Abstract
The absence of clinical tools to evaluate individual variation in the pace of aging represents a major impediment to understanding aging and maximizing health throughout life. The human lens is an ideal tissue for quantitative assessment of molecular aging in vivo. Long-lived proteins in lens fiber cells are expressed during fetal life, do not undergo turnover, accumulate molecular alterations throughout life, and are optically accessible in vivo. We used quasi-elastic light scattering (QLS) to measure age-dependent signals in lenses of healthy human subjects. Age-dependent QLS signal changes detected in vivo recapitulated time-dependent changes in hydrodynamic radius, protein polydispersity, and supramolecular order of human lens proteins during long-term incubation (~1 year) and in response to sustained oxidation (~2.5 months) in vitro. Our findings demonstrate that QLS analysis of human lens proteins provides a practical technique for noninvasive assessment of molecular aging in vivo.
Keywords: Molecular aging, Human, Lens, Crystallin, Protein aggregation
Public health planning and medical management of an increasingly aged population depend on availability of methods and markers to accurately track human aging. Chronological age is an inadequate metric to assess individual variation in the pace of biological aging (1–3). The absence of clinical tools and metrics to quantitatively evaluate individual variation in aging represents a major impediment to understanding aging and maximizing health throughout life (4–6).
Structural and functional deterioration of biomolecules, cells, tissues, and organs is a key feature of aging (7–9). Age-dependent biological alterations accrue at different rates in different species (10). Moreover, variation in the pace and patterning of biological aging in individual members of the same species deviates from fixed linkage to chronological age (1–3). In humans, aging-related phenotypes are detectable in early midlife, increase with advancing age, and correlate with chronic disease morbidity and mortality (7,11,12). While numerous aging-related metrics and composite panels have been proposed and tested (13–17), no single biomarker has been identified or noninvasive method developed that can accurately measure and track biological aging in individual humans (6,18).
A small number of long-lived proteins in humans afford potential as biomarkers of biological aging (19–21). These persistent proteins are expressed and maintained in nonregenerating somatic organs (skeletal muscle, heart, brain) (22), extracellular matrix connective tissues (tendon, cartilage, dentin) (19), and the protein-rich cytosol in postmitotic fiber cells in the lens of the eye (19,23) (Figure 1A). Of these bodily compartments, only the lens contains optically accessible proteins that remain extant and accrue aging-related molecular alterations throughout life (Figure 1B and C). Moreover, clinically detectable lens pathology in adults correlates with increased mortality in a manner that is not explained by known risk factors (24–27). Finally, from a biomedical engineering perspective, the lens represents an ideal target tissue for noninvasive evaluation in vivo (28).
Figure 1.
Long-lived proteins in the human body. (A) A small number of proteins are expressed during development and remain extant throughout life. These long-lived proteins include: collagen, the most abundant protein in the body, found in bone, tendon, cartilage, muscle, intervertebral discs, skin, and cornea; enamel and dentin proteins in teeth; nuclear pore proteins (nucleoporins) in neurons in the brain; elastin in heart, lung, and blood vessels; and the crystallins (α, β, γ), cytosolic proteins in the lens of the eye. (B) Diagrammatic representation of the human lens in transverse section (left) and magnified coronal section at the equator (right). (C) Long-lived structural proteins are subject to cumulative molecular damage, posttranslational alterations, and supramolecular reorganization, including pathogenic protein aggregation. Collectively, these molecular pathologies impair organelle, cellular, and tissue homeostasis; degrade structural and physiological functions; and accelerate biological aging.
The lens is a living, adaptively deformable, optically transparent tissue that resides in the anterior chamber of the eye (Figure 1B) (29). The primary function of the lens is to focus visible light on the retina. The bulk of the lens is composed of long-lived fiber cells (29): modified epithelial cells that are devoid of cellular organelles and arranged in tightly packed, concentric growth shells and radial columns that surround a central core of primary fibers, the embryonic nucleus (30,31). A monolayer of epithelial progenitor cells on the anterior surface of the lens mitotically divide and terminally differentiate into successive layers of elongated postmitotic fiber cells (32,33). Successive strata of secondary fibers comprise the fetal, juvenile, and adult nucleus and deep layers of the cortex. Much like tree rings and mollusk shell bands, each lens fiber growth shell represents a unique time point in the life history of the organism.
Long-lived proteins maintained in the cytosol of primary fiber cells in the central nucleus of the lens originate during fetal life (34,35). These cells contain the highest concentration of protein in the human body (~35% wet weight) of which ~90% is α-, β-, and γ-crystallins (36,37). The high concentration, short-range order, and molecular arrangement of cytosolic proteins in mature fiber cells provide the biophysical basis for the transparency, refractive power, and intrinsic light scattering of the lens (38–42). Terminal differentiation of lens fibers renders these long-lived postmitotic cells incapable of further protein synthesis or turnover (43). Within the nucleus of the lens where the oldest fiber cells reside, lens protein that was expressed before birth remains extant for life. Consequently, these long-lived proteins accrue posttranslational modifications (44,45) and supramolecular alterations throughout life (46,47). A wide range of aging-related posttranslational modifications have been detected in human lens proteins, including proteolysis (truncation), deamidation, oxidation, glycation, methylation, phosphorylation, acetylation, carbamylation, thiolation, disulfide cross-linking, radicalization and adduct formation, conformational alterations, and aggregation (36,48–53). Cumulative changes in the molecular structure and supramolecular organization of long-lived proteins lead to altered hydrodynamic size, commonly manifesting in aggregation, and progressively alter the physical, optical, and functional properties of the lens (40–42). Subcataractous lenticular protein aggregation can be noninvasively detected in vivo using advanced spectroscopic methods such as quasi-elastic light scattering (QLS). Experimental and clinical applications of QLS include detection and tracking of supramolecular changes in lens proteins ex vivo (54–56) and in vivo (57–62) that are not possible with conventional techniques such as slit lamp biomicroscopy, Scheimpflug imaging, retroillumination, lens densitometry, or optical coherence tomography.
Here we show that QLS autocorrelation spectroscopy can be used to detect aging-dependent changes in human lens proteins in vivo, which cannot be detected using conventional ophthalmic imaging techniques, and that these effects are recapitulated with time-dependent oxidation in vitro. While previous in vivo QLS work has focused on using lenticular QLS to investigate onset of cataract development, here we demonstrate feasibility of using light scattering of long-lived lens proteins as a biomarker of human age-related damage generally.
