Abstract
Background
It is unknown whether moderate-to-vigorous physical activity (MVPA) in bouts of <10 minutes protects against disability risks or if only 10 minutes bouts of MVPA is critical. Additionally, it is unclear whether light physical activity (LPA) or its accumulation patterns is associated with functional disability.
Methods
A total of 1,687 adults aged ≥65 years and without functional disability at baseline were followed up for 6 years. Functional disability was identified using the database of Japan’s Long-term Care Insurance System. Physical activity was measured using a tri-axial accelerometer secured to the waist.
Results
Functional disability was identified in 274 participants (16.2%). When examined as quartiles, higher levels of all MVPA measures were dose-dependently associated with lower risk of functional disability. Associations of MVPA in ≥10 and <10 minutes bouts remained significant in a mutually adjusted model. Neither total LPA nor LPA in bout of ≥10 minutes, but LPA in bouts of <10 minutes was associated with functional disability. Analyses using restricted cubic spline functions showed that associations of all MVPA measures and LPA in bouts of <10 minutes with functional disability were linear (p for nonlinear >.05). The hazard ratios (HRs; 95% confidence interval [CI]) for functional disability per 10 minutes increment of total MVPA and LPA in bout of <10 minutes were 0.86 (0.81–0.92) and 0.96 (0.93–0.99), respectively.
Conclusions
Higher MVPA, regardless accumulation patterns, or LPA in bouts of <10 minutes was associated with lower risk of functional disability in a linear dose–response manner in older adults.
Keywords: Accelerometry, Long-term care needs, Risk factors
As worldwide population aging continues, the number of older adults with functional disability will grow considerably and result in significant challenges for social, economic, and health systems (1). Among Japan’s population aged 65 and over, the number of disabled people certified for Long-term Care Insurance (LTCI) almost tripled from 2.18 million in 2000 to 6.08 million by 2015 (2). Identifying the risk and protective factors for the onset of functional disability is urgently needed in developing preventive strategies for Japan and other countries with rapidly aging populations.
To achieve substantial health benefits in older adults, public health guidelines traditionally recommend accumulating moderate-to-vigorous physical activity (MVPA) in bouts of ≥10 minutes (3,4). However, recent evidence from studies using accelerometer-assessed physical activity has shown that MVPA accumulated in bouts of <10 minutes is associated with favorable health outcomes, including all-cause mortality (5,6), suggesting that bouts of any length of MVPA may confer health benefits. It is unclear whether MVPA in bouts of <10 minutes protects against disability risks or if only 10 minutes bouts of MVPA is critical. To our knowledge, no prospective studies have yet explored how MVPA accumulated in different bouts relates to risk of functional disability.
Light physical activity (LPA) accounts for a considerable proportion of physically active time in older adults (7). However, it is less clear whether lower-intensity physical activity is associated with reduced risk of functional disability in such age groups (8,9), and there is a lack of evidence on health benefits of LPA accumulated in different bout lengths. Moreover, the shapes of dose–response curves for physical activity and disability have not been investigated in detail. Accordingly, in a 6-year prospective cohort of older Japanese adults, the purposes of this study were: (i) to investigate the independent associations of MVPA in bouts of ≥10 and <10 minutes with incident functional disability, hypothesizing MVPA in bouts of <10 minutes are associated with a lower risk of functional disability, independent of MVPA in bouts of ≥10 minutes, and vice versa (ii) to examine the associations of LPA and its accumulation patterns with risk of functional disability, hypothesizing higher levels of LPA, regardless the accumulated patterns, is associated with lower risk of functional disability, and (iii) to examined dose–response associations using restricted cubic splines with assumption that observed associations are in a linear dose–response manner.
Methods
Participants
The design of the Sasaguri Genkimon Study (SGS) is described in detail elsewhere (10). The SGS is an ongoing community-based prospective study in Sasaguri, a suburban town in Fukuoka, Japan, aiming to explore risk and protective factors related to long-term care needs. Briefly, at the end of January 2011, 4,979 Sasaguri residents aged ≥65 years or older and not certified as requiring long-term care according to the LTCI system met the SGS inclusion criteria. After excluding subjects who had died or moved out of the district (n = 66) by the onset of the study, 4,913 subjects were invited to participate and 2,629 consented. Of these, participants were excluded from the present study because: (i) being identified as requiring LTCI before the date of their baseline assessment, conducted from May to August 2011 (n = 9); (ii) with self-reported medical history of dementia or Parkinson’s disease (n = 15); (iii) without valid accelerometer data (n = 858); and (iv) with missing data on other covariates (n = 60) at baseline. The final sample comprised 1,687 adults. Supplementary Table 1 shows the characteristics of included participants and excluded subjects due to without valid accelerometer data in present study.
Written informed consent was obtained from all subjects. This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the Institute of Health Science, Kyushu University.
Functional Disability
Functional disability was identified using the nationally uniform database of the LTCI system and data were provided by the municipal government office. LTCI is mandatory social insurance and every Japanese adult aged 65 or older is eligible for the benefits based strictly on physical and mental disability (11,12). Certification of LTCI has been reported in detail elsewhere (11). Briefly, upon the request of the older person or the caregiver, a trained local government official visits the home to evaluate the applicant’s long-term care needs using nationally standardized questionnaire on current physical and mental status, including paralysis and limitation of joint movement, movement and balance, complex movement, conditions requiring special assistance, conditions requiring assistance with activities of daily living/instrumental activities of daily living, communication and cognition, and behavioral problems. A computer-based standardized scoring system is used to calculate scores for physical and mental function and estimate the amount of time required for care in eight categories (grooming and bathing, eating, using the toilet, transferring, assistance with instrumental activities of daily living, behavioral problems, rehabilitation, and medical services). Finally, a local Certification Committee of Needed Long-Term Care (comprising physicians, nurses, and other experts in health and social services) decide whether an older adult should be certified as requiring long-term care and assign the care needs at one of seven levels (support level, 1–2; care level, 1–5). The levels of LTCI certification have been shown to be highly correlated with the Barthel Index (Spearman’s ρ = −0.86) and moderately correlated with Mini-Mental State Examination (MMSE) scores (Spearman’s ρ = −0.42) (13). We defined functional disability as the onset of long-term care needs at the first level (support level 1) or above (9,14,15). Participants were followed up from the date of the baseline survey until being ascertained as needing long-term care, death, loss to follow up because of moving out of town, or March 31, 2017, whichever came first. Information on death or moving out of the town was provided by the Sasaguri municipal government office using the resident registration system.
