”, “moderate”, or “most of the time” whereas those answering “rarely or none of the time” were classified with lower fatigue. All analyses were conducted using SAS version 9.4.
Results
Overall, the sample ranged in age from 60 to 108 years, with nearly 55% women. Participants were generally highly educated (84% with more than a high-school education), overweight (mean BMI = 27.4 ± 5.0 kg/m2), and few currently smoked (4%). On average, the cohort was better functioning (mean SPPB 10.2 ± 2.7) and nearly 94% self-reported good or better health (Table 1). After adjustment for age, sex, and field center, the residual genetic heritability of perceived physical fatigability was 0.263 (p = 6.6 × 10−9). Overall, mean PFS physical scores were 1.6-fold greater from the youngest age strata (11.0) to the oldest age strata (28.6), p < .0001 (Figure 1). Furthermore, the prevalence of higher perceived physical fatigability was greater with age (Figure 2), from 27.9% in the 60–69 year old strata to 89.5% for participants aged 90–108 (p < .0001). When stratified by sex, women reported greater mean PFS physical scores (Figure 1). Also, a larger proportion of women had higher perceived physical fatigability than men in each age strata, including a 5% difference in 60–69 (p = .04), 8% difference in 70–79 (p = .02), 21% difference in 80–89 (p = .001), and 12% difference in 90–108 (p = .0003) with p-values for sex differences accounting for family relatedness and adjusted for field center (Figure 2).
Table 1.
Sample Characteristics By Age Strata: The Long Life Family Study
| Overall [N = 2355] | 60–69 years [n = 1000] | 70–79 years [n = 837] | 80–89 years [n = 252] | 90–108 years [n = 266] | |
|---|---|---|---|---|---|
| Demographic | |||||
| Age, years | 73.7 ± 10.5a | 65.0 ± 2.8 | 73.7 ± 2.8 | 84.2 ± .2.9 | 96.2 ± 4.0 |
| Sex, women | 54.7 [1289] | 56.9 [569] | 50.5 [423] | 55.2 [139] | 59.4 [158] |
| Currently married | 69.0 [1624] | 79.3 [793] | 77.3 [647] | 56.8 [143] | 15.4 [41] |
| Education level | |||||
| Less than high school | 7.3 [172] | 4.1 [41] | 7.5 [63] | 7.9 [20] | 18.1 [48] |
| High school or equivalent | 8.4 [197] | 3.5 [35] | 6.1 [51] | 22.2 [56] | 20.8 [55] |
| More than high school | 84.3 [1983] | 92.4 [923] | 86.4 [722] | 69.8 [176] | 61.1 [162] |
| Lifestyle and anthropometric | |||||
| Smoking status | |||||
| Current | 4.0 [93] | 5.0 [50] | 4.1 [34] | 2.4 [6] | 1.1 [3] |
| Former | 39.0 [913] | 36.2 [360] | 44.0 [367] | 36.6 [91] | 35.9 [95] |
| Never | 57.1 [1337] | 58.8 [584] | 52.0 [434] | 61.0 [152] | 63.0 [167] |
| Total MET-hours per day | 36.3 ± 7.2 [2306] | 37.7 ± 6.9 [985] | 37.5 ± 6.8 [818] | 34.6 ± 7.0 [246] | 29.1 ± 4.8 [257] |
| Body mass index, kg/m2 | 27.4 ± 5.0 [2229] | 27.8 ± 5.3 [960] | 27.6 ± 4.9 [808] | 27.0 ± 4.8 [233] | 25.9 ± 4.2 [228] |
| Waist circumference, cm | 96.7 ± 13.3 [2225] | 96.5 ± 13.7 [959] | 97.7 ± 13.3 [809] | 96.8 ± 12.2 [234] | 93.8 ± 11.6 [223] |
| Waist-to-height ratio | 0.58 ± 0.08 [2211] | 0.57 ± 0.08 [958] | 0.58 ± 0.08 [807] | 0.60 ± 0.08 [229] | 0.60 ± 0.07 [217] |
| Physical function | |||||
| ADL difficulty | 28.0 [660] | 3.3 [33] | 6.5 [54] | 19.1 [48] | 62.6 [166] |
| IADL dependence | 20.1 [474] | 4.6 [46] | 7.1 [59] | 18.9 [47] | 60.6 [154] |
| Grip strength, kg | 29.2 ± 11.3 [2239] | 32.6 ± 10.8 [959] | 30.5 ± 10.2 [807] | 23.0 ± 8.8 [236] | 16.6 ± 7.0 [237] |
| SPPB score, 0–12 | 10.2 ± 2.7 [2248] | 11.2 ± 1.4 [966] | 10.8 ± 1.7 [811] | 8.7 ± 2.6 [234] | 5.1 ± 3.1 237] |
