Summary
DNA methyltransferase 3A (DNMT3A) is the most commonly mutated gene in clonal hematopoiesis (CH). Somatic DNMT3A mutations arise in hematopoietic stem cells (HSCs) many years before malignancies develop, but difficulties in comparing their impact before malignancy with wild-type cells have limited understanding of their contributions to transformation. To circumvent this limitation, we derived normal and DNMT3A-mutant lymphoblastoid cell lines from a germline mosaic individual in whom these cells co-existed for nearly six decades. Mutant cells dominated the blood system but not other tissues. Deep sequencing revealed similar mutational burdens and signatures in normal and mutant clones, while epigenetic profiling uncovered focal erosion of DNA methylation at oncogenic regulatory regions in mutant clones. These regions overlapped with those sensitive to DNMT3A loss after DNMT3A ablation in HSCs, and in leukemia samples. These results suggest DNMT3A maintains a conserved DNA methylation pattern whose erosion provides a distinct competitive advantage to hematopoietic cells.
eTOC
A human germline-mosaic for WT and DNMT3A-mutant cells reveals a marked advantage for DNMT3A mutant cells in the hematopoietic system compared to epithelial cells. Deep sequencing suggests very early tissue advantage driven by DNA methylation loss at conserved loci rather than increased mutation burden.
INTRODUCTION
DNA Methyltransferase 3A (DNMT3A) is a critical tumor suppressor in the hematopoietic system (Ley et al., 2010; Yang et al., 2015), mutations in which are found in malignancies of nearly every hematopoietic lineage. Study of patients with acute myeloid leukemia (AML) have shown that DNMT3A mutation can arise decades before the malignancy in hematopoietic stem cells (HSCs) creating a pre-leukemic pool (Jan et al., 2012; Shlush et al., 2017). Murine studies have shown that Dnmt3a−/− HSCs have enhanced self-renewal capacity (Challen et al., 2011; Challen et al., 2014; Jeong et al., 2018), providing fertile ground for second pro-oncogenic hits that drive frank malignancy (Brunetti et al., 2017).
Somatic DNMT3A mutations are also the most common driver of age-related clonal hematopoiesis (CH), in which specific HSC clones dominate blood. CH is associated with a greatly increased risk of hematologic malignancy and all-cause mortality (Jaiswal and Libby, 2020; Luis et al., 2019). CH is thought to represent decades-long competition between HSC clones bearing different somatic mutations and WT clones, which increasingly manifests with age.
Other tissues also experience clonal competition among stem and progenitor cells. In skin and esophagus, these clones are spatially constrained and many clones simultaneous expand (Martincorena et al., 2018; Martincorena et al., 2015; Yokoyama et al., 2019. Importantly, mutations in a limited set of genes drive clonal competition in different tissues. While common in hematologic malignances and CH, DNMT3A mutations are essentially absent from aging skin and esophagus as well as associated malignancies; instead, TP53 and NOTCH mutations dominate, indicating context specificity of the advantage conferred by mutations.
While clonal competition is increasingly recognized as a key feature of normal aging, it also likely occurs earlier in life. The breadth of mosaicism across tissues should reflect that stage of development when mutations arise (Freed et al., 2014). Whether particular mutations confer an advantage within specific tissues is unknown, nor are the mechanisms of clonal dominance.
Here, we explore the advantages conferred by mutations in DNMT3A. Utilizing multiple clones from an individual who is a life-long mosaic for DNMT3A-mutant and WT cells, we examined the long-term impact of DNMT3A mutations across lineages, offering insights into the mechanisms of clonal competition in multiple tissues.
RESULTS
Lifelong somatic mosaicism with DNMT3A and WT cells in an adult
The mosaic individual was identified because 4 of his 14 offspring were diagnosed with the dominant Tatton-Brown-Rahmen syndrome (TBRS) characterized by overgrowth and developmental delay (Tatton-Brown et al., 2014; Xin et al., 2017). The 4 affected offspring all had classic features of TBRS and a heterozygous DNMT3AR771Q mutation, while other siblings had two WT alleles. The DNMT3AR771Q mutation, which falls in the catalytic methyltransferase domain, has been observed in leukemia (Hou et al., 2012; Ribeiro et al., 2012) as well as clonal hematopoiesis (Watson et al., 2020) and thus is likely pathogenic. The dominant association of TBRS and the allelic segregation pattern suggested the individual was a somatic mosaic. He was born in 1955, has normal blood counts, does not present with overgrowth (height 5’8; 32% percentile/weight 258 lbs; 90% percentile) or facial features associated with TBRS (Supplementary Fig. 1A- and B (Xin et al., 2017)).
Expansion of DNMT3A mutant cells is unique to the blood lineage
Given the role of DNMT3A in regulation of stem cell differentiation and the prevalence of mutations in the hematopoietic lineage (Jaiswal et al., 2014; Sanders et al., 2018), we explored whether DNMT3A-mutant cells would have similar advantages in the blood and other lineages in this individual. We evaluated the level of DNMT3AR771Q mosaicism in peripheral blood (PB) collected on three separate occasions, in lymphoblastoid cells (LCLs) generated from isolated B-cells, and in other available tissues using deep sequencing of a PCR-amplified region. Remarkably, almost 100% of the blood cells from this individual were DNMT3AR771Q/+ (variant allele frequency (VAF) of nearly 0.5) across all available time points covering a period of 7 years. In contrast, the proportion of mutant cells was substantially lower in epidermal cells derived from eyebrow hair bulbs (0.022%; VAF: 0.011), saliva (8%; VAF: 0.04), and urine (20%; VAF: 0.1; Fig. 1A), all of which primarily contain epithelial cells (immune cell contamination cannot be completely excluded). In the LCL pool, the VAF, at 0.35, was lower than the PB. Since B-lymphocytes are the source of the LCLs, they may represent cells that developed in the late teens or early twenties. Therefore, the lower VAF in the LCL pool compared to PB suggests that the ratios of WT/mutant were different when the individual was younger (Lee-Six et al., 2018). We also used the proportion of offspring carrying the DNMT3A mutant allele to estimate the level of germline mosaicism (acknowledging the caveat of a small sample size). As one heterozygous mutant spermatogonial stem cell (diploid) undergoes two sequential meiotic divisions to form 2 wildtype (WT) and 2 mutant spermatozoa (haploid), we deduce that 4 of 14 total offspring affected (Supplementary Fig. 1A–B and (Xin et al., 2017)) indicates potential cellular mosaicism of 57% in the individual’s germline (equivalent to a VAF of 0.29; with the caveat of small sample size). The observation that epithelial, hematopoietic, and germ cells, which arise from different germ layers, all harbor DNMT3A-mutant cells indicates that these lineages shared a common ancestor, but expansion mainly occurred in the blood.
Figure 1. DNMT3A mutant cells dominates the blood without increase in mutation number or spectrum.
(A) Variant allele frequency (VAF) of DNMT3A c.2312G>A substitution (DNMT3AR771Q) measured by amplicon sequencing. 3 separate peripheral blood measurements were taken at the indicated years. (B) Summary of the number of base substitutions (covered ≥3X) for WT and LCL clones (C) Mutational signatures for WT and MUT clones that comprised at least 10% of the total signature classification. (D) Top: phylogenetic tree of the LCL clones constructed by identifying shared mutations. Branch lengths are proportional to the number of somatic mutations unique to each clone. DNMT3A-MUT clones are red. Bottom: zoom in of the x-axis (E) Phylogenetic tree of the LCL clones reflecting their representation in the PB with the VAF of key mutations indicated on the branches.