Physical Activity Measures
Physical activity was objectively measured using a tri-axial accelerometer (Active Style Pro HJA-350IT, Omron Healthcare, Kyoto, Japan). Participants were asked to wear the accelerometer on either side of their waist for 7 consecutive days and remove it for sleeping or any water activities.
Intensity of activities were directly estimated as metabolic equivalent (MET) every 60 seconds using established algorithms that could classify locomotive and nonlocomotive activities (16), and the accuracy of the MET estimated by the Active Style Pro has been validated with the Douglas bag method (16,17). The SAS macro program provided by the National Cancer Institute was used to compute nonwear time (18) with modifications based on our accelerometer (19). Nonwear time was defined as a consecutive period of no activity (estimated activity intensity equal to 0 MET) for at least 60 minutes, allowing for 2 minutes of activities when the intensity rose to 1.0 MET. Only participants with ≥4 valid wear days (≥10 hours of wear time per day) were included in the analysis (20).
The cutoff points used to define LPA and MVPA were 1.6–2.9 METs and ≥3 METs, respectively. MVPA was also categorized as bouts of ≥10 minutes (accumulated in sustained bouts ≥10 minutes with an allowance for up to 2 minutes below threshold) and <10 minutes. Given that no cutoffs reference for LPA bout duration currently exists and majority of LPA (60.3%) were accumulated in <10 bouts in the present study, LPA was also divided into bouts of ≥10 and <10 minutes. Time in MVPA and LPA was averaged over the number of valid days and expressed as minute/day.
Covariates Measures
Information on age and sex was obtained from the municipality office. Years of formal education, living alone (yes or no), current smoking and drinking status (yes or no), and fall experience in the previous year (yes or no) were obtained using a questionnaire. Body mass and height were measured using conventional scales, and body mass index (BMI) was calculated by dividing the body mass (kg) by height (m) squared (kg/m2). Multimorbidity was defined as the presence of two or more among 13 chronic diseases: hypertension, stroke, heart disease, diabetes mellitus, hyperlipidemia, respiratory disease, digestive disease, kidney disease, osteoarthritis or rheumatism, trauma fracture, cancer, ear disease, and eye disease. The presence of chronic diseases was self-reported on the questionnaire. Cognitive function was measured with the Japanese version of the MMSE. MMSE scores range from 0 to 30 with higher scores indicating better cognitive function. Cognitive impairment was defined as an MMSE score <24 (21). We also used a single question of “Do you often walk continuously for 15 minutes?” with answer of yes/no (22) to define low extremity limitation to rule out the confounding effect of unmeasured functional disability.
Statistical Analysis
Baseline characteristics were described using means (standard deviation [SD]), medians (interquartile range [IQR]), or proportions across the sex-specific quartiles of the total MVPA. Trends across quartiles were tested using the Jonckheere–Terpstra trend test for continuous variables and Cochran–Armitage trend test for categorical variables.
The cumulative incidence of functional disability in the overall sample was plotted using Kaplan–Meier estimates. Cox proportional hazard models were used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for functional disability according to sex-specific quartiles of each physical activity measure. Model 1 was adjusted for sex, age, and accelerometer wear time. Model 2 was additionally adjusted for education, BMI, living alone, cognitive impairment, multimorbidity, smoking, and drinking. Model 3 also included MVPA or LPA in bouts of ≥10 minutes and <10 minutes to examine whether physical activities accumulated in different bout durations were associated with functional disability independent of each other. We tested interactions of sex and age with each physical activity measure to examine the potential moderation effect.
To demonstrate the shape of the dose–response curves for each physical activity measure (as continuous variable) and functional disability and to test possible nonlinear associations, restricted cubic splines functions were added to the fully-adjusted models, with three knots placed at the 5th, 50th, and 95th percentiles (23,24). The lowest value of each physical activity variable was set as the reference. The linearity of the dose–response association was evaluated using a likelihood ratio test. We also ran the models with four knots to test whether the sharps of the dose–response curves were sensitive to the number of knots. No meaningful differences were evident between three and four knots, so we selected models using three knots to maximize the statistical power (24). HRs and 95% CIs for functional disability were also estimated for each 10 minutes increment of each physical activity measure if a linear association was observed.
In the sensitivity analysis, we excluded 44 participants who were certified as requiring long-term care in the first year of follow-up and 5 participants with an MMSE score of <18 at baseline. Standard Cox proportional hazard models tend to overestimate the risk of event of interest because participants who died before the development of event were censored, particularly in cohorts of older adults (25). Analyses were repeated using the Fine and Grey extension of the Cox model as an additional sensitivity analysis to account for death as a competing risk (26). All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC). We used the LGTPHCURV9 macro to compute cubic splines (27). A significance level was set at two-sided α = 0.05.
