Abstract
Cognitive impairment in the aging population is quickly becoming a health care priority, for which currently no disease-modifying treatment is available. Multiple domains of cognition decline with age even in the absence of neurodegenerative diseases. The cellular and molecular changes leading to cognitive decline with age remain elusive. Synaptobrevin-2 (Syb2), the major vesicular SNAP receptor protein, highly expressed in the cerebral cortex and hippocampus, is essential for synaptic transmission. We have analyzed Syb2 protein levels in mice and found a decrease with age. To investigate the functional consequences of lower Syb2 expression, we have used adult Syb2 heterozygous mice (Syb2+/−) with reduced Syb2 levels. This allowed us to mimic the age-related decrease of Syb2 in the brain in order to selectively test its effects on learning and memory. Our results show that Syb2+/− animals have impaired learning and memory skills and they perform worse with age in the radial arm water maze assay. Syb2+/− hippocampal neurons have reduced synaptic plasticity with reduced release probability and impaired long-term potentiation in the CA1 region. Syb2+/− neurons also have lower vesicular release rates when compared to WT controls. These results indicate that reduced Syb2 expression with age is sufficient to cause cognitive impairment.
Keywords: Brain aging, Neurotransmitter release, Hippocampus, Cognitive impairment, Synaptic plasticity
Aging is associated with progressive deterioration of physiological integrity that causes functional deficits leading to a decrease in quality of life. Cognitive decline with memory impairment and other changes in nervous system functions, like processing speed and executive functions are common complains associated with aging (1). Decline in many well-described memory parameters such as working memory, long-term episodic memory, and spatial recollection have been shown to decrease with age (2–4). Unlike dementias such as Alzheimer’s disease, age-related cognitive impairment is not accompanied by massive neuronal loss (5–8). Unfortunately, age-related changes in the brain are multifactorial and the confounding factors, even in well-controlled aging animal studies (9,10), make it extremely difficult to properly identify the relative importance of specific proteins or factors in the process leading to cognitive impairment. For this reason, despite extensive research in the field, the exact mechanisms underlying age-related cognitive decline are still not well understood. As the population of adults over 65 is predicted to double in the United States by 2050 (4), it has become essential to identify the key factors leading to dementia and cognitive decline during aging if we are to counter these devastating age-related disorders.
Synapses are the elementary building blocks of the nervous system and synaptic function is essential for rapid information propagation and processing in the brain. Synaptic plasticity, the ability of the synapse to change its strength in response to stimulus intensity, is critical in learning and memory storage (11–15). Synaptic dysfunction has emerged as a leading cause of cognitive impairment seen in many neurological diseases (16–18). Synaptic vesicles release neurotransmitters that allow the transfer of signals from one neuron to the next. Dysregulation or dysfunction of the components of the synaptic vesicle release machinery can have profound consequences on information transfer in the brain leading to significant deficits. For effective communication between neurons, action potentials arrive at a presynaptic terminal and cause an influx of calcium ions that bind to synaptotagmin which then interacts with the SNARE (soluble NSF attachment protein receptor) complex and triggers fast synaptic vesicle release of neurotransmitter. Proteins that make up the SNARE complex drive membrane fusion and are essential for neurotransmitter release (19,20). At neuronal synapses, the SNARE complex is composed of three proteins: syntaxin-1, SNAP-25, and synaptobrevin-2 (Syb2).
Age-related changes in the brain are multifactorial and the confounding factors even in well-controlled aging animal studies make it extremely difficult to properly identify the relative importance of a specific protein or factor in the process leading to cognitive impairment. Researchers have shown that expression of a number of synaptic proteins reduces with age (21–23), although the exact mechanism of this reduction is not well understood. Interestingly, a recent human study (24) indicated that cognitive performance in elderly adults was associated with the levels of synaptobrevin-2—a key protein of the SNARE complex in synaptic transmitter release.
Synaptobrevin-2 (also known as vesicle associated membrane protein VAMP2) is especially interesting because it is the major vesicular SNARE protein in the brain. Syb2 has also been shown to be essential for fast vesicle exocytosis (25–31) and endocytosis (32). Syb2 is highly expressed in the hippocampus; a region that is essential for normal learning and memory function, and this led us to question if Syb2 downregulation plays a role in age-related memory decline. We hypothesized that reduced Syb2 expression mediates age-related cognitive decline. To test this hypothesis, we used Syb2+/− knockout animals to model brain aging. Although Syb2 homozygous knockout mice are neonatal lethal, heterozygous animals survive and are fertile. We assessed the learning and memory ability of the animals using behavioral assays, neuronal circuitry and long-term potentiation (LTP) assays and in vitro synaptic strength assays.
Methods
Animals
We used VAMP2 heterozygous (Syb2+/−) knockout animals that were generated as previously described (30). These mice were maintained by breeding Syb2+/− with 129/SV (WT; The Jackson Laboratories) mice. Animals were kept on a 12-hour light/dark cycle in the Rodent Barrier Facility at the University of Oklahoma Health Sciences Center (OUHSC) and fed standard rodent chow (D12450B Research Diets Inc., New Brunswick, NJ) ad libitum. Experiments were conducted according to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and our protocols were approved by the Institutional Animal Care and Use Committee of OUHSC.
Genotyping
Syb2+/− cohorts were genotyped as previously described in (30). A small piece of the tail was digested using a REDEXTRACT-N-Amp Tissue PCR kit (Sigma Aldrich, St. Louis, MO). Primers and polymerase chain reaction conditions were used as previously described (30).
Chemicals
The chemicals used in this study were purchased from the following sources: FM1-43, B-27 supplement and minimal essential medium (Invitrogen, Carlsbad, CA), Matrigel and fetal bovine serum (ThermoFisher, Waltham, MA), and transferrin (Millipore, Billerica, MA). All additional chemicals were purchased from Sigma Aldrich.
Western Blotting
To quantify the amount of Syb2, half brains from controls (WT) and Syb2+/− KO mice from different age groups were lysed in radioimmunoprecipitation assay buffer (Sigma Aldrich) containing protease inhibitor cocktail (Roche diagnostics, Mannheim, Germany) and protein concentration was determined using a DC protein quantification assay kit (BioRad). All antibodies used in Western blots were from commercial sources with well-established and public validation data as indicated by the catalog numbers and referenced to the Antibody Registry.
Equal amounts of total protein (20 µg) from each brain lysate were separated on a 10% bis-tris gel (Novex), blotted on to a nitrocellulose membrane (BioRad). The membrane was then blocked and sequentially probed with primary antibodies for Syb2 (rabbit polyclonal anti-synaptobrevin-2; Synaptic Systems Cat# 104 202, RRID: AB_887810) and actin (mouse anti-actin; Millipore Cat# MAB1501, RRID:AB_2223041) in blocking buffer (1:2,000 in 1% bovine serum albumin blocking buffer) overnight. The membrane was then washed and incubated with secondary antibody, donkey anti-rabbit, 800CW (LI-COR Biosciences Cat# 926–32213, RRID:AB_621848) for Syb2 and goat anti-mouse 680CW for actin (LI-COR Biosciences Cat# 827–08364, RRID:AB_10793856) (1:10,000 in tris buffered saline with tween) for 1 hour at room temperature. The membrane was visualized using an odyssey imager (Li-Cor, Lincoln NE). Syb2 levels (pixel densities) were analyzed and normalized to actin.
