Abstract
Quantification of biological aging is of interest in gerontology as a means to surveil aging rates in the population and to evaluate the effects of interventions to increase healthy life span. Analysis of proposed methods to quantify biological aging has focused on samples of midlife or mixed-age adults in the West. Research is needed to test whether quantifications of biological aging can differentiate aging rates among older adults and if quantifications of biological aging developed in Western samples can differentiate aging rates in non-Western populations. We conducted analysis of Klemera-Doubal method (KDM) Biological Age and homeostatic dysregulation measures of biological aging developed in the U.S. NHANES and tested in a sample of older Taiwanese adults in the Social Environment and Biomarkers of Aging Study. We conducted analysis of physical and cognitive function and mortality, comparing quantifications of biological aging to a biomarker index based on norms within our analysis sample and to participants’ ratings of their own health. Results showed that quantifications of biological aging (a) predicted differences in physical and cognitive function and in mortality risk among Taiwanese older adults and (b) performed as well as a traditional biomarker index and participant self-rated health for prediction of these outcomes.
Keywords: Biomarkers, Physical performance, Cognitive decline, Mortality
Biological aging is the gradual and progressive decline in the integrity of bodily systems that occurs with advancing chronological age (1). Processes of biological aging are thought to represent a modifiable cause of age-related disease, disability, and mortality (2). The etiology of biological aging is thought to involve an accumulation of molecular changes occurring at the cellular level, which in turn leads to declining system integrity (3,4). Studies in animals suggest that interventions modifying these cellular-level changes may prevent a range of age-related disease and extend healthy life span (5,6). A barrier to conducting human trials of these therapies is the length of human life spans; because aging-related health declines in humans unfold over decades, follow-up needed to test prevention or delay of chronic disease or disability is cost- and time-prohibitive. If it were possible to measure biological aging, such measures could serve as surrogate endpoints, accelerating testing to translate therapies to slow aging from animals to humans.
Among measurement methods proposed to quantify biological aging, methods that use data from blood chemistry tests and physiological assessments already conducted in routine clinical care are appealing for their scalability. A growing number of studies suggest that proposed quantifications of biological aging derived from this type of data can predict differences in health, function, and life span (7–11), including in individuals still too young to have age-related disease (12). But most of these studies have focused on midlife adults or mixed-age samples, although some previous studies have reported analyses focused on older adults (10,13,14). Much research in gerontology is focused on older adults. Studies are therefore needed to test whether proposed quantifications of biological aging can differentiate patterns of aging within aged populations. Specifically, can proposed measures of biological aging differentiate older adults in terms of functional status and survival?
A second issue is that most biological aging research has been conducted in Western samples. Two studies considering Western and non-Western populations have raised questions about cross-population utility of quantifications of biological aging (7,15). More data are needed to address this uncertainty. A specific question is whether measures of biological aging developed in one population can be applied to study aging processes in a different population.
We conducted a study to test quantifications of biological aging developed from analysis of a U.S.-based mixed-age sample in a sample of Taiwanese older adults in the Social Environment and Biomarkers of Aging Study (SEBAS). SEBAS is a sub-study of the Taiwan Longitudinal Study of Aging, a nationally representative longitudinal study of Taiwanese older adults aged 54+ in 2000 (16). We conducted analysis of two proposed methods to quantify biological aging, the Klemera-Doubal Method (KDM) Biological Age (17) and homeostatic dysregulation (18). We tested associations between these measures of biological aging and measures of physical and cognitive function, and the risk of death. We compared effect sizes from these analyses to effect sizes from two alternative measures: a biomarker index based on norms within the SEBAS sample (19) and participant ratings of their own health.
Methods
Data
We analyzed data from SEBAS, a nationally representative longitudinal study of Taiwanese older adults aged 54+ in 2000 (16). SEBAS is based on a subsample of the Taiwan Longitudinal Study of Aging, which began in 1989. SEBAS survey assessments were conducted in 2000, when 1,023 participants were interviewed and completed a health exam, and again in 2006, when 639 of the original participants were interviewed and completed a health exam.