Results
The mean age at baseline was 73.3 (SD, 6.0) years and 37.8% of the participants were men. The mean accelerometer wear time was 839.1 (SD, 105.3) minutes/day. The medians of total MVPA and MVPA in bouts of ≥10 minutes and <10 minutes were 38.1 (IQR, 19.3–60.8), 8.7 (IQR, 1.4–24.1), and 24.0 (IQR, 13–38.4) minutes/day, respectively. The mean time of LPA was 332.0 (SD, 98.0) minutes/day. The median (IQR) within sex-specific quartiles of each physical activity measure is shown in Supplementary Table 2. Table 1 shows the participants’ baseline characteristics by quartiles of total MVPA. Participants accumulating more time in MVPA were younger and more educated, had lower BMI, multimorbidity, fall experience, low extremity limitation, and cognitive impairment, and were less likely to smoke though more likely to drink.
Table 1.
Baseline Participant Characteristic by Quartile of Total MVPA
| Quartile 1 (low) | Quartile 2 | Quartile 3 | Quartile 4 (high) | p for trend | |
|---|---|---|---|---|---|
| (n = 420) | (n = 420) | (n = 424) | (n = 423) | ||
| Men, % | 37.9 | 38.1 | 37.5 | 37.8 | .95 |
| Age, years | 77.5 ± 6.3 | 73.6 ± 5.7 | 72 ± 5 | 70.3 ± 4.6 | <.0001 |
| Education, years | 10.6 ± 2.4 | 11.1 ± 2.4 | 11.4 ± 2.5 | 11.3 ± 2.4 | <.0001 |
| Living alone, % | 13.3 | 14.3 | 13.7 | 11.4 | .37 |
| BMI, kg/m2 | 23.2 ± 3.3 | 23.4 ± 3.3 | 23.2 ± 3.1 | 22.8 ± 2.7 | <.05 |
| Multimorbidity, % | 61.2 | 49.8 | 43.4 | 33.6 | <.0001 |
| Fall experience in the past year, % | 24.8 | 16.7 | 17.7 | 18.2 | <.05 |
| Low extremity limitation, % | 25.0 | 13.1 | 7.3 | 5.0 | <.0001 |
| Cognitive impairment, % | 9.1 | 6.0 | 4.0 | 2.4 | <.0001 |
| Current smoker, % | 9.5 | 9.1 | 6.8 | 3.8 | <.001 |
| Current drinker, % | 32.1 | 37.9 | 39.6 | 45.4 | <.0001 |
| Accelerometer wear time, min/d | 816.9 ± 107.3 | 834.3 ± 102.5 | 843.5 ± 100.5 | 861.7 ± 106.2 | <.0001 |
| Total MVPA, min/d | 10.2 (5.7 - 14.7) | 28.0 (23.7 - 33.7) | 48.4 (42.8 - 54.9) | 84 (70.3 - 100.8) | <.0001 |
| MVPA in bouts of ≥10 min, min/d | 0 (0 - 1.7) | 5.0 (1.4 - 10.5) | 15.0 (7.5 - 23.5) | 38.1 (22.6 - 56.6) | <.0001 |
| MVPA in bouts of 1–9 min, min/d | 8.8 (4.9 - 12.9) | 22.3 (17 - 26.5) | 33.0 (23.3 - 40.4) | 47.2 (32 - 60.9) | <.0001 |
| Total LPA, min/day | 280.4 ± 93.8 | 327.6 ± 86 | 349.8 ± 96.1 | 370 ± 92.8 | <.0001 |
| LPA in bouts of ≥10 min, min/d | 122.6 ± 73.3 | 140.9 ± 66.9 | 147.8 ± 73.6 | 143.5 ± 67.3 | <.0001 |
| LPA in bouts of 1–9 min, min/d | 157.8 ± 42 | 186.6 ± 40.2 | 201.9 ± 41.8 | 226.5 ± 45.3 | <.0001 |
Note: Continuous variables are represented as mean ± standard deviation or median (IQR).
The quartile cut points were 16.9, 34.8, and 57.8 min/d for men and 20.6, 40.0, and 64.1 min/d for women.
BMI = body mass index; LPA = light physical activity; MVPA = moderate-to-vigorous physical activity.
During a median follow-up of 5.8 years, 274 participants developed functional disability, 91 died prior to experiencing the event, and 47 moved out of town. Cumulative incidence curve for the risk of functional disability was shown in Supplementary Figure 1.
Table 2 shows the associations between MVPA measures and incidence of functional disability. There were inverse associations across the quartiles of each MVPA measure, with lower risk in higher quartiles in model 1 (p for trend <.0001). Further adjustment for potential confounders minimally affected the estimates and CIs (model 2). The multivariable-adjusted HRs for functional disability in the highest quartiles of total MVPA and MVPA in bouts of ≥10 and <10 minutes were 0.39 (95% CI, 0.25–0.62; p for trend <.0001), 0.45 (95% CI, 0.29–0.69; p for trend <.001), and 0.45 (95% CI, 0.29–0.70; p for trend <.0001), respectively, compared with the lowest quartiles in each measure. After mutual adjustment in model 3, associations of MVPA in bouts of ≥10 minutes and <10 minutes were attenuated, but all remained significant.
Table 2.