DNMT3A mutation does not increase the burden or spectrum of mutations
In CH, DNMT3A mutant cells are disproportionately represented in the blood, but the timing of mutation acquisition cannot be determined, impeding comparisons of WT and mutant cells. Here, WT and mutant clones from a life-long mosaic offered a unique opportunity for such analysis. From the LCL pool, we isolated multiple WT and DNMT3AR771Q/+ clones (hereafter DNMT3A-MUT) and characterized in depth 3 WT and 5 mutant clones.
We first considered the possibility that the tumor suppressor activity of DNMT3A may, like that of TP53, derive in part from an ability to suppress mutations, as suggested for the common AML-associated DNMT3AR882 mutation (Guryanova et al., 2016), and for TET2, which is involved in demethylation (Pan et al., 2017; Yang et al., 2015). We subjected the LCL clones to whole genome sequencing (WGS), identifying base substitutions, insertions and deletions and compared the number of lesions. The total mutation burden was similar across WT and mutant clones (sampling variants > 0.1 VAF). While the total number of mutations in myeloid cells from individuals of similar ages is expected to be around 1000 (~15 mutations per year x 60 years (Lee-Six et al., 2018)), the LCL clones exhibited roughly double that number, consistent with their B-cell origin in which activation-induced deaminase (AID) activity leads to increased mutational burden (Fig. 1B and Supplemental Fig. 1D) (Zhang et al., 2019).
Different mutational processes generate distinct signatures (Chalmers et al., 2017); therefore, we considered the possibility that DNMT3A mutation could alter mutation type, even in the absence of an increased mutation rate. We observed similar mutational signatures in both WT and mutant cells, although we cannot preclude that with more clones some signatures would have shown differences. Specifically, we found signatures associated with normal aging (1 and 5) (Alexandrov et al., 2015), and signature 9, associated with AID activity in B-cells, the origin for LCLs. Finally, we observed enrichment of mutational signatures 8 and 16, patterns with unknown etiology frequently observed in cancer (Fig. 1C and Supplemental Fig. 1E–F). Together these data indicate that increased mutagenicity is unlikely to be a driving force in DNMT3A-mutant malignancies.
DNMT3A-mutant cells evolved polyclonally and dominate PB without reaching fixation
Every cell division generates 1–2 de novo mutations and each mutation can serve as a barcode enabling inferences regarding lineal relationships between cells. The WGS of WT and mutant LCL clones thus enable the distinction of mutations shared and private between LCL clones serving as building blocks to disentangle their developmental relationships. Our phylogenetic tree revealed two distinct lineages of WT and mutant clones. The branch lengths indicate the approximate timing of clonal divergence, based on the number of mutations under assumptions of constant mutation rates; short sub-branching indicates recent segregation, possibly during the in vitro LCL derivation process (WT2-WT3, MUT1-MUT2) (Fig. 1D). Indeed, WT2/WT3 and MUT1/MUT2 shared the same B-cell receptor (BCR), suggesting recent divergence (Supplemental Table 1). The long branches separating WT and mutant clones indicate very early separation, with all clones sharing at maximum 30 mutations. This separation is consistent with B-cells being generated from multiple HSCs very early during development. Furthermore, the mutant clones shared only three mutations, including in DNMT3A. Mutations identified after WGS of PB revealed the absence of other major mutant clones. These results indicate that the original DNMT3A-mutant clone generated multiple independent lineages, which then evolved in parallel for nearly 60 years.
Next, we examined the representation of the LCL clones within the PB (from WGS). Mutations distinguishing WT and mutant lineages were validated by amplicon sequencing in the PB and available tissues (Supplemental Fig. 1G and Supplemental Table 2). Although, we could isolate WT clones from the LCL pool, sequencing of bulk PB, revealed that all variants shared between mutant LCLs clones were present in the PB, with the DNMT3A mutation being the most abundant. Moreover, one particular sub-branch (represented by MUT LCL clones 3,4,5) was over-represented in the PB, indicated by the truncal mutation with the highest VAF at 0.45 (Figure 1E). Thus, while multiple sub-clones were established many cell generations ago with descendants of both mutant branches being present in PB, one of the mutant branches ultimately became dominant. The PB was screened for secondary mutations, but no obvious drivers could explain the expansion of this clone (Fig. 1E, Supplemental Table 3 (Deposited in Mendeley) and Supplemental Fig. 1H) (Grove and Vassiliou, 2014). Together, these results demonstrate that both WT and mutant clones seed the hematopoietic system, giving rise to further sub-clones identifiable in B-cell descendants. DNMT3A mutant HSCs likely expanded contributing most of the cells in the active PB during the sampling period. The continued presence of WT and mutant clones in the B-cell pool likely reflects establishment during childhood and a lack of clonal advantage in the B-cell compartment.
DNMT3AR771Q leads to an increase in the number and length of hypomethylated regions
Next, we examined differences in DNA methylation that could explain the competitive advantage of DNMT3A mutant clones. We performed whole-genome bisulfite sequencing (WGBS) on 2 WT and 2 DNMT3A-MUT LCL clones (>7x depth). We detected a 2.3% global decrease in DNA methylation in the DNMT3AR771Q heterozygous mutant cells (Fig. 2A). Complete ablation of DNMT3A, as well as some characterized point mutations, results in focally reduced methylation (Challen et al., 2014; Gu et al., 2018; Spencer et al., 2017). Thus, we identified regions with low methylation (hypomethylated regions, or HMRs) in each genotype. Based on the mean HMR methylation, we identified differentially methylated regions (DMRs) between WT and mutant LCLs. Most of the >20,000 DMRs lost methylation in mutants in regions of already low methylation (10%−40%) (Supplemental Fig. 2A). To examine if methylation changes in LCLs (B-cell derived) overlapped with those in PB, we performed WGBS on PB DNA and compared it to previously published WT data (Heyn et al., 2012). Although, we did not detect global DNA methylation differences, we did identify hypo- and hyper-DMRs (Supplemental Fig. 2B–C). For these specific PB DMRs, we calculated the average DNA methylation in the LCLs. The magnitude of DNA methylation loss is greater (~7.4%) than those that gain methylation (~2%) (Supplemental Fig. 2D). These data show DNA methylation was reduced in a conserved pattern across both PB and LCLs, suggesting the lower DNA methylation in HSCs is conveyed across multiple lineages.