Materials and Methods
Quasi-Elastic Light Scattering
The instrument incorporated two low-intensity near-infrared diode lasers (λ = 780 nm). The first laser (Laser 1, Figure 2A) was configured as a pencil beam (focal point diameter = 65 μm). The second laser (Laser 2, Figure 2A) generated a slit beam (focal point width, 100 μm; length, 1 cm) that was used to confirm sampling localization. During alignment, both lasers were automatically alternated such that the two lasers were never activated at the same time. In addition, the laser beams diverge significantly before reaching the retina, thus decreasing focal energy deposition in this ocular tissue and increasing patient safety during clinical examinations. The average power of the pencil beam laser during alignment was <200 μW at 15 Hz pulse repetition rate. The average power of the slit beam laser during alignment was 150 μW at 15 Hz pulse repetition rate. During QLS measurements, the slit beam laser was switched off and the pencil beam was operated as a continuous wave laser (power, <400 μW). The pencil beam from Laser 1 passed through a beam splitter and was focused in the sample of interest (ie, in the cuvette in vitro, in the living human lens in vivo). Backscattered light from the target was collected at a fixed angle by a lens doublet and passed through a pinhole mirror and was delivered by a multimode fiber to a single-photon avalanche photodiode (C30902SH/DTC, Perkin Elmer). A CCD-driven piezo mirror tracking system maintains the selected acquisition location and automatically compensates for motion artifact, including voluntary and involuntary (saccadic, pulsatile) eye movements. An integrated CCD camera automatically recorded digital images of the target sampling region at the time of acquisition. For in vivo studies, these images were used for offline confirmation of the sampling region. Autocorrelation functions were recorded by a multi-tau, real-time digital correlator (Flex99OEM-480, Correlator.com). Polystyrene beads (3000 Series Nanosphere, Thermo Scientific) and fluorescent nanoparticles (Fluoro-Max, Thermo Scientific) were used to evaluate analytical performance. The FDA has determined that the QLS instrument is a nonsignificant risk device.
Figure 2.
Quasi-elastic light scattering (QLS) instrument and performance characteristics. (A) Optical diagram of QLS instrument. CL, cylindrical lens; BS, beam splitter; GL, GRIN lens. (B) Particle size distributions extracted from the mean autocorrelation functions obtained by QLS analysis of 50-nm radius polystyrene beads in water. Calculated mean hydrodynamic radius (48 nm) closely approximates mean bead radius. (C) Scattering intensity of light increases linearly with concentration of 50-nm radius polystyrene beads suspended in water. Each point represents mean value ± SD. (D) Correlation time increases linearly with polystyrene bead radius. Each point represents mean value ± SD.
Human Lens Protein Extract
A water-soluble lens protein extract was prepared from dissected lenses obtained from postmortem human eyes (National Disease Research Interchange). Individual lenses, one donor per experiment, were homogenized in a Wheaton tissue homogenizer using 1 mL of 0.22 µm filtered sterile Millipore water. The homogenate was centrifuged at 100,000g for 60 minutes at 4°C in a Beckman Optima TL-100. The resulting supernatant (defined as water-soluble human lens protein extract, hLPE) was collected and diluted in sterile phosphate-buffered saline (PBS, without added calcium or magnesium, pH 7.4) to a final concentration of 1 mg/mL. Protein concentration was quantitated using a bicinchoninic acid assay (Pierce). The hLPE preparations were aliquoted into individual sealed glass cylindrical cuvettes and incubated under sterile conditions at 37.4°C. Incubation was conducted in the dark to minimize photodynamic effects.
Human Lens Protein Oxidation
An established oxidation protocol based on cyclic Fenton chemistry was used for these studies (63). A stock solution of 1 mM iron (III) chloride hexahydrate (FeCl3·6H2O), 3 mM ethylenediamine tetraacetic acid (EDTA), and 150 mM phosphate-free sodium chloride (NaCl) was added to the protein (1:10 by volume) for final concentrations of 0.1 mM iron (III), 0.3 mM EDTA, and 2.5 mg/mL human lens protein. Hydrogen peroxide (30% stock; 1 mM final concentration) was added to the reaction mixture to initiate oxidation.
Protein Gel Electrophoresis
Bis-Tris sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE; 4–12%; Novex NuPAGE, Thermo Scientific) was used to separate water-soluble human lens proteins under denaturing, nonreducing conditions. Freshly prepared hLPE was adjusted to a stock concentration of 2.5 mg/mL in PBS (without added calcium or magnesium) and loaded at 10 μg per lane. Protein staining was conducted using Coomassie Blue dye (Colloidal Blue Staining Kit, Life Technologies).
Transmission Electron Microscopy
Transmission electron microscopy was conducted on incubated hLPE samples using a JEOL 1200EX operated at 80 kV. The protein samples were spotted at a concentration of 0.1 mg/mL on 150-mesh copper grids (Electron Microscopy Sciences). The grids were placed on top of a 12 μL sample droplet for 60 seconds, then washed for 30 seconds with water twice. The washed grids were then stained with 1% uranyl formate solution for 30–60 seconds.
Human Subjects
Clinical QLS evaluations were conducted at Boston Children’s Hospital, Boston, MA, as a cross-sectional study with prospective enrollment. A total of 34 subjects (18 males, 16 females; mean age ± SD: 23.9 ± 16.3 years) without history or evidence of eye disease were enrolled by consecutive recruitment from eligible subjects who presented at the Department of Ophthalmology. Male and female study participants were matched for number and age (males: N = 18, mean age ± SD: 24.6 ± 17.5 years; females: N = 16, mean age ± SD: 23.0 ± 15.3 years). Informed consent was obtained from each study participant, and in the case of pediatric subjects, a legal guardian. Patients older than 2 years of age and capable of cooperating for the duration of a slit lamp examination were considered eligible for the study. Patients with ocular conditions that might be associated with anterior segment abnormalities were excluded. Each subject was evaluated for narrow-angle glaucoma and questioned regarding contraindications prior to topical administration of tropicamide 1% (Bausch & Lomb) and phenylephrine 2.5% (Akorn, Inc.) to induce transient mydriasis and cycloplegia. Data collection and storage were compliant with HIPAA regulations. Study procedures, protocol, and study forms were approved and authorized for use by the Institutional Review Board at Boston Children’s Hospital in accordance with tenets of the Declaration of Helsinki.
QLS Data Acquisition, Processing, and Filtering
QLS measures the temporal correlation in intensity fluctuations of monochromatic light scattered by particles smaller than the wavelength of light (Rayleigh scattering). The second-order correlation function (equation 1) is recorded during clinical or experiment acquisition and can be utilized to extract diffusion coefficients and particle size distributions (64,65) according to the equation:
| (1) |
where G(2)(τ) is the autocorrelation function at delay time τ, I is the light intensity, and δI is the intensity fluctuation. QLS enables a quantitative assessment of changes in particle sizes with nanometer resolution. In general, both correlation time and scattering intensity increase as particle size increases.
Replicate autocorrelation functions (median = 11 replicates; acquisition time per replicate, 220 ms) were acquired from the nucleus of the right lens in each study subject. Recorded CCD images were obtained for each acquired QLS autocorrelation function and reviewed for evidence of blinking, motion, eyelash interference, off-target fixation, or other obvious artifacts. If detected, the corresponding autocorrelation function was rejected from further analysis. Autocorrelation functions with amplitude greater than 2, that did not monotonically decrease, or with amplitudes >2σ from the mean value were rejected. Correlation times that deviated ≥100% from the mean or varied >2σ were rejected. Time-averaged intensity values that varied >2σ from the mean were rejected. Extraction of size distribution profiles was based on the method of truncated singular value decomposition (Dynals 2.0, Alango Ltd, Israel) (66). Extraction of particle size distributions from the autocorrelation function is a mathematically challenging problem as its exact solution might not exist, and there might be multiple approximate solutions to the problem with different values of errors (67–69). Singular value decomposition is a linear algebraic technique that allows computation of potential solutions to the problems such as particle size calculation. A special technique of truncating unwanted components is utilized to increase the accuracy and error stability of the obtained solution by filtering out solutions that lie outside the desired range—nonnegative components in this case (67).
