Abstract
The prevalence of brain pathologies increases with age and cognitive and physical functions worsen over the lifetime. It is unclear whether these processes show a similar increase with age. We studied the association of markers for brain pathology cognitive and physical functions with age in 288 cognitively normal individuals aged 60–102 years selected from the cross-sectional EMIF-AD PreclinAD and 90+ Study at the Amsterdam UMC. An abnormal score was consistent with a score below the 5th percentile in the 60- to 70-year-old individuals. Prevalence of abnormal scores was estimated using Generalized Estimating Equations (GEE) models. The prevalence of abnormal handgrip strength, the Digit Symbol Substitution Test, and hippocampal volume showed the fastest increase with age and abnormal MMSE score, muscle mass, and amyloid aggregation the lowest. The increase in prevalence of abnormal markers was partly dependent on sex, level of education, and amyloid aggregation. We did not find a consistent pattern in which markers of brain pathology cognitive and physical processes became abnormal with age.
Keywords: Biomarkers, Brain aging, Cognitive function, Human aging
Aging is an inevitable process during which different pathologies arise and multiple body systems lose their functionality. The prevalences of brain pathologies, such as cortical atrophy, amyloid aggregation, the pathological hallmark of Alzheimer’s disease (AD), and vascular pathology, are strongly dependent on age (1,2). Examples of functions that worsen with aging are cognitive performance and muscle strength, which are both important negative determinants of independent living and quality of life (3–5).
The sequence in which these different processes become abnormal with aging is still largely unknown. Earlier literature found that temporal relationships between cognitive and physical functions are dependent on the used measures (6,7). Furthermore, brain pathologies such as amyloid aggregation and cortical atrophy seem to precede cognitive decline (2,8). None of these studies examine the age relation of brain pathology markers and cognitive and physical markers in the same cohort with a wide age range, including nonagenarians and centenarians. Furthermore, aging patterns of these markers might differ between males and females and low and highly educated individuals as sex and educational level are factors known to influence cognitive or physical status (4,9).
In the present study, we determined the association of brain pathology cognitive and physical markers with age in a cognitively normal population aged 60–102 years to explore differences in aging patterns between markers and the effect of sex, educational level, and amyloid status on aging patterns.
Method
Study Sample
We selected individuals with normal cognition from the European Medical Information Framework for AD (EMIF-AD) PreclinAD study and the EMIF-AD 90+ Study included at the Amsterdam University Medical Centers (UMC). Both studies were part of the Innovative Medicine Initiative (IMI) EMIF-AD project and used similar data collection protocols (10,11) (www.emif.eu). The PreclinAD study aimed to identify risk factors for amyloid pathology in cognitively normal individuals aged 60 years and older, and the EMIF-AD 90+ Study aimed to identify factors associated with resilience to cognitive impairment in individuals aged 90 years and older. In both studies, normal cognition was determined based on the absence of dementia or mild cognitive impairment (MCI) and a score of 0 points on the global Clinical Dementia Rating (CDR) scale (12). For the PreclinAD study individuals also had to score >22 points on the Telephone Interview for Cognitive Status modified (TICS-m) and a score >−1.5 SD of age-adjusted normative data on the delayed recall of the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) 10 words test (13,14). For the EMIF-AD 90+ Study, cognitively normal individuals also had to score ≥26 points on the mini-mental state examination (MMSE). Three individuals from the EMIF-AD 90+ Study, who were determined to be cognitively normal after extensive cognitive testing, with a MMSE < 26 points were also included. Exclusion criteria for both studies were the presence of a neurological, systemic, or psychiatric disorder that could cause cognitive impairment. Recruitment of the PreclinAD study was between December 2014 and August 2016. We included 99 monozygotic twin pairs, one dizygotic twin pair, and four single individuals selected from the Netherlands Twin Registry (15). Recruitment of the EMIF-AD 90+ Study was between June 2016 and July 2018 and took place via advertisement, general practitioners, and the 100-plus Study (16), resulting in 84 individuals with normal cognition in the EMIF-AD 90+ Study. Both studies were approved by the Medical Ethical Committee of the Amsterdam UMC and all individuals gave written informed consent.
Aging Markers
Brain pathology markers
Hippocampal and white matter hyperintensity volume
To assess brain atrophy, we determined hippocampal volume and as measure of vascular brain damage, we used white matter hyperintensity (WMH) volume. Brain MRI-scans were performed on a single Philips 3T Achieva scanner using an 8-channel head coil. Isotropic structural three-dimensional (3D) T1-weighted images were acquired using a sagittal turbo field echo sequence (1.00 mm3 isotropic voxels, repetition time = 7.9 ms, echo time = 4.5 ms, and flip angle = 8°) and 3D sagittal fluid-attenuated inversion recovery (FLAIR) sequences (1.12 mm3 isotropic voxels, repetition time = 4800 ms, echo time = 279 ms, and inversion time = 1650 ms) were acquired for the WMH segmentation. The MRI-scans were visually inspected for incidental findings by a neuroradiologist. Volumetric segmentation of the 3D T1 images was performed with the FreeSurfer image analysis suite, which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/). Details of this procedure were described previously (17). Briefly, the automated procedure includes motion correction, removal of nonbrain tissue, automated Talairach transformation, and segmentation of the subcortical white matter and deep gray matter volumetric structures (including hippocampus, amygdala, caudate, putamen, and ventricles). Hippocampal volume was computed from the segmented images in native space and mean hippocampal volume was calculated by averaging the left and right side. WMH segmentation was performed by using a previously established algorithm based on a three-level Gaussian mixture model to model healthy tissues and lesions (18). Both the FreeSurfer and WMH segmentations were visually inspected. Reasons for exclusions are described in Supplementary Table S1. In the present analyses, we computed the percentage of hippocampal and WMH volume relative to total intracranial volume (TIV, which is the sum of grey matter, white matter, and cerebrospinal fluid), in order to correct for head size.