Radial Arm Water Maze (spatial learning and memory assay): We tested spatial learning and memory using a modified radial arm water maze (RAWM) protocol previously described (33). Briefly, an eight-arm radial maze is filled with an opaque fluid (water with food coloring). A platform was submerged below the surface of the water (hidden platform) in one of the arms. Animals are trained eight times a day for 3 days to find the hidden platform. The animals are placed in an arm without the platform and allowed to swim and find the platform. Animals which could not find the platform at the end of the 60 seconds were carefully guided to the platform. After Day 3, the animals were returned to their home cages and left undisturbed for one week. At the end of this week, a probe test was performed. The animals were put back in the RAWM (four trials per animal) to test how well they still remembered the location of the platform. Animal movements in the maze were recorded by a camera and tracked using an automated tracking system (Noldus Ethovision XT 11, Wageningen, The Netherlands). The swim speed, distance covered, time to target, and errors were recorded. An error was counted when the animal’s full body length entered into an arm which did not have the platform.
Electrophysiology
LTP measurements
To measure the effects of reduced Syb2 expression on LTP, the cellular mechanism of learning and memory, we used a modified protocol previously described (34). Brains were carefully removed from euthanized mice and put in ice-cold oxygenated artificial cerebrospinal fluid solution containing (in mmol/L: NaCl 126, KCl 2.5, NaH2PO4 1.25, MgCl2 2, CaCl2 2, NaHCO3 26, glucose 10, pyruvic acid 2, ascorbic acid 0.4) for approximately 1 minute. Appropriate portions of the brain were trimmed away and the brain was then glued to an ice-cold stage and placed in a HM650V vibrating microtome (Thermo Scientific, Burlington, ON) filled with chilled cold oxygenated slicing solution containing (in mmol/L): sucrose 240, NaCl 25, KCl 2.5, NaH2PO4 1.25, NaHCO3 26, ascorbic acid 0.4, glucose 10, MgCl2 10, pyruvic acid 2. The brain was sliced horizontally and hippocampal slices of thickness 350 µm were collected and transferred to a recovery chamber containing oxygenated artificial cerebrospinal fluid for 1 hour. To record, the slice was transferred to and positioned on a P5002A multi-electrode array (Alpha MED Scientific Inc., Osaka, Japan). The chamber was perfused with oxygenated artificial cerebrospinal fluid at a rate of 2mL/min, at 32°C. Field excitatory postsynaptic potentials (fEPSPs) were generated in the CA1 region of the hippocampus by stimulating downstream electrodes in the CA1 and CA3 regions of the hippocampus along the Schaffer collateral pathway. Input/output curves (I/O curves) were generated by applying increasing stimulus currents to the pathway from 0 to 100µA and recording the responses. The threshold stimulus for generating fEPSPs was determined as 40%–50% of the stimulus strength needed to generate the maximum fEPSP amplitude during the I/O curve measurement. The slice was stimulated once every 30 seconds until a stable baseline lasting at least 10 minutes was observed. LTP was induced using 100 high-frequency stimulation pulses at 100 Hz applied four times with 30-second intervals. Then, we resumed baseline stimulation and recorded fEPSPs for at least 60 more minutes. Finally, we recorded another I/O curve generated as described above. For all recordings, we used the MED-64 system and Mobius software (Alpha MED Scientific Inc). Potentiation was calculated as the percent increase of the mean fEPSP descending slope (10–90 section) after high-frequency stimulation and normalized to the mean fEPSP descending slope of baseline recordings during 3 minutes prior tetanus. In order to calculate the paired-pulse facilitation (PPF) ratio, we induced a second identical stimulus 50 ms after the first for each step of the LTP recording. We then measured the ratio of the second response to the first over time. This ratio gives us a measure of neural facilitation in these brain sections.
Hippocampal cultures
Syb2+/− pups were generated by breeding heterozygous knockout animals with wild-type animals. Hippocampi from P0-P2 mice were dissociated using trypsin (5 mg/mL for 10 minutes at 37°C), and plated onto coverslips coated with Matrigel. Neurons were cultured for at least 14 days in vitro in minimal essential media containing 5 g/L glucose, 0.1 g/L transferrin, 0.25 g/L insulin, 0.3 g/L glutamine, 5%–10% FBS, 2% B-27 supplement, and 1 µM cytosine arabinoside as previously described in detail (32,35,36).
Synaptic Release Recording by FM Imaging
Live fluorescence imaging experiments were carried out using a modified protocol as previously described (32). Briefly, a modified Tyrode solution containing (in mmol/L): 150 NaCl, 2.5 KCl, 2 MgCl2.6H2O 10 glucose, 10 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid, and 2 CaCl2.2H2O at pH 7.4 was used as bath solution for cultured neurons. Synaptic boutons in these cultures were loaded with FM1-43 (8 µM; Molecular Probes, Eugene, OR) for 90 seconds in a hyperkalemic solution of 90 mM K+ (bath Tyrode solution with concentrations of NaCl and KCl changed to 60 and 90 mM, respectively) before being washed away with calcium-free Tyrode bath solution to minimize spontaneous release. All staining and washing protocols were performed in the presence of 10 µM 6-cyano-7-nitro-quinoxaline-2,3-dione (CNQX) and 50 µM (2R)-amino-5-phosphonopentanoate) (AP-5) to prevent recurrent activity. After the wash, synaptic boutons (about 1 µm2) were selected (at least 100 boutons per coverslip). Synaptic vesicle fusion and release was induced by gravity perfusion of Tyrode solution. Full synaptic recycling, was initiated by perfusing FM1-43-loaded synapses with high K+ Tyrode solution for 60 seconds before being washed with calcium-free Tyrode for another 60 seconds. This process was repeated four more times (to ensure complete release of all fluorescence). The loss of fluorescence in the selected synaptic boutons (measure of synaptic release) was captured by a cooled CCD camera (Roper Scientific, Trenton, NJ) and analyzed using Metafluor Software (Universal Imaging, Downingtown, PA). The data for release rate was then normalized to starting fluorescence intensity to help compare large and small synapses for release properties as expressed in the histograms.
Statistics
Significance was calculated using Student’s t test, repeated-measures (RM) two-way analysis of variance (ANOVA) with p values (p < .05 marked as *, p < .01 as ** for RAWM, LTP) and Kolmogorov–Smirnov test (K–S test, Fluorescence assays). Results are displayed as Mean ± SEM.