Statistics
We used a linear mixed-effects model with random intercept for each subject to test age dependence (70). We tested for inhomogeneity of within-subject and between-subject variability using a Breusch–Pagan test for heteroscedasticity (71).
Results
QLS Instrument and Analytical Characterization
Operational and analytical testing were conducted using aqueous suspensions of polystyrene beads. Repeated QLS assessment of polystyrene bead size standards yielded an extracted hydrodynamic radius of 48 nm in close agreement with nominal bead radius (50 nm; Figure 2B). We confirmed linearity of increasing average scattering intensity with increasing particle concentration (R2 = 1.00; Figure 2C) and linear dependence of correlation time versus particle size (R2 = 0.99; Figure 2D).
Molecular Aging in Healthy Humans Detected by QLS In Vivo
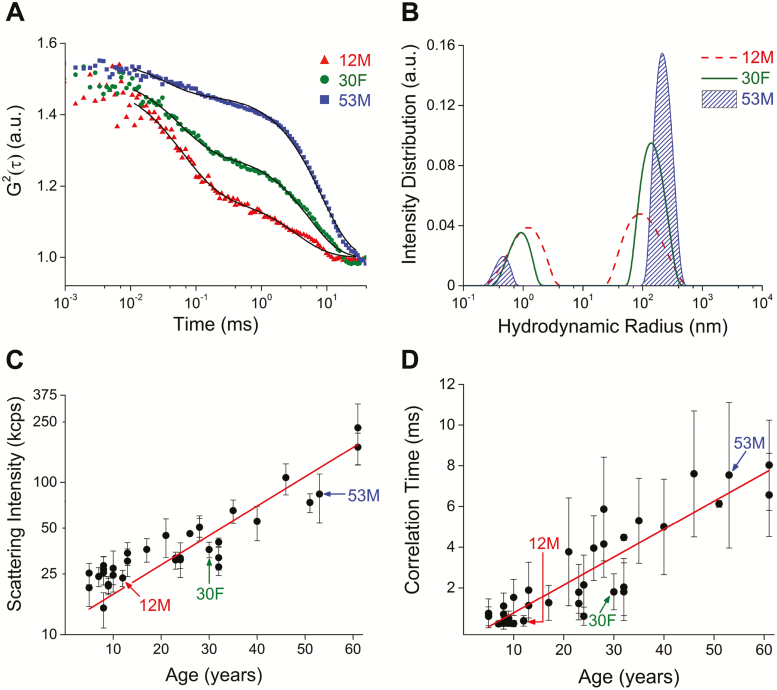
Next, we tested the analytical performance of the QLS instrument to detect changes in light scattering in lenses of 34 healthy human subjects (18 males, 16 females; 5–61 years; mean age, SD: 23.9 ± 16.3 years). Subjects were pretreated with a topical mydriatic and sympathomimetic to facilitate QLS assessment through a dilated pupil. Multiple QLS autocorrelation functions were automatically acquired in the lens nucleus at a predetermined distance from the anterior capsule of the right lens in each subject (median = 11 replicates; acquisition time, 220 ms per replicate). Movement artifacts were minimized by integrated eye tracking and dynamic piezo mirror systems. A parfocal CCD camera image of the acquisition location was automatically captured coincident with each acquired autocorrelation function and digitally stored for postacquisition confirmation of the target region within the lens. A lens viscosity of 5.74 cP was assumed for computing hydrodynamic size distributions (72). Correlation functions and hydrodynamic radius size distributions obtained from three representative subjects of different ages (12, 30, 53 years) revealed two intensity distribution peaks with an age-dependent shift toward larger scattering components (Figure 3A and B). Mean hydrodynamic radii for the larger scattering centers were 88 nm in the 12-year-old subject, 135 nm in the 30-year-old subject, and 208 nm in the 53-year-old-subject (Figure 3B). Mean hydrodynamic radii for smaller scattering centers were on the order of 1 nm for all three subjects (Figure 3B). We observed an age-dependent exponential increase in scattering intensity (R2 = 0.83, calculated for log-transformed data; Figure 3C) and linear increase in correlation time across all 34 subjects (R2 = 0.81; Figure 3D).
Figure 3.
Molecular aging detected by quasi-elastic light scattering (QLS) in vivo. (A) Representative QLS autocorrelation functions obtained from three healthy human subjects (12-year-old male, 30-year-old female, 53-year-old male). (B) Shift in hydrodynamic radius for the same subjects. (C) Increase in light scattering intensity as a function of age in healthy human subjects (N = 34; 18 males, 16 females). Data collected from nuclear region of the lens. Red line, exponential fit model, R2 = 0.83 (calculated for log-transformed data). (D) Change in correlation time observed with increasing age. Red line, linear fit model, R2 = 0.81.
Our results support the following model for jth observation of the ith subject:
where the parameters, and , are measures of within-subject and between-subject degree of inhomogeneity, respectively. This model is equivalent to a Breusch–Pagan test for heteroscedasticity (71) and can be used to estimate two-level residuals from mixed-effects regression analysis (70) of QLS metrics acquired from individuals of the same and differing ages across the human life span. For scattering intensity, the between-subject variability accounted for 57.9% of the total variability (p < .0001), whereas for correlation time, the between-subject variability accounted for 21.5% of the total variability (p = .0009). For scattering intensity, both within-subject and between-subject variance correlated with age (F = 39.9, p < .0001; F = 13.5, p = .0009). Similarly, for correlation time, the within-subject variance correlated with age (F = 64.6, p < .0001).
QLS Detects and Tracks Time-Dependent Changes in Human Lens Protein During Long-term (~1 Year) Incubation In Vitro
Next, we investigated the molecular origin of aging-related QLS signal changes detected in vivo by examining time-dependent QLS signal changes measured longitudinally during long-term incubation of water-soluble hLPE. We utilized this closed system as a cell-free analog of the human lens fiber cytosol (73). Single-donor hLPE samples were used to control for between-donor variability. We monitored QLS signal changes longitudinally during 322 days of continuous incubation in vitro at 37.4°C in a light-sealed incubator (Figure 4). The hLPE samples did not visibly precipitate during this incubation period. QLS measurements recorded during long-term incubation of hLPE revealed a biphasic effect in both scattering intensity (Figure 4A) and correlation time (Figure 4B). For scattering intensity, the apparent inflection time point was 58.7 days, as calculated by fitting experimental data to a piecewise exponential function with two segments (R2 = 0.99, Figure 4A). For correlation time, a piecewise linear function with two segments and selected intersection point was used (Figure 4B, R2 = 0.99). A small decrease in correlation time was observed prior to the inflection point (red arrow, Figure 4B). After 58.7 days, scattering intensity increased exponentially and correlation time increased linearly with time for the remainder of the experiment (Figure 4A and B). To calculate corresponding size distribution profiles, we assumed a viscosity of 1 cP corresponding to PBS. Extracted hydrodynamic radius values indicated a time-dependent shift toward increasing molecular size of the hLPE samples (Figure 4C). Mean hydrodynamic radii for the primary peak at 56, 154, 303 days were 20, 42, 65 nm, respectively (Figure 4C).