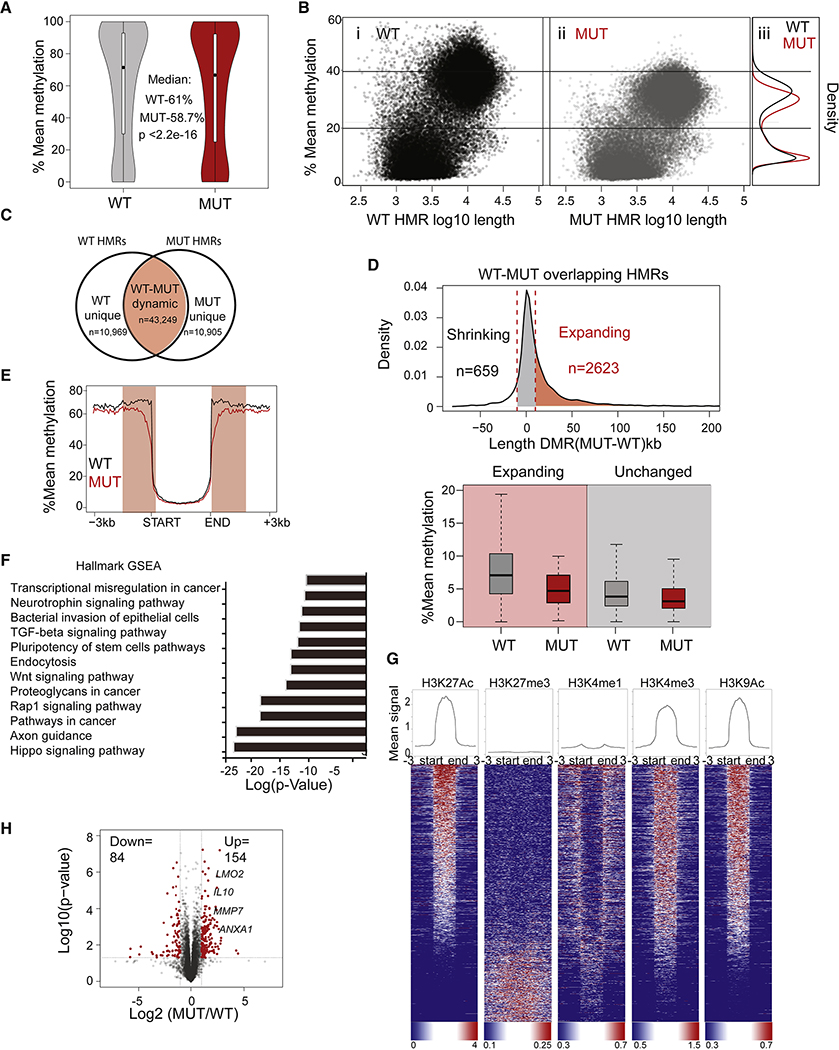
Figure 2. Loss of DNMT3A leads to focal hypomethylation and expansion of low methylation regions.
(A) Violin plots of genome-wide mean methylation of single CpGs grouped from 2 WT (gray) and 2 DNMT3A-MUT clones (red). (B) Scatter plot depicting WT (i) and DNMT3A-MUT LCLs (ii). Density plots (iii) depicting the distribution of the mean methylation of the WT and MUT hypomethylated regions (HMRs). (C) Venn diagram showing overlap of WT and DNMT3A-MUT HMRs. Overlapping, dynamic HMRs are regions that change either in methylation or length in MUT LCLs (D) Top: Density plot distribution depicting the change in HMR length for dynamic HMRs with mean methylation <10% and length> 2.5 kb. Delta length is the change in base pairs (bp) for these dynamic HMRs. Marked are DNMT3A-MUT HMRs that expanded in length (red) or were unchanged (gray). Bottom: Box plot of mean DNA methylation. (E) Mean methylation across canyons (>3 Kb and <10% methylation) for WT LCLs. (F) Enrichment based on p-value score for the identified GSEA pathways after the expanding HMRs (from D) were annotated using HOMER. (G) Quantification of signal (top) and heatmap enrichment (bottom) of the indicated histone marks across the expanding regions from LCLs (from Fig. 2D). (H) RNA-seq analysis for 3 WT (each in duplicate) and 4 DNMT3A-MUT clones (in duplicate). Differentially expressed genes between WT and MUT LCLs based on RNAseq fold-change and p-values. (H) Gene Ontology pathway enrichment of differentially expressed genes (fold>2, p<0.05) between WT and MUT clones plotted based on log transformed p-value enrichment score.
In absence of DNMT3A, lower methylation reflects a failure to establish DNA methylation. In order to identify sites at which DNMT3A is required, we analyzed the number, methylation level, and length of HMRs. Plotting WT HMRs based on mean methylation and length (Fig. 2B; Supplemental Fig 2E) revealed two groups: intermediate methylated HMRs (mean methylation of ~40%) and low methylated regions (LMRs, mean ~10%). WT-defined HMRs showed reduced methylation and increased length in mutant cells, indicating that DNMT3AR771Q preferentially impacts regions with low baseline methylation.
To classify HMRs based on their difference or overlap across the LCLs, we grouped all HMRs into unique HMRs (genotype-distinct regions of low methylation) and to dynamic HMRs. Dynamic HMRs overlap in both genotypes (Fig. 2C) but changed in length or methylation level in mutant LCLs. Remarkably, when we plotted these two groups of HMRs for WT-LCLs (Supplemental Fig. 2F), the dynamic regions clustered together in regions of lower methylation and longer length (above 2.5 Kb) and nearly 80% of these regions expanded in the context of a DNMT3A mutation (Fig. 2D). These observations demonstrate that methylation erosion is greatest at the edges of large methylation nadirs (canyons) (Fig. 2E) as seen in mice (Jeong et al., 2014).
DNA methylation erosion is concentrated in accessible regions near cancer-related genes
To identify the context of methylation loss in mutant cells, we performed gene ontology analysis of genes near DMRs, which revealed enhanced representation for pathways involved in cancer initiation and progression (Supplemental Fig. 2G,H). Examples of genes in expanding canyons include HOX family members, RUNX2, and LMO2, all implicated in hematologic malignancies (McCormack et al., 2010; Rawat et al., 2012; Sood et al., 2017) (Supplemental Fig. 2I).
Expanded canyons in mutant LCLs were primarily associated with active chromatin marks (Fig. 2G) as seen in murine HSCs (Jeong et al., 2014). Transcriptional analysis showed more genes up- than downregulated in DNMT3A-MUT LCLs (Supplemental Table 4 (Deposited in Mendeley); Fig. 2H). Although gene expression differences did not correlate well with changes in DNA methylation (Supplemental Fig. 2J), multiple genes implicated in cancer and cell cycle progression were upregulated (Supplemental Fig. 2K), including some with promoter hypomethylation in mutant cells (e.g. LMO2 and HOXB7; Supplemental Fig. 2H–I; Supplemental Table 5 and 6 (Deposited in Mendeley). Although the mosaic individual has not developed a hematological malignancy, the DNMT3A-mutant LCLs display increased expression of pro-growth genes possibly explaining cellular expansion.
Conserved DNA methylation reduction with DNMT3A loss
To determine if the DNA methylation changes identified in LCLs reflect events that occur in HSCs, we ablated DNMT3A in CD34+ HSPCs enriched from umbilical cord blood (Supplemental Fig. 3A–B and Supplementary Table 7 (Deposited in Mendeley)). After expansion for 3 months in NOD-SCID mice we performed WGBS on DNMT3A knockout (KO) and control CD34+ HSPCs (Fig. 3A, Supplemental Fig. 3C–D) (Gundry et al., 2016). As in LCLs, DNA methylation was slightly globally decreased in KO cells (Fig. 3B). Methylation loss was greatest at low and intermediate methylated regions (Supplemental Fig. 3E). DNMT3A is thought to establish methylation as HSCs differentiate. Thus, we speculated that regions with reduced methylation in CD34+ KO cells would overlap with those identified in mutant LCLs. Indeed, although the LCLs have a heterozygous DNMT3A mutation, 58% of the hypomethylated-DMRs (hypo-DMRs) identified in LCLs overlapped with those from the CD34+ KO cells (Fig. 3C–D).
