Abstract
Background
Evidence suggests that short and long sleep durations are potential lifestyle factors associated with cardiovascular disease (CVD). Research on sleep duration and CVD risk is limited by use of self-report sleep measures, homogeneous populations, and studies on individual CVD risk factors. For women, risk of CVD and inadequate sleep duration increases with age. We hypothesized that accelerometer-measured sleep duration was associated with 10-year predicted probability of future CVD risk in a cohort of aging women.
Methods
This cross-sectional analysis included 3,367 older women (mean age 78.9 years; 53.3% White), from the Objective Physical Activity and Cardiovascular Health Study, ancillary study to the Women’s Health Initiative. Women wore ActiGraph GT3X+ accelerometers on the hip for 24 hours/7 days. A 10-year predicted probability of future CVD risk, the Reynolds Risk Score (RRS), was computed using age, systolic blood pressure, high-sensitivity C-reactive protein (CRP), total and HDL cholesterol, diabetes mellitus status, smoking status, and family history of CVD. Average nightly sleep duration was derived from accelerometer data. Adjusted linear regression models investigated the association between sleep duration and RRS.
Results
Results suggested a U-shaped relationship between sleep duration and RRS, with both short and long sleep associated with higher RRS (p < .001). The association remained significant after adjustments for race/ethnicity, education, lifestyle factors, and health status indicators.
Conclusion
In older women, actigraphy-ascertained sleep duration was associated with a 10-year predicted probability of future CVD risk. This study supports sleep duration as a modifiable risk factor for CVD in older women.
Keywords: Sleep, Cardiovascular, Accelerometers, Successful aging
Cardiovascular disease (CVD) poses serious risk to aging women in the United States (1). According to the American Heart Association (AHA), 30% of women have some form of CVD (1). Women >80 years have a higher incidence of CVD events than men (1). Modifiable risk factors for CVD include: obesity, physical inactivity, elevated total cholesterol, diabetes mellitus (DM), smoking, and hypertension (2).
Evidence supports both short (<7 hours) and long (>9 hours) sleep duration as potential lifestyle risk factors for CVD (3). Cross-sectional and prospective studies demonstrate that self-reported short sleep is a predictor of incident cardiometabolic conditions such as DM, hypertension, and obesity (4–6). A meta-analysis found that among 249,324 adults, self-reported short sleepers (<7 hours) had a 50% increase in risk of developing or dying from coronary heart disease (CHD) when compared with normal sleepers (7–8 hours) (4). Self-reported long sleepers (>8 hours) experienced a 38% increased risk of CHD versus normal sleepers (4). Evidence of the association between sleep duration and CVD risk is constrained by the difficulty of obtaining objective estimates of sleep duration in large prospective cohorts (4). However, emerging evidence utilizing objective measures show abnormal sleep duration is associated with increased risk of subclinical CVD (7), peripheral artery disease (8), and when paired with poor sleep quality, increased risk of incident CVD (9).
Previous studies were limited by their reliance on self-reported measures of sleep duration in study samples primarily comprised of middle-aged non-Hispanic White adults (10). While CVD incidence is higher in older women, and in African American and non-White Hispanic populations (1), most studies on sleep and CVD risk have not adequately included women with representation from these groups (3,11). According to the AHA Scientific Statement on sleep duration and CVD, inclusion of understudied populations, such as aging, African American, and non-White Hispanic women, and use of objective measures of sleep, should be priorities for future research (3). Previous studies examined the relationship between sleep duration and individual CVD risk factors, such as systolic blood pressure (SBP) and body mass index (BMI), with meta-analyses drawing conclusions about overall cardiometabolic risk (4,10,11). Composite risk scores, such as the Reynolds Risk Score (RRS), use a multivariate approach to sum weighted individual risk factors to estimate 10-year risk of future CVD (12). In the clinical setting, composite risk scores are the conventional approach to predict future probability of CVD risk, determine need for further testing (eg, stress testing), and initiate therapeutic intervention (13). No previous studies have assessed the relationship between sleep duration and a composite risk score.
The objective of this paper is to examine the relationship between accelerometer-measured sleep duration and 10-year predicted probability of future CVD risk in a large racially/ethnically diverse cohort of older women.
Methods
Women included in the study were enrolled in the Objective Physical Activity and Cardiovascular Health (OPACH) study, an ancillary study to the Women’s Health Initiative (WHI). The OPACH study aimed to prospectively examine the relationship between accelerometer-measured PA and CVD incidence in older women. Information on the objectives, recruitment, and assessment of the WHI and OPACH study have been published (14). Between 2012 and 2013, women participating in the WHI Long Life Study (LLS) completed in-home assessments of factors associated with healthy aging and cardiovascular health. Data collection included fasting blood draws, personal health and lifestyle questionnaires, anthropometric measurements, and blood pressure assessments. A subset of LLS participants (N = 7,048) enrolled in the OPACH study and were asked to wear GT3x accelerometers on their waist for 24 hours/7 days. Participants completed sleep logs. Of the consented participants, 6,489 women returned their devices with accelerometer data. Of these, 3,369 met eligibility criteria for inclusion in this analysis with at least three nights of valid accelerometer data, a completed sleep log overlapping with accelerometer nights, and fasting blood data. There were statistically significant differences in the age and proportion of race/ethnicity groups between the analytic sample and women in the entire OPACH study (N = 6,489). The women included in this analysis were slightly older (p < .01), and the sample had a larger proportion of White women and a smaller proportion of African American women (p < .001). There were no significant differences in self-reported sleep duration, smoking status, or self-rated health between the analytic sample and women in the OPACH cohort. Women in the analytic sample had significantly lower BMIs (27.9 vs 28.6 kg/m2; p < .001) and higher physical functioning scores (69.2 vs 67.6; p < .05).