Amyloid BPND
All individuals underwent amyloid positron emission tomography (PET) imaging using a specific fibrillary amyloid β radiotracer, [18F]flutemetamol, which was produced by General Electric (GE) Healthcare at the Cyclotron Research Centre of the University of Liège (Liège, Belgium). PET scans were performed on a Philips Ingenuity TF PET-MRI. The emission scan was performed in two parts starting with a 30 minute dynamic scan simultaneously with the bolus intravenous injection of 185 MBq [18F]flutemetamol, followed by a 20 minute scan performed 90–110 minutes after the injection. Immediately before these two scans, a T1-weighted gradient echo pulse MRI-scan was obtained for attenuation correction of the PET scan. The dynamic data were analyzed on a voxel-by-voxel level using the basis function approach of the Simplified Reference Tissue Model (SRTM) with cerebellar grey matter as reference tissue to determine amyloid nondisplaceable binding potential (BPND) (19,20). Global amyloid BPND was determined by determining the volume-weighted average BPND of the frontal (superior, middle, and inferior frontal gyrus), parietal (posterior cingulate, superior parietal gyrus, postcentral gyrus, and inferolateral remainder of parietal lobe), and temporal regions (parahippocampal gyrus, hippocampus, medial temporal lobe, superior, middle, and inferior temporal gyrus) (21).
Cognitive markers
Cognitive tests were administered by a trained neuropsychologist. As a measure for global cognition, we used the MMSE (range: 0–30 points), for processing speed the Digit Symbol Substitution Test (DSST, range: 0–93 points) from the Wechsler Adult Intelligence Scale-Revised (22) and for memory the CERAD 10 words test immediate recall total score over three trials (CERAD memory test, range: 0–30 words).
Physical markers
Grip strength of the dominant hand was measured twice with a hand dynamometer (Jamar hand dynamometer; Sammons Preston, Inc., Bolingbrook, IL) and the highest score in kilograms was used in the analyses (23). Skeletal muscle mass (further described as muscle mass) was measured in kilograms using a Bioelectrical Impedance Analysis (BIA; InBody 770 or S10; Biospace Co., Ltd, Seoul, Korea) (24). For the analyses, we divided muscle mass by height (kg/m2).
Statistical Analyses
To quantify brain pathology cognitive and physical abnormalities and to be able to compare their associations with age, each marker measuring these processes was dichotomized normal and abnormal based on the value of the worst 5th percentile in the youngest age group (60–70 years old) (25) or on a previously established cut point (0.26 for amyloid BPND) (26). The cut point value was included in the abnormal group. For handgrip strength and muscle mass, sex-specific cut points were used because maximal levels in young adulthood are higher in males than in females (4). Frequencies of marker abnormality were reported in the total study sample and per 10-year age groups (60–70, 70–80, 80–90, and ≥ 90 yr). The association between age (independent variable) and the proportions of abnormality per aging marker (dependent variable) was estimated using Generalized Estimating Equations (GEE). GEE models were chosen because they compute robust standard errors estimates that are consistent even when the “working” correlation matrix is incorrectly specified. We used GEE models with a logit link function for binary outcomes and assumed an exchangeable correlation to account for correlation within twin individuals, and including a random effect for family dependencies. Based on the estimated model, we simulated the estimated frequencies. Per marker, we tested interactions of age with sex, age with educational level, which was dichotomized on the median years of education (=10 yr), and age with amyloid status. If the interaction was significant, stratified analyses for sex, educational level, or amyloid status were performed including the interaction term in the model. If the interaction was not significant, stratified analyses for sex, educational level, or amyloid status were performed including sex, educational level, or amyloid status as covariate in the model.
Across the age range, we determined at what ages the simulated, estimated frequencies were significantly different between the stratified groups for sex, educational level, and amyloid status and smoothed the p-values to minimize statistical noise.
The p-value threshold for significance was set at .05. All analyses were performed in R-Studio version 1.1.414 with R version 3.4.3 (27). The “Zelig” package was used for GEE (28,29).
Results
We included 288 individuals (56.9% female) who were on average 77.1 (SD: 12.1, range: 60.3–102.2) years old and had 11.3 (SD: 2.9) years of education (Table 1). For 253 (87.8%) individuals, amyloid PET was available and of these individuals, 54 (21.3%) had an abnormal amyloid status based on the cut point. Reasons for missing variables are presented in Supplementary Table S1. Cut points are presented in Table 2.
Table 1.
Characteristics of Study Sample
| Total sample | 60–70 yr | 70–80 yr | 80–90 yr | ≥90 yr | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N* | Mean (SD) | N* | Mean (SD) | N* | Mean (SD) | N* | Mean (SD) | N* | Mean (SD) | |
| Age, yr | 288 | 77.1 (12.1) | 106 | 64.6 (2.8) | 73 | 74.2 (2.9) | 22 | 85.0 (2.6) | 87 | 92.8 (2.8) |
| Female† | 288 | 164 (56.9) | 106 | 52.0 (49.1) | 73 | 48 (65.8) | 22 | 16 (72.7) | 87 | 48 (55.2) |
| Education, yr | 288 | 11.3 (2.9) | 106 | 11.8 (2.6) | 73 | 11.3 (2.7) | 22 | 9.8 (1.9) | 87 | 11.2 (3.4) |
| HCV, mm3 | 263 | 3508 (583) | 105 | 3865 (392) | 71 | 3651 (509) | 21 | 3101 (394) | 66 | 2914 (397) |
| Amyloid BPND | 253 | 0.2 (0.2) | 100 | 0.1 (0.1) | 70 | 0.2 (0.1) | 19 | 0.2 (0.1) | 64 | 0.3 (0.3) |
| WMHV‡, mm3 | 263 | 3844 (13624) | 104 | 1333 (2507) | 71 | 3383 (7800) | 21 | 13113 (17812) | 67 | 20480 (24123) |
| MMSE, points | 288 | 28.8 (1.3) | 106 | 29.1 (1.1) | 73 | 29.0 (1.2) | 22 | 28.6 (1.0) | 87 | 28.4 (1.5) |
| Memory§, words | 283 | 20.8 (3.8) | 106 | 22.9 (2.8) | 73 | 21.7 (2.7) | 22 | 19.2 (2.8) | 82 | 17.7 (3.8) |
| DSST, points | 273 | 41.0 (13.4) | 106 | 50.0 (10.3) | 72 | 41.7 (11.1) | 22 | 34.3 (9.2) | 73 | 29.4 (10.3) |
| HGS, kg | ||||||||||
| Males | 123 | 35.4 (12.4) | 54 | 43.8 (7.8) | 25 | 38.7 (10.7) | 6 | 28.9 (8.5) | 38 | 22.5 (6.7) |
| Females | 163 | 22.2 (8.8) | 52 | 28.6 (8.5) | 48 | 25.1 (4.5) | 16 | 20.1 (5.2) | 47 | 12.8 (4.2) |
| Muscle mass, kg/m2 | ||||||||||
| Males | 88 | 10.6 (0.9) | 30 | 11.2 (0.9) | 22 | 10.9 (0.6) | 6 | 10.1 (0.6) | 30 | 9.9 (0.6) |
| Females | 122 | 8.9 (0.8) | 28 | 9.2 (0.9) | 45 | 9.1 (0.7) | 12 | 8.6 (0.6) | 37 | 8.5 (0.8) |
Notes: Values are presented as mean (SD) unless stated otherwise.