Associations Between MVPA Measures and Functional Disability
| No. of Events/ Subjects | Incidence Rate per 1,000 Person-Years | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |||
| Total MVPA | ||||||||
| Quartile 1 (low) | 137/420 | 72.7 | 1.00 | 1.00 | ||||
| Quartile 2 | 71/420 | 32.9 | 0.70 (0.52–0.94) | .02 | 0.70 (0.52–0.95) | .02 | ||
| Quartile 3 | 39/424 | 16.7 | 0.43 (0.29–0.62) | <.0001 | 0.44 (0.30–0.65) | <.0001 | ||
| Quartile 4 (high) | 27/423 | 11.5 | 0.37 (0.24–0.57) | <.0001 | 0.39 (0.25–0.62) | <.0001 | ||
| p for trend | <.0001 | <.0001 | ||||||
| MVPA in bouts of ≥10 min | ||||||||
| Quartile 1 (low) | 122/416 | 64.3 | 1.00 | 1.00 | 1.00 | |||
| Quartile 2 | 65/425 | 29.0 | 0.62 (0.46–0.85) | .003 | 0.58 (0.42–0.79) | .0007 | 0.68 (0.49–0.95) | .02 |
| Quartile 3 | 55/423 | 24.2 | 0.65 (0.47–0.91) | .01 | 0.66 (0.46–0.93) | .02 | 0.87 (0.60–1.25) | .44 |
| Quartile 4 (high) | 32/423 | 13.8 | 0.44 (0.29–0.66) | <.0001 | 0.45 (0.29–0.69) | .0002 | 0.59 (0.38–0.92) | .02 |
| p for trend | <.0001 | .0003 | .047 | |||||
| MVPA in bouts of 1–9 min | ||||||||
| Quartile 1 (low) | 132/420 | 69.4 | 1.00 | 1.00 | 1.00 | |||
| Quartile 2 | 73/421 | 33.5 | 0.84 (0.62–1.14) | .26 | 0.85 (0.63–1.15) | .30 | 0.87 (0.64–1.18) | .38 |
| Quartile 3 | 40/422 | 17.5 | 0.51 (0.35–0.74) | .0004 | 0.52 (0.36–0.76) | .0007 | 0.54 (0.37–0.79) | .002 |
| Quartile 4 (high) | 29/424 | 12.3 | 0.43 (0.28–0.66) | .0001 | 0.45 (0.29–0.70) | .0004 | 0.50 (0.32–0.78) | .002 |
| p for trend | <.0001 | <.0001 | .0001 |
Note: Model 1 adjusted for age, sex, and wear time.
Model 2 adjusted for education, body mass index, living alone, cognitive impairment, multimorbidity, low extremity limitation, smoking, drinking plus factors in model 1.
Model 3 adjusted for other MVPA variables (MVPA in bouts of ≥10 or <10 min) plus factors in model 2.
The quartile cut points were: total MVPA, 16.9, 34.8, and 57.8 min/d for men and 20.6, 40.0, and 64.1 min/d for women; MVPA in bouts of ≥10 min, 1.6, 9.0, 27.9 min/d for men and 1.4, 8.6, and 21.9 min/d for women; MVPA in bouts of <10 min, 10.0, 19.0, and 30.8 min/d for men and 15.5, 27.8, and 42.6 min/d for women.
CI = confidence interval; HR = hazard ratio; MVPA = moderate-to-vigorous physical activity; PY = person-year.
Total LPA or LPA in bouts of ≥10 minutes was not significantly associated with functional disability in any model (Table 3). On the other hand, LPA in bouts of <10 minutes was significantly inversely associated with risk of functional disability in model 1 (p for trend = .01). In model 2, multivariable-adjusted HRs for functional disability in the highest quartiles of LPA in bouts of <10 minutes were 0.65 (95% CI, 0.43–0.99; p for trend = .03). Associations of LPA in bouts of <10 minutes bouts remained significant after mutually adjusting for LPA in bouts of ≥10 minutes.
Table 3.
Associations Between LPA Measures and Functional Disability
| No. of Events/ Subjects | Incidence Rate Per 1,000 Person-Years | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |||
| Total LPA | ||||||||
| Quartile 1 (low) | 96/421 | 46.9 | 1.00 | 1.00 | ||||
| Quartile 2 | 65/422 | 30.2 | 0.87 (0.63–1.20) | .41 | 0.88 (0.64–1.22) | .45 | ||
| Quartile 3 | 63/421 | 28.0 | 0.89 (0.64–1.23) | .48 | 0.90 (0.65–1.25) | .53 | ||
| Quartile 4 (high) | 50/423 | 22.0 | 0.76 (0.53–1.10) | .14 | 0.78 (0.54–1.13) | .18 | ||
| p for trend | .17 | .22 | ||||||
| LPA in bouts of ≥10 min | ||||||||
| Quartile 1 (low) | 76/421 | 36.1 | 1.00 | 1.00 | 1.00 | |||
| Quartile 2 | 67/421 | 30.6 | 1.02 (0.74–1.42) | .89 | 1.07 (0.77–1.49) | .70 | 1.14 (0.81–1.59) | .46 |
| Quartile 3 | 58/422 | 26.3 | 0.83 (0.59–1.17) | .28 | 0.86 (0.61–1.22) | .40 | 0.96 (0.67–1.38) | .84 |
| Quartile 4 (high) | 73/423 | 32.8 | 1.06 (0.77–1.47) | .72 | 1.06 (0.77–1.48) | .71 | 1.17 (0.84–1.63) | .37 |
| p for trend | .97 | .99 | .54 | |||||
| LPA in bouts of 1–9 min | ||||||||
| Quartile 1 (low) | 115/421 | 58.5 | 1.00 | 1.00 | 1.00 | |||
| Quartile 2 | 66/422 | 30.3 | 0.66 (0.48–0.90) | .009 | 0.64 (0.46–0.87) | .01 | 0.63 (0.45–0.86) | .004 |
| Quartile 3 | 53/421 | 23.3 | 0.68 (0.48–0.97) | .03 | 0.69 (0.49–0.99) | .04 | 0.68 (0.48–0.98) | .04 |
| Quartile 4 (high) | 40/423 | 17.4 | 0.63 (0.42–0.94) | .03 | 0.65 (0.43–0.99) | .046 | 0.64 (0.42–0.98) | .04 |
| p for trend | .01 | .03 | .03 |
Note: Model 1 adjusted for age, sex, and wear time.