| Usual gait speed, m/s | 1.0 ± 0.3 [2193] | 1.1 ± 0.2 [944] | 1.1 ± 0.2 [787] | 0.89 ± 0.55 [226] | 0.58 ± 0.22 [236] |
| Cognitive function | |||||
| MMSE score, 0–30 | 28.4 ± 2.9 [2265] | 29.2 ± 1.7 [964] | 28.9 ± 1.8 [813] | 27.6 ± 3.1 [240] | 24.7 ± 5.0 [248] |
| Digit symbol substitution score, 0–100 | 44.5 ± 13.6 [2168] | 51.3 ± 11.2 [952] | 43.6 ± 10.3 [792] | 36.5 ± 12.2 [222] | 25.0 ± 12.1 [202] |
| Health conditions | |||||
| Fair or poor self-rated health | 4.1 [97] | 2.1 [21] | 3.0 [25] | 1.6 [4] | 5.4 [14] |
| Diabetesb | 9.9 [232] | 8.3 [83] | 12.4 [104] | 13.9 [35] | 15.8 [42] |
| Fasting glucose, mg/dL | 100.2 ± 22.3 [2167] | 99.4 ± 23.4 [935] | 101.0 ± 21.4 [784] | 101.3 ± 21.3 [230] | 99.3 ± 21.1 [218] |
| Hypertensionc | 60.3 [1566] | 54.1 [567] | 64.8 [588] | 65.6 [187] | 62.8[224] |
| Cancer history, excluding skin | 18.3 [43] | 12.3 [123] | 22.1 [185] | 26.2 [66] | 21.1 [56] |
| Depressive symptoms (CES-D) score, 0–30 | 3.3 ± 3.5 [2271] | 2.9 ± 3.3 [979] | 3.0 ± 3.3 [824] | 4.2 ± 4.1 [230] | 4.7 ± 3.9 [227] |
Note: MET = Metabolic Equivalent of Task from the Framingham Physical Activity Index; ADL = activities of daily living; IADL = instrumental activities of daily living; SPPB = Short Physical Performance Battery score; MMSE = Mini-Mental State Examination; CES-D = Center for Epidemiologic Studies—Depression Scale score.
aMean ± SD [n] or % [n].
b
Diabetes defined according to American Diabetes Association 2018 classification (hemoglobin A1c ≥6.5).
cHypertension defined as systolic blood pressure ≥140 mmHg and diastolic blood pressure ≥80 mmHg.
Figure 1.
Mean Pittsburgh Fatigability Scale physical scores by age strata and sex in the Long Life Family Study. ap-values between sex are adjusted for field center and account for family relatedness.
Figure 2.
Prevalence (%) of higher perceived physical fatigability by age strata and sex in the Long Life Family Study. Note: Higher perceived physical fatigability defined as Pittsburgh Fatigability Scale score ≥15; ap-values between sex are adjusted for field center and account for family relatedness.
Supplementary Table 1 presents the evaluation of the potential risk factors associated with higher versus lower perceived physical fatigability, adjusted for sex, field center and accounting for family relatedness. Higher perceived physical fatigability was associated with being currently unmarried in the 60–69 age strata (p = .01) and having less than a high-school education in the 70–79 strata (p = .02). No demographic characteristics remained associated with higher perceived physical fatigability across any age strata after multiple-comparisons adjustment (21 tests). Across all age strata, those with higher perceived physical fatigability had lower physical activity scores (p < .002). Furthermore, those with higher perceived physical fatigability had greater BMI, waist circumference, and waist-to-height ratio, but only in the two youngest age strata (p < .0001) after adjustment for multiple comparisons.
In adjusted analyses, those with higher perceived physical fatigability had worse SPPB scores, slower gait speed, and more ADL difficulty in every age strata (p < .0001 for all). Additionally, those with higher perceived physical fatigability also had worse grip strength in the 80–89 strata only, and IADL dependence in some (70–79 and 90–108), but not all, age strata (Supplementary Table 1).