BPND = nondisplaceable binding potential; CERAD = Consortium for the Establishment of a Registry for Alzheimer’s Disease; DSST = Digit Symbol Substitution Test; HGS = handgrip strength; HCV = hippocampal volume, mean of left and right hippocampus; kg = kilogram; mm = millimeter; MMSE = mini-mental state examination; SD = standard deviation; WMHV = white matter hyperintensities volume.
*N: Sample size per aging marker and per age group.
†Presented as number (%).
‡Presented as median (IQR).
§Assessed with CERAD memory test total score over three trials.
Table 2.
Cut Points and Observed Frequencies of Abnormal Values According to Age
| Cut point* | Total sample | 60–70 yr | 70–80 yr | 80–90 yr | ≥90 yr | |
|---|---|---|---|---|---|---|
| HCV | 0.2 mm3 | 74 (28.2) | 6 (5.7) | 7 (9.9) | 15 (71.4) | 46 (70.8) |
| Amyloid BPND | 0.26 | 54 (21.3) | 8 (8.0) | 14 (20.0) | 6 (31.6) | 26 (40.6) |
| WMHV | 1.1 mm3 | 60 (23.0) | 6 (5.8) | 8 (11.3) | 8 (38.1) | 38 (58.5) |
| MMSE | 27.0 points | 45 (15.6) | 10 (9.4) | 8 (11.0) | 4 (18.2) | 23 (26.4) |
| Memory† | 18.0 words | 74 (26.1) | 8 (7.5) | 8 (11.0) | 10 (45.5) | 48 (58.5) |
| DSST | 36.0 points | 100 (36.6) | 6 (5.7) | 24 (33.3) | 14 (63.6) | 56 (76.7) |
| HGS | F: 19.6 kg M: 34.1 kg |
111 (38.8) | 6 (5.7) | 12 (16.4) | 10 (45.5) | 83 (97.6) |
| Muscle mass | F: 8.0 kg/m2 M: 9.6 kg/m2 |
32 (15.2) | 4 (6.9) | 5 (7.5) | 3 (16.7) | 20 (29.9) |
Notes: Except for the cut points, values are presented as numbers (% of total valid measurements).
BPND = nondisplaceable binding potential; CERAD = Consortium for the Establishment of a Registry for Alzheimer’s Disease; DSST = Digit Symbol Substitution Test; F = females; HGS = handgrip strength; HCV = hippocampal volume; M = males; MMSE = mini-mental state examination; WMHV = white matter hyperintensities volume.
*Cut points for abnormality were based on the value of the worst 5th percentile in the youngest age group (60–70 years old) (25) or an amyloid BPND equal to or above a previously defined cut point (26). The cut point value was included in the abnormal group.
†Assessed with CERAD memory test total score over three trials.
Effect of Age on Aging Marker Abnormality
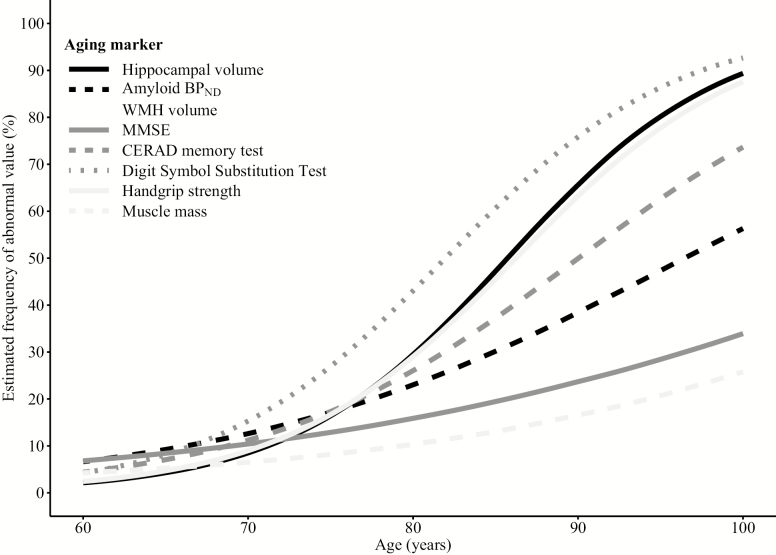
All markers showed higher observed and estimated frequencies of abnormality with older age (Tables 2 and 3). The associations with age differed among the markers (Figure 1). At age 80 and 100 years, muscle mass and MMSE had the lowest prevalence of abnormality (80 yr: 13.8%–15.8%; 100 yr: 33.4%–42.4%) and DSST and handgrip strength the highest (80 yr: 43.0%–44.9%; 100 yr: 92.7%–98.2%). The prevalence of abnormality of the other aging markers was 20%–30% at age 80 years and at age 100 years 89.3% for hippocampal volume, 73.6%–75.7% for the CERAD memory test and WMH volume, and 56.4% for amyloid BPND.
Table 3.