Results
Synaptobrevin-2 Protein Levels Decrease With Age
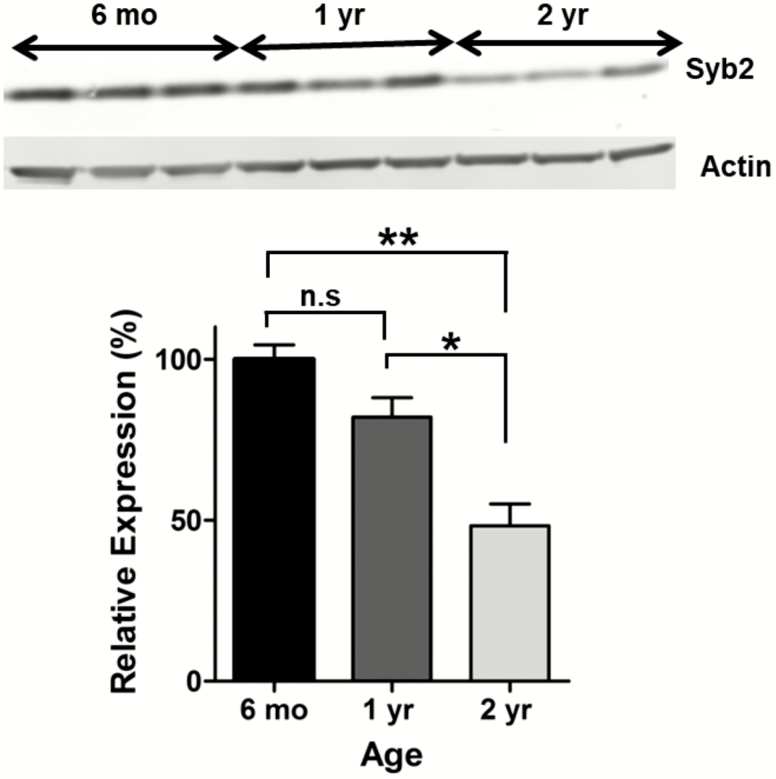
In order to quantify changes of Syb2 levels with age, we performed Western blotting assays in young, adult, and old mice. We found that Syb2 levels decrease with age as 1-year-old mice express 82.02 ± 5.99% and 2-year-old mice express 48.25 ± 6.78% of the Syb2 levels in 6-month-old mice (Figure 1; n = 3 for each group; p = .0021, one-way ANOVA). We also compared the different age groups individually and while there was a strong trend of the 1-year-old mice having reduced Syb2 than young 6-month olds, this was not significant (p = .07; t test). However, old (2-year-old) mice had significantly reduced Syb2 than 6-month old and 1-year mice (p = .003 and p = .02 respectively).
Figure 1.
Synaptobrevin-2 (syb2) expression with age. Bar graph showing that syb2 levels decrease with age as 1-year-old mice (n = 3) express 82.02 ± 5.99% and 2-year-old mice (n = 3) express 48.25 ± 6.78% of the syb2 levels expressed in 6-month-old mice (n = 3) (one-way analysis of variance, Tukey’s multiple comparison, p = .002). While there was a trend of 1-year-old mice expressing less syb2 than 6-month-old controls, this difference was not that significant (p = .07).
Reduced Syb2 Expression Causes Impaired Learning and Memory
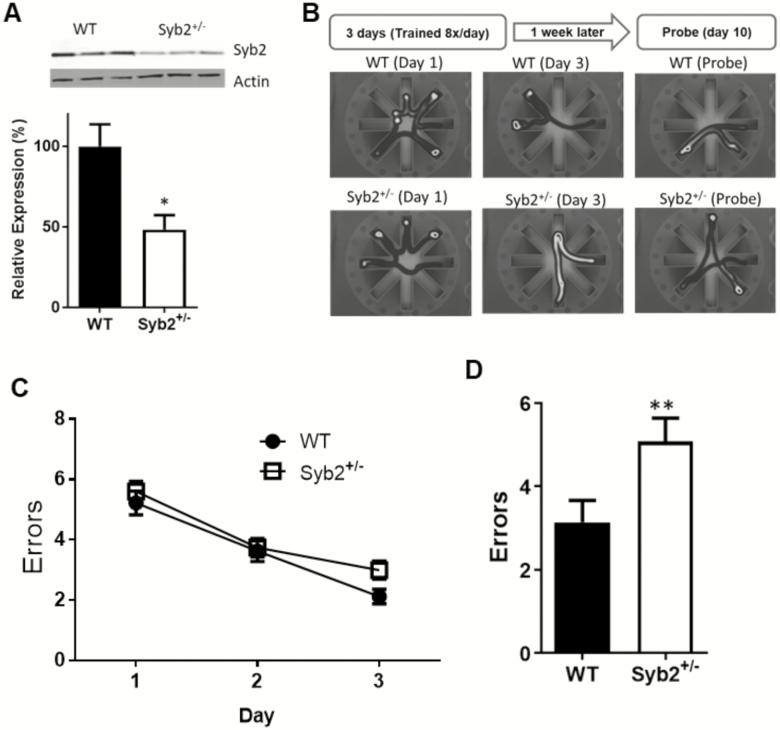
To test selectively the effects of Syb2 on brain function, we used Syb2 +/− mice at various ages. First, we performed Western blot experiments confirming that adult (1-year-old) Syb2+/− animals express approximately 50% less Syb2 than WT controls (Figure 2A). To assess spatial learning and memory in Syb2+/− animals, we tested them in the Radial Arm Water Maze (RAWM) assay. We used a 3-day training protocol (described in details in Methods and as published (36)) to compare how well the animals learned during the acquisition phase and how well they remember 1 week later during the probe phase.
Figure 2.
Spatial learning and memory impairment in adult synaptobrevin-2+/− mice. (A) Western blot showing syb2+/− animals express about 50% less syb2 than wild-type (WT) controls in the brain (WT = 100 ± 13.7, n = 4; syb2+/− = 48.5 ± 8.94, n = 4; p = .02). (B) Illustration of experimental procedure and representative paths of WT and Syb2+/− mice in the radial arm water maze. (C) Graph depicts errors made during acquisition phase. There was no significant difference between the groups here (on Day 3; WT = 2.12 ± 0.24; Syb2+/− = 3.0 ± 0.31; p = .1; two-way analysis of variance) (D) On the probe day, syb2+/− animals performed significantly worse than WT, with more errors (5.08 ± 0.56) when compared to WT animals (3.14 ± 0.52). p = .015 Student’s t test.
In the RAWM, while there was no significant difference in the performance of Syb2+/− animals and WT controls in the acquisition phase (Figure 2B), but during the probe task Syb2+/− animals made significantly more errors (Figure 2C; 5.08 ± 0.56 errors; n = 21) than WT controls (3.14 ± 0.52; n = 16; p = .015; two-way ANOVA).
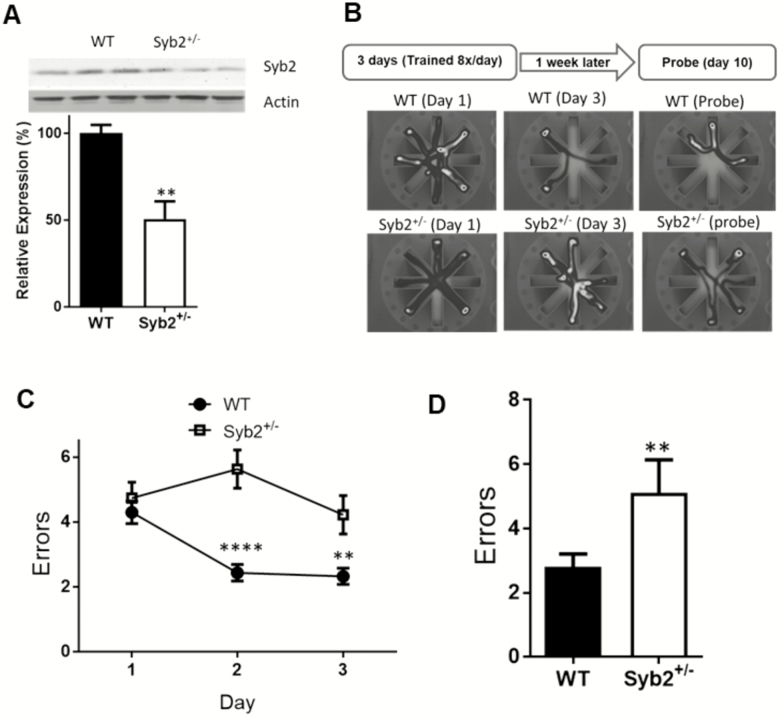
We also performed the RAWM task on aged (2-year-old) animals. We confirmed using Western blots that at 2 years of age, Syb2+/− animals express about 50% less Syb2 than WT control (Figure 3A). During the acquisition, while the WT controls learned the task over the 3 days, the aged Syb2+/− animals did not adequately learn the task as indicated by an almost flat learning curve and by the fact that by Day 3 they were making significantly more errors than WT controls (Syb2+/− = 4.21 ± 0.6, n = 7, WT = 2.32 ± 0.25, n = 14; p < .01; Figure 3B). This can also be seen on the probe day, as the aged Syb2+/− animals perform significantly worse (5.11 ± 1.0 errors; n = 7) than aged WT controls (2.8 ± 0.40; n = 14; p = .01, two-way ANOVA).