MMSE scores were lower among those with higher perceived fatigability in the 70–79 (p = .002), 80–89 (p = .05), and 90–108 (p = .001) age strata, but no difference was found among higher and lower fatigability in the youngest age strata. Higher perceived physical fatigability was also associated with lower DSST scores in the three younger strata, but the oldest strata did not have a large enough sample to model these data (Supplementary Table 1).
Depressive symptomatology was low overall, but those with higher perceived physical fatigability had consistently significantly higher depression symptoms in all age strata. Those self-reporting fair or poor health had higher perceived physical fatigability in the 60–69 and 70–79 age strata. Hypertension was not associated with having higher perceived physical fatigability across age strata. Participants with higher perceived physical fatigability had greater prevalence of diabetes, and higher mean fasting glucose only in the age strata under 80 years old (p < .02). Although those with higher perceived physical fatigability had greater cancer rates, we found no significant differences across age strata (Supplementary Table 1).
The multivariable analysis (Table 2) identified the following risk factors that remained associated with higher perceived physical fatigability: older age (p = .001), being a women (p = .0004), having greater depressive symptoms (p < .0001), higher BMI (p < .0001), lower physical activity (p < .0001), having difficulty with ADLs (p < .0001), slower gait speed (p = .02), worse physical function (p = .03), and lower digit symbol substation test scores (p = .04). The multivariable model with global fatigue as the outcome (Supplementary Table 2) did not have age and sex remain in the model as risk factors associated with higher fatigue. Furthermore, there were no sex differences across the age strata for the single-item CES-D measure of global fatigue, except for a trend toward significance in the 80–89 age strata (Supplementary Figure 1).
Table 2.
Multivariable Analysis to Examine Potential Risk Factors of Higher Perceived Physical Fatigability Using the Pittsburgh Fatigability Scale: The Long Life Family Study
| β coefficienta | p-value | |
|---|---|---|
| Demographic | ||
| Age, years | 0.04 | .005 |
| Sex, women | 0.27 | .0004 |
| Lifestyle and anthropometric | ||
| Total MET hours per day | –0.07 | <.0001 |
| Body mass index, kg/m2 | 0.11 | <.0001 |
| Physical function | ||
| ADL difficulty | 0.84 | <.0001 |
| SPPB score, 0–12 | –0.14 | .03 |
| Usual gait speed, m/s | –1.06 | .02 |
| Cognitive function | ||
| Digit symbol substitution score, 0–100 | –0.02 | .04 |
| Health conditions | ||
| Depressive symptoms (CES-D) score, 0–30 | 0.13 | <.0001 |
Note: ADL = activities of daily living; CES-D = Center for Epidemiologic Studies—Depression Scale score; MET = Metabolic Equivalent of Tasks from the Framingham Physical Activity Index; SPPB = Short Physical Performance Battery score.
aModel adjusted for field center and account for family relatedness.
Discussion
The results of this cross-sectional analysis of the LLFS cohort suggest that higher perceived physical fatigability is more prevalent for women than men, greater across older age strata, and associated with lower levels of physical activity, health conditions, and several measures of physical and cognitive function. Despite LLFS being a healthier cohort enriched for exceptional longevity, the prevalence of higher perceived physical fatigability was considerable at 42% overall for those age 60–108 years, and ranged from 28% for those 60–69 years to nearly 90% for those age 90–108 years. In a nationally representative birth cohort from the United Kingdom, Cooper and colleagues (16) reported prevalence rates of higher perceived physical fatigability of 49.4% and 37% for women and men, respectively, at age 68, slightly higher, but overall consistent with our 60–69 and 70–79 age strata of older adults enriched for longevity.
Our sensitivity analyses with a single-item global fatigue measure as the outcome in the multivariable model in place of perceived physical fatigability failed to detect an age association similarly to several other studies with global fatigue outcomes (12,14,31–36). Furthermore, the global fatigue measure in this study showed lower prevalence rates (by about one-third) across age strata compared with the strong stepwise associations seen with higher perceived physical fatigability. The marked differences in findings corroborate the hypothesis that, due to self-pacing, general measures of fatigue may not be sensitive enough to capture the relationship between age and the impact of fatigue (37). Thus, measuring perceived physical fatigability, specifically with the PFS instead of with a single-item fatigue measure allowed us to better assess the degree to which older adults were physically limited due to tiredness (5,8,37).