Estimated Frequencies of Abnormal Values According to Age
| Age, yr | HCV | Amyloid BPND | WMHV | MMSE | Memory* | DSST | HGS | Muscle mass |
|---|---|---|---|---|---|---|---|---|
| 60 | 2.1 (0.7–5.0) | 6.7 (3.1–12.5) | 3.1 (1.1–7.1) | 6.8 (3.3–12.2) | 4.4 (1.9–9.1) | 4.1 (2.0–7.6) | 1.3 (0.3–3.5) | 3.6 (1.3–8.9) |
| 65 | 4.2 (1.7–8.6) | 9.1 (5.1–14.9) | 5.4 (2.3–10.4) | 8.3 (4.5–13.7) | 7.1 (3.7–12.1) | 8.1 (4.7–12.7) | 3.4 (1.1–7.8) | 5.1 (2.1–10.5) |
| 70 | 8.6 (4.4–14.9) | 12.6 (7.9–18.4) | 8.8 (4.8–14.5) | 10.4 (6.3–15.1) | 11.2 (6.6–17.2) | 15.2 (10.4–21.6) | 8.8 (4.2–16.3) | 7.3 (3.5–12.3) |
| 75 | 16.5 (10.6–24.1) | 17.2 (12.0–23.6) | 14.6 (9.2–21.7) | 12.9 (8.9–17.7) | 17.4 (11.8–23.9) | 26.9 (20.5–34.6) | 21.9 (13.6–33.0) | 10.1 (6.1–15.5) |
| 80 | 29.6 (21.4–38.9) | 23.0 (17.4–29.3) | 23.5 (16.7–31.4) | 15.8 (11.6–20.8) | 26.1 (20.3–32.8) | 43.0 (35.0–51.2) | 44.9 (34.4–56.6) | 13.8 (9.3–19.4) |
| 85 | 47.3 (37.5–56.7) | 30.3 (23.5–37.6) | 35.5 (27.5–43.9) | 19.5 (14.5–25.4) | 37.1 (30.2–44.9) | 60.5 (50.3–69.7) | 70.5 (61.0–79.6) | 18.9 (13.7–25.1) |
| 90 | 65.6 (54.8–75.1) | 38.3 (29.5–48.0) | 49.6 (39.4–60.1) | 23.5 (17.1–31.4) | 49.9 (41.2–58.8) | 75.9 (65.7–84.0) | 87.3 (79.8–92.9) | 25.4 (18.4–34.3) |
| 95 | 79.9 (67.8–87.8) | 47.4 (35.9–59.9) | 63.7 (51.3–75.5) | 28.3 (18.9–38.3) | 62.9 (51.9–73.0) | 86.3 (78.1–92.3) | 95.1 (90.6–97.9) | 33.4 (21.3–46.5) |
| 100 | 89.3 (80.7–95.2) | 56.4 (41.7–70.2) | 75.7 (63.0–86.4) | 33.4 (20.9–47.1) | 73.6 (61.0–84.0) | 92.7 (86.6–96.7) | 98.2 (95.7–99.4) | 42.4 (26.9–59.1) |
Notes: Values are presented as % (95% confidence interval). Aging markers were defined as abnormal based on the value of the worst 5th percentile in the youngest age group (60–70 years old) (25) or an amyloid BPND equal to or above a previously defined cut point (26). The cut point value was included in the abnormal group.
BPND = nondisplaceable binding potential; CERAD = Consortium for the Establishment of a Registry for Alzheimer’s Disease; DSST = Digit Symbol Substitution Test; HGS = handgrip strength; HCV = hippocampal volume; MMSE = mini-mental state examination; WMHV = white matter hyperintensities volume.
*Assessed with CERAD memory test total score over three trials.
Figure 1.
Effect of age on aging marker abnormality. Aging markers were defined as abnormal based on the value of the worst 5th percentile in the youngest age group (60–70 years old) (25) or an amyloid BPND equal to or above a previously defined cut point (26). The cut point value was included in the abnormal group. BPND = nondisplaceable binding potential; CERAD = Consortium for the Establishment of a Registry for Alzheimer’s Disease; MMSE = mini-mental state examination; WMH = white matter hyperintensities.
Effect of Sex on Aging Marker Abnormality
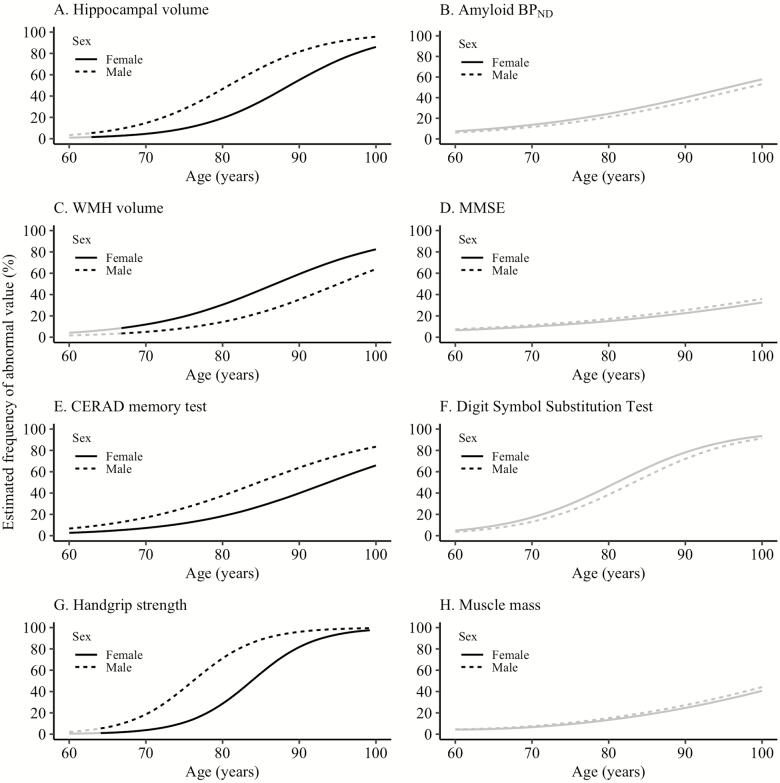
From age 62.3 years onwards, hippocampal volume abnormality was more common in males than in females (confidence interval [CI] at age 77.1 years [=mean age in the total study sample] for males: 22.5–48.5, for females: 6.8–22.0) and from age 66.2 years onwards WMH volume abnormality was more common in females than in males (CI at age 77.1 years for females: 15.2–33.0, for males: 4.9–19.3; Figure 2, Supplementary Tables S2 and S5). Across the age range, CERAD memory test abnormality was more common in males than in females (CI at age 77.1 years for males: 21.4–40.6, for females: 8.8–21.5) and handgrip strength abnormality was more common in males than in females from age 64.1–99.3 years (CI at age 77.1 years for males: 37.1–73.7, for females: 7.9–30.0). The association between age and amyloid BPND, MMSE, DSST, and muscle mass abnormality showed no differences between males and females.
Figure 2.
Effect of sex on aging marker abnormality. The black line indicates at which ages the estimated frequency of abnormality is significantly different between the two groups. Aging markers were defined as abnormal based on the value of the worst 5th percentile in the youngest age group (60–70 years old) (25) or an amyloid BPND equal to or above a previously defined cut point (26). The cut point value was included in the abnormal group. BPND = nondisplaceable binding potential; CERAD = Consortium for the Establishment of a Registry for Alzheimer’s Disease; MMSE = mini-mental state examination; WMH = white matter hyperintensities.