Figure 3.
Spatial learning and memory impairment in old synaptobrevib2+/− animals. (A) Western blot showing 2-year-old syb2+/− animals still express approximately 50% less syb2 than wild-type (WT) controls in the brain (WT = 100 ± 4.83, n = 3; syb2+/− = 50.71 ± 10.1, n = 3; p = .01; Student’s t test). (B) Illustration of radial arm water maze (RAWM) assay and representative heat map data of WT and syb2+/− mice at different days of the trial. (C) Graph depicts errors made during acquisition phase. Syb2+/− animals make significantly more errors than WT mice on Day 2 (syb2+/− = 5.63 ± 0.59, n = 7, WT = 2.43 ± 0.25, n = 14; p < .001) and Day 3 (syb2+/− = 4.21±0.6, n = 7, WT = 2.32 ± 0.25, n = 14; p < .01) of the acquisition (two-way analysis of variance). (D) On the probe day, syb2+/− animals again performed significantly worse, making more errors (5.11 ± 1.03, n = 7) when compared to WT animals (2.8 ± 0.4, n = 14); p = .014; Student’s t test.
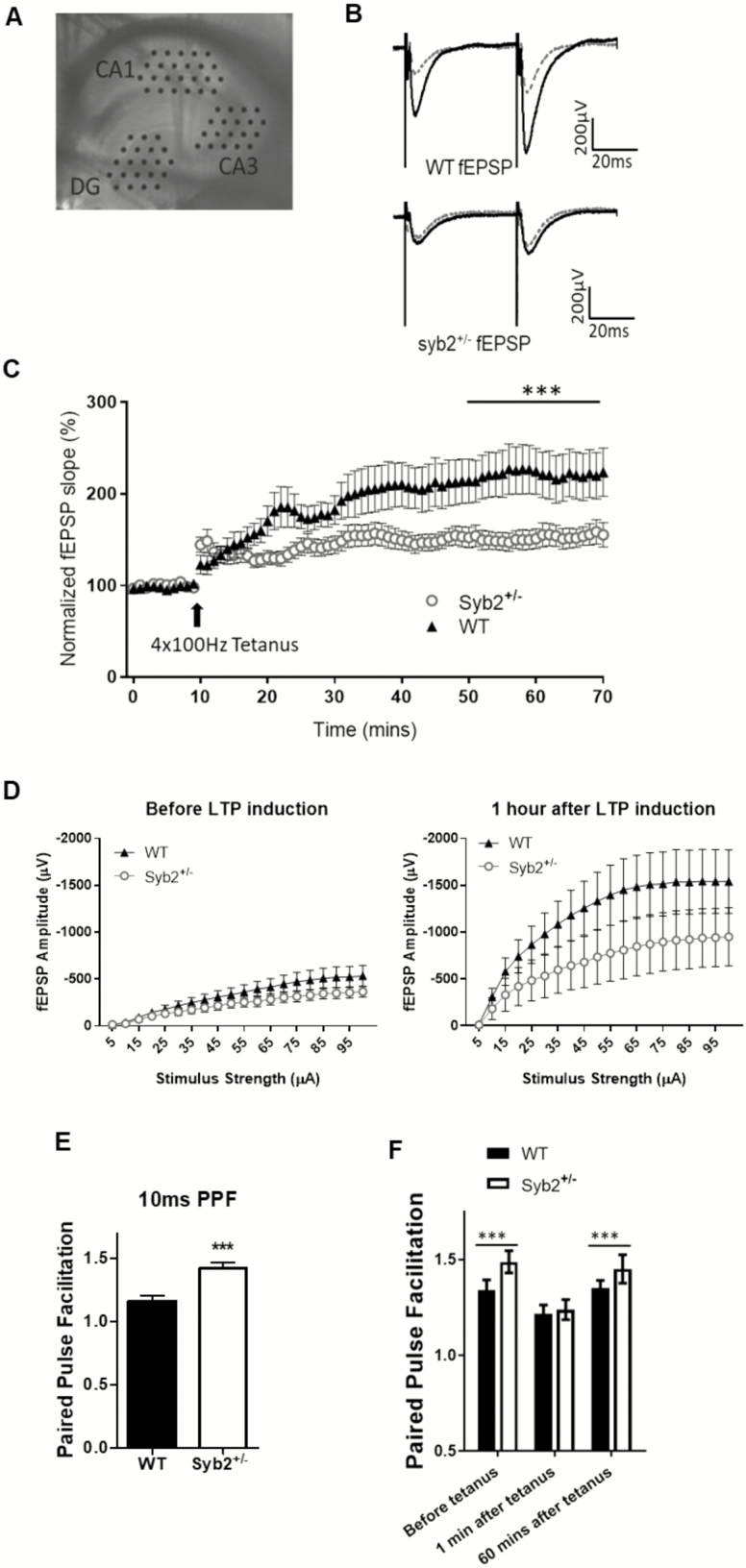
Synaptobrevin-2 Deficiency Causes Impaired Synaptic Plasticity
In order to understand the mechanism by which reduced Syb2 expression leads to impaired learning and memory seen in the RAWM, we performed various ex-vivo assays to measure synaptic plasticity in 1-year-old animals. LTP is a long lasting increase in the strength of a synapse that is typically induced by high-frequency stimulations. We collected brain slices from 1-year-old mice and probed the Schaffer collateral pathway from the CA3 to CA1 regions of the hippocampus (Figure 4A). This pathway has been implicated in spatial learning and memory (37,38) and is the most relevant pathway based on the spatial behavioral tasks the animals performed during the behavior training. We stimulated this pathway in both groups of animals to get a baseline recording for 10 minutes before inducing LTP by tetanic stimulation. Returning to the baseline stimulus, it is clearly observable that the response in both groups has potentiated and it lasted for at least 60 minutes, indicating that both groups are able to undergo LTP. However, analysis of the last 10 minutes of the LTP experiments showed impaired maintenance of hippocampal synaptic plasticity of Syb2+/− animals compared to WT controls (Figure 4B and C; WT = 220.4 ± 0.804% normalized slope versus Syb2+/− = 153.9 ± 0.726; p = .029).
Figure 4.