Additionally, unlike our global fatigue measure, women, as expected, had higher PFS physical scores and a greater prevalence of higher perceived physical fatigability compared to men. This corroborates and extends findings from several studies that have identified higher prevalence and severity of fatigue in women (12,13,36,38–41). Several hypotheses exist to explain this sex difference, namely that endocrine, stress-related, and socio-contextual factors may account for this disparity (42,43). Women are affected by disability-related health outcomes in late life at higher rates than men (44). This disproportionate burden of disability on women coupled with longer lifespan, may also account for differences in fatigability status by sex (45). Future research will focus on the etiology of higher perceived physical fatigability to understand the greater risk in women.
All measures of physical function included in these analyses were significantly associated with higher perceived physical fatigability. SPPB, ADL difficulty, IADL dependence, and gait speed had particularly strong relationships with fatigability across all age strata. Previous research has linked fatigability to gait speed and other objective measures of physical function, but ours is the first to identify ADL difficulty and IADL dependence as significant predictors of higher perceived physical fatigability in older adults. Our findings concur with the existing fatigability research that has identified a cross-sectional association between fatigability and function (6,13). Our findings also support new research showing that the PFS is sensitive to change and can predict subsequent decline in physical function (10).
Worse cognitive functioning was associated with higher perceived physical fatigability in this cohort. Specifically, our findings for the MSSE scores are similar to data from the oldest participants (age 85 years) in the Jerusalem Longitudinal Cohort Study (13). Lower DSST was significantly associated with higher perceived physical fatigability in the two youngest age strata. Psychomotor speed, measured by the DSST, captures the relationship between physical movement and conscious cognitive processing, and has previously been associated with two established correlates of physical fatigability: gait speed and physical activity (46,47). Therefore, although the exact nature of this association is not well-understood, it is likely that a relationship exists between worse cognitive function and higher perceived physical fatigability.
Overall, the 80–89 and 90–108 age strata yielded fewer significant relationships with higher perceived physical fatigability than the younger age strata. This may be indicative of a change in etiology of fatigability with aging—perhaps it becomes more idiopathic with age, and previously protective factors have a smaller effect—but it may also be a result of some of the study’s limitations. The 80–89 and 90–108 age strata had much smaller sample sizes than either the 60–69 or 70–79 age strata, with only 28 participants meeting the lower perceived physical fatigability criteria in the oldest strata. We also cannot rule out a cohort effect, as the oldest people are from the proband generation whereas most of the younger participants come from the offspring generation. Additionally, bias due to the LLFS cohort being enriched for exceptional survival coupled with a healthy survivor effect may have an impact on our findings in the ≥80 year-old participants.
Due to the family study design of the LLFS, we were also able to estimate the contribution of genetics by measuring the heritability of perceived physical fatigability in older adults. We found that 26.3% of the variation in perceived physical fatigability in this cohort could be attributed to additive genetic factors, indicating moderate heritability in this population. Thus, there appears to be significant genetic contributions to perceived physical fatigability in older adults, which is independent of the effects of age and sex. This is in agreement with the fatigue literature in a large cohort in the United Kingdom, which reported significant SNP-based heritability estimates for tiredness in women and men (48) and with a measure of fatigue in a smaller cohort of twins in Australia (49). The current results on perceived physical fatigability add to the evidence that an underlying genetic etiology of the body’s predisposition to fatigue exists rather than being solely due to one’s perception of tiredness or ability to self-pace. As this work shows, fatigability is a multifaceted construct and correlated with many demographic, physiological, and functional characteristics, and some of these factors may potentially share genetic overlap (48). Future research in the LLFS cohort will include whole-genome linkage and genome-wide association testing to understand the etiology of perceived physical fatigability.
A few general limitations of this study exist. Since these analyses are cross-sectional, we are unable to assess the causal direction of the relationships with higher perceived physical fatigability. The LLFS is comprised almost entirely of white families, and is a cohort enriched for exceptional longevity, so these results may not be widely generalizable. Our sample is healthier than the average population, which may limit our power in detecting differences for diseases such as cancer that have been shown to be associated with fatigability in a recent study (50). Strengths of this study include the large sample size of the LLFS, particularly in the oldest age strata. Use of the PFS, a validated measure of perceived physical fatigability, allowed us to examine this novel trait in a large cohort across a wide age range of older adults. Although other epidemiological studies have examined the correlates of fatigue in older adults, we are the first to present a cross-sectional analysis of the risk factors associated with higher perceived physical fatigability.