Analysis focused on SEBAS participants who provided blood samples at the year 2000 assessment and for whom data on all biomarkers were available (n = 951, Table 1). Analyses of physical performance and cognitive function were further restricted to the subsample of these individuals re-interviewed and examined in 2006 (n = 598–668 for physical performance; n = 689 for cognitive function; Table 1). Mortality follow-up was conducted through 2015. Survival analysis was conducted using two intervals. First, to match analysis of physical and cognitive functioning, we conducted survival analysis with follow-up through 2006 (n = 166 deaths). Second, to take full advantage of available data, we conducted survival analysis with follow-up through 2015 (n = 451 deaths).
Table 1.
Descriptive Statistics
| Variables | Mean | SD | n | Correlation With CA |
|---|---|---|---|---|
| Chronological age in 2000 | 67.74 | 8.39 | 951 | - |
| KDM Biological Age in 2000 | 67.74 | 12.87 | 951 | 0.67 |
| Homeostatic dysregulation in 2000 | 4.10 | 0.86 | 951 | 0.23 |
| Physiological dysregulation in 2000 | 5.43 | 2.79 | 951 | 0.24 |
| Self-rated health in 2000 | 2.93 | 0.99 | 951 | 0.07 |
| Chair-stand speed in 2006 | 0.48 | 0.18 | 598 | -0.33 |
| Walk speed in 2006 | 0.77 | 0.28 | 661 | -0.43 |
| Grip strength in 2006 | 25.68 | 10.14 | 668 | -0.33 |
| Peak expiratory flow in 2006 | 296.59 | 124.34 | 660 | -0.29 |
| Cognitive assessment in 2000 | 17.11 | 3.14 | 689 | -0.30 |
| Cognitive assessment in 2006 | 15.54 | 3.93 | 689 | -0.38 |
Note: CA = cognitive assessment; KDM = Klemera-Doubal Method.
Measures
We analyzed two proposed measures of biological aging and two additional measures to provide effect size comparisons. The biological aging measures were the KDM Biological Age and homeostatic dysregulation. These are individual-level measures that combine information from multiple clinical biomarkers to quantify decline in system integrity. We compared these two proposed quantifications of biological aging to two established methods for differentiating health and aging in older adults: a composite biomarker index counting the number of biomarkers with extreme values within the analysis sample and a survey item measuring self-rated health.
KDM Biological Age and homeostatic dysregulation
KDM Biological Age and homeostatic dysregulation are algorithm-based measures that combine information on the integrity of multiple organ systems in the body (17,18,20). Biological aging measurements made with these algorithms are predictive of morbidity, mortality, and indicators of healthspan in young and older populations (12,13,18,20–22). We calculated KDM Biological Age and homeostatic dysregulation based on a panel of 11 biomarkers measuring system integrity, including cardiovascular, renal, hepatic, immune, and metabolic function: albumin, blood urea nitrogen, creatinine, C-reactive protein, cytomegalovirus optical density, glycated hemoglobin, total cholesterol, white blood cell count, lymphocyte percent, mean corpuscular volume, and systolic blood pressure (Supplementary Table 1). Biomarkers were selected on the basis of their inclusion in published analyses of biological age (20,22,23) and availability in the SEBAS data. The biomarkers included in the three previous algorithms and their overlap in the SEBAS analysis are reported in Supplementary Table 2. We conducted analysis to estimate parameters for the KDM Biological Age and homeostatic dysregulation algorithms in data from NHANES III and continuous NHANES panels spanning 1999–2016 (Supplementary Methods).
The KDM Biological Age algorithm is derived from a series of regressions of individual biomarkers on chronological age in a reference population. Following previous work (20,24), we formed this reference population from NHANES participants aged 30–75 years who were not pregnant (N = 38,765, 49% male). An individual’s KDM Biological Age prediction corresponds to the chronological age at which her/his physiology would be approximately normal in the NHANES reference sample.
The homeostatic dysregulation algorithm is based on Mahalanobis distance (25) for a panel of biomarkers. Following previous work (23,24), we computed the distance based on a comparison of SEBAS participants to a reference population composed of NHANES participants aged 20–30 years who were not obese, not pregnant, and for whom all biomarkers fell within the clinically normal range (N = 502, 24% male). An individual’s homeostatic dysregulation value quantifies how different their physiology is from this young, healthy NHANES sample.