Reduced synaptic potentiation of hippocampal CA1 synapses in synaptobrevin-2+/− mice. (A) Picture of hippocampal slice in MED 64 probe with electrodes positioned on the dentate gyrus (DG), CA3, and CA1 regions. (B) Representative traces for syb2+/− and wild-type (WT) before (grey line) and after (black traces) long-term potentiation (LTP) induction. (C) Normalized fEPSP for both syb+/− (◯; n = 8 animals) and WT (▲; n = 7 animals) depicting syb2+/− animals have impaired LTP (p < .001; two-way repeated-measures [RM] analysis of variance [ANOVA]). (D) Input–output curves depicting syb2+/− animals have a significantly muted response to a range of stimuli after LTP induction (p < .014; two-way RM ANOVA). (E) Ratio of amplitudes in paired-pulse facilitation (PPF) at 10 ms for both WT and syb2+/− animals. Syb2+/− animals have a significantly higher PPF ratio than WT controls at 1.42 ± 0.04 and 1.16 ± 0.05, respectively; p < .001; two-tailed t test. (F) Ratio of amplitudes showing that syb2+/− neurons also have a higher PPF ratio at 50 ms before LTP induction (1.49 ± 0.008 and 1.34 ± 0.003, respectively) and 1 hour after (1.45 ± 0.003 and 1.35 ± 0.002, respectively; p < .001; two-way ANOVA).
To gain better insight of the mechanism how Syb2 deficiency interferes with LTP, we also measured the basic physiological properties of the Shaffer collateral pathway. We recorded the Input–Output characteristics of synaptic responses to a range of stimuli (0–100 µA) before and after LTP. This test allows us to see how LTP affects the general properties of synaptic release. Before LTP, there was no significant difference in activity between WT and Syb2+/− groups at any stimulation strength. However, after LTP, while the response for both groups increases significantly, syb2+/− neurons had a relatively reduced response when compared to WT controls (Figure 4D), suggesting that reduced Syb2+/− expression causes reduced synaptic plasticity. We also looked at the PPF ratio in these slices. For this, we measured the ratio of the response between two pulses 10 and 50 ms apart. Right before tetanus induction, ratio of fEPSP responses 10 ms apart showed that Syb2+/− neurons had a significantly higher PPF ratio than WT controls (Figure 4E; 1.42 ± 0.04 and 1.16 ± 0.05, respectively; p < .001; two-tailed t test). We also measured the PPF ratio 50 ms apart during the 10 minutes before tetanus, immediately after tetanus and 60 minutes after tetanus. Syb2+/− neurons had a significantly higher PPF ratio than WT control neurons (1.49 ± 0.008 and 1.34 ± 0.003, respectively) before and 1 hour after LTP induction (1.45 ± 0.003 and 1.35 ± 0.002, respectively) (p < .001 two-way ANOVA) (Figure 4F). These results suggest that Syb2+/− animals have reduced vesicle release probability when compared to WT controls. The value of PPF was around 1.25 for both WT and Syb2 +/− groups immediately after tetanus as the synapses were in the state of post-tetanic potentiation, a temporary state that lasted about 2–3 minutes in our experiments (Figure 4F).
Synaptobrevin-2+/− Neurons Have Reduced Synaptic Release Rates
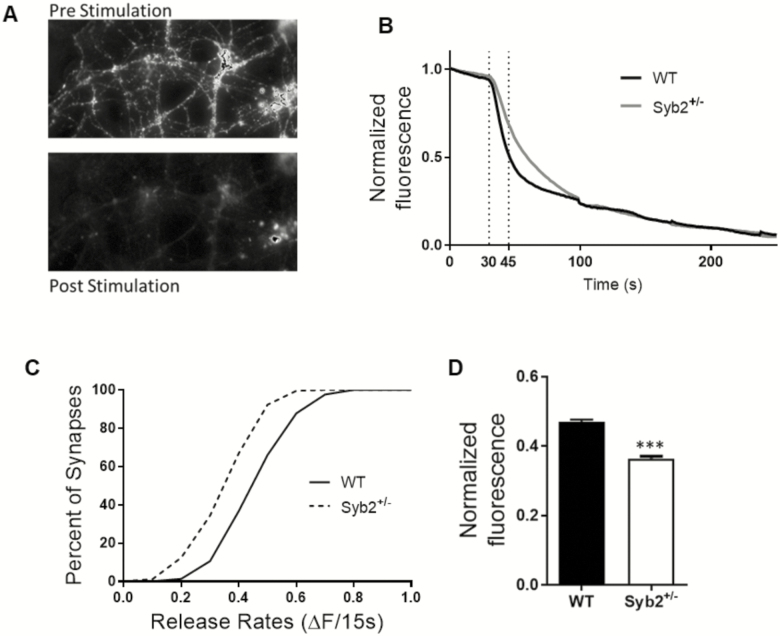
Because our electrophysiological data strongly indicated that reduced Syb2 levels lead to lower release probability, we investigated the effects of reducing Syb2 expression on synaptic vesicle release with a more sensitive and direct method. Using primary neuronal cultures, we performed fluorescent imaging assay, when synapses were labeled with the fluorescent dye, FM 1–43 and synaptic recycling was induced with strong depolarization via application of 90 mM KCl containing modified Tyrode solution. The loss of fluorescence was recorded as a measure of synaptic vesicle release (Figure 5A and B). This assay showed that Syb2+/− neurons released significantly less fluorescence during the first 15 seconds of release than WT neurons (p < .001; K–S test; Figure 5C). Syb2+/− neurons released approximately 20% less fluorescence during the first 15 seconds) (Syb2+/− = 0.364 ± 0.006, WT = 0.47 ± 0.006; Mann–Whitney U = 1421; p < .0001; Figure 5D). This indicates that Syb2+/− neurons have a reduced synaptic release rate than WT controls. However, there was no difference in recycling pool sizes as the total amount of fluorescence loss was equal between the WT and Syb2+/− neurons (data not shown), indicating similar number of vesicles released after four rounds of depolarization.
Figure 5.
Synaptobrevin-2+/− synapses have reduced vesicular release rates. (A) Representative images of the synapses loaded with FM1-43 dye, showing fluorescence before and after four rounds of depolarization. (B) Representative traces of WT and syb2+/− neurons showing the loss of fluorescence during the first depolarizing stimulus. (C) Cumulative histogram summarizes the distribution of the rate of synaptic vesicle release in the first 15 seconds for syb2+/− (n = 9; 805 synapses, dotted red line) and WT (n = 11; 1,001 synapses, solid black line). Note that syb2+/− synapses have a slower release rate than WT neurons (p < .0001; Kolmogorov–Smirnov test). (D) Bar graph showing syb2+/− neurons release approximately 20% less fluorescence than WT controls during the first 15 seconds of 90 mMK+ Tyrode application (syb2+/− = 0.364 ± 0.006, WT = 0.47 ± 0.006; Mann–Whitney U = 1,421; p < .0001).