In conclusion, perceived physical fatigability is highly prevalent in older adults, particularly women, and strongly associated with age, physical and cognitive function, physical activity, and a number of health conditions. The identification of risk factors associated with higher perceived physical fatigability can inform the development of targeted interventions for those most at risk for higher fatigability, including older women, older adults with depression, and those who are less physically active. Future research should aim to examine perceived physical fatigability prospectively in order to determine whether fatigability is a cause or consequence of declining physical function.
Supplementary Material
Acknowledgments
Preliminary findings were presented at the 2018 Gerontological Society of America Annual Scientific Meeting, Boston, Massachusetts, November 2018.
Funding
This work was supported by the National Institute on Aging (NIA U01 AG023712, U01 AG023744 U01 AG023746, U01 AG023749, and U01 AG023755). Additionally, the Claude D. Pepper Older Americans Independence Center, Research Registry and Developmental Pilot Grant (NIH P30 AG024827), and the Intramural Research Program, National Institute on Aging supported N.W.G. to develop the Pittsburgh Fatigability Scale. The Epidemiology of Aging training grant at the University of Pittsburgh (NIA T32 AG000181) supported T.G. Furthermore, a career development award from the Pittsburgh Claude D. Pepper Older Americans Independence Center (P30 AG024827) and National Institute of Health/National Institute on Aging (K01 AG057726) supported A.J.S.
Conflict of InterestNone declared.
References
- 1. Gill TM, Desai MM, Gahbauer EA, Holford TR, Williams CS. Restricted activity among community-living older persons: incidence, precipitants, and health care utilization. Ann Intern Med. 2001;135:313–321. doi: 10.7326/0003-4819-135-5-200109040-00007 [DOI] [PubMed] [Google Scholar]
- 2. Leveille SG, Fried L, Guralnik JM. Disabling symptoms: what do older women report? J Gen Intern Med. 2002;17:766–773. doi: 10.1046/j.1525-1497.2002.20229.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Avlund K. Fatigue in older adults: an early indicator of the aging process? Aging Clin Exp Res. 2010;22:100–115. doi: 10.1007/bf03324782 [DOI] [PubMed] [Google Scholar]
- 4. Zengarini E, Ruggiero C, Pérez-Zepeda MU, et al. Fatigue: relevance and implications in the aging population. Exp Gerontol. 2015;70:78–83. doi: 10.1016/j.exger.2015.07.011 [DOI] [PubMed] [Google Scholar]
- 5. Eldadah BA. Fatigue and fatigability in older adults. PM R. 2010;2:406–413. doi: 10.1016/j.pmrj.2010.03.022 [DOI] [PubMed] [Google Scholar]
- 6. Simonsick EM, Schrack JA, Glynn NW, Ferrucci L. Assessing fatigability in mobility-intact older adults. J Am Geriatr Soc. 2014;62:347–351. doi: 10.1111/jgs.12638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wasson E, Rosso AL, Santanasto AJ, et al. Neural correlates of perceived physical and mental fatigability in older adults: a pilot study. Exp Gerontol. 2019;115:139–147. doi: 10.1016/j.exger.2018.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Glynn NW, Santanasto AJ, Simonsick EM, et al. The Pittsburgh Fatigability Scale for older adults: development and validation. J Am Geriatr Soc. 2015;63:130–135. doi: 10.1111/jgs.13191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simonsick EM, Glynn NW, Jerome GJ, Shardell M, Schrack JA, Ferrucci L. Fatigued, but not frail: perceived fatigability as a marker of impending decline in mobility-intact older adults. J Am Geriatr Soc. 2016;64:1287–1292. doi: 10.1111/jgs.14138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simonsick EM, Schrack JA, Santanasto AJ, Studenski SA, Ferrucci L, Glynn NW. Pittsburgh Fatigability Scale: one-page predictor of mobility decline in mobility-intact older adults. J Am Geriatr Soc. 2018;66:2092–2096. doi: 10.1111/jgs.15531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim I, Hacker E, Ferrans CE, Horswill C, Park C, Kapella M. Evaluation of fatigability measurement: integrative review. Geriatr Nurs. 2018;39:39–47. doi: 10.1016/j.gerinurse.2017.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Junghaenel DU, Christodoulou C, Lai JS, Stone AA. Demographic correlates of fatigue in the US general population: results from the patient-reported outcomes measurement information system (PROMIS) initiative. J Psychosom Res. 2011;71:117–123. doi: 10.1016/j.jpsychores.2011.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moreh E, Jacobs JM, Stessman J. Fatigue, function, and mortality in older adults. J Gerontol A Biol Sci Med Sci. 2010;65:887–895. doi: 10.1093/gerona/glq064 [DOI] [PubMed] [Google Scholar]