Figure 3. Hypomethylated regions are conserved upon DNMT3A loss or mutation.
(A) Experimental schematic: CD34 expressing cells (CD34+) were enriched from umbilical cord blood and used to knockout DNMT3A with CRISPR-cas9 (CD34+DNMT3A-KO). Controls (Con-CD34) were electroporated with cas9 only. 3 months after transplantation in NSG mice CD34+ cells were sorted and used for WGBS. (B) Violin plots of genome-wide mean methylation of single CpG dinucleotides, grouped from 2 transplant experiments for CD34+ cells from KO and controls. (C) Differentially hypomethylated HMRs (hypo-DMRs) were those with lower methylation in KO vs controls. The Venn diagram shows the number of HMRs that overlap between the CD34 and LCL data sets. (D) Box plot depicting the mean methylation of all regions defined as hypo-DMRs in KO HSPCs compared to controls and their mean methylation in WT and mutant LCLs. (E) Loci that decreased in methylation in somatic mutant-DNMT3AR882H AMLs relative to DNMT3A-WT AMLs were identified as AML-hypomethylated DMRs (hypo-DMRs). For these same loci we plotted the mean methylation of in the CD34+ WT and KO cells. (F) Histogram depicting the frequency of methylation values in CD34-KO compared to controls for the AML-hypomethylated DMRs (G) As in E but plotting the LCLs. (H) Histogram depicting the frequency of methylation values in LCL-MUT compared to LCL-WT.
Remarkably, when we compared hypo-DMRs from a published analysis of TBRS (Supplemental Fig. 3C) (Spencer et al., 2017) and AML samples to those from HSPCs (Fig. 3E–F) and LCLs (Fig. 3G–H), we again observed consistent hypomethylation patterns. Yet, many of the hypermethylated-DMRs (hyper-DMRs) in AML were not altered in LCL clones (Supplemental Fig. 3G), consistent with the concept that the hypermethylation seen in DNMT3A-mutated AML is a consequence of malignant transformation rather than the DNMT3A mutation (Spencer et al., 2017). These results show that reduced DNMT3A activity leads to lower DNA methylation in a conserved pattern across stem cells, blood lineages and malignancy.
Loss of DNMT3A activity impairs DNA methylation gains at lineage-specific loci
Expansion is one features of dynamic HMRs in mutant LCLs. Surprisingly, in KO HSPCs we observed only a minor expansion in dynamic HMR length (Fig. 4A and Supplemental Fig. 4A). In comparison, the majority (~71%) of these regions increased in length in mutant LCLs (Fig. 4B–C, Supplemental Fig. 4B). Methylation may have been eroded in the LCLs over their ~60 years of cell divisions compared to only 3 months for the HSPCs. Alternatively, it is possible that the HMRs in mutant LCLs do not expand per se, but instead fail to gain active lineage-specific methylation normally acquired in WT cells.
Figure 4. Loss of DNMT3A prevents gain of differentiation-associated methylation and promotes HMR expansion.
(A) Scatter plot depicting the length of control HSPCs HMRs compared to KO HMRs. (B) Scatter plot depicting the length of the same loci defined as CD34+DNMT3A-KO hypo-DMRs in the WT-LCL compared to DNMT3A-MUT LCLs. (C) Density plot depicting the distribution of regions that increase in length as calculated of the delta length of the CD34+ regions in the scatter plot in B (MUT:red and WT:gray). (D) Violin plot of methylation values at regions defined as hypermethylated in the WT-LCLs (red) compared to control CD34 (gray). (E) Methylation distribution for hyper-DMR regions between WT-LCLs and control CD34 (displayed as 500 bp upstream and downstream).
To test how lineage-specific gains of methylation affect HMR length, we defined regions that gained methylation in WT-LCLs compared to WT HSPCs (LCL-hyper DMRs, Fig 4 D). We plotted the mean methylation of these regions and compared them across samples and genotypes (Fig. 4E). Our data show that methylation increased inside and at the edges of LCL-hyper DMRs compared to WT HSPCs, as expected from lineage-specific DNA methylation gain. However, compared to mutant LCLs, these regions appeared to expand substantially in length (Fig. 4E and Supplemental Fig. 4C). These results indicate that the longer HMR length in mutant LCLs (B-cell linage), is partially due to their inability to gain DNMT3A-mediated methylation during differentiation.
DISCUSSION
To address how clonal dominance is achieved by cells with mutated DNMT3A, we evaluated mutation burden and DNA methylation changes in DNMT3A-mutant cells and their normal counterparts from a mosaic individual. Epithelial, hematopoietic, and germ cells all harbored the DNMT3A mutation, indicating that the mutation occurred early in embryogenesis, likely at either the 4 or 8 cell stage. We cannot preclude that neutral drift accounts for the variation in mosaicism in the germline and epithelial tissues; nevertheless, the potential enrichment of DNMT3AR771Q/+ cells in the germline warrants further exploration and has implications for the prevalence of TBRS. The enrichment of mutant cells in the PB to nearly 100% argues for a particular advantage in the blood.
While the DNMT3A-mutant clones have persisted for six decades without transformation, mutant LCLs exhibited some features associated with leukemogenesis such as hypomethylation and upregulation of putative oncogenes. Our study shows that nearly the entire PB of an individual can be derived from a single DNMT3A-mutant clone without obligate progression to leukemia. The epigenetic changes caused by DNMT3A mutation likely impede differentiation, whereas other genetic events are likely required for transformation (Gilliland and Griffin, 2002). In the case of blood composed entirely of DNMT3A-mutant cells, it is possible that DNMT3A-mutant sub-clones compete with each other creating a stable hematopoietic system. Nevertheless, mice reconstituted with Dnmt3a−/−or Dnmt3aR878H HSCs do eventually all succumb to hematologic disease (Celik et al., 2015; Dai et al., 2017; Mayle et al., 2015); in at least some cases additional driver mutations were shown to be acquired, as enforced presence of additional drivers increases the efficiency and uniformity of transformation (Yang et al., 2016).
The development of genomic instability, such as in the paradigm of TP53 mutation, is a key hallmark of cancer (Hanahan and Weinberg, 2011) thought to enable acquisition of additional “hits”. Our data argue against a major contribution of genetic instability from DNMT3A mutations, and instead suggest that a stereotyped pattern of DNA methylation erosion arises upon DNMT3A loss or mutation. We suggest these DNA methylation changes creates a cancer-poised epigenomic landscape that permits high expression of cancer-associated and stem cell self-renewal genes. Thus, although mutation of DNMT3A alone does not suffice for transformation, it enables indefinite expansion and promotes malignant transformation once additional mutations occur. This study reinforces the concept that, analogous to p53 for genome integrity, DNMT3A maintains epigenome integrity, loss of which may be considered a “hallmark” of hematologic malignancies.
Limitations of Study
While we conclude that the study subject incurred a DNMT3A mutation during embryogenesis and the mutant cells contributed disproportionally to the blood, we cannot be certain which stage the mutation occurred. Furthermore, sampling of other tissues could reveal other tissues with biased contribution. Excess blood contribution may not necessarily occur in other mosaic individuals. While we conclude that this particular DNMT3A mutation (R771Q) does not cause genome instability, our analysis is limited to the number of clones we were able to study, and it is conceivable that different types of DNMT3A mutation would affect mutation acquisition.
STAR Methods
Resource availability
Lead Contact
Further information and requests of reagents can be directed to the Lead Contact, Margaret Goodell (goodell@bcm.edu).