Discussion
The central finding of the study is that reduced Syb2 expression accelerates age-related memory decline. It is well known that learning and memory decline with age. Thus, our first step was showing that our Syb2 model of aging followed the same trend and understanding the role of Syb2 in this process. Our data showed that Syb2+/− mice, which expressed ≈50% of the Syb2 in age-matched controls, displayed impaired learning, and memory at 1 year of age when compared to WT controls. This phenotype got worse at 2 years of age as these animals were still expressing around 50% Syb2 as aged matched old WT controls. Old (24 months) Syb2+/− mice expressed about 30% of the Syb2 levels seen in young (6 months) mice. We see this as the major reason for their markedly deteriorating performance in spatial learning and memory tests. Indeed, when we tested 6-month-old Syb2+/− mice in the RAWM assay, there was no significance between 6-month-old Syb2+/− mice and WT age-matched controls in either the acquisition or probe phases of the RAWM assay (Supplementary Figure 1B and C). When we measured Syb2 protein expression in these young mice, we saw that Syb2+/− animals express 72 ± 4.62% of age-matched WT Syb2 levels (Supplementary Figure 1A; p = .013; Student’s t test). This was a higher protein expression than we saw in 2-year-old WT mice. Therefore, the relatively well-maintained Syb2 expression levels at 6 months explain why we do not see a significant difference at this age between the WT and Syb2+/− mice. The syb2 protein level in the 6-month-old animals is most likely still sufficient to support normal neurotransmission and synaptic plasticity. On the other hand, expression level of Syb2 in 1-year-old Syb2+/− mice was sufficiently reduced to induce a phenotype. One-year-old Syb2+/− mice also showed impaired plasticity in the Input–Output and LTP assays when compared to WT controls. Syb2+/− mice also demonstrated increased PPF ratio when compared to controls at both 10 and 50 ms stimulation intervals. Higher PPF ratios are indicative of reduced synaptic vesicle release probability. The happens because, when the second stimulus arrives, some transient Ca2+ from the first stimulus will still be present within the terminal, which will add to the strength of the second stimulus by increasing the release probability. According to our model, Syb2+/− neurons retained more vesicles during the first pulse (due to impairments in vesicular release) and this in combination with the increased calcium load resulting in elevated release probability allows Syb2+/− neurons to release more vesicles at second pulse of PPF than they were able to during the first stimulus. The PPF for both groups was reduced immediately after LTP induction. As there was no difference in PPF during post-tetanic facilitation between syb2+/− and WT CA1 synapses (Figure 4F), which we interpret that both groups received similarly strong tetanic stimuli during the 100 Hz trains. Interestingly, when we quantified the synaptic short-term depression of Shaffer collaterals during 100 Hz stimulation, we found no significant difference between WT and syb2 heterozygous slices (Supplementary Figure 2.). This is in good agreement with our results directly measuring vesicular release from hippocampal terminals using live fluorescence assays. In the FM dye assays, we also revealed that Syb2+/− hippocampal neurons had reduced synaptic release rates when compared to WT control neurons, however, there was no significant difference in the total recycling pool size between Syb2+/− and WT control neurons. Reduced vesicle release rates could be the mechanism behind the impaired LTP and plasticity seen in these animals. Studies have shown that fast vesicle recycling is essential for synaptic plasticity and LTP (39,40) and this made us question the role Syb2 plays in regulating synaptic pools of vesicles. Reduced Syb2 expression causing reduced release rates will affect general plasticity and LTP which in turn leads to learning and memory deficits. The total amount of recycling vesicles was not significantly different between WT and Syb2+/− neurons leading us to conclude that reduced synaptic vesicle release rates, caused by decreased release probability is the mechanism behind the impaired spatial learning and memory phenotype and the number of recycling vesicles is not affected by reduction in Syb2.
Synaptobrevin is an evolutionary conserved synaptic protein in neurotransmission (41). The critical role of Syb2 in normal synaptic function has been well established in the mouse brain after the introduction of Syb2 KO mice (30,32). However, the effects of age-related changes in Syb2 expression have remained elusive. This is the first study to our best knowledge to show that reduced expression of Syb2 leads to learning and memory deficits, implicating it in age-related cognitive decline. Syb2 is the most abundant synaptic vesicle protein, with an average of 70 copies present at a single small synaptic vesicle (42). This may explain our finding that more than 50% reduction in syb2 levels is necessary to cause a learning impairment. We propose that there is a critical Syb2 level threshold (around 40% of young WT expression levels) at which symptoms of cognitive impairment manifest. Indeed, a recent human study (24) described a positive correlation of better cognitive performance and maintained Syb2 levels in elderly adults. Patients with the lowest Syb2 levels had higher prevalence of dementia (24).
It is evident that this research is only the starting point for better understanding the role of syb2 in the aging process and cognitive decline. Just to mention a few caveats of the study. First, although our study clearly showed that reduced Syb2 expression which causes decreased synaptic release probability and reduced vesicle release rates are possible mechanisms behind impaired learning and memory seen with aging, more research is required to properly describe and definitely prove the role of Syb2 in learning and memory. Second, although the list of synaptic proteins altered with age is long (9,10,43), very few as syb2 is seen consistently downregulated (21,44–46). Syb2 levels were decreased in the brain of aged rats independently of DHA supplementation (47) in contrast to some other synaptic proteins like fodrin-α, synaptopodin, postsynaptic density protein 95, synaptic vesicle glycoprotein 2B, synaptosomal-associated protein-25, synaptosomal-associated protein-α, N-methyl-D-aspartate receptor subunit epsilon-2 precursor, AMPA2, AP2, which were further reduced when brain DHA was depleted by diet. In other studies, synaptosomal-associated protein-25 (SNAP-25), synaptotagmin-1, synaptophysin, and syntaxin were not significantly different in the synaptosomal preparations between young (6 months) and aged (27-month-old) rats (48,49). In addition, the aged rats with severe cognitive impairment had levels of these synaptic proteins that were similar to those of the aged rats with preserved cognitive function (49). Analysis of hippocampal RNA from male Fischer rats confirms that neither brain-derived neurotrophic factor mRNA nor trkB mRNA levels change with age (50). Moreover, the distribution of SNAP-25, a target-SNARE located in synapses, did not change with age in this study (50). These findings suggest that age-related neuropathological changes, including hippocampal atrophy, are not the result of changes in hippocampal brain-derived neurotrophic factor or trkB mRNA expression. Interestingly, in Alzheimer’s disease, loss of synaptophysin, syntaxin, and SNAP-25 is a late-stage phenomenon associated with moderate to severe clinical grades of dementia and occurring only at pathological Braak5/6 stages with profound degeneration (51).
The most relevant translational benefit of our study, therefore, could be by exploring if enhancing or better maintaining Syb2 levels alone in the elderly adults will be sufficient to prevent or slow down cognitive decline. For example, could overexpressing Syb2 improve learning and memory? A transgenic mouse model which overexpresses Syb2 could be used to answer this question and also provide an in-vivo demonstration of the effects of overexpressing Syb2. It is known that as we age, the brain as well as other organs become less efficient and age is associated with a shift of regulatory processes at various levels from epigenetics to hormone levels (4,52–54). Antiaging interventions could provide further insight about the processes leading to lower syb2 levels with age: For instance, caloric restriction has been a well-studied antiaging paradigm and one could speculate how it may influence the level of syb2 as it was shown to enhance dopaminergic activity in substantia nigra and the striatum in rats (55) circulating factors in primates (56) and the GH/IGF-1 pathway in rodents (57,58). Caloric restriction may limit also the damage to syb2 through alleviating oxidative damage to proteins (59). These may be possible mechanisms behind the reduction of Syb2 with age. On the other hand, one possible scenario for the role of syb2 in aging that decreased syb2 levels will reduce exocytosis in all organs where this protein is needed for vesicular release. This is not necessarily the case, though, as regulation of syb2 expression can vary among the various tissues. For instance, no syb2 change was reported in lacrimal glands of aged rats (60). More research need to be conducted to show exactly how and why Syb2 levels reduce with age, and to explore avenues for either preventing Syb2 levels to decrease or to restore Syb2 to aged neurons and rescuing them from synaptic dysfunction.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
We are grateful for the Presbyterian Health Foundation’s Bridge grant and the College of Medicine Alumni Association Award (to F.D.) as well as for the OCNS Student Pilot grant (to A.O.). The research results discussed in this publication were also made possible in part by funding through the Health Research award for our project from the Oklahoma Center for the Advancement of Science and Technology (HR13-051 to F.D.) and the support of National Institutes of Health P20GM104934 CoBRE Pilot grant (to F.D.) and the support through the Oklahoma Nathan Shock Center award 3P30AG050911-04S1 from National Institute of Aging of the National Institutes of Health.