Sleep duration was assessed with the hip-worn accelerometer (Actigraph GT3X+; Pensacola, FL). Participants wore the accelerometer secured to their hip with a belt, 24 hours/7 consecutive days, except when bathing or swimming. Participants completed a daily sleep log recording time in-bed and out-of-bed. Although wrist-worn accelerometers are standard for sleep assessment, hip-worn accelerometers are the norm for PA studies like OPACH (15,16). Hip accelerometers have been validated for assessment of total sleep duration against the gold standard polysomnography (16). Accelerometer estimated sleep duration is considered more accurate than self-reported sleep duration or sleep logs alone (17).
Sleep duration data were processed using ActiLife version 6.11 software. Collected at 30 Hz, raw data were condensed to 60-second epoch Agilegraph Data Files (AGD) with the low frequency extension filter applied. Participant AGD files were coded using a standard protocol. This protocol is aligned with the Society of Behavioral Sleep Medicine actigraphy methods guidelines and draws from a protocol shown previously to have high interraterreliability (18). A trained coder identified the primary sleep period for each night, by determining the participant’s in-bed time and out of bed time, using the participant sleep logs and a visual review of the data for each night the participant wore the device. The Cole-Kripke algorithm (19), built into the Actilife software, was applied to the identified primary sleep periods, and classified each minute of the sleep period as “sleep” or “wake.” Estimates of nightly sleep duration were derived from the summing of epochs classified as sleep by the Cole-Kripke algorithm (minutes/night) during the defined primary sleep period. Sleep duration was averages across the nights of data (ranging from 3 to 7) to get an average nightly sleep duration. Because a well-established definition of short (short: <6 or <7 hours) or long (long: >9 or >10 hours) sleep duration does not exist, we retained sleep duration as a continuous variable in our analysis. To be included in the present analysis, we required at least three nights of wear. Previous sleep studies demonstrate that one night of accelerometer wear is not sufficient to assess regular sleep habits (20). Additionally, cardiovascular cohort studies have defined valid wear as three nights (21).
The RRS is an estimate of 10-year predicted probability of future CVD risk with demonstrated accuracy in the prediction of future CVD occurrence in women (12). RRS was calculated using age, SBP, CRP, total and HDL cholesterol, DM, smoking status, and family history of myocardial infarction. Based on the results of a previous study conducted in the WHI cohort, we chose the RRS, which demonstrated better prediction of actual CVD events, than the Framingham ATP-III Score (22). Hemoglobin A1c was not assessed in the OPACH study and was not included in the RRS calculation (for those who report a DM diagnosis).
Fasting blood samples were obtained at the home visit. Participants fasted for 12 hours prior to the blood draw. Samples were centrifuged within 2 hours of blood draw and shipped overnight to the Fred Hutchinson Cancer Research Center Specimen Processing Laboratory (Seattle, WA). An aliquot for each participant was sent from the WHI Biorepository to the University of Minnesota Fairview ARDL Laboratory for CVD biomarker testing. The biomarkers required in the RRS were obtained from these test results. Resting blood pressure was measured after a 5-minute rest period using an aneroid sphygmomanometer and cuff and the average of two readings was recorded. DM status, smoking status, and family history of premature myocardial infarction were assessed using the LLS questionnaire.
LLS and WHI questionnaires ascertained participant demographics and information on lifestyle factors and health status. Participants self-identified race/ethnicity (White, Black, Hispanic), education (high school education or less, some college, college graduate or more), self-reported alcohol intake over the past 3 months (nondrinkers, <1 drink/week, 1–4 drinks/week, or >5 drinks/week), sleep disturbances (WHI Insomnia Rating Scale (WHIIRS) (23)), sleep medication use over the past 4 months (none, ≤ 2 times per week, ≥ 3 times per week), physical functioning (10-item subscale from the RAND 36-Item Health Survey (24)), depression (Burnam screening algorithm (25)), self-rated health (at least good or fair/poor), and the number of non-CVD chronic conditions (summed to create a comorbidity index score (26) from: cerebrovascular disease, cancer, hip fractures, osteoarthritis, chronic obstructive pulmonary disease, cognitive impairment, sensory impairment, history of frequent falls, and urinary incontinence). BMI (kg/m2) was calculated using height (cm) and weight (kg) measured using a stadiometer and scale. From the GT3x acccelerometer wear, total daily minutes of PA (minutes/day) during waking hours was calculated as > 1.6 metabolic equivalent (METs) (≥19 counts/15 seconds). Details of this accelerometer processing have previously published (27). Overall, rates of missing data on covariates were low (range 0%–7.6%). For covariates with <3% missing data (education, sleep disturbances, sleep medication use, physical functioning, depression, and BMI) mean imputation was used for missing values. For self-reported alcohol intake and self-rated health (>3% missing), missing indicator variables were assigned.
Statistical Analysis
Participant characteristics were described and then compared across sleep duration categories using one-way analysis of variances for continuous variables and chi-square tests for proportions.
Progressively adjusted linear regression models and marginal means plots were used to evaluate the relationship between average nightly sleep duration and RRS. The first model adjusted for race/ethnicity and education. The second model included additional adjustment for lifestyle factors including: alcohol intake, PA, sleep disturbance score, and sleep medications. The final model included adjustment for health status indicators including: BMI, physical functioning, comorbidity index score, depression, and self-rated health.
Age was not included in the series of models as it is a component of the RRS. Adjustment for a variable included in the RRS could lead to over adjustment. To explore if the relationship between RRS and sleep was consistent across age groups we completed a sensitivity analysis testing for interaction (Age × Sleep Duration) and by stratifying our final model on the mean age (78.9 years). In an additional sensitivity analysis, we explored if the relationship between RRS and sleep differed across racial/ethnic groups by testing for interaction (Race/Ethnicity × Sleep Duration) and running our final model on race/ethnicity stratified samples.
The literature suggests both short and long sleep durations are related to increased risk for CVD. Therefore, we tested the relationship for nonlinearity by including a quadratic term for sleep duration in this series of models. Additionally, we conducted a complete case analysis and evaluated whether the magnitude of our effects differed in models that included only individuals with complete data versus those who had missing covariate data. All analyses were performed using R statistical software version 3.1.1 (28).