Materials availability
The LCL pool and clones are available from Andrew Crosby’s laboratory, University of Exeter.
Data and code availability
The code generated in this study for visualization of hypomethylated regions is deposited in GitHub. Datasets of CD34 WGBS data supporting this manuscript are deposited in GEO (GSE150515). Source data from the LCLs have not been deposited in a public repository due to subject consent. Instead, we have included processed data of base substitutions identified (Table S3 related to the data in figure 1 ), DMRs (Table S4 related to the data in figure 2), and RNAseq (Table S5–6 related to data in figure 3 and 4 ) from the LCLs in the supplemental tables deposited in Mendeley (DOI 10.17632/rp3y5snpkk.1). Also deposited in Mendeley is a summary table of the DNMT3A exon 10 deletion information (Supplemental table 7 related to figure 3).
Experimental Model and Subject Details
Sample collection
All tissue samples collected for this study were collected with IRB approvals (DDC clinic -FWA 00013820, University of Arizona 10–0050-01, Baylor College of Medicine H-40209, H-20911).
Generation of lymphoblastoid cell lines and single clone isolation
We collected peripheral blood from a 58-year-old man with a mosaic germline mutation in the gene DNMT3A, and from his blood established lymphoblastoid cell lines (LCLs) using standard protocols at UK Public Health. LCLs were incubated in RPMI media with 15% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S) at 5% CO2 in a 37°C incubator. To generate single clones from the LCL pool, we plated single cells in 96 well u-bottom plates on top of irradiated pool cells. Clones were collected and validated with Sanger sequencing.
Cell culture and isolation of human CD34+ cells
Cell isolation and electroporation were done as previously described (Gundry et al., 2016). In brief: human CD34+ cord blood cells from discarded individual donor umbilical cords (MD Anderson Cord Blood Bank) were collected and enriched from mononuclear cells using CD34 microbeads and Auto-MACS Pro (Miltenyi Biotec) following manufacturer’s instructions. Purity of sorted cord blood CD34+ cells was assessed following Auto-MACS enrichment using flow-cytometry. Isolated cells were then cultured in StemSpan SFEM II (Stem Cell Technologies) supplemented with 100ng/ml FLT3L (Peprotech), 100ng/ml thrombopoietin (Peprotech) and 100ng/ml stem cell factor (Peprotech) for a maximum of 48 hours prior to electroporation. Guide synthesis was done as previously described using ribonucleoprotein (RNP) delivery of dual guides targeting exon 10 of DNMT3A. Following, Cas9-sgRNA RNPs, 1 μg of either in-vitro transcribed sgRNA was incubated with 1.5 μg Cas9 for 30 minutes at room temperature. CD34+ samples 1.5–2.5 × 105 cells were then electroporated using the Neon Transfection System.
Method Details
Eyebrow hair blub DNA isolation
Eyebrow hair follicles (~5) plucked from the described individual were used for DNA isolation using a modified method of QIAamp® DNA Mini Kit (Qiagen) (Weitzel et al., 2018). Briefly, hair follicles were digested at 55°C for 1 hour using cell lysis buffer (10mM Tris- HCl pH 8.0, 10mM EDTA pH 8.0, 100mM NaCl, 40 mM DTT, 2% SDS and 250 μg/mL Proteinase K (Fisher Healthcare). Following, equal volume of buffer AL (Qiagen) and of 100% ethanol were added to cell lysate. Next, column loading and washes were according to the manufacturer’s instructions (Castillo D, 2016).
In vivo transplantation of CD34+ cord blood cells
Adult (6–8 weeks of age) NOD-SCID gamma (NSG) mice were conditioned with sub-lethal (2.5 Gy) total-body irradiation and transplanted with electroporated 1.5–2.5 × 105 CD34+ cord-blood cells 6 hours post electroporation. Three months following transplantation BM cells were collected and human CD34+ cells were sorted by flow cytometry. Conformation of DNMT3A-knockout efficiency at genomic level was done by amplicon sequencing on genomic DNA isolated from either CD34+ CAS9 only electroporated cells or CD34+ following DNMT3A-knockout. The same genomic DNA was also used for WGBS.
Mutation discovery and tree building from whole genome sequencing
We used mutation-calling pipelines to discover all somatic changes in whole genome data (Lee-Six et al., 2018). Briefly, we used CaVEMan to identify single base somatic substitutions genome-wide. For mutation calling we have filtered out variants supported by less than 4 sequencing read fragments, all the sample were sequenced to an average coverage of 30–40x. This approach calls mutations at a VAF of 0.1 and higher. Mutations were called against a matched normal sample and annotated using ANNOVAR. For the calling of trinucleotide pattern of mutational signatures we used the R package MutationalPatterns and followed the guidelines (Blokzijl et al., 2016). We used the COSMIC mutational signatures enrichment tool to identify the patterns most enriched in our LCLs (above 50% similarity) and presented those with frequency above 10%. Phylogenetic trees were built by an approach described previously (Moore et al., 2020).
Calculation of mutations variant allele frequency
We obtained extracted DNA from the LCL pool, peripheral blood, urine and saliva from the previously published study Xin, et al, 2016 (Baylor College of Medicine H-40209) we isolated DNA and subjected it to PCR using the primers listed in Key Resources. Nextera libraries were generated from the PCR product using the manufacturer’s protocol for the Nextera XT DNA library prep kit (Illumina). Each PCR-amplicon was sequenced using Illumina Nextseq 500 sequencer to a coverage >10,000 reads.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| CD34 microbeads | Miltenyi Biotec | Cat # 130–046-702 |
| Anti human CD19 | BD | Cat # 341103 |
| Mouse anti-Human, FITC, Clone: 8G12 | BD | Cat # 348053 |
| StemSpan SFEM II | Stemcell technologies | Cat # 9655 |
| Biological Samples | ||
| Newborn umbilical cord | (Gundry et al., 2016) and this manuscript | The MD Anderson Cord Blood Bank |
| Saliva from described individual | (Xin et al., 2017) | N\A |
| Urine from described individual | (Xin et al., 2017) | N\A |
| Peripheral blood from described individual | (Xin et al., 2017) and this manuscript | N\A |
| Eyebrow hair plucks from described individual | This manuscript | N\A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Human Recombinant FLT3L 100ug | Peprotech | Cat # 30019100UG |
| Human Recombinant SCF 100ug | Peprotech | Cat # 30007100UG |
| Recombinant animal-free murine thrombopoietin (TPO) 50ug | Peprotech | Cat # AF-315–14 |
| Alt-R® S.p. Cas9 Nuclease V3, 100 μg | IDT | Cat #1081058 |
| Proteinase-K | Fisher Healthcare | BP1700–50 |
| Critical Commercial Assays | ||
| Whole genome bisulfite sequencing kit | Illumina | https://www.illumina.com/content/dam/illumina-marketing/documents/products/appnotes/appnotemethylseqwgbs.pdf |
| Accel-NGS® Methyl-Seq DNA Library Kit | Swift | Cat # 30024/ 30096 |
| EZ DNA Methylation-Lightning Kits | ZYMO RESEARCH | D5030 |