We applied KDM Biological Age and homeostatic dysregulation algorithms developed in the NHANES database to compute biological aging measures in the SEBAS dataset (KDM Biological Age range 35–144, M = 67.74, SD = 12.87; homeostatic dysregulation range 1.67–6.87, M = 4.10, SD = 0.86; Table 1). Summary statistics for SEBAS biomarkers are reported in Supplementary Table 1. For analysis of KDM Biological Age, we calculated the difference between participants’ biological and chronological ages and standardized the resulting values to have M = 0, SD = 1, with higher values indicating more advanced biological age. For analysis of homeostatic dysregulation, we first regressed homeostatic dysregulation on chronological age and predicted residual values. We then standardized these residual values to have M = 0, SD = 1, with higher values indicating greater homeostatic dysregulation.
For comparison purposes, we also conducted analysis of two alternative measures, physiological dysregulation - a biomarker health index previously constructed from the SEBAS database - and self-rated health.
Physiological dysregulation
We constructed the physiological dysregulation (PD) biomarker index from 24 biomarkers according to the procedure used in previous SEBAS analysis (19,26). Briefly, for each biomarker, participants were classified as being in a high-risk or normal-risk group based on the distributions of the biomarkers in the SEBAS cohort. High risk was defined as the top and/or bottom quintile or decile depending on the markers (Supplementary Table 1). The PD biomarker index was calculated by summing the number of high-risk biomarkers across the set of 24 (M = 5.43, SD = 2.79, Table 1). Physiological dysregulation was standardized to have M = 0, SD = 1 for analysis.
In addition to this 24-biomarker measure of physiological dysregulation used in previous SEBAS analysis (19), we conducted analysis of two additional PD measures. These two additional PD measures comprise the 11 biomarkers included in the KDM Biological Age and homeostatic dysregulation measures. One was defined based on cut-points established within SEBAS. The other was defined based on cut-points established within the NHANES. We report effect sizes for the original PD measure in the main text and for these alternative measures in Supplementary Tables 4–9.
Self-rated health
At the time of survey in 2000, respondents were asked (in Chinese) to rate their health on a 1–5 scale as excellent, good, average, not so good, or poor (M = 2.93, SD = 0.99, Table 1). Self-rated health was standardized to have M = 0, SD = 1 for analysis, with higher values indicating worse self-rated health.
To evaluate criterion validity of biological aging measures and comparison measures, we tested associations with measures of physical performance, cognitive function, and survival.
Physical performance
SEBAS administered four in-home tests of physical performance in 2006: a chair-stand test, walk-speed test, grip strength test, and peak-expiratory-flow test. The chair-stand test was conducted with arms folded across the chest and consisted of standing up and sitting down five times in a row as quickly as possible. Chair-stand speed (stand/s) was adjusted for chair height (27) (M = 0.48, SD = 0.18, Table 1). Walk-speed (m/s) was measured as the faster of two trials walking three meters at normal speed, starting in a standing position (M = 0.77 meters per second, SD = 0.28 Table 1). Grip strength was measured as the highest value across three trials on each hand using the North Coast hydraulic hand dynamometer (M = 25.68 kg, SD = 10.14, Table 1). Peak-expiratory-flow, a measure of lung function, was assessed using the TruZone peak flow meter (M = 296.59 L/m, SD = 124, Table 1). Physical functioning measures were standardized to have M = 0, SD = 1 for analysis with higher values indicating higher levels of physical functioning. For grip strength, standardization was conducted separately for men and women.
Cognitive functioning
Cognitive assessments were conducted in 2000 and 2006 using a set of cognitive and memory tasks derived from the Short Portable Mental Status Questionnaire (28), the Rey Auditory Verbal Learning Test (29), and a modified Digits Backward test (30). Each task was scored following published guidelines (31) and scores were summed to compute an overall cognitive score ranging from 0 to 24. If a respondent did not answer an item, it was coded as incorrect, or 0. The mean score in the analytic sample was 17.1 in 2000 (SD = 3.14, Table 1), and 15.5 in 2006 (SD = 3.93, Table 1). The cognitive functioning measures were standardized to have M = 0, SD = 1 for analysis, with higher scores indicating higher cognitive functioning.
Mortality
Mortality follow-up of SEBAS participants was conducted through linkage to the Ministry of Health and Welfare death certificate registration system. Primary analysis of mortality focused on follow-up through 2006, when functional testing was conducted. Additional analysis of survival through 2015 is reported in Supplementary Table 9.