Model 2 adjusted for education, body mass index, living alone, cognitive impairment, multimorbidity, low extremity limitation, smoking, drinking plus factors in model 1.
Model 3 adjusted for other LPA variables (LPA in bouts of ≥10 or <10 min) plus factors in model 2.
The quartile cut points were: total LPA, 217.3, 276.4, and 336.0 min/d for men and 300.7, 364.0, and 428.4 min/d for women; LPA in bouts of ≥10 min, 56.6, 91.4, and 132.9 min/d for men and 112.3, 157.8, and 201.8 min/d for women; LPA in bouts of <10 min: 145.8, 175.7, and 209.0 min/d for men, 171.7, 202.0, and 233.4 min/d for women.
CI = confidence interval; HR = hazard ratio; LPA = light physical activity.
The association of MVPA and LPA measures with functional disability did not vary by age and sex (interaction p > .05). However, the association of MVPA in bouts of <10 minutes differed between men and women (interaction p < .01). Despite heterogeneity in the association, estimates for both sexes were consistent in direction. The HRs (95% CIs) for the higher versus lowest quartiles were 0.90 (0.53–1.52), 0.26 (0.12–0.60), and 0.35 (0.15–0.83) in men (p for trend <.001) and 0.82 (0.56–1.20), 0.66 (0.42–1.03), and 0.50 (0.30–0.85) in women (p for trend <.01).
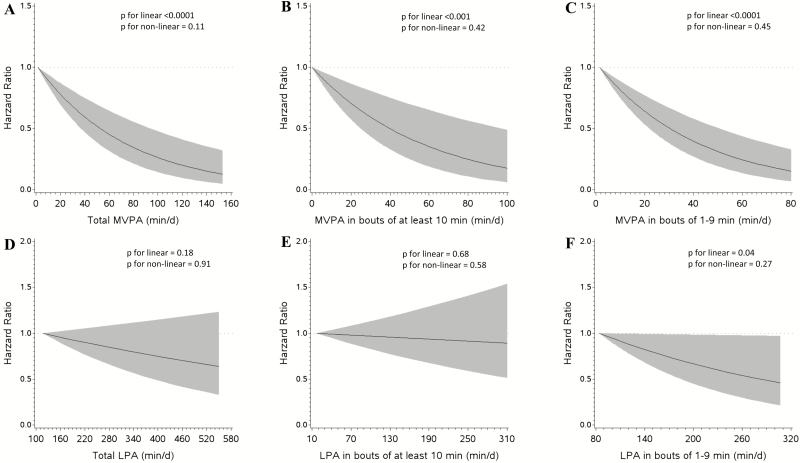
Through the use of restricted cubic splines, all MVPA measures were associated with functional disability in a linear, dose-dependent manner in the fully-adjusted models: total MVPA (p for linear <.0001, p for nonlinear = .11; Figure 1A), MVPA in bouts of ≥10 minutes (p for linear <.001, p for nonlinear = .42; Figure 1B), and MVPA in bouts of <10 minutes (p for linear <.0001, p for nonlinear = .45; Figure 1C). The HRs (95% CIs) for functional disability per 10 minutes increment of total MVPA and MVPA in bouts of ≥10 and <10 minutes were 0.86 (0.81–0.92), 0.88 (0.81–0.97), and 0.79 (0.72–0.87), respectively.
Figure 1.
Dose–response associations of MVPA measures and LPA with functional disability. Models were fit for each physical activity measure using cubic restricted splines, with three knots at the 5th, 50th, and 95th percentiles. Lowest value of each physical activity was set as the reference. Results are trimmed at the 1st and 99th percentiles and reported as HRs (black line) and 95% CIs (shaded area). CI = confidence interval; HR = hazard ratio; LPA = light physical activity; MVPA = moderate-to-vigorous physical activity.
The restricted cubic splines for total LPA (Figure 1D) and LPA in bouts of ≥10 minutes (Figure 1E) did not display either linear or nonlinear associations with functional disability. LPA in bouts of <10 minutes was associated with functional disability in a linear, dose–response manner (p for linear = .04, p for nonlinear = .27; Figure 1F). For every 10 minutes increase in LPA in bouts of <10 minutes, the HR was 0.96 (95% CI, 0.93–0.99).
In the sensitivity analyses, excluding participants certified as having functional disability in the first year of follow-up (n = 44) and those with MMSE scores <18 (n = 5) (Supplementary Table 3), or accounting for competing risk of death (Supplementary Table 4) did not alter the results for MVPA measures. The dose–response association for LPA in bouts of <10 minutes (Supplementary Table 5) was attenuated but remained significant in model 2 in analysis of excluding participants who had a functional disability event in the first year of follow and those with MMSE scores <18. Whereas the dose–response association of LPA in bouts of <10 was attenuated after accounting for the risk of death (Supplementary Table 6). The HRs (95% CIs) in the competing risk model for the higher versus lowest quartiles were 0.66 (0.47–0.91), 0.71 (0.50–1.02), and 0.68 (0.44–1.04; p for trend = .06).
Discussion
In this prospective study of older Japanese adults, higher levels of accelerometer-measured total MVPA were significantly associated with decreased risk of functional disability in a linear dose–response manner. Importantly, MVPA in bouts of ≥10 and <10 minutes was independently associated with lower risk of functional disability. In the other hand, only LPA accumulated in bouts of <10 minutes was significantly associated with risk of functional disability. Taken together, these findings suggest that increasing total daily MVPA time irrespective of the way in which it is accumulated and LPA in short bouts may be beneficial in reducing the risk of functional disability in older people.