Effect of Educational Level on Aging Marker Abnormality
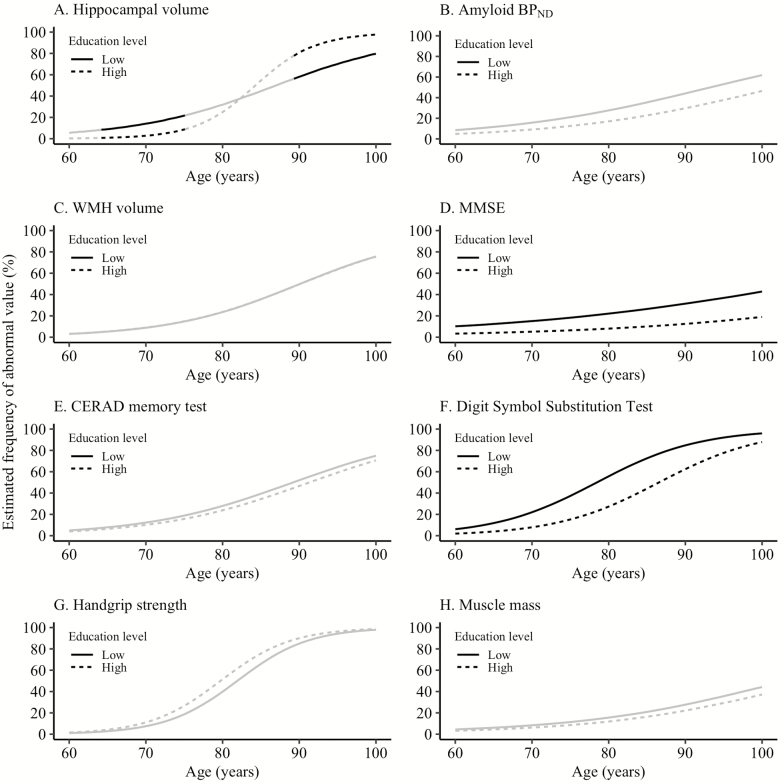
Abnormal hippocampal volume was more common in individuals with a low educational level between age 64.2 and 74.7 years but less common in individuals above age 89.0 years (p-value for interaction: <0.01; Figure 3, Supplementary Tables S3 and S6). MMSE and DSST abnormality were more common in individuals with a low compared with high educational level across the age range (MMSE CI at age 77.1 yr for individuals with a low education level: 13.0–27.8, for individuals with a high educational level: 3.6–12.5; DSST CI at age 77.1 yr for individuals with a low educational level: 34.4–56.9, for individuals with a high educational: 11.6–29.3). The association between age and amyloid BPND, WMH volume, the CERAD memory test, handgrip strength, and muscle mass abnormality did not differ between individuals with a low and high educational level.
Figure 3.
Effect of educational level on aging marker abnormality. The black line indicates at which ages the estimated frequency of abnormality is significantly different between the two groups. Aging markers were defined as abnormal based on the value of the worst 5th percentile in the youngest age group (60–70 years old) (25) or an amyloid BPND equal to or above a previously defined cut point (26). Low educational level: ≤ 10 years; high educational level: > 10 years. BPND = nondisplaceable binding potential; CERAD = Consortium for the Establishment of a Registry for Alzheimer’s Disease; MMSE = mini-mental state examination; WMH = white matter hyperintensities.
Effect of Amyloid Status on Aging Marker Abnormality
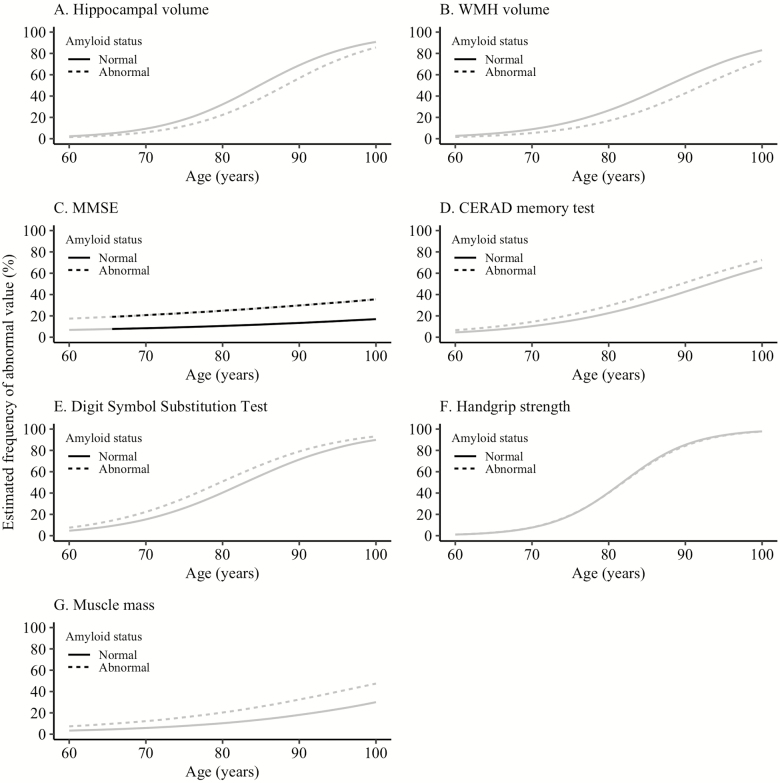
Cognitive measures were more often impaired in individuals with abnormal amyloid than in individuals with normal amyloid, although this was only statistically significant for the MMSE from age 65.2 years onwards (Figure 4, Supplementary Tables S4 and S7). The brain pathology and physical markers were not differently associated with age for individuals with normal compared with abnormal amyloid.
Figure 4.
Effect of amyloid status on aging marker abnormality. The black line indicates at which ages the estimated frequency of abnormality is significantly different between the two groups. Aging markers were defined as abnormal based on the value of the worst 5th percentile in the youngest age group (60–70 years old) (25) or an amyloid BPND equal to or above a previously defined cut point (26). BPND = nondisplaceable binding potential; CERAD = Consortium for the Establishment of a Registry for Alzheimer’s Disease; MMSE = mini-mental state examination; WMH = white matter hyperintensities.
