Abstract
Background:
Dysregulated bone turnover is an important clinical manifestation of multiple myeloma (MM), and 30% of patients present with hypercalcemia. Serum calcium levels are routinely monitored using total calcium measurements corrected for albumin. However, myeloma-related paraproteins may bind calcium, confounding these measurements.
Patients and Methods:
We retrospectively analyzed correlation between corrected calcium and ionized calcium in a sample of patients with MM and a control sample of patients with breast or non-small cell lung cancers (n = 200). Multiple linear regression was used to identify variables affecting corrected calcium measurements.
Results:
Correlation between corrected calcium and ionized calcium was stronger in the control group compared to the MM group (Spearman correlation coefficient 0.85 versus 0.76, respectively). Sensitivity of corrected calcium in identifying hypercalcemia defined by elevated ionized calcium was 36% in patients with MM and 76% in the control group. Multiple linear regression did not reveal variables significantly influencing corrected calcium in the MM group, although serum paraprotein trended toward significance (p = 0.09).
Conclusion:
Ionized calcium may be better than corrected calcium for detecting hypercalcemia in patients with MM. Additional analyses are needed to better quantify the clinical impact of paraprotein calcium-binding.
Keywords: hypercalcemia of malignancy, oncologic emergency, myeloma bone disease, M-protein, paraprotein
Microabstract
Hypercalcemia is a common manifestation of multiple myeloma (MM). Myeloma paraproteins may confound albumin-corrected total calcium. We retrospectively analyzed corrected and ionized calcium in 100 patients with MM and 100 patients with other malignancies (control). Corrected calcium to ionized calcium correlation was stronger in the control group. Ionized calcium may be better than corrected calcium for detecting hypercalcemia in MM.
Introduction
Multiple myeloma (MM) is a hematologic malignancy of plasma cell proliferation, accounting for 18% of hematologic malignancies and 1.8% of all cancers [1]. Dysregulated bone turnover is a hallmark of MM, and approximately 30% of patients present with hypercalcemia [2]. The complex pathogenesis for this process is driven by interactions between MM cells and the bone marrow microenvironment, leading to the secretion of a variety of osteoclast-activating and osteoblast-inhibiting factors with resultant disproportionate bone resorption [3, 4].
Hypercalcemia accompanied by signs of plasma cell-related bone disease is a diagnostic marker of MM [5, 6, 7]. As many patients with MM present with both hypercalcemia and hypoalbuminemia [8, 9], and given albumin’s binding capacity for calcium [8, 10], the National Comprehensive Cancer Network recommends the use of total serum calcium measurements corrected for serum albumin concentrations in this population [5]. Since free calcium concentrations represent the physiologically-active proportion of calcium in the serum [11, 12, 13], such correction may better represent the state of a patient’s calcium homeostasis. The modified Orrell equation, referred to commonly in the United States as the corrected calcium equation, applies a correction factor to account for the binding of calcium to albumin, based on an estimated 0.8mg/dL of calcium bound to each 1g/dL of albumin in serum [14].
While its use in clinical practice is widespread [15, 16], the modified Orrell equation is one of many that have been proposed for total calcium correction [17]. These formulas have not been assessed for precision or reliability, and in some populations have shown poor ability to detect calcium disturbances when compared to ionized calcium measurements [10, 18, 19], particularly in patients with renal dysfunction [15]. Additionally, in patients with MM, paraproteins secreted by malignant plasma cells are known to have calcium-binding properties [20, 21, 22, 23, 24]. Calcium binding by paraproteins may confound corrected calcium calculations, as the correction factor in the modified Orrell equation does not account for calcium binding by other serum proteins. Using corrected calcium for monitoring calcium levels in patients with MM may therefore result in underdiagnosis of hypercalcemia if not confirmed by ionized calcium measurements. In this retrospective study, we investigated whether there is correlation between corrected calcium and ionized calcium measurements in a sample of patients with MM and a sample of patients with hypercalcemia related to other malignancies.
Methods
Study design and enrollment
A single-center retrospective cohort study of adults with hypercalcemia and MM, breast cancer, or non-small cell lung cancer (NSCLC) treated at the University of Texas MD Anderson Cancer Center between January 2005 and December 2015 was performed with approval of the institutional review board. Breast cancer and NSCLC were chosen for inclusion in a pooled control cohort based on similar incidence of hypercalcemia to patients with MM [25, 26] to indirectly compare patients with and without serum paraprotein calcium-binding effect.