Analysis
We tested associations of KDM Biological Age, homeostatic dysregulation, physiological dysregulation, and self-rated health with measures of physical performance and cognitive functioning using linear regression. We estimated two models. The first model included covariates for chronological age in 2000 and sex. The second model included body mass index in 2000 as an additional covariate. For analysis of cognitive test data, we also fitted a third model that included the participant’s cognitive test score in 2000 as a covariate. The coefficient estimated for the biological aging variable from this third model tests the association between measured aging and cognitive decline between 2000 and 2006. We report effect sizes as standardized regression coefficients (interpretable as Pearson’s r). We tested associations of KDM Biological Age, homeostatic dysregulation, physiological dysregulation, and self-rated health with survival using Cox proportional hazard models. To test whether each measure of aging improved prediction of measures of functioning and mortality over and above chronological age alone, we computed Harrell’s concordance index (32). Harrell’s concordance index is measured as the proportion of time that the model correctly orders survival times for pairs of respondents among all possible pairs. Higher values indicate better prediction.
Results
SEBAS participants with older chronological age had older KDM Biological Ages, higher levels of homeostatic dysregulation, higher scores on physiological dysregulation, and poorer self-rated health (r = .07– .67, Table 1). The correlation was highest for KDM Biological Age, which includes chronological age in the calculation formula, and lowest for self-rated health. We next evaluated correlation among the different aging measures. We first regressed all measures on chronological age and sex and computed residual values. We then computed correlations among these residuals (Supplementary Table 3). Participants with more advanced KDM Biological Age also tended to have higher levels of homeostatic dysregulation (r = .68) and more physiological dysregulation (r = .42), but no association with self-rated health (r = .06).
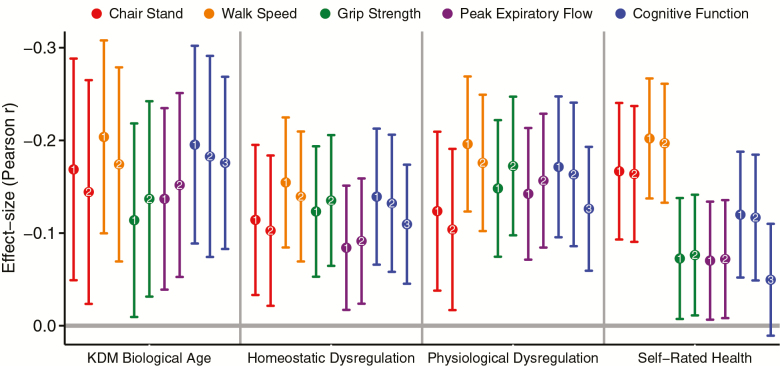
SEBAS participants with more advanced KDM Biological Age and higher levels of homeostatic dysregulation performed worse on tests of physical performance. Effect sizes were small (r = .08–.20), but similar in analysis of physiological dysregulation and self-rated health (r = .07–.20). When we repeated the analysis including body mass index in 2000 as a covariate, effect sizes were substantively similar (Supplementary Tables 4–7). Effect sizes with 95% confidence intervals are plotted in Figure 1. Full results from regression analysis are reported in Supplementary Tables 4–7.
Figure 1.
Effect sizes for associations of aging measures with tests of physical performance, cognitive functioning, and cognitive decline. Plotted points are standardized regression coefficients reflecting effect sizes interpretable as Pearson’s r. Error bars show 95% confidence intervals. Points are labeled as reflecting effect sizes from different regression models. The first model, denoted with the number 1, included chronological age and sex as covariates. The second model, denoted with the number 2, included chronological age, sex, and body mass index in 2000 as covariates. For analysis of cognitive function (in blue), we report a third model, denoted with the number 3. This model estimates an effect size for cognitive decline by including the participant’s cognitive test score in 2000 as a covariate in the model predicting cognitive test score in 2006. Full results from regression models are reported in Supplementary Tables 4–8.