| TruSeq RNA Library Preparation Kit v2, Set A (48 samples, 12 indexes | Illumina | Cat # RS-122- |
| Nextera XT DNA Library Preparation Kit | Illumina | Cat # FC-1311096 |
| QIAamp® DNA Mini Kit | QIAGEN | 51304 |
| Deposited Data | ||
| LCL base substitution Supplemental Table 3 related to Fig 1 | Mendeley | DOI: 10.17632/rp3y5snpkk.1 |
| WGBS of CD34 HSPCs following KO of DNMT3A Raw and analyzed data | This manuscript | GSE150515 |
| FPKM values from RNAseq of WT and MUT LCL clones related to Fig 2, see Supplemental Table 4 | This manuscript | DOI: 10.17632/rp3y5snpkk.1 |
| DMRs from LCLs related to Fig 4, see Supplemental Table 5 | This manuscript | DOI: 10.17632/rp3y5snpkk.1 |
| ChIP-seq data for LCL cell line GM12478 | ENCODE | GSE29611 |
| Experimental Models: Cell Lines | ||
| LCL clones | This manuscript | N/A |
| Experimental Models: Organisms/Strains | ||
| Adult 8 weeks NOD scid gamma NSG mice | The Jackson laboratory | NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ |
| Oligonucleotides | ||
| sgRNA for DNMT3A: AGGUGGCCAGCAGCCGCGCG UGACACUGCCAAGGCCGUGG | (Gundry et al., 2016) | SYNTHEGO Gene Knockout Kit v2 |
| Oligos used for amplicon sequencing of DNMT3A or point mutations | This manuscript | Supplemental Table 2 related to Figure 1 |
| Software and Algorithms | ||
| Bowtie2 | (Langmead and Salzberg, 2012) | http://bowtiebio.sourceforge.net/bowtie2/index.shtml |
| Samtools | (Li et al., 2009) | http://samtools.sourceforge.net/ |
| CaVEMan | (Jones et al., 2016) | https://github.com/cancerit/CaVEMan |
| Hisat2 | (Kim et al., 2019) | http://daehwankimlab.github.io/hisat2/ |
| Cufflinks | (Trapnell et al., 2012) | https://github.com/cole-trapnelllab/cufflinks |
| WALT | (Chen et al., 2016) | https://github.com/smithlabcode/walt |
| HOMER | (Heinz et al., 2010) | http://homer.ucsd.edu/homer/motif/ |
| MutationalPatterns R package | (Maura et al., 2019) | https://bioconductor.org/packages/release/bioc/html/MutationalPatterns.html |
| ANNOVAR | (Wang et al., 2010) | https://docopenbio.readthedocs.io/projects/annovar/en/latest/ |
| Algorithms and code for DNA methylation visualization | This manuscript | https://github.com/jaimemrb/methylPlot |
| Docker container, SNV (CaVEMan) and indel (cgpPindel) calling | (Sanders et al., 2018) | https://github.com/cancerit/dockstorecgpwgs |
| Software and Singularity container for variant annotation and filtering after applying the cgpWGS Docker container | (Sanders et al., 2018) | https://github.com/MathijsSanders/SangerLCMFiltering |
| Other | ||
| StemSpan SFEM II | Stemcell technologies | Cat # 9655 |
B- cell receptor identification
In order to identify the immunoglobulin heavy chain V(D)J rearrangements and CDR3 sequences present in each clone, we extracted reads in which one read maps to an IGHV gene and the paired read maps to an IGHJ gene. We then filtered the read pairs to those that include at least one of the reads clipped internal to the putative rearrangement. For each specific V/J gene combination we identified the full recombined DNA sequence (greatest weight path through a DeBruijn graph) and submitted this as an IgBLAST (v1.15.0) query using IMGT gene sequences as the database (www.imgt.org, downloaded 03/20/2020). For each sample we report the top hit IGHV, IGHD and IGHJ genes and the CDR3 sequence.
RNA sequencing and analysis
RNA was extracted using RNeasy micro kit (Qiagen) and quantified using Nanodrop. We prepared the Truseq stranded mRNA library using the manufacturer’s instructions (Illumina). Libraries were sequenced using Nextseq 500 sequencer. Paired-end RNA-seq reads were mapped to human genome (hg19) using Hisat 2. We calculated the Fragments Per Kilobase of exon per million fragments mapped (FPKM) values using Cufflinks 2.2.1 (Trapnell et al., 2012). Genes were defined as significant with FPKM>=10, a fold change >=2 and with student t-test <=0.05.
Whole Genome Bisulfite Sequencing (WGBS) and Analysis
We extracted DNA from cell pellets using DNeasy kit (Qiagen 69504). For the LCLs and CD34 cells, we used 100 ng DNA to prepare WGBS libraries with the TruSeq DNA methylation kit according to the manufacturer’s instruction (Illumina). Libraries were sequenced using an IlluminaNextseq 500 sequencer. For each WGBS profile, we used WALT (Chen et al., 2016) default parameters to get aligned bisulfite–treated reads to the human genome (hg19). Duplicates were removed using duplicate-remover as part of the WALT pipeline. We then used methcounts to calculate the methylation ratio of each CpG covered with at least 5 reads. Replicates of each genotype (WT or DNMT3A-MUT) were merged for downstream analysis. For CD34+ cells biological triplicates were merged for downstream analysis for CD34+ control or CD34+DBNT3A-KO (GSE150515). For PB samples we sued 100 ng of DNA and used Accel-NGS Methyl-Seq DNA Library Kit according to the manufacturer’s instruction (Swift).
Analysis and definition of hypomethylated regions (HMRs)
To identify HMRs in the LCL or PB WGBS data we used default settings. Briefly, a two-state hidden Markov model-based method was used for the HMR calling (Song et al., 2013). This model compares one state representing HMRs to highly methylated background from the same genomic DNA source. We then calculated the mean methylation of HMRs using the methylation ratio for each CpG (Coverage 5x per) as calculated above. To identify HMRs that changed in DNMT3A-MUT cells compared to WT, we pooled all HMRs from both genotypes. Overlapping or proximal HMRs that were within 200 bp from each other were merged. Within those overlapping HMRs, we defined DNA methylation canyons as areas with less than 10% mean methylation and >3kb length. We divided canyons into 200 non-overlapping genomic bins and plotted mean methylation in each bin.
Gene ontology and gene enrichment
Based on HOMER functional enrichment analysis, we assigned HMRs or differentially methylated regions (DMRs; defined as an HMR with 30% minimal difference in DNA methylation mean) to corresponding genes (Heinz et al., 2010). We used Enrichr and GSEA for gene enrichment analysis and gene ontology (GO) term enrichment for RNAseq data and other genomic loci (Chen et al., 2013; Subramanian et al., 2005).
ChIP-seq analysis
We created heatmaps for all expanding canyons (=>3 kb), as well as for H3K27ac, H3K27me3, H3K4me1, and H3K4me3 datasets acquired from ENCODE (GEO: GSE29611). Each row represents a canyon and the +/− 3kb flanking the canyon edges. Rows are ranked by the average occupancy of H3K27ac, indicating the relative activity of each canyon where the topmost rows correspond to the most active canyons. We determined the signal at each mark by dividing canyon loci into 200 bins and calculating the average density of reads per bin normalized to the total number of million mapped reads (rpm/bp). Metaplots indicate the average signal density for all rows in each bin.
Statistical Analysis
For comparisons involving two groups, unpaired Student’s t tests (two-tailed) or non-parametric tests were used. See the figure legends or methods details for specifics.