A list of patients with MM, breast cancer, or NSCLC was generated based on the dates above. Screening and collection of demographics and clinical data occurred via chart review. Adults with a diagnosis of MM, breast cancer, or NSCLC were included if there were measurements of total serum calcium, serum albumin, and ionized calcium collected within a single 24-hour period. Patients were excluded if there was documented administration of any calcium-modifying agent, including calcitonin, bisphosphonates, denosumab, one liter or more of normal saline, or any anticancer agent (including dexamethasone) within 5 drug half-lives prior to laboratory measurements [27, 28, 29, 30, 31], or any anticancer therapy (including dexamethasone) within 30 days prior to laboratory measurements. Target enrollment for each group was 50 patients with hypercalcemia and 50 patients with normal or low serum calcium, for a total of 200 patients. Hypercalcemia was defined as total serum calcium or corrected calcium > 11mg/dL or ionized calcium ≥ 5.33mg/dL (1.33mmol/L). Low serum calcium was defined as total serum calcium or corrected calcium < 8.4mg/dL or ionized calcium < 4.53mg/dL (1.13mmol/L). The cutoff values defining hypercalcemia for total and corrected calcium were based on the International Myeloma Working Group guidelines [7]; other cutoffs were based on institutional laboratory reference ranges. Calcium measurements during the study period were performed using ABL 90 Flex analyzers (Radiometer Medical ApS, Brønshøj, Denmark).
Outcomes and statistical analysis
The primary objective was to determine the correlation between corrected calcium and ionized calcium in each group using the Spearman correlation coefficient. To illustrate correlation, scatterplots of calcium measurements were fitted with locally-weighted smoothing regression curves [32]. Secondary analyses included sensitivity and specificity of corrected calcium in detecting hypercalcemia using the ranges defined above, where a true hypercalcemia was defined by elevated ionized calcium ≥ 5.33mg/dL. Additionally, multiple linear regression was performed to assess the influence of several myeloma-related variables (serum albumin, phosphorus, creatinine, paraprotein, free kappa light chains, free lambda light chains, and MM immunoglobulin type) on corrected calcium measurements. Continuous variables were compared between patient groups via two-sample t-test (or Mann-Whitney test) and discrete variables were evaluated for association via Fisher’s exact test (or Chi-square test). Statistical significance was defined as p-value < 0.05. Statistical software SAS 9.3 (SAS, Cary, NC), R, and S-Plus 8.2 (TIBCO Software Inc., Palo Alto, CA) were used for the analyses.
Results
Screening identified 100 patients per group meeting criteria for analysis. Each group included 50 patients with hypercalcemia and 50 normocalcemic patients. Patient demographics and laboratory measurements are presented in Table 1. Statistically significant differences were noted between patients in the MM and control groups in sex (46% female vs 76%, respectively), body mass index (28kg/m2 vs 26kg/m2), serum creatinine (1mg/dL vs 0.9mg/dL), serum phosphorus (3.7mg/dL vs 3.4mg/dL), and serum albumin (3.8g/dL vs 3.25g/dL). Measurements of serum paraprotein in patients with MM drawn within 60 days of calcium measurements were available for 78% of patients; median serum paraprotein was 0.5g/dL.
Table 1.
Patient demographics and laboratory measurements.*
| Multiple myeloma n = 100 | Control n = 100 | p-value | |
|---|---|---|---|
| Age (years) | 61 (55-69) | 65 (54-72) | 0.09 |
| Female, n (%) | 46 (46) | 76 (76) | < 0.01 |
| BMI (kg/m2) | 28 (25-32) | 26 (22-30) | 0.02 |
| Creatinine (mg/dL)** | 1 (0.8-1.5) | 0.9 (0.7-1.1) | < 0.01 |
| Phosphorus (mg/dL)**† | 3.7 (3-4.4) | 3.4 (2.7-4) | 0.047 |
| Albumin (g/dL)** | 3.8 (3.3-4.1) | 3.3 (2.7-3.9) | < 0.01 |
| Ionized calcium | 5.17 (4.81-5.45) mg/dL | 5.09 (4.45-5.69) mg/dL | 0.64 |
| 1.29 (1.2-1.36) mmol/L | 1.27 (1.11-1.42) mmol/L | ||
| Corrected calcium (mg/dL) | 9.8 (9.1-10.8) | 9.8 (9.1-11.6) | 0.29 |
| Paraprotein (g/dL)ठ| 0.5 (0.2-1.6) | N/A | N/A |
| Free kappa light chains (mg/dL)‡ | 1.27 (0.5-2.95) | N/A | N/A |
| Free lambda light chains (mg/dL)‡ | 1.58 (0.9-10) | N/A | N/A |
IQR, interquartile range; BMI, body mass index; N/A, not applicable
Median (IQR), unless noted otherwise
Measured within 24 hours of calcium measurements.