Discussion
The prevalence of abnormality increased with age for all brain pathology cognitive and physical markers in cognitively normal individuals aged 60–102 years. The increase in prevalence of abnormal markers with age was partly dependent on sex, level of education, and amyloid aggregation. The aging pattern differed between markers of the same process, in other words, the three brain pathology markers did not show a similar aging pattern, neither did the markers measuring the cognitive or physical process. Also, there was no clear order in which markers of different processes became abnormal with higher age.
With regard to the brain pathology markers, hippocampal atrophy was almost inevitable with higher age while amyloid aggregation was not. This difference is consistent with a study performed in 1246 cognitively normal individuals aged 30–95 years, which showed that the age-related loss of hippocampal volume is to some extent independent of amyloid aggregation and might indicate a process of typical aging (30). Additionally, hippocampal atrophy at older age is probably also related to other brain pathologies than amyloid aggregation, for example, hippocampal sclerosis, argyrophilic grain disease, and vascular pathology (31,32). In the present study, WMH showed a higher prevalence of abnormality with age than amyloid aggregation, but prevalences were still lower than for hippocampal volume, which furthermore implicates that age-related hippocampal atrophy is probably driven by multiple pathologies. Additionally, the considerable number of oldest-old individuals without amyloid aggregation suggests that a subset of the oldest-old may have protective factors against amyloid aggregation, for example, the absence of the APOE-4 allele (33).
Also, the age-related change of markers measuring the cognitive and physical processes differed. With regard to the cognitive markers, processing speed showed the highest age-related decrease, followed by memory and subsequently global cognition, which is in line with previous literature indicating that memory and processing speed are the main cognitive domains affected in normal cognitive aging (34,35). A higher age-related increase of abnormal muscle strength compared with abnormal muscle mass has been shown before and suggests an important role for alterations in muscle quality to explain the age-related increase in abnormal muscle strength (36).
In this study of cognitively normal individuals, we did not find a sequence in which abnormal markers of one process proceeded abnormality of markers measuring another process. For example, abnormality of brain pathology markers did not clearly precede abnormality of the cognitive and physical markers. It might be that with older age, a composite of different brain pathologies explains the age-related increase of abnormal cognitive functioning (37), including brain pathologies that are not included in the present study, such as infarcts, tangles, arteriosclerosis, and hippocampal sclerosis.
The age-related pattern of abnormal handgrip strength seemed to precede the age-related pattern of abnormal global cognition. An earlier study that tested the temporal relationships between cognitive and physical performance also found that weaker handgrip strength at baseline was associated with steeper decline in global cognition (7,38). This suggests that for these specific markers, age-related abnormality of physical function proceeds abnormal cognitive function in a cognitively normal population.
Effect of Sex
We observed across almost the entire age range a higher prevalence of abnormal hippocampal volume and memory for males and a higher prevalence of WMH volume for females, which is in line with previous literature (1,30,39). We now show that these differences persist in cognitively normal individuals over 90 years old. The higher prevalence of handgrip strength abnormality in males compared with females aged 64–99 years is in line with one study (40) but in contrast to another (41), which may be explained by different methods to define cut points in these studies. Overall, our results indicate that the aging process is dependent on sex. Possible underlying mechanisms include differences in risk factor exposure and cognitive activities throughout the life course and hormonal effects (42,43).
The association of amyloid abnormality with age was similar for males and females across the age range, which is also in line with previous literature but in contrast to what might be expected based on the higher dementia prevalence in females than males (2,44). This discrepancy may be explained by a higher prevalence of other neuropathologies in females, leading to faster cognitive decline in females with the same amyloid burden as males (45,46). Unfortunately, our sample size was too small to formally test for a three-way interaction or stratified analysis including both amyloid status and sex. Further research with larger sample sizes will enable verification of this hypothesis. Furthermore, the higher dementia prevalence in females might also be largely explained by the longer life expectancy in females (47), which makes a similar abnormal amyloid prevalence between males and females less surprising.
Effect of educational level
We found that across the age range, individuals with a low level of education had more frequently abnormal scores for global cognition and processing speed, which is in line with previous literature (9,48). Individuals below age 75 years with a low educational level had more often hippocampal atrophy than individuals below age 75 years with a high educational level. This is in line with a previous study showing that shorter education was associated with lower hippocampal volume across the age range 17 to 87 years (49). Above age 90 years, individuals with a high educational level, however, had more often hippocampal atrophy compared with individuals with a low education level. It is possible that over age 90 years, highly educated individuals may be better able to maintain normal cognition despite hippocampal atrophy than individuals with a low level of education. Future research focusing on individuals over age 90 years with both normal and impaired cognition might clarify this aspect.
Effect of Amyloid Status
Individuals with an abnormal amyloid status had higher frequencies of abnormal global cognition and tended to have higher frequencies of abnormal memory and processing speed, although differences were subtle. The number of individuals with abnormal amyloid may have been too small to detect a statistically significant difference.
Implications
As our data show that all markers worsen with aging, this raises the question whether age-adjusted cut points should be used in daily practice. This is commonly done for cognitive tests and for the visual assessment of hippocampal volume with the medial temporal lobe atrophy (MTA) score, but not for the other aging markers. Ideally, cut points are not based on the age-related mean level of functioning, but based on their predictive value for negative outcomes. This is especially preferable in older individuals as multiple functions decline with age, potentially leading to an underestimation of impairments when cut points based on the age-related mean level of functioning are used.
Strengths and Limitations
A potential limitation of our study is that we estimated the aging pattern based on cross-sectional data. The younger individuals in our study may not survive up to high age or they will not meet the inclusion criteria when they are older, for example, because they have developed cognitive deficits. As a consequence, the effects of age we observed may have been underestimated, as the older individuals represent the group that remained normal. On the other hand, later birth cohorts show a better overall health, which might lead to overestimations in age-related changes (50). As such, more longitudinal data are needed to further study aging trajectories into very old ages in more detail. Another limitation is our definition of abnormality, which was based on the individuals aged 60–70 years (25), except for amyloid pathology. We chose this method as no formal cut point that is unrelated to age exists for the other markers. However, this method does not consider the associations with age before age 60 years and the possibility that the abnormality prevalence differs between the markers in individuals aged 60–70 years. Additionally, these cut points may not be related to negative outcomes which might reduce their clinical relevance. Furthermore, individuals had to have a certain level of physical function to be able to participate in our study which may have led to selection bias and thereby to an underestimation of abnormality prevalence. These selection criteria were the same for the individuals used to define abnormality as for the total study sample, which makes an underestimation of abnormality prevalence less likely. However, these criteria may explain the lower estimated amyloid abnormality prevalence we observed around age 80 years in our study compared with population-based cohorts (23% compared with 30%–40% with overlapping confidence intervals) (2), because cognitively normal individuals around age 80 years with abnormal amyloid show lower performance on memory tests compared with cognitively normal individuals with normal amyloid (51).