Results
Characteristics of the study sample according to sleep duration quartiles are presented in Table 1. The analytic sample had an average nightly sleep duration of 8.16 hours. Women were on average 78.9 years old, and 53.3% were White. Almost 80% of participants had some college education and approximately 60% reported drinking some alcohol on a weekly basis. The average sleep disturbance score was 6.3 out of 20. The mean BMI for the cohort was 27.9 kg/m2. Over 46% of women reported ≥2 comorbidities, but 84% reported at least good self-rated health. In the cohort, RRS ranged from 0.8 to 94.3, with an average estimated risk of 12.5%. OPACH Participant characteristics according to racial ethnic group are presented in Supplementary Table 1.
Table 1.
OPACH Participant Characteristics by Sleep Duration Quartiles (N = 3,367)
| Average Nightly Sleep Duration Quartiles* | |||||
|---|---|---|---|---|---|
| Overall N = 3,367 | 1 (short) | 2 | 3 | 4 (long) | |
| Characteristics (number missing) | Mean ± SD or n (%) | ||||
| Age, years (0) | 78.9 ± 6.7 | 77.7 ± 6.6 | 78.5 ± 6.5 | 79.6 ± 6.7 | 79.8 ± 6.7 |
| 63–69 | 334 (9.9) | 106 (12.6) | 97 (11.5) | 65 (7.7) | 66 (7.8) |
| 70–79 | 1,295 (38.5) | 372 (44.2) | 329 (39.2) | 301 (35.6) | 293 (34.8) |
| 80–89 | 1,589 (47.2) | 341 (40.5) | 386 (45.9) | 429 (50.8) | 433 (51.5) |
| ≥90 | 149 (4.4) | 22 (2.6) | 28 (3.3) | 50 (5.9) | 49 (5.8) |
| Race/ethnicity (0) | |||||
| White | 1,795 (53.3) | 370 (44.0) | 480 (57.1) | 487 (57.6) | 458 (54.5) |
| Black | 1,007 (29.9) | 311 (37.0) | 216 (25.7) | 223 (26.4) | 257 (30.5) |
| Hispanic | 565 (16.8) | 160 (19.0) | 144 (17.1) | 135 (16.0) | 126 (15.0) |
| Education (18) | |||||
| High school or less | 694 (20.6) | 166 (19.8) | 164 (19.6) | 177 (21.1) | 187 (22.4) |
| Some college | 1,299 (38.6) | 319 (38.1) | 317 (37.9) | 343 (40.8) | 320 (38.3) |
| College graduate | 1,356 (40.3) | 353 (42.1) | 355 (42.5) | 320 (38.1) | 328 (39.3) |
| Lifestyle Factors | |||||
| Alcohol Intake (244) | |||||
| Nondrinker | 1,166 (34.6) | 301 (38.6) | 271 (34.6) | 261 (33.3) | 333 (42.9) |
| <1 drink/week | 1,055 (31.3) | 284 (36.5) | 264 (33.7) | 280 (35.8) | 227 (29.2) |
| 1–4 drinks/week | 538 (16.0) | 125 (16.0) | 128 (16.3) | 155 (19.8) | 130 (16.7) |
| ≥5 drinks/week | 364 (10.8) | 69 (8.9) | 121 (15.4) | 87 (11.1) | 87 (11.2) |
| Physical Activity (0) | |||||
| Minutes/day | 344.4 ± 97.7 | 381.2 ± 103.5 | 358.9 ± 89.7 | 336.4 ± 88.1 | 300.9 ± 90.2 |
| Sleep Disturbances (58) | 6.3 ± 4.5 | 5.9± 4.5 | 6.1 ± 4.3 | 6.4 ± 4.4 | 6.8 ± 4.6 |
| Sleep Medication Use (20) | |||||
| None | 2,747 (81.6) | 726 (86.7) | 680 (81.7) | 669 (79.5) | 672 (80.3) |
| ≤2 times/week | 394 (11.7) | 75 (9.0) | 100 (12.0) | 121 (14.4) | 98 (11.7) |
| ≥3 times/week | 206 (6.1) | 36 (4.3) | 52 (6.3) | 51 (6.1) | 67 (8.0) |
| Health Status Indicators | |||||
| BMI, kg/m 2 (24) | 27.9 ± 5.7 | 28.5 ± 6.0 | 27.7 ± 5.5 | 27.6 ± 5.5 | 27.7 ± 5.5 |
| Physical Functioning (54) | |||||
| 0–100 score | 84.7 ± 17.1 | 85.7 ± 16.6 | 85.8 ± 16.3 | 84.9 ± 16.1 | 82.5 ± 19.1 |
| Depression Score (0–1) (89) | 0.03 ± 0.1 | 0.03 ± 0.1 | 0.03 ± 0.1 | 0.03 ± 0.1 | 0.04 ± 0.1 |
| Self-rated Health (255) | |||||
| At least good | 2,823 (83.8) | 717 (92.6) | 727 (93.1) | 705 (90.0) | 674 (87.1) |
| Comorbidity Index (0) | |||||
| Zero conditions | 609 (18.1) | 172 (20.4) | 169 (20.1) | 151 (17.9) | 117 (13.9) |
| One condition | 1,178 (35.0) | 289 (34.4) | 298 (35.5) | 301 (35.6) | 290 (34.5) |
| Two or more conditions | 1,580 (46.9) | 380 (45.2) | 373 (44.4) | 393 (46.5) | 434 (51.6) |
| Reynolds Risk Score (%) (0) | 12.5 ± 10.9 | 11.5 ± 10.1 | 11.6 ± 10.0 | 12.8 ± 10.6 | 14.1 ± 12.4 |
Note: Numbers do not sum to total due to missing data. BMI = Body mass index.