Figure 4.
Quasi-elastic light scattering (QLS) detects time-dependent changes in human lens protein during long-term (~1 year) incubation in vitro. (A) Increase in light scattering intensity during long-term (~1 year) incubation of human lens protein in vitro. Red line, exponential fit model (R2 = 0.99). (B) Increase in correlation time during long-term (~1 year) incubation in vitro. Red line, linear fit model (R2 = 0.99). Black arrow, calculated time of inflection. Red arrow, apparent reduction in correlation time. (C) Mean particle size distribution increases with incubation time.
Oxidation Accelerates Time-Dependent QLS changes in Human Lens Protein During Long-term Incubation In Vitro
Next, we examined time-dependent effects of oxidation on QLS signals obtained from hLPE during long-term incubation in vitro (Figure 5). Compared to control hLPE specimens (N = 3), hLPE exposed to oxidation (N = 3) demonstrated a rapid increase in scattering intensity that continued at an accelerated rate during 77 days of incubation (Figure 5A). Analysis of correlation time demonstrated a more complex pattern of QLS signal changes (Figure 5B). Both control and oxidized samples exhibited an initial decrease in correlation time consistent with our previous results (Figure 5B). However, the rate of change of the initial decrease in correlation time of hLPE under oxidative conditions was strikingly different compared to the control specimens (Figure 5B, red arrow). Following the initial decrease, the correlation time of the control sample stabilized for approximately 80 days (Figure 5B), consistent with the 1-year experiment (Figure 4B). Whereas, following an accelerated decrease until Day 5, the correlation time of the oxidized sample increased linearly with time (Figure 5B). These results suggest that the particle size in the oxidized hLPE sample was increasing rapidly compared to the control sample.
Figure 5.
Oxidation accelerates time-dependent quasi-elastic light scattering (QLS) changes in human lens protein during long-term incubation in vitro. (A) Change in light scattering intensity in control and oxidized human lens protein samples as a function of incubation time in vitro. (B) Change in correlation time in control and oxidized human lens protein samples as a function of incubation time. Error bars represent SD. Red arrow, apparent reduction in correlation time. (C–E) Protein gel electrophoresis (left panels), gel band densitometry plots (middle panels), and transmission electron microscope (TEM) images (right panels) comparing control and oxidized human lens protein specimens at representative time points during incubation (C, Day 0; D, Day 36; E, Day 77). Gel band densitometry plots are normalized to total signal for each sample. Arrow, high-molecular-weight (HMW) lens protein. Box, lens protein bands corresponding to α-crystallins. Histogram color code: violet = 0–11 kDa; blue = 12–32 kDa; green = 33–89 kDa; orange = 90–255 kDa; red > 255 kDa. Scale bar (TEM) = 100 nm.
We performed nonreducing SDS–PAGE with aliquots of hLPE sampled at selected time points (Day 0, 36, 77; Figure 5C–E) to investigate the supramolecular reorganization suggested by the QLS results. The band density corresponding to α-crystallin (~20 kDa; black box; Figure 5C–E) at Day 0 was consistent between the control and oxidized sample (Figure 5C). Between Day 0 and Day 36 in the oxidized sample there was a decrease in protein abundance at 20 kDa and an increase in protein of mass >255 kDa, whereas the control sample remained stable at 20 kDa (Figure 5D). Transmission electron microscope (TEM) images indicate that at Day 36 the control sample had started to form small aggregates, although these were degraded upon treatment with SDS during electrophoresis (Figure 5D). The large aggregates observed with TEM for the oxidized sample were resistant to SDS and so were present at >255 kDa in the corresponding nonreducing SDS–PAGE gel (Figure 5D). At Day 77, the control sample contained a higher proportion of SDS-resistant aggregates relative to earlier time points (8% of total protein with mass >255 kDa, Figure 5E). The TEM images of the oxidized sample at Day 77 showed further aggregation compared to Day 36. However, these further aggregates were largely susceptible to SDS degradation, as no further decrease in signal at 20 kDa was observed with SDS–PAGE, and there was only a modest increase in high-molecular-mass protein species (Figure 5E).
Discussion
Highly conserved crystallin proteins are expressed in lens fiber cells before birth, remain extant throughout life, and accumulate posttranslational modifications as a function of aging. A wide range of aging-related posttranslational modifications have been documented in human lens proteins (36,48–53). A shared consequence of these aberrant modifications for the ordered, transparent lens proteins is altered hydrodynamic radius. Subcataractous light scattering signals acquired using QLS from the nuclear region of the lens could therefore reflect aging-dependent molecular changes in these long-lived proteins integrated over a lifetime. Current putative biomarker test batteries of aging incorporating composite metrics have been developed to track biological aging in humans (16,17,74,75). However, many of these indices are far removed from the underlying molecular mechanisms of aging. In this study, we showed that QLS can be used to detect time-dependent changes in long-lived lens proteins during long-term incubation in vitro and aging-dependent changes in these same proteins in the lenses of human subjects in vivo.
The QLS instrument used in this study was designed for rapid, reliable, and automatic acquisition of multiple autocorrelation data sets in the nuclear subregion of the lens in children and adults. We utilized a unique instrument design that automatically detected the anterior capsule interface and localized the QLS acquisition volume in the central nucleus of the lens. Detection of modifications to long-lived proteins in the central nuclear region of the lens reflects aging-dependent molecular changes integrated over a lifetime. In order to show feasibility of our QLS instrument to measure molecular aging, we first investigated the origin of time-dependent alterations in QLS signals obtained from hLPE recorded during long-term incubation (~1 year) and in response to biologically relevant oxidative stress. Interestingly, the shape of the size distributions and observed changes (shifts to larger sizes) of isolated lens proteins incubated at 37°C in vitro for 56, 154, and 303 days were similar to three representative human patients aged 12, 30, and 53 years (Figures 3B and 4C). These data suggest that isolated soluble lens proteins undergo accelerated aging and associated supramolecular reorganization when incubated in vitro, and that these effects can be accurately measured and recapitulated in aging human lenses measured in vivo. Biologically relevant oxidative stress was used to induce accelerated aggregation as measured by QLS and confirmed with SDS–PAGE and TEM. Oxidized protein species accumulate in the aging human lens (76,77). Cumulative oxidative damage in the lens is postulated as a major driver of cataract formation and presbyopia (23), the leading causes of human blindness and visual disability (78). While the increase in protein size over time in both control and oxidized in vitro samples was expected (79), it is not clear what the initial decrease (5 days in oxidized samples, ~40 days in controls) in QLS correlation time represents in both oxidized and control in vitro samples (Figures 4B and 5B). Further investigation is required to determine if this sustained initial decrease in correlation time in vitro represents a drop in molecular size in these samples, and to determine whether this effect has any relevance for in vivo lens tissue, for instance in response to acute oxidative stress.