Data available for 70% of multiple myeloma group patients and 97% of control group patients.
Scatterplots of corrected calcium and ionized calcium with locally-weighted smoothing regression are shown in Figure 1. The Spearman correlation coefficient was 0.76 (p < 0.001 for testing if the coefficient is significantly different from 0) in the MM group and 0.85 (p < 0.001) in the control group. The sensitivity of corrected calcium in identifying ionized calcium-defined hypercalcemia was 36% and 72% in the MM and control groups, respectively. The specificity was 91% and 88% in the MM and control groups, respectively. Multiple linear regression did not identify any variable significantly correlating with corrected calcium in either group. P-values for all included variables were > 0.1 except for paraprotein in the MM model (p = 0.09) and phosphorus in the control model (p = 0.05).
Figure 1.
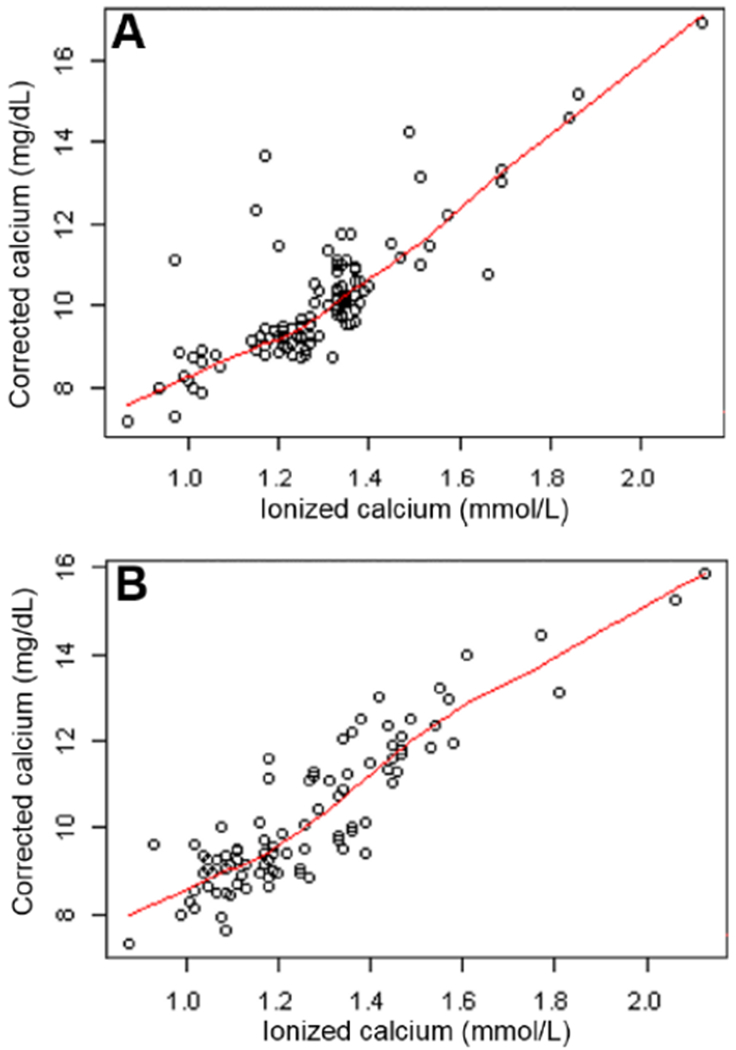
Corrected calcium vs ionized calcium in multiple myeloma (A) and control (B) groups with locally weighted smoothing regression.