*Quartile cutpoints for average nightly sleep duration (minutes), Q1 = 221.2–444.1, Q2 = 444.2–488.3, Q3 = 488.4–531.4, Q4 = 531.5–780.5.
Women in the shortest sleep duration quartile (<7.4 hours) were significantly younger and more likely to be African American or Hispanic compared to women with longer sleep durations. Education did not differ across sleep quartiles. Women in the short sleep quartile (<7.4 hours) also had higher levels of daily PA and reported lower sleep disturbance scores than longer sleepers.
Women in the longest sleep duration quartile (>8.9 hours) had the highest sleep disturbance scores, sleep medication use, depression scores, and proportion with two or more comorbidities. Additionally, physical functioning scores, total PA, and the proportion of the sample reporting good to excellent self-rated health were lowest among women in the long sleep duration quartile (>8.9 hours).
Sleep Duration and Reynolds Risk Score
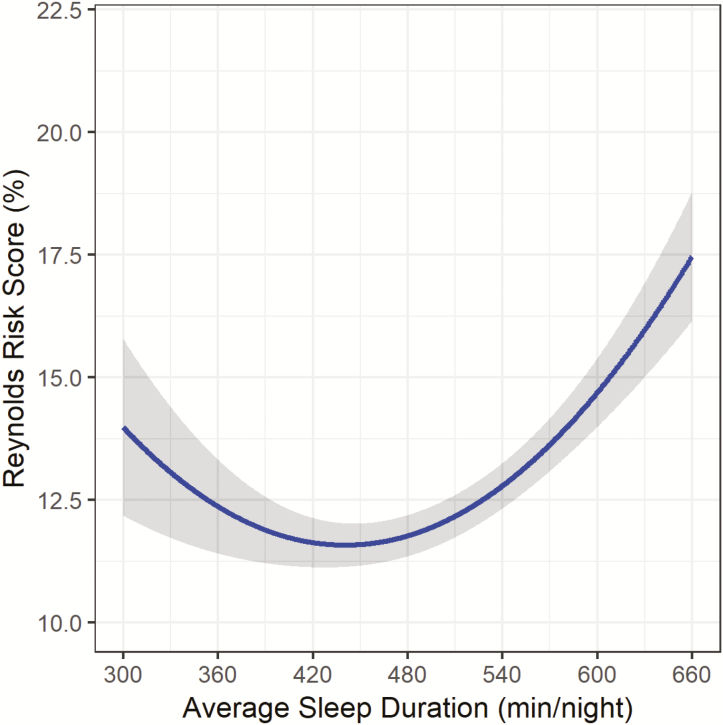
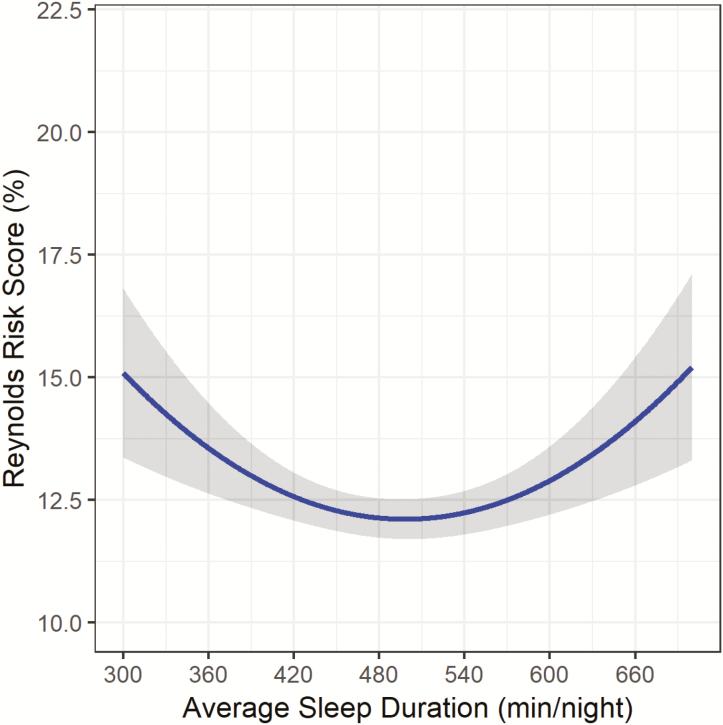
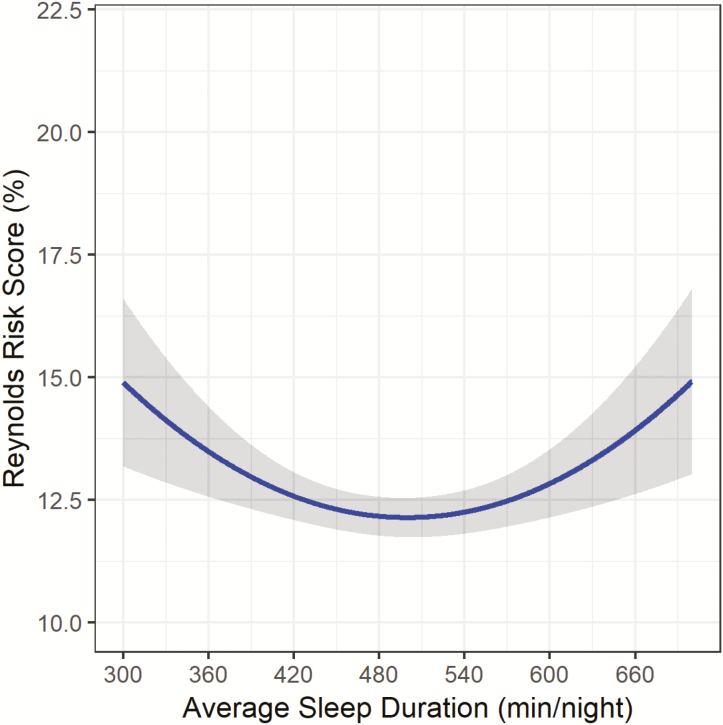
After minimal adjustment for race/ethnicity and education, both sleep duration and the quadratic sleep duration term were significantly related to RRS (Figure 1; sleep duration: B: −7.86, SE: 1.61, p < .001; sleep duration (2) B: 8.92, SE: 1.61, p < .001). The relationship appears to be U-shaped, with both short and long sleep durations associated with higher RRS as compared to the middle of the sleep duration distribution. After adjustment for lifestyle factors (model 2) and health status indicators (model 3), the quadratic sleep duration term remained significantly associated with RRS (Figure 2; sleep duration (2) B: 6.28, SE: 1.63, p < .001; and Figure 3; sleep duration (2) B: 6.30, SE: 1.67, p < .001).