In line with an early meta-analysis based on self-reported data (28), findings from the present study show that higher accelerometer-measured MVPA was associated with lower risk of functional disability. This result is in agreement with those from two recent studies using accelerometer data. In a study of 1,680 subjects aged 49 years or older with or at high risk of knee osteoarthritis, Dunlop et al. found a significant association between increasing levels of MVPA and reduced risk of incident disability in a 2-year follow-up (8). Another 2-year prospective study of 693 older Japanese adults with chronic pain reported that greater MVPA time was associated with lower risk of functional disability (9). However, evidence from those studies was limited to populations with specific medical conditions; the relatively short follow-up periods raise concerns of reverse causality. With a longer follow-up, the findings from the present study demonstrate that this association is consistent in an older population with various health conditions.
Previous evidence (mostly from cross-sectional studies) suggests that MVPA accumulated in bouts <10 minutes is favorably associated with cardiometabolic risk factors (29–32). Two recent studies also linked MVPA patterns to the risk of mortality showing that the mortality benefit from accumulating MVPA in bouts of <10 minutes was similar to that from accumulation in bouts ≥10 minutes (5,6). A key contribution of the present investigation was extending previous studies by providing prospective evidence that accumulating MVPA in any bouts protected against the risk of functional disability. Our findings support the newly released scientific report produced by the U.S. 2018 Physical Activity Guidelines Advisory Committee, which suggested that bouts of MVPA of any duration may be included in the accumulated total amount of MVPA (33). Given that older adults are the least active age group and with shorter MVPA times in 10-minute bouts, encouraging greater time in total MVPA by incorporating short bouts of <10 minutes may have broad public health implications.
Dose–response analyses using restricted cubic spline functions showed that all MVPA measures were associated with the risk of functional disability in a linear dose–response manner. These results suggest there is no specific threshold for reducing the risk of functional disability, and more benefits occur with increasing MVPA, regardless of the accumulation pattern.
Recent prospective evidence has linked accelerometer-measured LPA with all-cause mortality (6,34,35) and incidence of cardiovascular diseases (36) in older adults. However, it is unclear whether LPA or its accumulation patterns is associated with functional disability. To our knowledge, only two earlier prospective investigations have examined the association between total LPA and disability, but yielded inconsistent findings (8,9). No relationship between LPA and functional disability was reported in a Japanese cohort of older subjects with chronic pain (9). The other study found an association between greater time in LPA and reduced risk of onset of disability in adults with knee osteoarthritis or risk factors for knee osteoarthritis (8). In the present study, we observed neither a linear nor nonlinear association between total LPA and functional disability. When LPA was examined in different bout durations, LPA accumulated in bouts of <10 minutes was associated with lower risk of functional disability, suggesting a potential role of short bout LPA for maintaining functional independence in later life. Indeed, Jefferis et al. also observed similar health benefits of LPA in different bout durations in a population-based study of British older men although using all-cause mortality (6) and metabolic outcomes (32) as health outcomes. For instance, LPA in bouts of <10 minutes but not LPA in bouts of ≥10 was significantly associated with all-cause mortality (6). In addition, Jefferis et al. found LPA in bouts of <10 minutes was positively correlated with sedentary time in bouts of 1–15 minutes (r = .62), but inversely correlated with min spent in longer sedentary bout (eg, r = −.55 for sedentary bout of 31–60 minutes; r = −.67 for sedentary bout of ≥61 minutes) and the coefficients for the associations of LPA in bouts of <10 minutes with metabolic health outcomes were very similar to those for sedentary bouts of 1–15 minutes (32), suggesting that individuals who had more short bout LPA also frequently broke up sedentary time, thus the favorable association of LPA in bouts of <10 minutes may reflect the benefits of breaking up of prolonged sedentary time, which has been linked with adverse health outcomes including all-cause mortality (37) and cardiovascular disease (38). However, interpreting the present results also warrants caution given the wide CIs surrounding the point estimates and the attenuated dose–response association of LPA in bouts of <10 minutes in sensitivity analyses. Particularly, the trend was attenuated to nonsignificant using the competing risk model, suggesting the protective effect against functional disability of LPA in bouts <10 minutes could be overestimated when ignoring the competing risk of death. Given the very limited number of studies to date, more research on this topic is warranted.
This study has a number of strengths, including the prospective cohort design and large sample of community-based older adults. Using an accelerometer allowed the present study to quantify MVPA and LPA in different specified bouts as the exposures. The limitations of this study should be acknowledged. First, it is known that limitations of accelerometers include their inability to detect some types of PA (eg, water activities and cycling) or provide contents of activity performed (eg, walking vs jogging) that would be of particular interest of public health recommendations. In addition, the accelerometer used in the present study was developed and validated in adults (aged 20–59 years) and has been shown to underestimate the MET of activities particularly for activity at higher intensity in older adults (39); thus, the amount of MVPA could be underestimated in this study. Second, because the older person must contact the municipal government to have the care needs officially certified (11), it is possible that some disabled individuals may fail to report, and this may have resulted in underestimation of the incidence of functional disability in this study. Third, the present findings may also have been influenced by reverse causation by uncertified functional disability at baseline. However, our main analyses controlled for low extremity limitation and other major confounders at baseline to minimize this concern. Furthermore, we obtained consistent results, except for an attenuation for the association between LPA in bouts of <10 minutes and functional disability, after excluding events occurring within the first year of follow-up, which also mitigates that concern. Fourth, although we collected a range of potential confounders, we were unable to fully exclude the possibility that our findings could be explained by residual or unmeasured confounding effects. Finally, we excluded a large proportion of participants mainly owing to lack of valid accelerometer data. Included participants had less fall experience, low extremity limitation, and cognitive impairment, although with higher rate of multimorbidity than those without valid accelerometer data. Thus, participants in the present study would have been more physically active than the general population, and the observed results may have underestimated the strength of the association between PA and disability. Moreover, this study was undertaken in a single Japanese town, which may also limit the generalizability of the findings.