A strength of the study is that we examined brain pathology markers in combination with cognitive and physical markers over a wide age range. This is unique as earlier literature examining aging patterns of different markers, did not include these three different processes in one study. Another strength is the unique approach we used to study aging patterns of various markers which allows exploring the aging process from different angles.
Conclusion
Markers measuring brain pathology cognitive and physical processes are differently susceptible for the aging process. There was no shared aging pattern between the markers measuring the same process and, in this cohort of cognitively normal individuals, we did not find a sequence in which the brain pathology cognitive and physical processes became abnormal with age.
An important difference was found for the age-related increase of hippocampal atrophy and amyloid aggregation as hippocampal atrophy was almost inevitable with aging while amyloid aggregation was not. Important future steps to enhance our understanding of the aging process might be different approaches to define cut points, preferably based on the association with negative outcomes of the aging markers, and the exploration of the same aging patterns in the general population without excluding individuals with cognitive impairment.
Funding
This work was supported by the EU/EFPIA Innovative Medicines Initiative Joint Undertaking EMIF grant agreement no. 115372. F.B. is supported by the NIHR biomedical research center at UCLH.
Conflict of Interest
The authors have no actual or potential conflicts of interest.
Supplementary Material
References
- 1. DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26(4):491–510. doi:10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 2. Jansen WJ, Ossenkoppele R, Knol DL, et al. ; Amyloid Biomarker Study Group. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313:1924–1938. doi: 10.1001/jama.2015.4668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harada CN, Love MCN, Triebel K. Normal cognitive aging. Clin Geriatr Med. 2013;29(4):737–752. doi:10.1016/j.cger.2013.07.002.Normal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cruz-Jentoft AJ, Bahat G, Bauer JM, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2018;0:1–16. doi:10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beenakker KGM, Ling CH, Meskers CGM, et al. Patterns of muscle strength loss with age in the general population and patients with a chronic inflammatory state. Ageing Res Rev. 2010;9(4):431–436. doi:10.1016/j.arr.2010.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clouston SAP, Brewster P, Kuh D, et al. The dynamic relationship between physical function and cognition in longitudinal aging cohorts. Epidemiol Rev. 2013;35(1):33–50. doi:10.1093/epirev/mxs004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stijntjes M, Aartsen MJ, Taekema DG, et al. Temporal relationship between cognitive and physical performance in middle-aged to oldest old people. J Gerontol - Ser A Biol Sci Med Sci. 2017;72(5):662–668. doi:10.1093/gerona/glw133 [DOI] [PubMed] [Google Scholar]
- 8. Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12(4):357–367. doi:10.1016/S1474-4422(13)70044-9 [DOI] [PubMed] [Google Scholar]
- 9. van Hooren SAH, Valentijn AM, Bosma H, Ponds RWHM, van Boxtel MPJ, Jolles J. Cognitive functioning in healthy older adults aged 64–81: a cohort study into the effects of age, sex, and education. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2007;14(1):40–54. doi:10.1080/138255890969483 [DOI] [PubMed] [Google Scholar]
- 10. Konijnenberg E, Carter SF, Ten Kate M, et al. The EMIF-AD PreclinAD study: study design and baseline cohort overview. Alzheimer’s Res Ther. 2018;10(1):1–12. doi:10.1186/s13195-018-0406-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Legdeur N, Badissi M, Carter SF, et al. Resilience to cognitive impairment in the oldest-old : design of the EMIF-AD 90 + study. BMC Geriatr. 2018;18(289):1–16. doi:10.1186/s12877-018-0984-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morris J. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi:10.1212/WNL.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- 13. Morris J, Heyman A, Mohs R, et al. The consortium to establish a registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assesment of Alzheimer’s disease. Neurology. 1989;39(9):1159. doi:10.1212/WNL.39.9.1159 [DOI] [PubMed] [Google Scholar]
- 14. Aebi C. Validierung Der Neuropsychologischen Testbatterie CERAD-NP: Eine Multi-Center Studie [Validation of the CERAD Neuropsychological Assessment Battery: A Multi-Centre Study]. Basel: University of Basel; 2002. [Google Scholar]
- 15. Boomsma DI, De Geus EJC, Vink JM, et al. Netherlands twin register: from twins to twin families. Twin Res Hum Genet. 2006;9(6):849–857. doi:10.1375/183242706779462426. [DOI] [PubMed] [Google Scholar]
- 16. Holstege H, Beker N, Dijkstra T, et al. The 100-plus Study of cognitively healthy centenarians: rationale, design and cohort description. Eur J Epidemiol. 2018;3:1229–1249. doi:10.1007/s10654-018-0451-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. [DOI] [PubMed] [Google Scholar]
- 18. Sudre CH, Cardoso MJ, Bouvy WH, Biessels GJ, Barnes J, Ourselin S. Bayesian Model selection for pathological neuroimaging data applied to white matter lesion segmentation. IEEE Trans Med Imaging. 2015;34(10):2079–2102. doi:10.1109/TMI.2015.2419072 [DOI] [PubMed] [Google Scholar]
- 19. Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage. 1997;6:279–287. doi:10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- 20. Wu Y, Carson RE. Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J Cereb Blood Flow Metab. 2002;22(12):1440–1452. doi:10.1097/01.WCB.0000033967.83623.34 [DOI] [PubMed] [Google Scholar]
- 21. Tolboom N, Yaqub M, van der Flier WM, et al. Detection of alzheimer pathology in vivo using both 11C-PIB and 18F-FDDNP PET. J Nucl Med. 2009;50(2):191–197. doi:10.2967/jnumed.108.056499 [DOI] [PubMed] [Google Scholar]
- 22. Wechsler D. Wechsler adult intelligence scale. revised manual (WAIS-R).; 1981. [Google Scholar]