Figure 1.
Sleep Duration and Reynolds Risk Score in the OPACH Sleep Cohort after minimal adjustment1. 1Model adjusted for race/ethnicity and education. **Gray shading represents 95% Confidence Interval.
Figure 2.
Sleep Duration and Reynolds Risk Score in the OPACH Sleep Cohort after further adjustment1. 1Model adjusted for race/ethnicity, education, alcohol intake, physical activity, sleep medications, and sleep disturbances. **Gray shading represents 95% Confidence Interval.
Figure 3.
Sleep Duration and Reynolds Risk Score in the OPACH Sleep Cohort after final adjustment1. 1Model adjusted for race/ethnicity, education, alcohol intake, physical activity, sleep medications, sleep disturbances, body mass index, physical functioning, depression, self-rated health, and comorbidity index. *Gray shading represents 95% Confidence Interval.
Figures 1–3 illustrate the u-shape of the relationship between sleep duration and 10-year predicted probability of future CVD risk, which persists throughout the series of progressive adjustments. The relationship between longer sleep and RRS is substantially diminished when the final model is adjusted for health status indicators. In the fully-adjusted model (Figure 3), the relationship remains significant, with both short and long sleep associated with higher RRS. Table 2 provides the estimated RRS from the series of models for each hour of average sleep duration per night. In the fully-adjusted model, 8 hours of sleep was associated with the lowest estimated RRS (12.17%). Every hour of sleep duration less than or greater than 8 hours was associated with a higher RRS, with 5 hours corresponding to an estimated RRS of 14.90% and a sleep duration of 11 hours associated with an estimated RRS of 13.92%. The relationship between longer sleep durations (>8 hours) and RRS was attenuated after adjustment while the relationship between shorter sleep durations (<8 hours) and RRS becomes stronger after the adjustments. When covariates were added to the models individually, the pattern of attenuation observed in the progressive model adjustments could not be attributed to one variable (Supplementary Table 2). The strongest significant lifestyle factor appeared to be total minutes of physical activity (p < .01), which accounted for a substantive change in the estimated RRS across the distribution of sleep duration. Of the health status indicators, the adjustments for physical functioning (p < .01) and comorbidity index (p < .01) accounted for considerable attenuation in the association between sleep duration and estimated RRS.
Table 2.
Estimated Reynolds Risk Score by Average Sleep Duration in OPACH Cohort
| Estimated Reynolds Risk Score | |||
|---|---|---|---|
| Sleep Duration Hours (min) | Model 1 | Model 2 | Model 3 |
| 5 h (300 min) | 13.98 (12.18, 15.79) | 15.09 (13.37, 16.82) | 14.90 (13.19, 16.61) |
| 6 h (360 min) | 12.37 (11.41, 13.33) | 13.56 (12.63, 14.48) | 13.49 (12.57, 14.41) |
| 7 h (420 min) | 11.63 (11.13, 12.13) | 12.57 (12.08, 13.06) | 12.58 (12.09, 13.07) |
| 8 h (480 min) | 11.77 (11.35, 12.19) | 12.13 (11.73, 12.53) | 12.17 (11.77, 12.57) |
| 9 h (540 min) | 12.79 (12.33, 13.25) | 12.24 (11.80, 12.69) | 12.25 (11.81, 12.70) |
| 10 h (600 min) | 14.69 (13.99, 15.39) | 12.90 (12.21, 13.60) | 12.84 (12.14, 13.53) |
| 11 h (660 min) | 17.47 (16.15, 18.78) | 14.11 (12.80, 15.42) | 13.92 (12.61, 15.22) |
| p Value a | <.001 | <.001 | <.001 |
Note: Model 1: Adjusted for race/ethnicity and education. min = minutes; h = hour.
Model 2: Adjusted for race/ethnicity, education, alcohol intake, physical activity, sleep disturbance score, and sleep medications.
Model 3: Adjusted for race/ethnicity, education, alcohol intake, physical activity, sleep disturbance score, sleep medications, body mass index (BMI), physical functioning, depression, self-rated health, and comorbidity index.
a p values presented for quadratic term in the model.