Conclusions
The present study clearly demonstrated that higher MVPA in bouts of ≥10 minutes or <10 minutes was associated in a linear dose–response manner with lower risk of functional disability in older Japanese adults. Accumulating LPA in bouts of <10 minutes was also associated with lower risk of functional disability. These findings suggest that increasing MVPA of any duration or accumulating more LPA in short bouts may protect against the onset of functional disability in older people.
Funding
This work was partly supported by a Health and Labour Sciences Research Grant of the Ministry of Health, Labour and Welfare of Japan (2013-Ninchisho-Ippan-004) to S.K., a research grant from the Mitsui Sumitomo Insurance Welfare Foundation to S.C., a grant from Sasaguri Town to S.K. (2011–2016), and Japan Society for the Promotion of Science KAKENHI Grant Number JP17K09146 to K.N. None of the funding sources had any role in the study design, data analysis, data interpretation, writing of the manuscript, or decision about submission.
Supplementary Material
Acknowledgments
The authors would like to thank Ms. Yuka Haeuchi, Dr. Yu Nofuji, Ms. Eri Shiokawa, and the municipal staff in the primary care-giving division in Sasaguri, who helped us coordinate the survey in the community.
Conflict of Interest
None reported.
References
- 1.World Health Organization. World report on ageing and health. Geneva, Swizerland: World Health Organization. 2015. https://apps.who.int/iris/bitstream/handle/10665/186463/9789240694811_eng.pdf?sequence=1. Accessed July 13, 2019. [Google Scholar]
- 2.Ministry of Health, Labour and Welfare. Long-term care insurance System of Japan 2016. https://www.mhlw.go.jp/english/policy/care-welfare/care-welfare-elderly/dl/ltcisj_e.pdf. Accessed July 13, 2019.
- 3.World Health Organization. Global recommendations on physical activity for health. Geneva, Switzerland: World Health Organization. 2010. https://apps.who.int/iris/bitstream/handle/10665/44399/9789241599979_eng.pdf?sequence=1. Accessed July 13, 2019. [Google Scholar]
- 4. Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. American college of sports medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c [DOI] [PubMed] [Google Scholar]
- 5. Saint-Maurice PF, Troiano RP, Matthews CE, et al. Moderate-to-vigorous physical activity and all-cause mortality: do bouts matter? J Am Heart Assoc. 2018;7:e007678. doi: 10.1161/jaha.117.007678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jefferis BJ, Parsons TJ, Sartini C, et al. Objectively measured physical activity, sedentary behaviour and all-cause mortality in older men: does volume of activity matter more than pattern of accumulation? Br J Sports Med. 2019;53:1013–1020. doi: 10.1136/bjsports-2017-098733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen T, Narazaki K, Honda T, et al. Tri-axial accelerometer-determined daily physical activity and sedentary behavior of suburban community-dwelling older Japanese adults. J Sports Sci Med. 2015;14:507–514. [PMC free article] [PubMed] [Google Scholar]
- 8. Dunlop DD, Song J, Semanik PA, et al. Relation of physical activity time to incident disability in community dwelling adults with or at risk of knee arthritis: prospective cohort study. BMJ. 2014;348:g2472. doi: 10.1136/bmj.g2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Makino K, Lee S, Lee S, et al. Daily physical activity and functional disability incidence in community-dwelling older adults with chronic pain: a prospective cohort study. Pain Med. 2019;20:1702–1710. doi: 10.1093/pm/pny263. [DOI] [PubMed] [Google Scholar]
- 10. Narazaki K, Nofuji Y, Honda T, Matsuo E, Yonemoto K, Kumagai S. Normative data for the Montreal cognitive assessment in a Japanese community-dwelling older population. Neuroepidemiology. 2013;40:23–29. doi: 10.1159/000339753 [DOI] [PubMed] [Google Scholar]
- 11. Tsutsui T, Muramatsu N. Care-needs certification in the long-term care insurance system of Japan. J Am Geriatr Soc. 2005;53:522–527. doi: 10.1111/j.1532-5415.2005.53175.x [DOI] [PubMed] [Google Scholar]
- 12. Tsutsui T, Muramatsu N. Japan’s universal long-term care system reform of 2005: containing costs and realizing a vision. J Am Geriatr Soc. 2007;55:1458–1463. doi: 10.1111/j.1532-5415.2007.01281.x [DOI] [PubMed] [Google Scholar]
- 13. Arai Y, Zarit SH, Kumamoto K, et al. Are there inequities in the assessment of dementia under Japan’s LTC insurance system? Int J Geriatr Psychiatry. 2003;18:346–352. doi: 10.1002/gps.836 [DOI] [PubMed] [Google Scholar]
- 14. Tomata Y, Watanabe T, Sugawara Y, et al. Dietary patterns and incident functional disability in elderly Japanese: the Ohsaki cohort 2006 study. J Gerontol A Biol Sci Med Sci. 2014;69:843–851. doi: 10.1093/gerona/glt182 [DOI] [PubMed] [Google Scholar]
- 15. Kondo N, Kawachi I, Hirai H, et al. Relative deprivation and incident functional disability among older Japanese women and men: prospective cohort study. J Epidemiol Community Health. 2009;63:461–467. doi: 10.1136/jech.2008.078642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oshima Y, Kawaguchi K, Tanaka S, et al. Classifying household and locomotive activities using a triaxial accelerometer. Gait Posture. 2010;31:370–374. doi: 10.1016/j.gaitpost.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 17. Ohkawara K, Oshima Y, Hikihara Y, Ishikawa-Takata K, Tabata I, Tanaka S. Real-time estimation of daily physical activity intensity by a triaxial accelerometer and a gravity-removal classification algorithm. Br J Nutr. 2011;105:1681–1691. doi: 10.1017/S0007114510005441 [DOI] [PubMed] [Google Scholar]
- 18. National Cancer Institute: SAS programs for analyzing NHANES 2003–2004 accelerometer data. https://epi.grants.cancer.gov/nhanes_pam/. Accessed October 8, 2015.