Discussion
Known calcium-binding properties of myelomatous plasma cell-derived paraproteins may confound calculation of albumin-corrected serum calcium [14, 20, 21, 22, 23, 24]. It is also possible that differences in the paraproteins secreted by each patient produce variable calcium-binding properties, further limiting the utility of calcium correction formulas applied uniformly to patients with MM. Given the high incidence of hypercalcemia in MM [2], costs and potential toxicities accompanying its management [33], and widespread use of corrected calcium for monitoring [15, 16], characterizing the effect of paraproteins on calcium correction may have important implications for clinical practice. In this study, the correlation coefficient between corrected calcium and ionized calcium was higher in patients with breast cancer or NSCLC (0.85) compared to those with MM (0.76), suggesting that corrected calcium may be less reliable for monitoring calcium levels in patients with MM. Additionally, the calculated sensitivity of corrected calcium in detecting ionized calcium-defined hypercalcemia was significantly lower in the MM group (36%) compared to the control group (72%), indicating that corrected calcium may falsely indicate normocalcemia more frequently in patients with MM. In the multiple linear regression models, serum paraprotein trended toward a significant impact on corrected calcium calculations in the MM group (p = 0.09), but no variables demonstrated statistically significant impact.
The study sample characteristics limit interpretation of these results. Surprisingly, most of the MM group appeared to have near-normal serum albumin (median 3.8g/dL) [34]. The relatively low average serum paraprotein concentrations in the MM group (median 0.5g/dL vs mean 1.39g/dL; interquartile range 0.20 to 1.60g/dL vs overall range 0.10 to 8.10g/dL) also indicate most patients with MM had relatively low disease burden. This was likely due to the strict exclusion criteria utilized, as patients with higher serum paraprotein levels may have been more likely to have recently received exclusionary medications. This is corroborated by the fact that 22% of patients with MM did not have paraprotein measured within 60 days of the index calcium measurements, indicating calcium levels and presentation did not warrant disease re-evaluation in these patients. It is possible that the effect size could be greater between calculated corrected calcium and ionized calcium in patients with more advanced disease and higher serum paraprotein concentrations.
Additionally, most patients with hypercalcemia had relatively mild serum calcium elevations [35]. In the MM group, 75% of patients had an ionized calcium value of 5.45mg/dL (1.36mmol/L) or lower, while 75% of control group patients had an ionized calcium value of 5.69mg/dL (1.42mmol/L) or lower. This may also have been due to treatment-related exclusion criteria. Additionally, as data were collected at a large referral center, patients may have received antihypercalcemic or antimyeloma therapy prior to presentation to our institution, although it should be noted that paraprotein clearance takes weeks despite immediate reduction of plasma cell burden with treatment [36]. The difference between corrected calcium and ionized calcium may be more pronounced in patients with more severe hypercalcemia. While the calculated sensitivity of corrected calcium was low in the MM group, this may have been influenced by the lack of equivalence between the corrected calcium and ionized calcium cutoffs used to define hypercalcemia (e.g., ionized calcium of 5.33mg/dL does not necessarily correspond to corrected calcium of 11mg/dL).
There are several additional limitations that must be considered. This was a retrospective analysis of a relatively small sample of patients treated at a single institution, limiting generalizability. Additionally, a consecutive sampling method was used. Substantial changes in clinical oncology practice pertaining to the disease states included in this study occurred over the period during which patients presented, which may have affected the incidence, presentation, and management of hypercalcemia in these patients.
The above limitations identify directions for future research. Similar analyses should be performed in patients stratified by calcium status (e.g., comparing patients with MM or breast or non-small cell lung cancers with hypercalcemia only) and in a case-control-matched patient sample. Given the trend toward significant impact of paraprotein on corrected calcium, prospective analysis of this effect in patients with more advanced disease and higher serum paraprotein concentrations may be of particular interest. In addition to their clinical utility, these analyses may be useful in identifying cost-savings opportunities based on the elimination of unnecessary or redundant laboratory measurements.
Conclusion
Ionized calcium may be better than corrected calcium for detecting hypercalcemia in patients with MM. However, additional analyses are needed to better understand the clinical implications of myeloma-related factors, such as paraprotein calcium binding.
Clinical Practice Points.