Conflict of Interest
None reported.
Acknowledgments
Syb2 KO mice were a kind gift from Dr. Thomas C. Südhof. We are grateful for the excellent technical help from Sima Asfa, Jennifer Fessler, and Kushal Thapa with the mouse husbandry and neuronal cultures. We thank Dr. Nikolett Szarka for her advice and practical help on establishing the cell cultures and polymerase chain reaction. We also thank Julie Farley and Matthew Mitschelen for their help with mouse husbandry and the establishment of behavioral assays. The advices on this project from the Drs. Robert E. Anderson, Beverley Greenwood-Van Meerveld, Jose Rizo-Rey, William E. Sonntag and Rheal Towner (Graduate School Advisory Committee members to A.O.), and Dr. Arlan Richardson are greatly appreciated.
References
- 1. Richardson K, Schoen M, French B, et al. Statins and cognitive function: a systematic review. Ann Intern Med. 2013;159:688–697. doi:10.7326/0003-4819-159-10-201311190-00007 [DOI] [PubMed] [Google Scholar]
- 2. Park DC, Festini SB.. Theories of memory and aging: a look at the past and a glimpse of the future. J Gerontol B Psychol Sci Soc Sci. 2017;72:82–90. doi:10.1093/geronb/gbw066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ward EV, Berry CJ, Shanks DR.. Age effects on explicit and implicit memory. Front Psychol. 2013;4:639. doi:10.3389/fpsyg.2013.00639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harada CN, Natelson Love MC, Triebel KL.. Normal cognitive aging. Clin Geriatr Med. 2013;29:737–752. doi:10.1016/j.cger.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. West MJ, Coleman PD, Flood DG, Troncoso JC.. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet. 1994;344:769–772. [DOI] [PubMed] [Google Scholar]
- 6. Gómez-Isla T, Price JL, McKeel DW Jr, Morris JC, Growdon JH, Hyman BT.. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. 1996;16:4491–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yankner BA, Lu T, Loerch P.. The aging brain. Annu Rev Pathol. 2008;3:41–66. doi:10.1146/annurev.pathmechdis.2.010506.092044 [DOI] [PubMed] [Google Scholar]
- 8. Pannese E. Morphological changes in nerve cells during normal aging. Brain Struct Funct. 2011;216:85–89. doi:10.1007/s00429-011-0308-y [DOI] [PubMed] [Google Scholar]
- 9. Martin SA, DeMuth TM, Miller KN, et al. Regional metabolic heterogeneity of the hippocampus is nonuniformly impacted by age and caloric restriction. Aging Cell. 2016;15:100–110. doi:10.1111/acel.12418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hascup ER, Wang F, Kopchick JJ, Bartke A.. Inflammatory and glutamatergic homeostasis are involved in successful aging. J Gerontol A Biol Sci Med Sci. 2016;71:281–289. doi:10.1093/gerona/glv010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bast T, da Silva BM, Morris RG.. Distinct contributions of hippocampal NMDA and AMPA receptors to encoding and retrieval of one-trial place memory. J Neurosci. 2005;25:5845–5856. doi:10.1523/JNEUROSCI.0698-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Whitlock JR, Heynen AJ, Shuler MG, Bear MF.. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi:10.1126/science.1128134 [DOI] [PubMed] [Google Scholar]
- 13. Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC.. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi:10.1126/science.1128657 [DOI] [PubMed] [Google Scholar]
- 14. Steele RJ, Morris RG.. Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus. 1999;9:118–136. doi:10.1002/(SICI)1098-1063(1999)9:2<118::AID-HIPO4>3.0.CO;2-8 [DOI] [PubMed] [Google Scholar]
- 15. Bliss TV, Lomo T.. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang B, Hu Q, Hearn MG, et al. Isoform-specific knockout of FE65 leads to impaired learning and memory. J Neurosci Res. 2004;75:12–24. doi:10.1002/jnr.10834 [DOI] [PubMed] [Google Scholar]
- 17. Baudouin SJ, Gaudias J, Gerharz S, et al. Shared synaptic pathophysiology in syndromic and nonsyndromic rodent models of autism. Science. 2012;338:128–132. doi:10.1126/science.1224159 [DOI] [PubMed] [Google Scholar]
- 18. Zoghbi HY, Bear MF.. Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harb Perspect Biol. 2012;4:a009886. doi:10.1101/cshperspect.a009886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sudhof TC, Rizo J.. Synaptic vesicle exocytosis. Cold Spring Harb Perspect Biol. 2011;3(12):a005637. doi:10.1101/cshperspect.a005637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rizo J, Rosenmund C.. Synaptic vesicle fusion. Nat Struct Mol Biol. 2008;15:665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. VanGuilder HD, Yan H, Farley JA, Sonntag WE, Freeman WM.. Aging alters the expression of neurotransmission-regulating proteins in the hippocampal synaptoproteome. J Neurochem. 2010;113:1577–1588. doi:10.1111/j.1471-4159.2010.06719.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu P, Smith PF, Darlington CL.. Glutamate receptor subunits expression in memory-associated brain structures: regional variations and effects of aging. Synapse. 2008;62:834–841. doi:10.1002/syn.20563 [DOI] [PubMed] [Google Scholar]
- 23. Canas PM, Duarte JM, Rodrigues RJ, Köfalvi A, Cunha RA.. Modification upon aging of the density of presynaptic modulation systems in the hippocampus. Neurobiol Aging. 2009;30:1877–1884. doi:10.1016/j.neurobiolaging.2008.01.003 [DOI] [PubMed] [Google Scholar]
- 24. Honer WG, Barr AM, Sawada K, et al. Cognitive reserve, presynaptic proteins and dementia in the elderly. Transl Psychiatry. 2012;2:e114. doi:10.1038/tp.2012.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Söllner T, Whiteheart SW, Brunner M, et al. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi:10.1038/362318a0 [DOI] [PubMed] [Google Scholar]
- 26. Hirling H, Scheller RH.. Phosphorylation of synaptic vesicle proteins: modulation of the alpha SNAP interaction with the core complex. Proc Natl Acad Sci USA. 1996;93:11945–11949. doi:10.1073/pnas.93.21.11945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Südhof TC, Rothman JE.. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi:10.1126/science.1161748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Link E, Edelmann L, Chou JH, et al. Tetanus toxin action: inhibition of neurotransmitter release linked to synaptobrevin proteolysis. Biochem Biophys Res Commun. 1992;189:1017–1023. [DOI] [PubMed] [Google Scholar]
- 29. Schiavo G, Benfenati F, Poulain B, et al. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832–835. doi:10.1038/359832a0 [DOI] [PubMed] [Google Scholar]
- 30. Schoch S, Deák F, Königstorfer A, et al. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science. 2001;294:1117–1122. doi:10.1126/science.1064335 [DOI] [PubMed] [Google Scholar]
- 31. Rizo J, Südhof TC.. The membrane fusion enigma: SNAREs, Sec1/Munc18 proteins, and their accomplices–guilty as charged?Annu Rev Cell Dev Biol. 2012;28:279–308. doi:10.1146/annurev-cellbio-101011-155818 [DOI] [PubMed] [Google Scholar]
- 32. Deák F, Schoch S, Liu X, Südhof TC, Kavalali ET.. Synaptobrevin is essential for fast synaptic-vesicle endocytosis. Nat Cell Biol. 2004;6:1102–1108. doi:10.1038/ncb1185 [DOI] [PubMed] [Google Scholar]