SEBAS participants with more advanced biological aging performed more poorly on tests of cognitive functioning and showed evidence of cognitive decline between 2000 and 2006. SEBAS participants with more advanced KDM Biological Age and higher levels of homeostatic dysregulation in 2000 performed worse on the test of cognitive functioning in 2006 (r = .13–.20). Effect sizes were similar in analyses of physiological dysregulation and self-rated health (r = .12–.17). Because cognitive functioning earlier in life is associated with healthier aging and individual differences in cognitive functioning are relatively stable across the life course (33,34), these associations could reflect reverse causation. Therefore, we conducted a second analysis focused on change in cognition between 2000 and 2006. We repeated the regression analysis, this time including participants’ scores on the same cognitive test in 2000 as a covariate. Effect sizes for KDM Biological Age (r = .17, CI = .08–.26) and homeostatic dysregulation (r = .11, CI =.04–.17) were modestly attenuated. Results were similar for physiological dysregulation (r = .12, CI = .05–.19). Self-rated health in 2000 was not associated with change in cognitive functioning between 2000 and 2006. Effect sizes for analysis of cognitive functioning are graphed in Figure 1. Full results from regression analysis are reported in Supplementary Table 8.
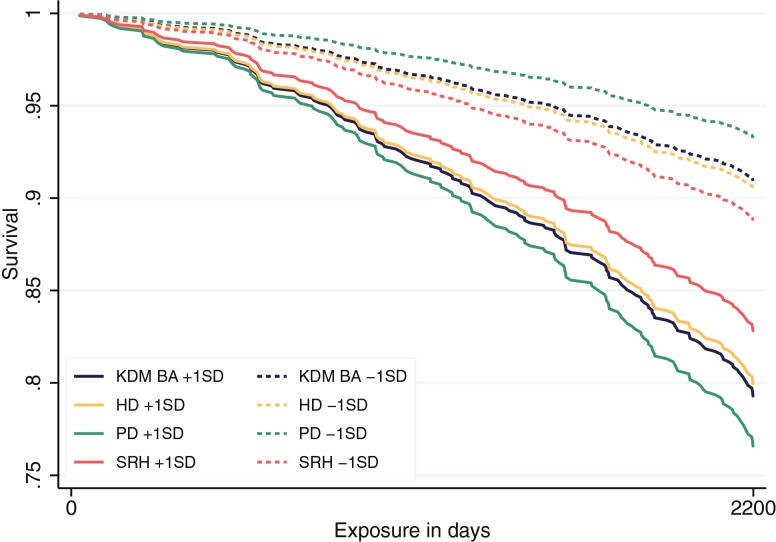
SEBAS participants with more advanced biological aging were at increased risk of death through follow-up in 2006 and 2015. By the time of follow-up in 2006, n = 161 participants had died (17%). Those with more advanced KDM Biological Age and higher levels of homeostatic dysregulation were at increased risk of death during 2000–2006 (KDM Biological Age HR = 1.56, CI = 1.35–1.81; homeostatic dysregulation HR = 1.50, CI = 1.27–1.76). Effect sizes were larger for physiological dysregulation (HR = 1.95, CI = 1.69–2.26) and smaller for self-rated health (HR = 1.26, CI = 1.07–1.48). By 2015, n = 421 had died (44%). Those with more advanced KDM Biological Age and higher levels of homeostatic dysregulation were at increased risk of death during 2000–2015 (KDM Biological Age HR = 1.59, CI = 1.43–1.77; homeostatic dysregulation HR = 1.54, CI = 1.39–1.71). Effect sizes were again larger for physiological dysregulation (HR = 1.81, CI = 1.64–1.98) and smaller for self-rated health (HR = 1.14, CI = 1.03–1.25). Resulting survival estimates from the 2000–2006 interval are graphed in Figure 2. Complete regression results are reported in Supplementary Table 9.
Figure 2.
Survival estimates from 2000 to 2006. Results are estimated from four separate Cox proportional hazard models of mortality over the 6-year follow-up period at 1 SD above and below the mean for Klemera-Doubal Method Biological Age (KDM BA), homeostatic dysregulation (HD), physiological dysregulation (PD), and self-rated health (SRH). Models control for chronological age and sex. Full results in Supplementary Table 9.