Variations by Race/Ethnicity
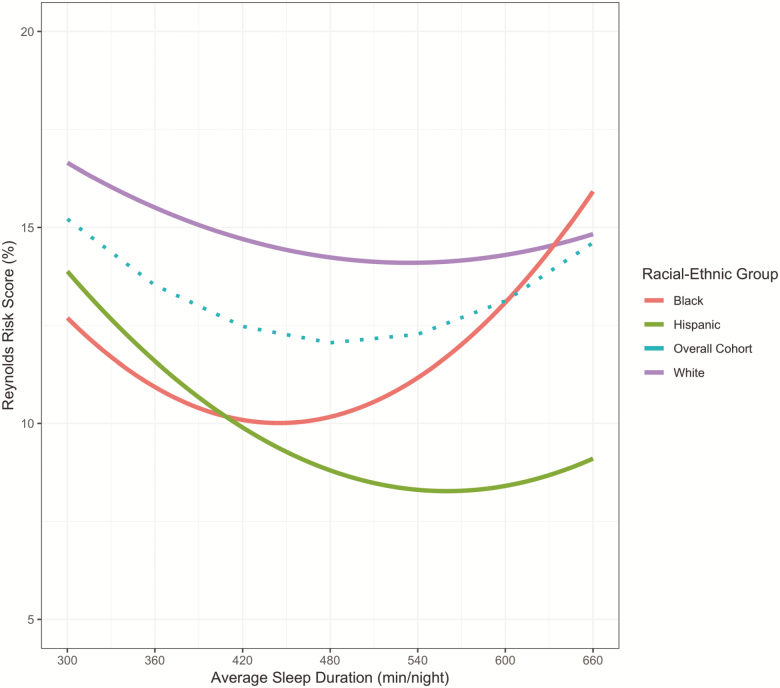
A significant interaction was observed in the association between sleep duration and RRS by race/ethnicity (p = .005). The race/ethnicity stratified analysis and the overall cohort association between sleep duration and 10-year predicted probability of future CVD risk, adjusted for lifestyle factors and health status indicators, are presented in Figure 4. At almost every average sleep duration, White women in the cohort had a higher mean RRS. Overall, the pattern of association were similar for White and Hispanic women, with shorter sleep durations associated with higher RRS, and lower RRS as sleep duration increases. However, for Black women, a U-shaped relationship was more prominent with both shorter and particularly longer sleep durations associated with higher predicted future CVD risk.
Figure 4.
Association of Sleep Duration and Reynolds Risk Score in the OPACH Sleep Cohort Stratified by Racial-Ethnic group. Model adjusted for race/ethnicity, education, alcohol intake, physical activity, sleep medications, sleep disturbances, body mass index, physical functioning, depression, self-rated health, and comorbidity, index.
Additional Sensitivity Analyses
Sensitivity analysis results showed that the relationship between RRS and sleep was consistent across age strata in our cohort. Age was not a significant effect modifier in the model, however the stratified analysis demonstrates that the relationship between sleep duration and RRS was not significant in the younger group (<80 years; sleep duration: B: −1.67, SE: 1.54, p = .28; sleep duration (2) B: 1.54, SE: 1.55, p = .32), but was significant in the older group of women (>80 years; sleep duration: B: −6.59, SE: 2.68, p = .01; sleep duration (2) B: 6.88, SE: 2.69, p = .01). Results of the complete case analyses showed that significance and magnitude of the associations remained fundamentally unchanged when only complete cases were included in the models.
Discussion
This is one of the largest studies to provide evidence that accelerometer-measured sleep duration is associated with a measure of 10-year predicted probability of future CVD risk in older women. The nature of the relationship between sleep duration and CVD risk remains a topic of investigation and our study results indicate the relationship appears to be nonlinear. Both shorter and longer sleep durations were associated with higher 10-year predicted future CVD risk, with variation across racial/ethnic groups.
Our analysis builds upon previous evidence. In The Sleep Heart Health Study, among 5,910 participants (aged >40), the odds of hypertension were 60% and 30% higher among those who reported sleeping <6 and >9 hours/night, respectively, versus those sleeping 7–8 hours/night (29). This reported U-shaped relationship between sleep duration and risk of hypertension is consistent with our study results. In The Wisconsin Sleep Cohort Study, among 1,024 adults, mean age 52.7 years, a U-shaped association between sleep duration and obesity was observed. Sleeping <7 and >7.5 hours per night, measured with polysomnography, resulted in an increased likelihood of obesity (30). Our results provide a novel contribution to the literature by assessing the relationship between sleep duration and the RRS, a clinically meaningful measure of predicted probability of future CVD. The RRS represents a measure of estimated future CVD based on the most predictive risk factors, not only aggregated, but also weighted to predict future risk. While examining sleep duration and individual risk factors may not sufficiently reflect an increased risk for CVD associated with short or long sleep duration, our study results show an association between sleep and predicted probability of future CVD that takes into account the interrelationships of each weighted risk factor, summed and then extrapolated to a 10-year probability.
This is the oldest cohort of women in which the relationship between accelerometer-measured sleep duration and CVD has been examined. In a previous WHI study among 86,329 women ages 50–79 (84% White), self-reported short (<5 hours) and long (>10 hours) sleep duration was associated with higher risk of incident CVD (19% and 37%), however results did not remain significant after adjustment (31). Our findings are similar to results of the minimally adjusted models in the aforementioned study; however, our sample is older and substantially more diverse (only 53.5% White). The long sleep quartile (>9 hours) in our study was made-up of the oldest older women (>80 years) in our sample. Older age may be an additional risk factor for long sleep that has not been sufficiently examined (32). Sleep might influence CVD risk factors and their integrated effect on predicted risk of a future CVD event differently depending on age, as demonstrated by our sensitivity analysis. Additional research is needed to better understand how sleep and age interrelate with propensity for CVD.
Literature on the detrimental effects of long sleep remains inconclusive, however some evidence indicates that long sleep is associated with increased risk of chronic conditions and mortality (33,34). We add to this literature, demonstrating that accelerometer-measured long sleep duration is related to increased 10-year predicted probability of CVD independent of comorbidities, sleep disturbances, depression, and other possible confounders that may explain this relationship. With cross-sectional data, we are not able to examine the pathways through which sleep duration is linked to predicted probability of CVD. Suggested biological pathways through which short sleep is related to cardiometabolic health include alterations in glucose metabolism (35), insulin resistance (36), and inflammation (37). Studies have shown that long sleep durations impacts gluco-regulatory function and systemic inflammation (38), however, the mechanisms through which long sleep relates to cardiometabolic health are not as clear.