- 14. Tsutsumimoto K, Doi T, Shimada H, et al. Self-reported exhaustion associated with physical activity among older adults. Geriatr Gerontol Int. 2016;16:625–630. doi: 10.1111/ggi.12528 [DOI] [PubMed] [Google Scholar]
- 15. Richardson CA, Glynn NW, Ferrucci LG, Mackey DC. Walking energetics, fatigability, and fatigue in older adults: the study of energy and aging pilot. J Gerontol A Biol Sci Med Sci. 2015;70:487–494. doi: 10.1093/gerona/glu146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cooper R, Popham M, Santanasto AJ, Hardy R, Glynn NW, Kuh D. Are BMI and inflammatory markers independently associated with physical fatigability in old age? Int J Obes (Lond). 2019;43:832–841. doi: 10.1038/s41366-018-0087-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wanigatunga AA, Simonsick EM, Zipunnikov V, et al. Perceived fatigability and objective physical activity in mid- to late-life. J Gerontol A Biol Sci Med Sci. 2018;73:630–635. doi: 10.1093/gerona/glx181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Urbanek JK, Zipunnikov V, Harris T, Crainiceanu C, Harezlak J, Glynn NW. Validation of gait characteristics extracted from raw accelerometry during walking against measures of physical function, mobility, fatigability, and fitness. J Gerontol A Biol Sci Med Sci. 2018;73:676–681. doi: 10.1093/gerona/glx174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sebastiani P, Hadley EC, Province M, et al. A family longevity selection score: ranking sibships by their longevity, size, and availability for study. Am J Epidemiol. 2009;170:1555–1562. doi: 10.1093/aje/kwp309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Newman AB, Glynn NW, Taylor CA, et al. Health and function of participants in the Long Life Family Study: a comparison with other cohorts. Aging (Albany NY). 2011;3:63–76. doi: 10.18632/aging.100242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Elo IT, Mykyta L, Sebastiani P, Christensen K, Glynn NW, Perls T. Age validation in the Long Life Family Study through a linkage to early-life census records. J Gerontol B Psychol Sci Soc Sci. 2013;68:580–585. doi: 10.1093/geronb/gbt033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kannel WB, Sorlie P. Some health benefits of physical activity. the framingham study. Arch Intern Med. 1979;139:857–861. [PubMed] [Google Scholar]
- 23. Ashwell M, Cole TJ, Dixon AK. Ratio of waist circumference to height is strong predictor of intra-abdominal fat. BMJ. 1996;313:559–560. doi: 10.1136/bmj.313.7056.559d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85 [DOI] [PubMed] [Google Scholar]
- 25. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi:10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 26. Wechsler D. Wechsler Adult Intelligence Scale-Revised. San Antonio: Psychological Corporation, 1981. [Google Scholar]
- 27. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes – 2018. Diabetes Care. 2018;41(Suppl 1):S13–S27. doi:10.2337/dc18-S002 [DOI] [PubMed] [Google Scholar]
- 28. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. doi:10.1177/014662167700100306 [Google Scholar]
- 29. Shugart YY, O’Connell JR, Wilson AF. An evaluation of the variance components approach: type I error, power and size of the estimated effect. Eur J Hum Genet. 2002;10:133–136. doi:10.1038/sj.ejhg.5200772 [DOI] [PubMed] [Google Scholar]
- 30. Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi:10.1086/301844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aggarwal VR, McBeth J, Zakrzewska JM, Lunt M, Macfarlane GJ. The epidemiology of chronic syndromes that are frequently unexplained: do they have common associated factors? Int J Epidemiol. 2006;35:468–476. doi: 10.1093/ije/dyi265 [DOI] [PubMed] [Google Scholar]
- 32. Morgan S, Henderson KM, Tapley A, et al. Investigation of fatigue by Australian general practice registrars: a cross-sectional study. J Prim Health Care. 2015;7:109–116. doi:10.1071/HC15109 [PubMed] [Google Scholar]