KDM Biological Age and homeostatic dysregulation both improved discrimination of mortality over and above a base model including chronological age and sex (base-model Harrell’s concordance index (HCI) = 0.725; KDM HCI = 0.755; HD HCI = 0.746). As in previous analysis, the SEBAS physiological dysregulation measure performed somewhat better (HCI = 0.793) and self-rated health performed less well (HCI = 0.732). The result for self-rated health was weaker than in a previous analysis in SEBAS that included more participants, a shorter follow-up interval, a different model specification, and measured discrimination using the AUC rather than the HCI (35).
Discussion
We conducted analysis of the KDM Biological Age and homeostatic dysregulation methods of quantification of biological aging in a sample of Taiwanese older adults participating in SEBAS. There were two main findings. First, biological aging measures developed using data from the U.S. NHANES prospectively predicted differences in functional status, cognitive decline, and mortality risk in a sample of Taiwanese older adults. Second, biological-aging-measure effect sizes were similar to effect sizes for a composite biomarker index developed within the analysis sample and a survey measure of self-rated health.
These findings address questions about clinical-biomarker-based quantifications of biological aging central to the application of these algorithms in clinical trials and etiologic studies. For clinical trials, a key question about biological aging algorithms is, “Can algorithms developed in one sample or one population be applied to another while retaining criterion validity?” If the validity of biological aging measures is restricted to people of a particular demographic profile or living in a particular place, then it will not be very useful in the context of a multi-site randomized trial. Two previous studies of clinical-biomarker-based quantifications of biological aging raised questions about cross-population validity of algorithms (7,15). In contrast, our study suggests reason for some confidence. We developed KDM Biological Age and homeostatic dysregulation algorithms from analysis of U.S. NHANES data and applied these algorithms to data from Taiwanese older adults. The resulting measures of biological aging were associated with measures of function and survival with effect sizes similar to those reported in studies of U.S. adults (9,24). These findings suggest that biological aging algorithms developed within the U.S. NHANES can generate estimates of biological aging with similar criterion validity in other populations. Additional replications are needed.
For etiologic studies, a key question about biological aging algorithms is whether they provide an adequate surrogate for processes of health decline in aging (36). The current standards in such studies include biomarker indices based on cut-points and participant’s subjective perceptions of their health status. Our findings suggest that algorithm methods proposed to quantify biological aging capture similar information about morbidity, disability, and mortality when compared with these alternatives. Given similar performance, the algorithm methods offer three advantages over biomarker cut-point and self-rated health alternatives. First, they are based on a reference external to the sample under study, and therefore, results are more directly comparable across samples. When we computed physiological dysregulation using NHANES reference data to form cut-points, effect sizes were somewhat smaller when compared with KDM and HD algorithms. Second, they capture similar information about aging processes at different life-course stages, permitting parallel application in young, midlife, and older adults. Third, because they are based on continuous distributions of biomarkers, they may be more sensitive to subtle differences in exposure.
We acknowledge several limitations. First, we analyzed two methods to quantify biological aging from clinical-biomarker data. But there are others. For example, we were unable to test the algorithm recently proposed by Liu and colleagues (9) because SEBAS data did not include two of the nine biomarkers included in that algorithm—alkaline phosphatase and red cell distribution width (Supplementary Table 2). Similarly, SEBAS does not have DNA methylation data from which to compute epigenetic clock measures of biological aging (37). These measures capture different information from the clinical-biomarker measures we analyzed (21). Second, we did not have access to a Taiwanese dataset comparable to the NHANES. As a result, we could not test if developing biological aging measures within a Taiwanese sample improved performance of the measures for prediction of functional status and mortality. However, effect sizes for the biological aging measures observed in the Taiwanese SEBAS sample are similar to effect sizes reported in U.S. samples. Third, SEBAS draws from the Taiwanese population, which enjoys a higher standard of living than much of the globe. Further analysis in low- and middle-income countries is needed. Fourth, our analysis was not powered to detect small differences in predictive accuracy between measures. For population surveillance applications, further testing in samples powered to detect small differences between measures is needed to establish if quantifications of biological aging can provide superior information to alternative measures.
Within the context of these limitations, our findings suggest promise for the expanded application of clinical-biomarker-based quantifications of biological aging in cohort and clinical studies within and outside the United States. One opportunity suggested by our findings is the potential for cross-national comparative studies of aging based on biomarker data. Given marked differences in patterns of age-related morbidity and longevity around the world and the growing availability of clinical-biomarker data from biosocial surveys, such comparisons represent a potent opportunity for gerontology.