- 23. Reijnierse EM, de Jong N, Trappenburg MC, et al. Assessment of maximal handgrip strength: how many attempts are needed? J Cachexia Sarcopenia Muscle. 2017;8(3):466–474. doi:10.1002/jcsm.12181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ling CHY, De Craen AJM, Slagboom PE, et al. Accuracy of direct segmental multi-frequency bioimpedance analysis in the assessment of total body and segmental body composition in middle-aged adult population. Clin Nutr. 2011;30:610–615. doi:10.1016/j.clnu.2011.04.001 [DOI] [PubMed] [Google Scholar]
- 25. Jack CR Jr, Wiste HJ, Weigand SD, et al. Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimers Dement. 2017;13:205–216. doi: 10.1016/j.jalz.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Collij L, Konijnenberg E, Reimand J, et al. Assessing amyloid pathology in cognitively normal subjects using [18F]Flutemetamol PET: comparing visual reads and quantitative methods. J Nucl Med. 2018:jnumed.118.211532. doi:10.2967/jnumed.118.211532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: https://www.r-project.org/. Published 2017. [Google Scholar]
- 28. Choirat C, Honaker J, Imai K, King G, Lau O.. Zelig: Everyone’s Statistical Software. Version 5.1.6 http://zeligproject.org/. Published 2018. [Google Scholar]
- 29. Imai K, King G, Lau O. Toward a common framework for statistical analysis and development. J Comput Graph Stat. 2008;17(4):892–913. doi:10.1198/106186008X384898 [Google Scholar]
- 30. Jack CR, Wiste HJ, Weigand SD, et al. Age, sex, and APOE ϵ4 effects on memory, brain structure, and β-Amyloid across the adult life Span. JAMA Neurol. 2015;72(5):511–519. doi:10.1001/jamaneurol.2014.4821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wirth M, Villeneuve S, Haase CM, et al. Associations between Alzheimer disease biomarkers, neurodegeneration, and cognition in cognitively normal older people. JAMA Neurol. 2013;70(12):1512–1519. doi:10.1001/jamaneurol.2013.4013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barkhof F, Polvikoski TM, van Straaten ECW, et al. The significance of medial temporal lobe atrophy: a postmortem MRI study in the very old. Neurology. 2007;69(15):1521–1527. doi:10.1212/01.wnl.0000277459.83543.99 [DOI] [PubMed] [Google Scholar]
- 33. Mattsson N, Groot C, Jansen WJ, et al. Prevalence of the apolipoprotein E ε4 allele in amyloid β positive subjects across the spectrum of Alzheimer’s disease. Alzheimer’s Dement J Alzheimer’s Assoc. 2018;0(0):1–12. doi:10.1016/J.JALZ.2018.02.009 [DOI] [PubMed] [Google Scholar]
- 34. Salthouse TA. Selective review of cognitive aging. J Int Neuropsychol Soc. 2010;16:754–760. doi: 10.1017/S1355617710000706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Christensen H. What cognitive changes can be expected with normal ageing? Aust N Z J Psychiatry. 2001;35:768–775. doi: 10.1046/j.1440-1614.2001.00966.x [DOI] [PubMed] [Google Scholar]
- 36. Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059 [DOI] [PubMed] [Google Scholar]
- 37. Power MC, Mormino E, Soldan A, et al. Combined neuropathological pathways account for age-related risk of dementia. 2018:1–13. doi:10.1002/ana.25246. [DOI] [PMC free article] [PubMed]
- 38. Taekema DG, Ling CH, Kurrle SE, et al. Temporal relationship between handgrip strength and cognitive performance in oldest old people. Age Ageing. 2012;41:506–512. doi: 10.1093/ageing/afs013 [DOI] [PubMed] [Google Scholar]
- 39. Welsh KA, Butters N, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology. 1994;44(4):609–614. doi: 10.1212/WNL.44.4.609 [DOI] [PubMed] [Google Scholar]
- 40. Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2015;95(5):1851–1860. doi:10.1152/japplphysiol.00246.2003 [DOI] [PubMed] [Google Scholar]
- 41. Dodds RM, Syddall HE, Cooper R, et al. Grip strength across the life course: normative data from twelve British studies. PLoS One. 2014;9:e113637. doi: 10.1371/journal.pone.0113637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin Epidemiol. 2014;6:37–48. doi: 10.2147/CLEP.S37929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Taekema DG, Ling CH, Blauw GJ, et al. Circulating levels of IGF1 are associated with muscle strength in middle-aged- and oldest-old women. Eur J Endocrinol. 2011;164:189–196. doi: 10.1530/EJE-10-0703 [DOI] [PubMed] [Google Scholar]
- 44. Buckley RF, Mormino EC, Amariglio RE, et al. ; Alzheimer’s Disease Neuroimaging Initiative; Australian Imaging, Biomarker and Lifestyle study of ageing; Harvard Aging Brain Study. Sex, amyloid, and APOE ε4 and risk of cognitive decline in preclinical Alzheimer’s disease: Findings from three well-characterized cohorts. Alzheimers Dement. 2018;14:1193–1203. doi: 10.1016/j.jalz.2018.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mofrad RB, Flier WM Van Der. Nature and implications of sex differences in AD pathology. Nat Rev Neurol. 2018; 15. doi: 10.1038/s41582-018-0115-7 [DOI] [PubMed] [Google Scholar]
- 46. Oveisgharan S, Arvanitakis Z, Yu L, Farfel J, Schneider JA, Bennett DA. Sex differences in Alzheimer’s disease and common neuropathologies of aging. Acta Neuropathol. 2018;136:887–900. doi: 10.1007/s00401-018-1920-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chêne G, Beiser A, Au R, et al. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimers Dement. 2015;11:310–320. doi: 10.1016/j.jalz.2013.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269:2386–2391. [PubMed] [Google Scholar]
- 49. Noble KG, Grieve SM, Korgaonkar MS, et al. Hippocampal volume varies with educational attainment across the life-span. Front Hum Neurosci. 2012;6(November):1–10. doi:10.3389/fnhum.2012.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Christensen K, Thinggaard M, Oksuzyan A, et al. Physical and cognitive functioning of people older than 90 years: a comparison of two Danish cohorts born 10 years apart. Lancet. 2013;382:1507–1513. doi: 10.1016/S0140-6736(13)60777-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jansen WJ, Ossenkoppele R, Tijms BM, et al. ; Amyloid Biomarker Study Group. Association of cerebral amyloid-β aggregation with cognitive functioning in persons without dementia. JAMA Psychiatry. 2018;75:84–95. doi: 10.1001/jamapsychiatry.2017.3391 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.