- 33. Fuhrer R, Wessely S. The epidemiology of fatigue and depression: a French primary-care study. Psychol Med. 1995;25:895–905. doi: 10.1017/s0033291700037387 [DOI] [PubMed] [Google Scholar]
- 34. Stone AA, Broderick JE, Schwartz JE, Schwarz N. Context effects in survey ratings of health, symptoms, and satisfaction. Med Care. 2008;46:662–667. doi: 10.1097/MLR.0b013e3181789387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bardel A, Wallander M-A, Wedel H, Svärdsudd K. Age-specific symptom prevalence in women 35–64 years old: a population-based study. BMC Public Health. 2009;9:37. doi:10.1186/1471-2458-9-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Engberg I, Segerstedt J, Waller G, Wennberg P, Eliasson M. Fatigue in the general population—associations to age, sex, socioeconomic status, physical activity, sitting time and self-rated health: the northern Sweden MONICA study 2014. BMC Public Health. 2017;17:654. doi:10.1186/s12889-017-4623-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alexander NB, Taffet GE, Horne FM, et al. Bedside-to-Bench conference: research agenda for idiopathic fatigue and aging. J Am Geriatr Soc. 2010;58:967–975. doi:10.1111/j.1532-5415.2010.02811.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vestergaard S, Nayfield SG, Patel KV, et al. Fatigue in a representative population of older persons and its association with functional impairment, functional limitation, and disability. J Gerontol A Biol Sci Med Sci. 2009;64:76–82. doi: 10.1093/gerona/gln017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mueller-Schotte S, Bleijenberg N, van der Schouw YT, Schuurmans MJ. Fatigue as a long-term risk factor for limitations in instrumental activities of daily living and/or mobility performance in older adults after 10 years. Clin Interv Aging. 2016;11:1579–1587. doi: 10.2147/CIA.S116741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mänty M, Rantanen T, Era P, Avlund K. Fatigue and depressive symptoms in older people. J Appl Gerontol. 2014;33:505–514. doi:10.1177/0733464812454011 [DOI] [PubMed] [Google Scholar]
- 41. Williamson RJ, Purcell S, Sterne A, et al. The relationship of fatigue to mental and physical health in a community sample. Soc Psychiatry Psychiatr Epidemiol. 2005;40:126–132. doi:10.1007/s00127-005-0858-5 [DOI] [PubMed] [Google Scholar]
- 42. Bensing JM, Hulsman RL, Karlein MGS. Gender differences in fatigue: biopsychosocial factors relating to fatigue in men and women. Med Care. 1999;37:1078–1083. doi:10.1097/00005650-199910000-00011 [DOI] [PubMed] [Google Scholar]
- 43. Ranjith G. Epidemiology of chronic fatigue syndrome. Occup Med (Lond). 2005;55:13–19. doi: 10.1093/occmed/kqi012 [DOI] [PubMed] [Google Scholar]
- 44. Murtagh K, Hubert HB. Gender differences in physical disability among an elderly cohort. Am J Pub Health. 2004;94:1406–1411. doi:10.2105/AJPH.94.8.1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Freedman VA, Wolf DA, Spillman BC. Disability-free life expectancy over 30 years: a growing female disadvantage in the US population. Am J Pub Health. 2016;106:1079–1085. doi:10.2105/AJPH.2016.303089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rosano C, Venkatraman VK, Guralnik J, et al. Psychomotor speed and functional brain MRI 2 years after completing a physical activity treatment. J Gerontol A Biol Sci Med Sci. 2010;65:639–647. doi:10.1093/gerona/glq038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13:881–889. doi: 10.1007/s12603-009-0246-z [DOI] [PubMed] [Google Scholar]
- 48. Deary V, Hagenaars SP, Harris SE, et al. Genetic contributions to self-reported tiredness. Mol Psychiatry. 2018;23:789–790. doi: 10.1038/mp.2017.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Corfield E, Martin N, Nyholt D. Familiality and heritability of fatigue in an Australian twin sample. Twin Research and Human Genetics.2017;20:208–215. doi:10.1017/thg.2017.22 [DOI] [PubMed] [Google Scholar]
- 50. Gresham G, Dy SM, Zipunnikov V, et al. Fatigability and endurance performance in cancer survivors: analyses from the Baltimore Longitudinal Study of Aging. Cancer. 2018;124:1279–1287. doi:10.1002/cncr.31238 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.