Funding
Support for this research was provided from the National Institute on Aging under grant numbers R01AG16661 and R01AG16790 and the Eunice Kennedy Shriver National Institute of Child Health and Human Development under grant number P2CHD047879. D.W.B. was supported in part by National Institute on Aging grant R21AG054846 and the Jacobs Foundation.
Conflict of Interest
None reported.
Supplementary Material
Acknowledgments
L.G. and D.W.B. conceived of the research question and conducted data analysis, with the assistance of D.G. L.G. wrote the first draft of the manuscript, with all authors contributing to rewriting and revision. N.G. secured funding for the data collection.
References
- 1. Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi:10.1016/j.cell.2005.01.027 [DOI] [PubMed] [Google Scholar]
- 2. Kaeberlein M. Longevity and aging. F1000Prime Rep. 2013;5:5. doi:10.12703/P5-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi:10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713. doi:10.1016/j.cell.2014.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaeberlein M, Rabinovitch PS, Martin GM. Healthy aging: the ultimate preventative medicine. Science. 2015;350:1191–1193. doi:10.1126/science.aad3267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fontana L, Kennedy BK, Longo VD, Seals D, Melov S. Medical research: treat ageing. Nature. 2014;511:405–407. doi:10.1038/511405a [DOI] [PubMed] [Google Scholar]
- 7. Mamoshina P, Kochetov K, Putin E, et al. Population specific biomarkers of human aging: a Big Data Study Using South Korean, Canadian, and Eastern European Patient Populations. J Gerontol A Biol Sci Med Sci. 2018;73:1482–1490. doi:10.1093/gerona/gly005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sebastiani P, Thyagarajan B, Sun F, et al. Biomarker signatures of aging. Aging Cell. 2017;16:329–338. doi:10.1111/acel.12557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu Z, Kuo PL, Horvath S, Crimmins E, Ferrucci L, Levine M. A new aging measure captures morbidity and mortality risk across diverse subpopulations from NHANES IV: a cohort study. PLoS Med. 2018;15:e1002718. doi:10.1371/journal.pmed.1002718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arbeev KG, Ukraintseva S V, Bagley O, et al. “Physiological Dysregulation” as a promising measure of robustness and resilience in studies of aging and a new indicator of preclinical disease. Journals Gerontol Ser A. 2018;74:462–468. doi:10.1093/gerona/gly136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mitnitski A, Collerton J, Martin-Ruiz C, et al. Age-related frailty and its association with biological markers of ageing. BMC Med. 2015;13:161. doi:10.1186/s12916-015-0400-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Belsky DW, Caspi A, Houts R, et al. Quantification of biological aging in young adults. Proc Natl Acad Sci U S A. 2015;112:E4104–E4110. doi:10.1073/pnas.1506264112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Q, Wang S, Milot E, et al. Homeostatic dysregulation proceeds in parallel in multiple physiological systems. Aging Cell. 2015;14:1103–1112. doi:10.1111/acel.12402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu Z, Chen X, Gill TM, Ma C, Crimmins EM, Levine ME. Associations of genetics, behaviors, and life course circumstances with a novel aging and healthspan measure: evidence from the Health and Retirement Study. PLoS Med. 2019;16:e1002827. doi:10.1371/journal.pmed.1002827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cohen AA, Morissette-Thomas V, Ferrucci L, Fried LP. Deep biomarkers of aging are population-dependent. Aging (Albany NY). 2016;8:2253–2255. doi:10.18632/aging.101034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cornman JC, Glei DA, Goldman N, et al. Cohort profile: the Social Environment and Biomarkers of Aging Study (SEBAS) in Taiwan. Int J Epidemiol. 2016;45:54–63. doi:10.1093/ije/dyu179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klemera P, Doubal S. A new approach to the concept and computation of biological age. Mech Ageing Dev. 2006;127:240–248. doi:10.1016/j.mad.2005.10.004 [DOI] [PubMed] [Google Scholar]