The primary strength of our study was the use of accelerometer-measured sleep duration. Previous cohort studies have relied upon one- or two-item self-reported measures to assess sleep duration. These measures pose concern as individuals are likely to report sleep times that align with what is perceived as acceptable and often over-report sleep durations (39). We employed additional data processing steps to ensure the accuracy of the data. The visual coding method is used in place of an automated algorithm, which has not been developed for older adult samples. This step is essential as older women have lower levels of activity throughout the day, which may lead to misclassifying sedentary time as sleep. Additionally, our sample was purposefully recruited to have larger numbers of racial/ethnic minorities including African Americans and Hispanics who report shorter sleep durations (40) and have higher prevalence of CVD risk factors (2). Prior studies that utilized objective assessment of sleep duration only compared two racial/ethnic groups with a limited proportion of women within these groups. A novelty of this study, which was not possible in prior studies, was to assess whether the association between sleep duration and 10-year predicted probability of CVD was consistent across racial/ethnic groups. The results of our sensitivity analysis demonstrate the sleep duration-RRS association does differ significantly across racial/ethnic groups. This finding further supports the need for future research to examine racial/ethnic differences in sleep disparities and to clarify further the relationship between sleep and future CVD risk.
This study has some limitations. The sleep duration that leads to the best health outcomes can vary by individual. Our study findings reflect the predicted probability of future CVD risk associated with average sleep durations across a large sample of women. Our study includes only postmenopausal women, limiting generalizability to the population as a whole. Further, sleep duration was assessed at one time point; therefore, the influence of sleep duration on estimated CVD risk over time cannot be evaluated. Our approach to estimating sleep duration using an accelerometer provides a more accurate estimate of sleep duration than self-reported measures, however separating sleep duration from time in bed continues to pose a challenge. As such, we are unable to determine if the association between long sleep and predicted probability of CVD risk is partially explained by more time in bed. For older women, longer periods of in bed time may not actually reflect time spent sleeping, but the presence of sleep disorders, comorbid conditions, depression, or reduced physical functioning (32). We attempted to address this possible misclassification bias by adjusting for these potential confounders in our models. In our sensitivity analysis, we explored racial/ethnic variations in the association between sleep duration and RRS, and found that there were differences in the association by racial/ethnic group. This additional analysis was exploratory and should be interpreted as such; however our findings warrant further investigation to better understand what factors may contribute to these differences by race/ethnicity. Lastly, while we included sleep medications and sleep disturbance in our analysis, we did not include a diagnosis of obstructive sleep apnea, which may be a risk factor for cardiometabolic conditions (41). Evidence indicates that the bulk of obstructive sleep apnea remains undiagnosed and untreated, particularly in the elderly adults, thus supporting the real-world applicability of our findings. Finally, it was beyond the scope of our study to examine sleep quality and predicted probability of future CVD risk.
This study supports growing evidence of an association between sleep duration and future CVD risk. As the incidence of CVD continues to rise in the aging population of women, it is important to identify additional risk factors to target for CVD prevention. While the length of sleep for optimal health is not completely clear at present, this study highlights the importance of sleep duration for cardiovascular health in the aging population.
Funding
This work was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health (grant number R01 HL105065 to A.Z.L.) and an American Heart Association training grant (award number 16PRE31100010 to K.M.F.).
Conflict of Interest
None reported.
Supplementary Material
Acknowledgments
The authors are thankful for the efforts of investigators and staff at the WHI clinical centers. A full list of WHI investigators can be found at the following site: www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf.
References
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics’2017 Update: A Report from the American Heart Association Circulation. 2017;135:e146–603. doi:10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013. ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American college of cardiology/American heart association task force on practice guidelines. J. Am. Coll. Cardiol. 2014;63:2935–2959. doi:10.1161/01.cir.0000437741.48606.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. St-Onge M-P, Grandner MA, Brown D, et al. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. 2016;134:e367–e386. doi:10.1161/CIR.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–1492. doi:10.1093/eurheartj/ehr007 [DOI] [PubMed] [Google Scholar]
- 5. Chaput J-P, McNeil J, Després J-P, Bouchard C, Tremblay A. Short sleep duration as a risk factor for the development of the metabolic syndrome in adults. Prev Med (Baltim). 2013;57:872–877. doi:10.1016/j.ypmed.2013.09.022 [DOI] [PubMed] [Google Scholar]
- 6. Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–839. doi:10.1161/01.HYP.0000217362.34748.e0 [DOI] [PubMed] [Google Scholar]
- 7. Aziz M, Ali SS, Das S, et al. Association of subjective and objective sleep duration as well as sleep quality with non-invasive markers of sub-clinical Cardiovascular Disease (CVD): a systematic review. J Atheroscler Thromb. 2017;24:208–226. doi:10.5551/jat.36194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nagayoshi M, Lutsey PL, Benkeser D, et al. Association of sleep apnea and sleep duration with peripheral artery disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2016;251:467–475. doi:10.1016/J.ATHEROSCLEROSIS.2016.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bertisch SM, Pollock BD, Mittleman MA, et al. Insomnia with objective short sleep duration and risk of incident cardiovascular disease and all-cause mortality: Sleep Heart Health Study. Sleep. 2018;41: zsy047. doi:10.1093/sleep/zsy047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best Pr Res Clin Endocrinol Metab. 2011;24:1–17. doi:10.1016/j.beem.2010.07.001.Sleep [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jackson CL, Redline S, Emmons KM. Sleep as a potential fundamental contributor to disparities in cardiovascular health. Annu Rev Public Health. 2015;36:417–440. doi:10.1146/annurev-publhealth-031914-122838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611–619. doi:10.1001/jama.297.6.611 [DOI] [PubMed] [Google Scholar]