Hypercalcemia is a common manifestation of newly-diagnosed and relapsed multiple myeloma (MM). In MM and other disease states, total serum calcium measurements are commonly corrected for binding by serum albumin. Myeloma-related paraproteins are known to bind calcium and may confound these calcium corrections, but the clinical relevance of this phenomenon is unknown. In this retrospective study, we found that corrected calcium and the gold standard ionized calcium were better correlated in a control group (n =100) of patients with breast cancer or non-small cell lung cancer compared to a group of patients with MM (n = 100). Additionally, the sensitivity of corrected calcium in detecting hypercalcemia defined by elevated ionized calcium was low in patients with MM compared to the control group. While multiple linear regression did not reveal specific factors affecting corrected calcium, this effect may be due to paraprotein calcium binding. These data suggest ionized calcium may be better than corrected calcium for identifying hypercalcemia in patients with MM, and have identified directions for future research. In particular, analysis of corrected calcium performance in patients with MM with significant disease burden is warranted.
Acknowledgments
Funding
This work was supported by the National Institutes of Health/National Cancer Institute under award number P30CA016672 and the Biostatistics Resource Group of The University of Texas MD Anderson Cancer Center.
Conflicts of Interest
HCL received research funding and consultancy fees from Amgen Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019. January;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Smith D, Yong K. Multiple myeloma. BMJ. 2013. June 26;346:f3863. doi: 10.1136/bmj.f3863. [DOI] [PubMed] [Google Scholar]
- 3.Raje N, Roodman GD. Advances in the biology and treatment of bone disease in multiple myeloma. Clin Cancer Res. 2011. March 15;17(6):1278–86. doi: 10.1158/1078-0432.CCR-10-1804. [DOI] [PubMed] [Google Scholar]
- 4.Xi H, An R, Li L, et al. Myeloma bone disease: Progress in pathogenesis. Prog Biophys Mol Biol. 2016. November;122(2):149–155. doi: 10.1016/j.pbiomolbio.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Kumar SK, Callander NS, Alsina M, et al. Multiple Myeloma, Version 3.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017. February;15(2):230–269. [DOI] [PubMed] [Google Scholar]
- 6.Moreau P, San Miguel J, Sonneveld P, et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;0:1–11. [DOI] [PubMed] [Google Scholar]
- 7.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014. November;15(12):e538–48. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 8.Chng WJ, Dispenzieri A, Chim CS, et al. IMWG consensus on risk stratification in multiple myeloma. Leukemia. 2014. February;28(2):269–77. doi: 10.1038/leu.2013.247. [DOI] [PubMed] [Google Scholar]
- 9.Goldner W Cancer-Related Hypercalcemia. J Oncol Pract. 2016. May;12(5):426–32. doi: 10.1200/JOP.2016.011155. [DOI] [PubMed] [Google Scholar]
- 10.Bushinsky DA, Monk RD. Electrolyte quintet: Calcium. Lancet. 1998. July 25;352(9124):306–11. [DOI] [PubMed] [Google Scholar]
- 11.McLean F, Hastings A. A biological method for the estimation of calcium ion concentration. J Biol Chem. 1934;107:337–50. [Google Scholar]
- 12.McLean F, Hastings A. The state of calcium in the fluids of the body. I. The conditions affecting the ionization of calcium. J Biol Chem. 1935;108:285–322. [Google Scholar]
- 13.Moore EW. Ionized calcium in normal serum, ultrafiltrates, and whole blood determined by ion-exchange electrodes. J Clin Invest. 1970. February;49(2):318–34. doi: 10.1172/JCI106241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Endres DB, Rude RK. Mineral and bone metabolism In: Burtis CA, Ashwood ER, editors. Tietz Textbok of Clinical Chemistry. Philadelphia: W. B. Saunders Company; 1999. p. 1395–1406. [Google Scholar]