- 18. Cohen AA, Milot E, Yong J, et al. A novel statistical approach shows evidence for multi-system physiological dysregulation during aging. Mech Ageing Dev. 2013;134:110–117. doi:10.1016/j.mad.2013.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cornman JC, Glei DA, Goldman N, Weinstein M. Physiological dysregulation, frailty, and risk of mortality among older adults. Res Aging. 2017;39:911–933. doi:10.1177/0164027516630794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levine ME. Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age? J Gerontol A Biol Sci Med Sci. 2013;68:667–674. doi:10.1093/gerona/gls233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Belsky DW, Moffitt TE, Cohen AA, et al. Eleven telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: do they measure the same thing? Am J Epidemiol. 2018;187:1220–1230. doi:10.1093/aje/kwx346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levine ME, Crimmins EM. Is 60 the new 50? examining changes in biological age over the past two decades. Demography. 2018;55:387–402. doi:10.1007/s13524-017-0644-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Belsky DW, Huffman KM, Pieper CF, Shalev I, Kraus WE. Change in the rate of biological aging in response to caloric restriction: CALERIE biobank analysis. Journals Gerontol Ser A. 2018;73:4–10. doi:10.1093/gerona/glx096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hastings W, Shalev I, Belsky D. Comparability of biological aging measures in the national health and nutrition examination study, 1999–2002. Psychoneuroendocrinology. 2019;106:171–178. https://www.sciencedirect.com/science/article/pii/S0306453018308084. Accessed May 2, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mahalanobis P. Mahalanobis distance. Proc Natl Acad Sci India. 1936;49:234–256. [Google Scholar]
- 26. Glei DA, Goldman N, Lin YH, Weinstein M. Relaxation practice and physiologic regulation in a national sample of older Taiwanese. J Altern Complement Med. 2012;18:653–661. doi:10.1089/acm.2010.0598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cornman JC, Glei D, Rodríguez G, Goldman N, Hurng BS, Weinstein M. Demographic and socioeconomic status differences in perceptions of difficulty with mobility in late life. J Gerontol B Psychol Sci Soc Sci. 2011;66:237–248. doi:10.1093/geronb/gbq087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–441. doi:10.1111/j.1532-5415.1975.tb00927.x [DOI] [PubMed] [Google Scholar]
- 29. Lezak M. Neuropsychological Assessment. 2nd ed Oxford, UK: Oxford University Press; 1983. https://books.google.com/books?hl=en&lr=&id=FroDVkVKA2EC&oi=fnd&pg=PA3&dq=Lezach+MD.+(1983).++Neuropsychological+Assessment,+2nd+Edition.+Oxford:+The+Oxford+University+Press.&ots=q6WhYRVn3T&sig=I88zZHo8riLd0XnW9bTMEJheD4c. Accessed April 10, 2018. [Google Scholar]
- 30. Wechsler D. WAIS-R Manual. New York, NY: Psychological Corporation; 1981. [Google Scholar]
- 31. Herzog AR, Wallace RB. Measures of cognitive functioning in the AHEAD Study. J Gerontol B Psychol Sci Soc Sci. 1997;52 Spec No:37–48. doi:10.1093/geronb/52b.special_issue.37 [DOI] [PubMed] [Google Scholar]
- 32. Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–2123. doi:10.1002/sim.1802 [DOI] [PubMed] [Google Scholar]
- 33. Batty GD, Deary IJ, Gottfredson LS. Premorbid (early life) IQ and later mortality risk: systematic review. Ann Epidemiol. 2007;17:278–288. doi:10.1016/j.annepidem.2006.07.010 [DOI] [PubMed] [Google Scholar]
- 34. Deary IJ, Whiteman MC, Starr JM, Whalley LJ, Fox HC. The impact of childhood intelligence on later life: following up the Scottish mental surveys of 1932 and 1947. J Pers Soc Psychol. 2004;86:130–147. doi:10.1037/0022-3514.86.1.130 [DOI] [PubMed] [Google Scholar]
- 35. Glei DA, Goldman N, Risques RA, et al. Predicting survival from telomere length versus conventional predictors: a Multinational Population-Based Cohort Study. PLoS One. 2016;11:e0152486. doi:10.1371/journal.pone.0152486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Glei DA, Goldman N, Rodríguez G, Weinstein M. Beyond self-reports: changes in biomarkers as predictors of mortality. Popul Dev Rev. 2014;40:331–360. doi:10.1111/j.1728-4457.2014.00676.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Horvath S, Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. 2018;19:371–384. doi:10.1038/s41576-018-0004-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.