Supplementary Material
Supplemental Table 1. List of the B-cell receptor sequence identified for each of the clones, related to Figure 1. List describes identity of each B-cell receptor per clone, highlighted are clones which share an identical B-cell receptor sequence
Supplemental Table 2. List of primers used to validate DNMT3A and other base substitutions in WT and mutant cells, related to Figure 1 and amplicon sequencing. List of primers used in the study
Highlights.
In a germline mosaic DNMT3AR771Q cells expand mainly in the blood lineage
DNMT3AR771Q does not alter mutation burden or signature in mutant cell clones
DNMT3A R771Q causes focal hypomethylation and expansion of active chromatin loci
Hypomethylation patterns overlap in aged clones and DNMT3AKO HSPCs
Acknowledgments
We thank Catherine Gillespie for editing and the Genomic and RNA Profiling Core at BCM. This work was supported by the Cancer Prevention and Research Institute of Texas, the Edward P. Evans Foundation, the Samuel Waxman Cancer Research Foundation, the Welcome Trust and the National Institutes of Health (DK092883, AG036695, CA183252, CA125123, CA242218), the MD Anderson Cord Blood Bank, and by Newlife Foundation for Disabled Children (Ref: SG/16-17/02, to ARJ, AHC and ELB) and by the Howard Hughes Medical Institute through the James H. Gilliam Fellowships for Advanced Study program. J Weitzel was supported by the Dr. Norman & Melinda Payson Professorship in Medical Oncology.
Footnotes
Declaration of Interests
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alexandrov LB, Jones PH, Wedge DC, Sale JE, Campbell PJ, Nik-Zainal S, and Stratton MR (2015). Clock-like mutational processes in human somatic cells. Nat Genet 47, 1402–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokzijl F, de Ligt J, Jager M, Sasselli V, Roerink S, Sasaki N, Huch M, Boymans S, Kuijk E, Prins P, et al. (2016). Tissue-specific mutation accumulation in human adult stem cells during life. Nature 538, 260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti L, Gundry MC, and Goodell MA (2017). DNMT3A in Leukemia. Cold Spring Harb Perspect Med 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo D, H.J., Sand S, O’Connor T, Clark C, Weitzel J. (2016). Well-groomed participants: eyebrow plucks as surrogates for biomarker samples and a viable source of constitutional DNA. In ASHG 2016 (Vancouver, Canada). [Google Scholar]
- Celik H, Mallaney C, Kothari A, Ostrander EL, Eultgen E, Martens A, Miller CA, Hundal J, Klco JM, and Challen GA (2015). Enforced differentiation of Dnmt3a-null bone marrow leads to failure with c-Kit mutations driving leukemic transformation. Blood 125, 619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challen GA, Sun D, Jeong M, Luo M, Jelinek J, Berg JS, Bock C, Vasanthakumar A, Gu H, Xi Y, et al. (2011). Dnmt3a is essential for hematopoietic stem cell differentiation. Nat Genet 44, 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challen GA, Sun D, Mayle A, Jeong M, Luo M, Rodriguez B, Mallaney C, Celik H, Yang L, Xia Z, et al. (2014). Dnmt3a and Dnmt3b have overlapping and distinct functions in hematopoietic stem cells. Cell Stem Cell 15, 350–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, Schrock A, Campbell B, Shlien A, Chmielecki J, et al. (2017). Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 9, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, Clark NR, and Ma’ayan A (2013). Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Smith AD, and Chen T (2016). WALT: fast and accurate read mapping for bisulfite sequencing. Bioinformatics 32, 3507–3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai YJ, Wang YY, Huang JY, Xia L, Shi XD, Xu J, Lu J, Su XB, Yang Y, Zhang WN, et al. (2017). Conditional knockin of Dnmt3a R878H initiates acute myeloid leukemia with mTOR pathway involvement. Proc Natl Acad Sci U S A 114, 5237–5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed D, Stevens EL, and Pevsner J (2014). Somatic mosaicism in the human genome. Genes (Basel) 5, 1064–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland DG, and Griffin JD (2002). The roles of FLT3 in hematopoiesis and leukemia. Blood 100, 1532–1542. [DOI] [PubMed] [Google Scholar]
- Grove CS, and Vassiliou GS (2014). Acute myeloid leukaemia: a paradigm for the clonal evolution of cancer? Dis Model Mech 7, 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu T, Lin X, Cullen SM, Luo M, Jeong M, Estecio M, Shen J, Hardikar S, Sun D, Su J, et al. (2018). DNMT3A and TET1 cooperate to regulate promoter epigenetic landscapes in mouse embryonic stem cells. Genome Biol 19, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundry MC, Brunetti L, Lin A, Mayle AE, Kitano A, Wagner D, Hsu JI, Hoegenauer KA, Rooney CM, Goodell MA, et al. (2016). Highly Efficient Genome Editing of Murine and Human Hematopoietic Progenitor Cells by CRISPR/Cas9. Cell Rep 17, 1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guryanova OA, Shank K, Spitzer B, Luciani L, Koche RP, Garrett-Bakelman FE, Ganzel C, Durham BH, Mohanty A, Hoermann G, et al. (2016). DNMT3A mutations promote anthracycline resistance in acute myeloid leukemia via impaired nucleosome remodeling. Nat Med 22, 1488–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, and Weinberg RA (2011). Hallmarks of cancer: the next generation. Cell 144, 646–674. [DOI] [PubMed] [Google Scholar]
- Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, and Glass CK (2010). Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 38, 576–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyn H, Li N, Ferreira HJ, Moran S, Pisano DG, Gomez A, Diez J, Sanchez-Mut JV, Setien F, Carmona FJ, et al. (2012). Distinct DNA methylomes of newborns and centenarians. Proc Natl Acad Sci U S A 109, 10522–10527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou HA, Kuo YY, Liu CY, Chou WC, Lee MC, Chen CY, Lin LI, Tseng MH, Huang CF, Chiang YC, et al. (2012). DNMT3A mutations in acute myeloid leukemia: stability during disease evolution and clinical implications. Blood 119, 559–568. [DOI] [PubMed] [Google Scholar]
- Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, et al. (2014). Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 371, 2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, and Libby P (2020). Clonal haematopoiesis: connecting ageing and inflammation in cardiovascular disease. Nat Rev Cardiol 17, 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan M, Snyder TM, Corces-Zimmerman MR, Vyas P, Weissman IL, Quake SR, and Majeti R (2012). Clonal evolution of preleukemic hematopoietic stem cells precedes human acute myeloid leukemia. Sci Transl Med 4, 149ra118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong M, Park HJ, Celik H, Ostrander EL, Reyes JM, Guzman A, Rodriguez B, Lei Y, Lee Y, Ding L, et al. (2018). Loss of Dnmt3a Immortalizes Hematopoietic Stem Cells In Vivo. Cell Rep 23, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong M, Sun D, Luo M, Huang Y, Challen GA, Rodriguez B, Zhang X, Chavez L, Wang H, Hannah R, et al. (2014). Large conserved domains of low DNA methylation maintained by Dnmt3a. Nat Genet 46, 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D, Raine KM, Davies H, Tarpey PS, Butler AP, Teague JW, Nik-Zainal S, and Campbell PJ (2016). cgpCaVEManWrapper: Simple Execution of CaVEMan in Order to Detect Somatic Single Nucleotide Variants in NGS Data . Curr Protoc Bioinformatics 56, 15 10 11–15 10 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Paggi JM, Park C, Bennett C, and Salzberg SL (2019). Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol 37, 907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, and Salzberg SL (2012). Fast gapped-read alignment with Bowtie 2. Nat Methods 9, 357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Six H, Obro NF, Shepherd MS, Grossmann S, Dawson K, Belmonte M, Osborne RJ, Huntly BJP, Martincorena I, Anderson E, et al. (2018). Population dynamics of normal human blood inferred from somatic mutations. Nature 561, 473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley TJ, Ding L, Walter MJ, McLellan MD, Lamprecht T, Larson DE, Kandoth C, Payton JE, Baty J, Welch J, et al. (2010). DNMT3A mutations in acute myeloid leukemia. N Engl J Med 363, 2424–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, and Genome Project Data Processing, S. (2009). The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis TC, Wilkinson AC, Beerman I, Jaiswal S, and Shlush LI (2019). Biological implications of clonal hematopoiesis. Exp Hematol 77, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maura F, Degasperi A, Nadeu F, Leongamornlert D, Davies H, Moore L, Royo R, Ziccheddu B, Puente XS, Avet-Loiseau H, et al. (2019). A practical guide for mutational signature analysis in hematological malignancies. Nat Commun 10, 2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayle A, Yang L, Rodriguez B, Zhou T, Chang E, Curry CV, Challen GA, Li W, Wheeler D, Rebel VI, et al. (2015). Dnmt3a loss predisposes murine hematopoietic stem cells to malignant transformation. Blood 125, 629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack MP, Young LF, Vasudevan S, de Graaf CA, Codrington R, Rabbitts TH, Jane SM, and Curtis DJ (2010). The Lmo2 oncogene initiates leukemia in mice by inducing thymocyte self-renewal. Science 327, 879–883. [DOI] [PubMed] [Google Scholar]
- Moore L, Leongamornlert D, Coorens THH, Sanders MA, Ellis P, Dentro SC, Dawson KJ, Butler T, Rahbari R, Mitchell TJ, et al. (2020). The mutational landscape of normal human endometrial epithelium. Nature 580, 640–646. [DOI] [PubMed] [Google Scholar]
- Pan F, Wingo TS, Zhao Z, Gao R, Makishima H, Qu G, Lin L, Yu M, Ortega JR, Wang J, et al. (2017). Tet2 loss leads to hypermutagenicity in haematopoietic stem/progenitor cells. Nat Commun 8, 15102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawat VP, Humphries RK, and Buske C (2012). Beyond Hox: the role of ParaHox genes in normal and malignant hematopoiesis. Blood 120, 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro AF, Pratcorona M, Erpelinck-Verschueren C, Rockova V, Sanders M, Abbas S, Figueroa ME, Zeilemaker A, Melnick A, Lowenberg B, et al. (2012). Mutant DNMT3A: a marker of poor prognosis in acute myeloid leukemia. Blood 119, 5824–5831. [DOI] [PubMed] [Google Scholar]
- Sanders MA, Chew E, Flensburg C, Zeilemaker A, Miller SE, Al Hinai AS, Bajel A, Luiken B, Rijken M, McLennan T, et al. (2018). MBD4 guards against methylation damage and germ line deficiency predisposes to clonal hematopoiesis and early-onset AML. Blood 132, 1526–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlush LI, Mitchell A, Heisler L, Abelson S, Ng SWK, Trotman-Grant A, Medeiros JJF, Rao-Bhatia A, Jaciw-Zurakowsky I, Marke R, et al. (2017). Tracing the origins of relapse in acute myeloid leukaemia to stem cells. Nature 547, 104–108. [DOI] [PubMed] [Google Scholar]
- Song Q, Decato B, Hong EE, Zhou M, Fang F, Qu J, Garvin T, Kessler M, Zhou J, and Smith AD (2013). A reference methylome database and analysis pipeline to facilitate integrative and comparative epigenomics. PLoS One 8, e81148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood R, Kamikubo Y, and Liu P (2017). Role of RUNX1 in hematological malignancies. Blood 129, 2070–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer DH, Russler-Germain DA, Ketkar S, Helton NM, Lamprecht TL, Fulton RS, Fronick CC, O’Laughlin M, Heath SE, Shinawi M, et al. (2017). CpG Island Hypermethylation Mediated by DNMT3A Is a Consequence of AML Progression. Cell 168, 801–816 e813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. (2005). Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102, 15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatton-Brown K, Seal S, Ruark E, Harmer J, Ramsay E, Del Vecchio Duarte S, Zachariou A, Hanks S, O’Brien E, Aksglaede L, et al. (2014). Mutations in the DNA methyltransferase gene DNMT3A cause an overgrowth syndrome with intellectual disability. Nat Genet 46, 385–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, and Pachter L (2012). Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 7, 562–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Li M, and Hakonarson H (2010). ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 38, e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CJ, Papula AL, Poon GYP, Wong WH, Young AL, Druley TE, Fisher DS, and Blundell JR (2020). The evolutionary dynamics and fitness landscape of clonal hematopoiesis. Science 367, 1449–1454. [DOI] [PubMed] [Google Scholar]
- Weitzel JN, Chao EC, Nehoray B, Van Tongeren LR, LaDuca H, Blazer KR, Slavin T, Facmg D, Pesaran T, Rybak C, et al. (2018). Somatic TP53 variants frequently confound germ-line testing results. Genet Med 20, 809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin B, Cruz Marino T, Szekely J, Leblanc J, Cechner K, Sency V, Wensel C, Barabas M, Therriault V, and Wang H (2017). Novel DNMT3A germline mutations are associated with inherited Tatton-Brown-Rahman syndrome. Clin Genet 91, 623–628. [DOI] [PubMed] [Google Scholar]
- Yang L, Rau R, and Goodell MA (2015). DNMT3A in haematological malignancies. Nat Rev Cancer 15, 152–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Rodriguez B, Mayle A, Park HJ, Lin X, Luo M, Jeong M, Curry CV, Kim SB, Ruau D, et al. (2016). DNMT3A Loss Drives Enhancer Hypomethylation in FLT3-ITD-Associated Leukemias. Cancer Cell 29, 922–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Dong X, Lee M, Maslov AY, Wang T, and Vijg J (2019). Single-cell whole-genome sequencing reveals the functional landscape of somatic mutations in B lymphocytes across the human lifespan. Proc Natl Acad Sci U S A 116, 9014–9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. List of the B-cell receptor sequence identified for each of the clones, related to Figure 1. List describes identity of each B-cell receptor per clone, highlighted are clones which share an identical B-cell receptor sequence
Supplemental Table 2. List of primers used to validate DNMT3A and other base substitutions in WT and mutant cells, related to Figure 1 and amplicon sequencing. List of primers used in the study
Data Availability Statement
The code generated in this study for visualization of hypomethylated regions is deposited in GitHub. Datasets of CD34 WGBS data supporting this manuscript are deposited in GEO (GSE150515). Source data from the LCLs have not been deposited in a public repository due to subject consent. Instead, we have included processed data of base substitutions identified (Table S3 related to the data in figure 1 ), DMRs (Table S4 related to the data in figure 2), and RNAseq (Table S5–6 related to data in figure 3 and 4 ) from the LCLs in the supplemental tables deposited in Mendeley (DOI 10.17632/rp3y5snpkk.1). Also deposited in Mendeley is a summary table of the DNMT3A exon 10 deletion information (Supplemental table 7 related to figure 3).