- 19. Honda T, Chen S, Yonemoto K, et al. Sedentary bout durations and metabolic syndrome among working adults: a prospective cohort study. BMC Public Health. 2016;16:888. doi: 10.1186/s12889-016-3570-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Trost SG, McIver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc. 2005;37(11 Suppl):S531–S543. doi: 10.1249/01.mss.0000185657.86065.98 [DOI] [PubMed] [Google Scholar]
- 21. Ideno Y, Takayama M, Hayashi K, Takagi H, Sugai Y. Evaluation of a Japanese version of the Mini-Mental State Examination in elderly persons. Geriatr Gerontol Int. 2012;12:310–316. doi: 10.1111/j.1447-0594.2011.00772.x [DOI] [PubMed] [Google Scholar]
- 22. Satake S, Senda K, Hong YJ, et al. Validity of the Kihon Checklist for assessing frailty status. Geriatr Gerontol Int. 2016;16:709–715. doi: 10.1111/ggi.12543 [DOI] [PubMed] [Google Scholar]
- 23. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504 [DOI] [PubMed] [Google Scholar]
- 24. Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29:1037–1057. doi: 10.1002/sim.3841 [DOI] [PubMed] [Google Scholar]
- 25. Berry SD, Ngo L, Samelson EJ, Kiel DP. Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc. 2010;58:783–787. doi: 10.1111/j.1532-5415.2010.02767.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. So Y, Lin G, Johnston G. Using the PHREG procedure to analyze competing-risks data. SAS Global Forum2015. Cary NC: SAS Institute Inc; 2015. http://support.sas.com/resources/papers/proceedings15/SAS1855-2015.pdf. Accessed July 30, 2019. [Google Scholar]
- 27. Li R, Hertzmark E, Louie M, Chen L, Spiegelman D. The SAS lgtphcurv9 macro Boston, MA: Channing Laboratory; 2011. https://www.hsph.harvard.edu/donna-spiegelman/software/lgtphcurv9/. Accessed July 1, 2019. [Google Scholar]
- 28. Paterson DH, Warburton DE. Physical activity and functional limitations in older adults: a systematic review related to Canada’s Physical Activity Guidelines. Int J Behav Nutr Phys Act. 2010;7:38. doi: 10.1186/1479-5868-7-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Glazer NL, Lyass A, Esliger DW, et al. Sustained and shorter bouts of physical activity are related to cardiovascular health. Med Sci Sports Exerc. 2013;45:109–115. doi: 10.1249/MSS.0b013e31826beae5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Clarke J, Janssen I. Sporadic and bouted physical activity and the metabolic syndrome in adults. Med Sci Sports Exerc. 2014;46:76–83. doi: 10.1249/MSS.0b013e31829f83a0 [DOI] [PubMed] [Google Scholar]
- 31. White DK, Gabriel KP, Kim Y, Lewis CE, Sternfeld B. Do short spurts of physical activity benefit cardiovascular health? The CARDIA Study. Med Sci Sports Exerc. 2015;47:2353–2358. doi: 10.1249/MSS.0000000000000662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jefferis BJ, Parsons TJ, Sartini C, et al. Does duration of physical activity bouts matter for adiposity and metabolic syndrome? A cross-sectional study of older British men. Int J Behav Nutr Phys Act. 2016;13:36. doi: 10.1186/s12966-016-0361-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA. 2018;320:2020–2028. doi: 10.1001/jama.2018.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ensrud KE, Blackwell TL, Cauley JA, et al. ; Osteoporotic Fractures in Men Study Group Objective measures of activity level and mortality in older men. J Am Geriatr Soc. 2014;62:2079–2087. doi: 10.1111/jgs.13101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. LaMonte MJ, Buchner DM, Rillamas-Sun E, et al. Accelerometer-measured physical activity and mortality in women aged 63 to 99. J Am Geriatr Soc. 2018;66:886–894. doi: 10.1111/jgs.15201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. LaCroix AZ, Bellettiere J, Rillamas-Sun E, et al. ; Women’s Health Initiative (WHI) Association of light physical activity measured by accelerometry and incidence of coronary heart disease and cardiovascular disease in older women. JAMA Netw Open. 2019;2:e190419. doi: 10.1001/jamanetworkopen.2019.0419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Diaz KM, Howard VJ, Hutto B, et al. Patterns of sedentary behavior and mortality in U.S. middle-aged and older adults: a National Cohort Study. Ann Intern Med. 2017;167:465–475. doi: 10.7326/M17-0212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bellettiere J, LaMonte MJ, Evenson KR, et al. Sedentary behavior and cardiovascular disease in older women: the objective physical activity and cardiovascular health (OPACH) study. Circulation. 2019;139:1036–1046. doi: 10.1161/circulationaha.118.035312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nagayoshi S, Oshima Y, Ando T, et al. Validity of estimating physical activity intensity using a triaxial accelerometer in healthy adults and older adults. BMJ Open Sport Exerc Med. 2019;5:e000592. doi: 10.1136/bmjsem-2019-000592 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.