- 15.Lian IA, Asberg A. Should total calcium be adjusted for albumin? A retrospective observational study of laboratory data from central Norway. BMJ Open. 2018. April 7;8(4):e017703. doi: 10.1136/bmjopen-2017-017703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hannan FM, Thakker RV. Investigating hypocalcaemia. BMJ. 2013. May 9;346:f2213. doi: 10.1136/bmj.f2213. [DOI] [PubMed] [Google Scholar]
- 17.Ladenson JH, Lewis JW, Boyd JC. Failure of total calcium corrected for protein, albumin, and pH to correctly assess free calcium status. J Clin Endocrinol Metab. 1978. June;46(6):986–93. doi: 10.1210/jcem-46-6-986. [DOI] [PubMed] [Google Scholar]
- 18.Ladenson JH, Lewis JW, McDonald JM, et al. Relationship of free and total calcium in hypercalcemic conditions. J Clin Endocrinol Metab. 1979. March;48(3):393–7. doi: 10.1210/jcem-48-3-393. [DOI] [PubMed] [Google Scholar]
- 19.Bowers GN Jr., Brassard C, Sena SF. Measurement of ionized calcium in serum with ion-selective electrodes: a mature technology that can meet the daily service needs. Clin Chem. 1986. August;32(8):1437–47. [PubMed] [Google Scholar]
- 20.Rawson AJ, Sunderman FW. Studies in serum electrolytes; the calcium-binding property of the serum proteins (multiple myeloma, lymphogranuloma venereum and sarcoidosis. J Clin Invest. 1948. January;27(1):82–90. [PubMed] [Google Scholar]
- 21.Annesley TM, Burritt MF, Kyle RA. Artifactual hypercalcemia in multiple myeloma. Mayo Clinic proceedings. 1982. September;57(9):572–5. [PubMed] [Google Scholar]
- 22.Lindgarde F, Zettervall O. Hypercalcemia and normal ionized serum calcium in a case of myelomatosis. Ann Intern Med. 1973. March;78(3):396–9. [DOI] [PubMed] [Google Scholar]
- 23.Merlini G, Fitzpatrick LA, Siris ES, et al. A human myeloma immunoglobulin G binding four moles of calcium associated with asymptomatic hypercalcemia. J Clin Immunol. 1984. May;4(3):185–96. [DOI] [PubMed] [Google Scholar]
- 24.Pearce CJ, Hine TJ, Peek K. Hypercalcaemia due to calcium binding by a polymeric IgA kappa-paraprotein. Ann Clin Biochem. 1991. May;28 ( Pt 3):229–34. doi: 10.1177/000456329102800305. [DOI] [PubMed] [Google Scholar]
- 25.Grill V, Martin TJ. Hypercalcemia of malignancy. Rev Endocr Metab Disord. 2000. November;1(4):253–63. [DOI] [PubMed] [Google Scholar]
- 26.Strewler GJ, Nissenson RA. Hypercalcemia in malignancy. West J Med. 1990. December;153(6):635–40. [PMC free article] [PubMed] [Google Scholar]
- 27.MIACALCIN- calcitonin salmon injection, solution. Canonsburg, PA: Mylan Institutional LLC; 2016. [Google Scholar]
- 28.BONIVA- ibandronate sodium injection, solution. San Francisco, CA: Genentech, Inc.; 2016. [Google Scholar]
- 29.PAMIDRONATE DISODIUM- pamidronate disodium injection, solution. Lake Forest, IL: Hospira Worldwide, Inc.; 2014. [Google Scholar]
- 30.ZOLEDRONIC ACID- zoledronic acid injection, solution. Eatontown, NJ: Heritage Pharmaceuticals, Inc.; 2016. [Google Scholar]
- 31.XGEVA- denosumab injection. Thousand Oaks, CA: Amgen, Inc.; 2016. [Google Scholar]
- 32.Cleveland W, Devlin S. Locally weighted regression: an approach to regression analysis by local fitting. J Am Stat Assoc. 1988;83(403):596–610. [Google Scholar]
- 33.Zagzag J, Hu MI, Fisher SB, et al. Hypercalcemia and cancer: Differential diagnosis and treatment. CA Cancer J Clin. 2018. September;68(5):377–386. doi: 10.3322/caac.21489. [DOI] [PubMed] [Google Scholar]
- 34.Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J Clin Oncol. 2015. September 10;33(26):2863–9. doi: 10.1200/JCO.2015.61.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart AF. Clinical practice. Hypercalcemia associated with cancer. N Engl J Med. 2005. January 27;352(4):373–9. doi: 10.1056/NEJMcp042806. [DOI] [PubMed] [Google Scholar]
- 36.Mills JR, Barnidge DR, Dispenzieri A, et al. High sensitivity blood-based M-protein detection in sCR patients with multiple myeloma. Blood Cancer J. 2017. August 25;7(8):e590. doi: 10.1038/bcj.2017.75. [DOI] [PMC free article] [PubMed] [Google Scholar]