- 33. Shukitt-Hale B, McEwen JJ, Szprengiel A, Joseph JA.. Effect of age on the radial arm water maze—a test of spatial learning and memory. Neurobiol Aging. 2004;25:223–229. doi:10.1016/S0197-4580(03)00041-1 [DOI] [PubMed] [Google Scholar]
- 34. Liu CC, Tsai CW, Deak F, et al. Deficiency in LRP6-mediated Wnt signaling contributes to synaptic abnormalities and amyloid pathology in Alzheimer’s disease. Neuron. 2014;84:63–77. doi:10.1016/j.neuron.2014.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Deák F, Shin OH, Kavalali ET, Südhof TC.. Structural determinants of synaptobrevin 2 function in synaptic vesicle fusion. J Neurosci. 2006;26:6668–6676. doi:10.1523/JNEUROSCI.5272-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Orock A, Logan S, Deak F.. Munc18-1 haploinsufficiency impairs learning and memory by reduced synaptic vesicular release in a model of Ohtahara syndrome. Mol Cell Neurosci. 2018;88:33–42. doi:10.1016/j.mcn.2017.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rehberg M, Kirschstein T, Guli X, et al. Functional metaplasticity of hippocampal schaffer collateral-ca1 synapses is reversed in chronically epileptic rats. Neural Plast. 2017;2017:8087401. doi:10.1155/2017/8087401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tsien JZ, Huerta PT, Tonegawa S.. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi:10.1016/S0092-8674(00)81827-9 [DOI] [PubMed] [Google Scholar]
- 39. Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi:10.1126/science.1067020 [DOI] [PubMed] [Google Scholar]
- 40. Ratnayaka A, Marra V, Bush D, Burden JJ, Branco T, Staras K.. Recruitment of resting vesicles into recycling pools supports NMDA receptor-dependent synaptic potentiation in cultured hippocampal neurons. J Physiol. 2012;590:1585–1597. doi:10.1113/jphysiol.2011.226688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Südhof TC, Baumert M, Perin MS, Jahn R.. A synaptic vesicle membrane protein is conserved from mammals to Drosophila. Neuron. 1989;2:1475–1481. [DOI] [PubMed] [Google Scholar]
- 42. Takamori S, Holt M, Stenius K, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi:10.1016/j.cell.2006.10.030 [DOI] [PubMed] [Google Scholar]
- 43. Masser DR, Bixler GV, Brucklacher RM, et al. Hippocampal subregions exhibit both distinct and shared transcriptomic responses to aging and nonneurodegenerative cognitive decline. J Gerontol A Biol Sci Med Sci. 2014;69:1311–1324. doi:10.1093/gerona/glu091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Berchtold NC, Coleman PD, Cribbs DH, Rogers J, Gillen DL, Cotman CW.. Synaptic genes are extensively downregulated across multiple brain regions in normal human aging and Alzheimer’s disease. Neurobiol Aging. 2013;34:1653–1661. doi:10.1016/j.neurobiolaging.2012.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tucsek Z, Noa Valcarcel-Ares M, Tarantini S, et al. Hypertension-induced synapse loss and impairment in synaptic plasticity in the mouse hippocampus mimics the aging phenotype: implications for the pathogenesis of vascular cognitive impairment. Geroscience. 2017;39(4):385–406. doi:10.1007/s11357-017-9981-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vallortigara J, Whitfield D, Quelch W, et al. Decreased levels of VAMP2 and monomeric alpha-synuclein correlate with duration of dementia. J Alzheimers Dis. 2016;50:101–110. doi: 10.3233/JAD-150707 [DOI] [PubMed] [Google Scholar]
- 47. Sidhu VK, Huang BX, Desai A, Kevala K, Kim HY.. Role of DHA in aging-related changes in mouse brain synaptic plasma membrane proteome. Neurobiol Aging. 2016;41:73–85. doi:10.1016/j.neurobiolaging.2016.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Iwamoto M, Hagishita T, Shoji-Kasai Y, Ando S, Tanaka Y.. Age-related changes in the levels of voltage-dependent calcium channels and other synaptic proteins in rat brain cortices. Neurosci Lett. 2004;366:277–281. doi:10.1016/j.neulet.2004.05.048 [DOI] [PubMed] [Google Scholar]
- 49. Nicolle MM, Gallagher M, McKinney M.. No loss of synaptic proteins in the hippocampus of aged, behaviorally impaired rats. Neurobiol Aging. 1999;20:343–348. doi:10.1016/S0197-4580(99)00054-8 [DOI] [PubMed] [Google Scholar]
- 50. Lapchak PA, Araujo DM, Beck KD, Finch CE, Johnson SA, Hefti F.. BDNF and trkB mRNA expression in the hippocampal formation of aging rats. Neurobiol Aging. 1993;14:121–126. doi:10.1016/0197-4580(93)90087-R [DOI] [PubMed] [Google Scholar]
- 51. Mukaetova-Ladinska EB, Garcia-Siera F, Hurt J, et al. Staging of cytoskeletal and beta-amyloid changes in human isocortex reveals biphasic synaptic protein response during progression of Alzheimer’s disease. Am J Pathol. 2000;157:623–636. doi:10.1016/S0002-9440(10)64573-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bishop NA, Lu T, Yankner BA.. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464:529–535. doi:10.1038/nature08983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Deak F, Sonntag WE.. Aging, synaptic dysfunction, and insulin-like growth factor (IGF)-1. J Gerontol A Biol Sci Med Sci. 2012;67:611–625. doi:10.1093/gerona/gls118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi:10.1186/gb-2013-14-10-r115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Salvatore MF, Terrebonne J, Cantu MA, et al. Dissociation of striatal dopamine and tyrosine hydroxylase expression from aging-related motor decline: evidence from calorie restriction intervention. J Gerontol A Biol Sci Med Sci. 2017;73:11–20. doi:10.1093/gerona/glx119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Csiszar A, Sosnowska D, Tucsek Z, et al. Circulating factors induced by caloric restriction in the nonhuman primate Macaca mulatta activate angiogenic processes in endothelial cells. J Gerontol A Biol Sci Med Sci. 2013;68:235–249. doi:10.1093/gerona/gls158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yamaza H, Komatsu T, To K, et al. Involvement of insulin-like growth factor-1 in the effect of caloric restriction: regulation of plasma adiponectin and leptin. J Gerontol A Biol Sci Med Sci. 2007;62: 27–33. doi:10.1093/gerona/62.1.27 [DOI] [PubMed] [Google Scholar]
- 58. Magnusson KR, Das SR, Kronemann D, Bartke A, Patrylo PR.. The effects of aging and genotype on NMDA receptor expression in growth hormone receptor knockout (GHRKO) mice. J Gerontol A Biol Sci Med Sci. 2011;66:607–619. doi:10.1093/gerona/glr024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Forster MJ, Sohal BH, Sohal RS.. Reversible effects of long-term caloric restriction on protein oxidative damage. J Gerontol A Biol Sci Med Sci. 2000;55:B522–B529. doi:10.1093/gerona/55.11.B522 [DOI] [PubMed] [Google Scholar]
- 60. Batista TM, Tomiyoshi LM, Dias AC, et al. Age-dependent changes in rat lacrimal gland anti-oxidant and vesicular related protein expression profiles. Mol Vis. 2012;18:194–202. http://www.molvis.org/molvis/v18/a22 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.