We subsequently deployed our QLS eye scanner in a clinical feasibility trial involving human subjects (N = 34) across a broad age range (5–61 years), from young children to older adults of both sexes. The results of this clinical study revealed aging-related QLS signals in the lenses of living humans that closely mirror time-dependent QLS changes observed during long-term incubation and oxidation of human lens protein in vitro. Data generated from pediatric subjects in the first decile of life are critical for longitudinal assessment of molecular aging across the life span. In this feasibility study of 34 male and female subjects aged 5 to 61 years of age, we showed that both scattering intensity and correlation time demonstrated significant aging-related dependencies with respect to first and second moments (within-subject mean and variance, respectively). Although the clinical component of this study was designed to ascertain technical feasibility, our results demonstrated that scattering intensity also exhibited significant aging-related dependence with respect to the between-subject variance. While the clinical component of this study was not designed for or powered to assess between-subject variance for correlation time, our results are consistent with and provide strong support for utilizing scattering intensity and correlation time assessed by QLS in the nucleus of the human lens as candidate biomarkers of molecular aging in vivo. Moreover, the expected variability observed in both QLS indices may provide other biologically relevant metrics of individual aging-related variation that capture increased local inhomogeneity in the oldest lens fibers (41,80). At this stage it is impossible to declare that the variation between individuals we observe with QLS in these subjects can differentiate biological from chronological aging. Thus, further investigation of the these and allied QLS-derived metrics is ongoing.
Study limitations include the small cohort size and cross-sectional design of the clinical component. However, our results provide scientific and feasibility validation that warrants further evaluation in multicenter clinical trials (cross-sectional and longitudinal) with the goal of generating normative data. While this objective is beyond the scope of the present study, the QLS changes that we detected in the lenses of living humans were sufficiently robust to allow detection of strongly age-dependent changes. A general analytical issue that warrants interpretive caution is use of the Stokes–Einstein relation (81,82) to determine hydrodynamic radii distribution profiles. Exact analyses using this relation are theoretically limited to dilute, optically isotropic suspensions of noninteracting, nonabsorbing spherical particles that are smaller than the wavelength of light. These conditions are not met in the human lens, and accordingly, we focused our quantitative analysis on specific indices (ie, scattering intensity, correlation time) that do not rely on the Stokes–Einstein relation. The presence of clinically significant cataract in the lens would render this technique inapplicable; therefore, subjects with cataract were excluded from our study. The findings in this study focus on the lens and thus raise questions about generalizability to other tissue compartments. This issue is constrained by the fact that there exist few proteins with extreme stability and longevity, and except for the lens crystallins, are not optically or biochemically accessible in living humans. Using posttranslational and supramolecular modifications of the crystallin proteins as an in vivo marker of molecular aging is analogous to measuring hemoglobin A1c (HbA1c) as an index of nonenzymatic glycosylation in diabetes mellitus (83–85)—with the caveat that HbA1c captures a specific posttranslational modification (ie, glycosylation) in the 3 months prior to sampling, whereas QLS analysis of scattering in the central nucleus of the lens detects the common consequence of a broad range of protein modifications (ie, altered hydrodynamic radius) integrated over a lifetime. Crucially, the lens fiber cell cytosol is not a restricted compartment, but rather is in biochemical contact with the rest of the body via lenticular circulation (86–88) of aqueous humor, the intraocular fluid produced by the ciliary epithelium from blood plasma through secretion, ultrafiltration, and diffusion (89). Further work is required to determine whether lens protein aggregation as measured by QLS is therefore a marker of posttranslational damage affecting other long-lived proteins, and by extension, the individual.
Our results show that the long-lived lens proteins time-dependently accumulate molecular alterations that increase hydrodynamic radius, protein polydispersity, and supramolecular order. Modified crystallins are retained in the fiber cell cytoplasm without turnover (43). Thus, the rationale for utilizing these extremely stable structural proteins as molecular clocks (21). Indeed, radiocarbon dating of crystallin proteins in the central nuclear region of the lens was recently used to establish the extreme life span and oldest age of Greenland sharks (Somniosus microcephalus), the longest living vertebrates known (90). We are not aware of another published study that has evaluated molecular changes in long-lived human proteins over an extended period (~1 year) or correlated specific aging-related mechanisms with corresponding molecular signals detected in vitro and validated in humans in vivo. In this context, it is important to emphasize that acquisition and analysis of these dynamic photon correlational spectroscopy metrics are not possible using static measurements acquired by retroillumination or other conventional densitometric techniques.
While the findings presented here establish the feasibility of using QLS assessment of the lens to evaluate molecular aging in vivo, establishing the scientific validity and clinical utility of this technique will require replication in cross-sectional and longitudinal trials involving diverse study populations across the human life span as well as concordance testing in laboratory animals with differing life spans, and in response to modulating factors (eg, genotype differences, dietary regimens, environmental exposures) that are known to alter biological aging. While our study focuses on temporal accrual of molecular alterations stemming from stochastic events, our results should not be interpreted to exclude genetic contributions to aging. Indeed, genetic and epigenetic contributions to aging pathways undoubtedly play a role in modulating homeostatic mechanisms (eg, reactive oxygen species reactivity, autophagy, proteostasis) that alter susceptibility, rate, and consequences of biomolecule damage (91–96).
The results reported here pave the way for a potentially transformative clinical tool for objective assessment and tracking of molecular aging in living humans. The framework for clinical implementation of QLS technology for this indication is similar to other recently adopted clinical biomarkers, including PET-PIB β-amyloid imaging for Alzheimer’s disease, DEXA bone densitometry for osteoporosis, and serum HbA1c blood tests for diabetes mellitus. While large test batteries incorporating composite metrics have been developed to track biological aging in humans (eg, (16)), these derivative indices are far removed from underlying molecular mechanisms of aging and are ill-suited for personalized longitudinal medical care. By contrast, QLS technology affords a rapid, noninvasive, objective technique for direct measurement of molecular alterations associated with aging that can be easily and safely implemented by a medical professional or allied health care provider at the point of care, thereby providing an individualized index of molecular aging that is available immediately after testing. Such a metric affords potential for precision medical care across the life span.
Funding
This work was supported by the Massachusetts Lions Eye Research Fund, the Boston University Alzheimer’s Disease Research Center through the National Institutes of Health (grant number P30-AG013846), and the Children’s Hospital Ophthalmology Foundation, Boston, MA.
Conflict of Interest
None reported.
Acknowledgments
The authors thank Robert H. Webb for helpful discussions and input on quasi-elastic light scattering instrument safety measures. Author contributions: O.M. and S.S. carried out the experiments, analyzed the data, and contributed to manuscript writing. D.M.L. and D.G.H. conducted the clinical study. O.M., S.S., C.A.B., and F.J.W. assisted with the clinical study. J.A.M. analyzed data and reviewed the manuscript. D.S.P. analyzed data and contributed to manuscript writing. K.J.W., R.D.M., and J.A.M. assisted with sample preparation. M.E. assisted with electron microscopy. Y.T., O.M., and S.S. performed statistical analysis. J.I.C., R.E.T., and D.G.H. provided critical review for scientific content and manuscript preparation. D.G.H. and L.E.G. designed the clinical study. L.E.G. organized the project (including instrument development, preclinical analyses, and clinical study), analyzed the data, and wrote the manuscript.