- 13. Grundy SM, Balady GJ, Criqui MH, et al. Guide to primary prevention of cardiovascular diseases. a statement for healthcare professionals from the Task Force on Risk Reduction. American Heart Association Science Advisory and Coordinating Committee. Circulation. 1997;95:2329–2331. doi:10.1161/01.cir.95.9.2329 [DOI] [PubMed] [Google Scholar]
- 14. LaCroix AZ, Rillamas-Sun E, Buchner D, et al. The objective physical activity and cardiovascular disease health in older women (OPACH) study. BMC Public Health. 2017;17:1–12. doi:10.1186/s12889-017-4065-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rosenberger ME, Haskell WL, Albinali F, Mota S, Nawyn J, Intille S. Estimating activity and sedentary behavior from an accelerometer on the hip or wrist. Med Sci Sports Exerc. 2013;45:964–975. doi:10.1249/MSS.0b013e31827f0d9c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Full KM, Kerr J, Grandner MA, et al. Validation of a physical activity accelerometer device worn on the hip and wrist against polysomnography. Sleep Health. 2018;4:209–216. doi:10.1016/j.sleh.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lockley SW, Skene DJ, Arendt J. Comparison between subjective and actigraphic measurement of sleep and sleep rhythms. J Sleep Res. 1999;8:175–183. doi:10.1046/j.1365-2869.1999.00155.x. [DOI] [PubMed] [Google Scholar]
- 18. Blackwell T, Ancoli-Israel S, Gehrman PR, Schneider JL, Pedula KL, Stone KL. Actigraphy scoring reliability in the study of osteoporotic fractures. Sleep. 2005;28:1599–1605. doi:10.1093/sleep/28.12.1599 [DOI] [PubMed] [Google Scholar]
- 19. Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15:461–469. doi:10.1093/sleep/15.5.461 [DOI] [PubMed] [Google Scholar]
- 20. Knutson KL, Rathouz PJ, Yan LL, Liu K, Lauderdale DS. Intra-individual daily and yearly variability in actigraphically recorded sleep measures: the CARDIA study. Sleep. 2007;30:793–796. doi:10.1093/sleep/30.6.793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lauderdale DS, Knutson KL, Yan LL, et al. Objectively measured sleep characteristics among early-middle-aged adults. Am J Epidemiol. 2006;164:5–16. doi:10.1093/aje/kwj199 [DOI] [PubMed] [Google Scholar]
- 22. Cook NR, Paynter NP, Eaton CB, et al. Comparison of the Framingham and Reynolds risk scores for global cardiovascular risk prediction in the multiethnic women’s health initiative. Circulation. 2012;125:1748–1756. doi:10.1161/CIRCULATIONAHA.111.075929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Levine DW, Kripke DF, Kaplan RM, et al. Reliability and validity of the Women’s Health Initiative Insomnia Rating Scale. Psychol Assess. 2003;15:137–148. doi:10.1037/1040-3590.15.2.137. [DOI] [PubMed] [Google Scholar]
- 24. Hays RD, Sherbourne CD, Mazel RM. The RAND 36-item health survey 1.0. Health Econ. 1993;2:217–227. doi:10.1002/hec.4730020305 [DOI] [PubMed] [Google Scholar]
- 25. Burnam MA, Wells KB, Leake B, Landsverk J. Development of a brief screening instrument for detecting depressive disorders. Med Care. 26:775–789. doi:10.2307/3765462 [DOI] [PubMed] [Google Scholar]
- 26. Rillamas-Sun E, LaCroix AZ, Bell CL, Ryckman K, Ockene JK, Wallace RB. The impact of multimorbidity and coronary disease comorbidity on physical function in women aged 80 years and older: the Women’s Health Initiative. Journals Gerontol Ser A Biol Sci Med Sci. 2016;71 (Suppl 1):S54–S61. doi:10.1093/gerona/glv059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. LaMonte MJ, Lewis CE, Buchner DM, et al. Both light intensity and moderate-to-vigorous physical activity measured by accelerometry are favorably associated with cardiometabolic risk factors in older women: the Objective Physical Activity and Cardiovascular Health (OPACH) Study. J Am Heart Assoc. 2017;6:e007064. doi:10.1161/(ISSN)2047–9980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. R Core Team. R: a language and environment for statistical computing 2013. http://www.r-project.org/.
- 29. Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29:1009–10014. doi:10.1093/sleep/29.8.1009 [DOI] [PubMed] [Google Scholar]
- 30. Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. Froguel P, ed. PLoS Med. 2004;1:e62. doi:10.1371/journal.pmed.0010062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sands-Lincoln M, Loucks EB, Lu B, et al. Sleep duration, insomnia, and coronary heart disease among postmenopausal women in the women’s health initiative. J Womens Heal. 2013;22:477–486. doi:10.1089/jwh.2012.3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Patel SR, Malhotra A, Gottlieb DJ, White DP, Hu FB. Correlates of long sleep duration. Sleep. 2006;29:881–889. doi:10.1016/j.biotechadv.2011.08.021.Secreted [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kripke DF, Langer RD, Elliot JA, Klauber M, Rex KM. Mortality related to actigraphic long and short sleep. Sleep Med. 2011;12:28–33. doi:10.1016/j.biotechadv.2011.08.021.Secreted [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ayas NT, White DP, Al-Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–384. doi:10.2337/diacare.26.2.380 [DOI] [PubMed] [Google Scholar]
- 35. Chaput JP, Després JP, Bouchard C, Tremblay A. Association of sleep duration with type 2 diabetes and impaired glucose tolerance. Diabetologia. 2007;50:2298–2304. doi:10.1007/s00125-007-0786-x [DOI] [PubMed] [Google Scholar]
- 36. Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99:2008–2019. doi:10.1152/japplphysiol.00660.2005 [DOI] [PubMed] [Google Scholar]
- 37. Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. doi:10.1016/j.pcad.2008.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ohkuma T, Fujii H, Iwase M, et al. Impact of sleep duration on obesity and the glycemic level in patients with type 2 diabetes: the Fukuoka Diabetes Registry. Diabetes Care. 2013;36:611–617. doi:10.2337/dc12-0904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Van Den Berg JF, Van Rooij FJ, Vos H, et al. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. J Sleep Res. 2008;17:295–302. doi:10.1111/j.1365-2869.2008.00638.x [DOI] [PubMed] [Google Scholar]
- 40. Hale L, Do DP. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30:1096–1103. doi:10.1093/sleep/30.9.1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182:269–277. doi:10.1164/rccm.200911-1746OC [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.