References
- 1. Herndon LA, Schmeissner PJ, Dudaronek JM, et al. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419:808–814. doi: 10.1038/nature01135 [DOI] [PubMed] [Google Scholar]
- 2. Kirkwood TB, Feder M, Finch CE, et al. What accounts for the wide variation in life span of genetically identical organisms reared in a constant environment? Mech Ageing Dev. 2005;126:439–443. doi: 10.1016/j.mad.2004.09.008 [DOI] [PubMed] [Google Scholar]
- 3. Hayflick L. Biological aging is no longer an unsolved problem. Ann NY Acad Sci. 2007;1100:1–13. doi: 10.1196/annals.1395.001 [DOI] [PubMed] [Google Scholar]
- 4. Johnson TE. Recent results: biomarkers of aging. Exp Gerontol. 2006;41:1243–1246. doi: 10.1016/j.exger.2006.09.006 [DOI] [PubMed] [Google Scholar]
- 5. Sanderson WC, Scherbov S. Demography. Remeasuring aging. Science. 2010;329:1287–1288. doi: 10.1126/science.1193647 [DOI] [PubMed] [Google Scholar]
- 6. Sprott RL. Biomarkers of aging and disease: introduction and definitions. Exp Gerontol. 2010;45:2–4. doi: 10.1016/j.exger.2009.07.008 [DOI] [PubMed] [Google Scholar]
- 7. Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027 [DOI] [PubMed] [Google Scholar]
- 8. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rose MR, Flatt T, Graves JL, et al. What is aging? Front Genet. 2012;3:134. doi: 10.3389/fgene.2012.00134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ingram DK, Nakamura E, Smucny D, Roth GS, Lane MA. Strategy for identifying biomarkers of aging in long-lived species. Exp Gerontol. 2001;36:1025–1034. doi: 10.1016/s0531-5565(01)00110-3 [DOI] [PubMed] [Google Scholar]
- 11. Flier JS, Underhill LH, McEwen BS. Protective and damaging effects of stress mediators. New Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307 [DOI] [PubMed] [Google Scholar]
- 12. Timiras PS. Physiological Basis of Aging and Geriatrics. Boca Raton, FL: CRC Press; 2007. [Google Scholar]
- 13. MacDonald SW, Dixon RA, Cohen AL, Hazlitt JE. Biological age and 12-year cognitive change in older adults: findings from the Victoria Longitudinal Study. Gerontology. 2004;50:64–81. doi: 10.1159/000075557 [DOI] [PubMed] [Google Scholar]
- 14. Levine ME. Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age? J Gerontol A Biol Sci Med Sci. 2013;68:667–674. doi: 10.1093/gerona/gls233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sanders JL, Minster RL, Barmada MM, et al. Heritability of and mortality prediction with a longevity phenotype: the healthy aging index. J Gerontol A Biol Sci Med Sci. 2014;69:479–485. doi: 10.1093/gerona/glt117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Belsky DW, Caspi A, Houts R, et al. Quantification of biological aging in young adults. Proc Natl Acad Sci USA. 2015;112:E4104-E4110. doi: 10.1073/pnas.1506264112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sebastiani P, Thyagarajan B, Sun F, et al. Biomarker signatures of aging. Aging Cell. 2017;16:329–338. doi: 10.1111/acel.12557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carnes BA. Can any biomarker predict the temporal behavior of aging? Gerontology. 2015;62:63–65. doi: 10.1159/000430912 [DOI] [PubMed] [Google Scholar]
- 19.Sell DR, Monnier VM. Aging of long lived proteins: extracellular matrix (collagens, elastins, proteoglycans) and lens crystallins. In: Terjung R, ed. Comprehensive Physiology - Handbook of Physiology, Aging. Bethesda, MD: American Physiological Society; 2011:235–305. [Google Scholar]
- 20. Toyama BH, Hetzer MW. Protein homeostasis: live long, won’t prosper. Nat Rev Mol Cell Biol. 2013;14:55–61. doi: 10.1038/nrm3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Truscott RJW, Schey KL, Friedrich MG. Old proteins in man: a field in its infancy. Trends Biochem Sci. 2016;41:654–664. doi: 10.1016/j.tibs.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Toyama BH, Savas JN, Park SK, et al. Identification of long-lived proteins reveals exceptional stability of essential cellular structures. Cell. 2013;154:971–982. doi: 10.1016/j.cell.2013.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Truscott RJ. Age-related nuclear cataract-oxidation is the key. Exp Eye Res. 2005;80:709–725. doi: 10.1016/j.exer.2004.12.007 [DOI] [PubMed] [Google Scholar]
- 24. Wang JJ, Mitchell P, Simpson JM, Cumming RG, Smith W. Visual impairment, age-related cataract, and mortality. Arch Ophthalmol. 2001;119:1186–1190. doi: 10.1001/archopht.119.8.1186 [DOI] [PubMed] [Google Scholar]
- 25. Knudtson MD, Klein BE, Klein R. Age-related eye disease, visual impairment, and survival: the Beaver Dam Eye Study. Arch Ophthalmol. 2006;124:243–249. doi: 10.1001/archopht.124.2.243 [DOI] [PubMed] [Google Scholar]
- 26. Khanna RC, Murthy GV, Giridhar P, et al. Cataract, visual impairment and long-term mortality in a rural cohort in India: the Andhra Pradesh Eye Disease Study. PLoS One. 2013;8:e78002. doi: 10.1371/journal.pone.0078002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tseng VL, Yu F, Lum F, Coleman AL. Cataract surgery and mortality in the United States Medicare population. Ophthalmology. 2016;123:1019–1026. doi: 10.1016/j.ophtha.2015.12.033 [DOI] [PubMed] [Google Scholar]
- 28. Pathai S, Shiels PG, Lawn SD, Cook C, Gilbert C. The eye as a model of ageing in translational research–molecular, epigenetic and clinical aspects. Ageing Res Rev. 2013;12:490–508. doi: 10.1016/j.arr.2012.11.002 [DOI] [PubMed] [Google Scholar]
- 29. Bloemendal H. The vertebrate eye lens. Science. 1977;197:127–138. doi: 10.1126/science.877544 [DOI] [PubMed] [Google Scholar]
- 30. Kuszak J, Costello M.. Embryology and Anatomy of Human Lenses. Duane’s Foundations of Clinical Ophthalmology. Philadelphia, PA: Lippincott Williams & Wilkins; 1992. [Google Scholar]
- 31. Augusteyn RC. On the growth and internal structure of the human lens. Exp Eye Res. 2010;90:643–654. doi: 10.1016/j.exer.2010.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19:134–153. doi: 10.1111/j.1432-0436.1981.tb01141.x [DOI] [PubMed] [Google Scholar]
- 33. Bassnett S. On the mechanism of organelle degradation in the vertebrate lens. Exp Eye Res. 2009;88:133–139. doi: 10.1016/j.exer.2008.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thomson JA, Augusteyn RC. Ontogeny of human lens crystallins. Exp Eye Res. 1985;40:393–410. doi: 10.1016/0014-4835(85)90152-6 [DOI] [PubMed] [Google Scholar]
- 35. McAvoy JW, Chamberlain CG, de Iongh RU, Hales AM, Lovicu FJ. Lens development. Eye (Lond). 1999;13 (Pt 3b):425–437. doi: 10.1038/eye.1999.117 [DOI] [PubMed] [Google Scholar]
- 36. Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86:407–485. doi: 10.1016/j.pbiomolbio.2003.11.012 [DOI] [PubMed] [Google Scholar]
- 37. Slingsby C, Wistow GJ, Clark AR. Evolution of crystallins for a role in the vertebrate eye lens. Protein Sci. 2013;22:367–380. doi: 10.1002/pro.2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Benedek GB. Theory of transparency of the eye. Appl Opt. 1971;10:459–473. doi: 10.1364/AO.10.000459 [DOI] [PubMed] [Google Scholar]
- 39. Delaye M, Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. Nature. 1983;302:415–417. doi: 10.1038/302415a0 [DOI] [PubMed] [Google Scholar]
- 40. Clark JI. Order and disorder in the transparent media of the eye. Exp Eye Res. 2004;78:427–432. doi: 10.1016/j.exer.2003.10.008 [DOI] [PubMed] [Google Scholar]
- 41. Schietroma C, Fain N, Zampighi LM, Lanzavecchia S, Zampighi GA. The structure of the cytoplasm of lens fibers as determined by conical tomography. Exp Eye Res. 2009;88:566–574. doi: 10.1016/j.exer.2008.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Costello MJ, Burette A, Weber M, et al. Electron tomography of fiber cell cytoplasm and dense cores of multilamellar bodies from human age-related nuclear cataracts. Exp Eye Res. 2012;101:72–81. doi: 10.1016/j.exer.2012.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lynnerup N, Kjeldsen H, Heegaard S, Jacobsen C, Heinemeier J. Radiocarbon dating of the human eye lens crystallines reveal proteins without carbon turnover throughout life. PLoS One. 2008;3:e1529. doi: 10.1371/journal.pone.0001529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Masters PM, Bada JL, Zigler JS Jr. Aspartic acid racemisation in the human lens during ageing and in cataract formation. Nature. 1977;268:71–73. doi: 10.1038/268071a0 [DOI] [PubMed] [Google Scholar]
- 45. Lampi KJ, Ma Z, Hanson SR, et al. Age-related changes in human lens crystallins identified by two-dimensional electrophoresis and mass spectrometry. Exp Eye Res. 1998;67:31–43. doi: 10.1006/exer.1998.0481 [DOI] [PubMed] [Google Scholar]
- 46. Truscott RJW. Macromolecular deterioration as the ultimate constraint on human lifespan. Ageing Res Rev. 2011;10:397–403. doi: 10.1016/j.arr.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 47. Michael R, Bron AJ. The ageing lens and cataract: a model of normal and pathological ageing. Philos Trans R Soc Lond B Biol Sci. 2011;366:1278–1292. doi: 10.1098/rstb.2010.0300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. MacCoss MJ, McDonald WH, Saraf A, et al. Shotgun identification of protein modifications from protein complexes and lens tissue. Proc Natl Acad Sci USA. 2002;99:7900–7905. doi: 10.1073/pnas.122231399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Harding JJ. Viewing molecular mechanisms of ageing through a lens. Ageing Res Rev. 2002;1:465–479. doi: 10.1016/s1568-1637(02)00012-0 [DOI] [PubMed] [Google Scholar]
- 51. Wilmarth PA, Tanner S, Dasari S, et al. Age-related changes in human crystallins determined from comparative analysis of post-translational modifications in young and aged lens: does deamidation contribute to crystallin insolubility? J Proteome Res. 2006;5:2554–2566. doi: 10.1021/pr050473a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hains PG, Truscott RJ. Post-translational modifications in the nuclear region of young, aged, and cataract human lenses. J Proteome Res. 2007;6:3935–3943. doi: 10.1021/pr070138h [DOI] [PubMed] [Google Scholar]
- 53. Schey KL, Wang Z, Friedrich M, Garland DL, Truscott RJ. Spatiotemporal changes in the human lens proteome: critical insights into long-lived proteins. Prog Retin Eye Res. 2019:100802. In press. doi: 10.1016/j.preteyeres.2019.100802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tanaka T, Benedek GB. Observation of protein diffusivity in intact human and bovine eye lenses with application to cataract. Invest Ophth Vis Sci. 1975;6:449–456. PMID: 1132941. [PubMed] [Google Scholar]
- 55. Jedziniak JA, Nicoli DF, Baram H, Benedek GB. Quantitative verification of the existence of high molecular weight protein aggregates in the intact normal human lens by light-scattering spectroscopy. Invest Ophthalmol Vis Sci. 1978;17:51–57. PMID: 621125. [PubMed] [Google Scholar]
- 56. Michiel M, Duprat E, Skouri-Panet F, et al. Aggregation of deamidated human betaB2-crystallin and incomplete rescue by alpha-crystallin chaperone. Exp Eye Res. 2010;90:688–698. doi: 10.1016/j.exer.2010.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Weiss JN, Rand LI, Gleason RE, Soeldner JS. Laser light scattering spectroscopy of in vivo human lenses. Invest Ophthalmol Vis Sci. 1984;25:594–598. PMID: 6715134. [PubMed] [Google Scholar]
- 58. Benedek GB, Chylack LT Jr, Libondi T, Magnante P, Pennett M. Quantitative detection of the molecular changes associated with early cataractogenesis in the living human lens using quasielastic light scattering. Curr Eye Res. 1987;6:1421–1432. doi: 10.3109/02713688709044506 [DOI] [PubMed] [Google Scholar]
- 59. Bursell SE, Baker RS, Weiss JN, Haughton JF, Rand LI. Clinical photon correlation spectroscopy evaluation of human diabetic lenses. Exp Eye Res. 1989;49:241–258. doi: 10.1016/0014-4835(89)90094-8 [DOI] [PubMed] [Google Scholar]
- 60. Thurston GM, Hayden DL, Burrows P, et al. Quasielastic light scattering study of the living human lens as a function of age. Curr Eye Res. 1997;16:197–207. doi: 10.1076/ceyr.16.3.197.15410 [DOI] [PubMed] [Google Scholar]
- 61. Datiles MB 3rd, Ansari RR, Suh KI, et al. Clinical detection of precataractous lens protein changes using dynamic light scattering. Arch Ophthalmol. 2008;126:1687–1693. doi: 10.1001/archophthalmol.2008.507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Datiles MB, Ansari RR, Yoshida J, et al. Longitudinal study of age-related cataract using dynamic light scattering: loss of α-crystallin leads to nuclear cataract development. Ophthalmology. 2016;123:248–254. doi: 10.1016/j.ophtha.2015.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zigler JS Jr, Huang QL, Du XY. Oxidative modification of lens crystallins by H2O2 and chelated iron. Free Radic Biol Med. 1989;7:499–505. doi: 10.1016/0891-5849(89)90025-7 [DOI] [PubMed] [Google Scholar]
- 64. Pecora R. Quasi-elastic light scattering from macromolecules. Annu Rev Biophys Bioeng. 1972;1:257–276. doi: 10.1146/annurev.bb.01.060172.001353 [DOI] [PubMed] [Google Scholar]
- 65. Berne BJ, Pecora R.. Dynamic Light Scattering with Applications to Chemistry, Biology, and Physics. New York, NY: John Wiley & Sons, Inc.; 1976. [Google Scholar]
- 66. Alango Ltd. DYNALS Software for Particle Size Distribution Analysis in Photon Correlation Spectroscopy Website http://www.softscientific.com/science/WhitePapers/dynals1/dynals100.htm. Updated March 2002. Accessed April 10, 2020.
- 67. Yuan X, Liu Z, Wang Y, et al. The non-negative truncated singular value decomposition for adaptive sampling of particle size distribution in dynamic light scattering inversion. J Quant Spectrosc RA. 2020;246:106917. doi: 10.1016/j.jqsrt.2020.106917 [DOI] [Google Scholar]
- 68.Provencher SW. A constrained regularization method for inverting data represented by linear algebraic or integral equations. Comput Phys Commun. 1982;27:213–227. doi: 10.1016/0010-4655(82)90173-4 [DOI] [Google Scholar]
- 69.Provencher SW. CONTIN: a general purpose constrained regularization program for inverting noisy linear algebraic and integral equations. Comput Phys Commun. 1982;27:229–242. doi: 10.1016/0010-4655(82)90174-6 [DOI] [Google Scholar]
- 70. Snijders TAB, Bosker RJ.. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. 2nd ed. London, UK: Sage Publishers; 2012. [Google Scholar]
- 71.Breusch TS, Pagan AR. A simple test for heteroscedasticity and random coefficient variation. Econometrica. 1979;47:1287–1294. doi: 10.2307/1911963 [DOI] [Google Scholar]
- 72. Thao MT, Perez D, Dillon J, Gaillard ER. Measuring the viscosity of whole bovine lens using a fiber optic oxygen sensing system. Mol Vis. 2014;20:125–131. PMID: 24505211. [PMC free article] [PubMed] [Google Scholar]
- 73. Zigler JS, Goosey J. Aging of protein molecules: lens crystallins as a model system. Trends Biochem Sci. 1981;6:133–136. doi: 10.1016/0968-0004(81)90050-5 [DOI] [Google Scholar]
- 74. Belsky DW, Moffitt TE, Cohen AA, et al. Eleven telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: do they measure the same thing? Am J Epidemiol. 2017;187:1220–1230. doi: 10.1093/aje/kwx346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hastings WJ, Shalev I, Belsky DW. Comparability of biological aging measures in the National Health and Nutrition Examination Study, 1999–2002. Psychoneuroendocrinology. 2019;106:171–178. doi: 10.1016/j.psyneuen.2019.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Garner MH, Spector A. Selective oxidation of cysteine and methionine in normal and senile cataractous lenses. Proc Natl Acad Sci USA. 1980;77:1274–1277. doi: 10.1073/pnas.77.3.1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Augusteyn R. Protein modification in cataract: possible oxidative mechanisms. In: Duncan G, ed. Mechanisms of Cataract Formation in the Human Lens. London, UK: Academic Press; 1981:71–115. [Google Scholar]
- 78. Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Brit J Ophthalmol. 2012;96:614–618. doi: 10.1136/bjophthalmol-2011-300539 [DOI] [PubMed] [Google Scholar]
- 79. Liu C, Pande J, Lomakin A, Ogun O, Benedek GB. Aggregation in aqueous solutions of bovine lens gamma-crystallins: special role of gamma(s). Invest Ophthalmol Vis Sci. 1998;39:1609–1619. PMID: 9699550. [PubMed] [Google Scholar]
- 80. Freel CD, Gilliland KO, Wesley Lane C, Giblin FJ, Costello MJ. Fourier analysis of cytoplasmic texture in nuclear fiber cells from transparent and cataractous human and animal lenses. Exp Eye Res. 2002;74:689–702. doi: 10.1006/exer.2001.1166 [DOI] [PubMed] [Google Scholar]
- 81. Einstein A. Über die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen. Annalen der Physik. 1905;4:549–560. doi: 10.1002/andp.19053220806 [DOI] [Google Scholar]
- 82. Sutherland W. LXXV. A dynamical theory of diffusion for non-electrolytes and the molecular mass of albumin. London, Edinburgh Dublin Philos Mag J Sci. 1905;9:781–785. doi: 10.1080/14786440509463331 [DOI] [Google Scholar]
- 83. Brownlee M, Vlassara H, Cerami A. Nonenzymatic glycosylation and the pathogenesis of diabetic complications. Ann Intern Med. 1984;101:527–537. doi: 10.7326/0003-4819-101-4-527 [DOI] [PubMed] [Google Scholar]
- 84. Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223–234. doi: 10.1146/annurev.med.46.1.223 [DOI] [PubMed] [Google Scholar]
- 85. Cohen MP. Diabetes and Protein Glycosylation: Measurement and Biologic Relevance. New York, NY: Springer Science & Business Media; 2012. [Google Scholar]
- 86. Mathias RT, Kistler J, Donaldson P. The lens circulation. J Membr Biol. 2007;216:1–16. doi: 10.1007/s00232-007-9019-y [DOI] [PubMed] [Google Scholar]
- 87. Donaldson PJ, Musil LS, Mathias RT. Point: A critical appraisal of the lens circulation model–an experimental paradigm for understanding the maintenance of lens transparency? Invest Ophthalmol Vis Sci. 2010;51:2303–2306. doi: 10.1167/iovs.10-5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Candia OA, Mathias R, Gerometta R. Fluid circulation determined in the isolated bovine lens. Invest Ophthalmol Vis Sci. 2012;53:7087–7096. doi: 10.1167/iovs.12-10295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Goel M, Picciani RG, Lee RK, Bhattacharya SK. Aqueous humor dynamics: a review. Open Ophthalmol J. 2010;4:52–59. doi: 10.2174/1874364101004010052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Nielsen J, Hedeholm RB, Heinemeier J, et al. Eye lens radiocarbon reveals centuries of longevity in the Greenland shark (Somniosus microcephalus). Science. 2016;353:702–704. doi: 10.1126/science.aaf1703 [DOI] [PubMed] [Google Scholar]
- 91. Finch CE, Tanzi RE. Genetics of aging. Science. 1997;278:407–411. doi: 10.1126/science.278.5337.407 [DOI] [PubMed] [Google Scholar]
- 92. Hayflick L. Entropy explains aging, genetic determinism explains longevity, and undefined terminology explains misunderstanding both. PLoS Genet. 2007;3:e220. doi: 10.1371/journal.pgen.0030220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kirkwood TB, Cordell HJ, Finch CE. Speed-bumps ahead for the genetics of later-life diseases. Trends Genet. 2011;27:387–388. doi: 10.1016/j.tig.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 94. Barzilai N, Guarente L, Kirkwood TB, Partridge L, Rando TA, Slagboom PE. The place of genetics in ageing research. Nat Rev Genet. 2012;13:589–594. doi: 10.1038/nrg3290 [DOI] [PubMed] [Google Scholar]
- 95. Brooks-Wilson AR. Genetics of healthy aging and longevity. Hum Genet. 2013;132:1323–1338. doi: 10.1007/s00439-013-1342-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Oshima J, Hisama FM, Martin GM. The biological basis of aging: implications for medical genetics. In: Rimoin DL, Pyeritz RE, Korf B, eds. Emery and Rimoin’s Principles and Practice of Medical Genetics and Genomics. Amsterdam, Netherlands: Elsevier; 2019:415–444. [Google Scholar]