Abstract
The development of new accelerators has given a new impetus to the development of new drugs and treatment technologies using boron neutron capture therapy (BNCT). We analyzed the current status and future directions of BNCT for cancer treatment, as well as the main issues related to its introduction. This review highlights the principles of BNCT and the key milestones in its development: new boron delivery drugs and different types of charged particle accelerators are described; several important aspects of BNCT implementation are discussed. BCNT could be used alone or in combination with chemotherapy and radiotherapy, and it is evaluated in light of the outlined issues. For the speedy implementation of BCNT in medical practice, it is necessary to develop more selective boron delivery agents and to generate an epithermal neutron beam with definite characteristics. Pharmacological companies and research laboratories should have access to accelerators for large‐scale screening of new, more specific boron delivery agents.
Keywords: boron compounds, Boron neutron capture therapy, cancer treatment, cancer, drug delivery
1. BACKGROUND
Given that morbidity and mortality of cancer continue to remain at relatively constant levels, we believe that a new cancer therapy, boron neutron capture therapy (BNCT), deserves to be further developed [1]. The prospects for such development and for the clinical implementation of BNCT are promising despite various problems. In particular, the speedy implementation of this method in clinical practice will require the development of more selective boron delivery agents and an epithermal neutron beam with suitable characteristics. This review addresses what in our view are the most important issues relating to the introduction of BNCT. The principles of BNCT and the key milestones in its development are described in details, covering the mechanism of BNCT‐induced cell death, the role of BNCT as a treatment modality for different cancers, the characteristics of the currently available boron preparations, and approaches to the delivery of isotope 10B to tumor cells.
2. BNCT
2.1. Historical aspects
The existence of the neutron was the first postulated in 1932 by Chadwick [2], who explored the properties of the penetrating radiation emitted from beryllium and boron when bombarded by the alpha particles of polonium. Subsequently, several researchers compared the effects of neutrons and X‐rays on normal and tumor tissues. In 1936, Locher [3] published a work about neutrons which had therapeutic possibilities. He proposed the principle of BNCT based on the selective concentration of boron in tumors and its irradiation by thermal neutrons. In this context it is to be noted that tumor tissue receives a higher radiation dose than normal tissue. In 1951, the first attempt at BNCT in a patient with malignant glioma was performed using the Brookhaven Graphite Research Reactor [4]. Thereafter other attempts were made to use BNCT for treatment of cancer patients, but serious adverse effects were encountered, including radiodermatoses of the scalp, cerebral edema, intractable shock, and brain necrosis. Because of poor neutron penetration in deeply seated tumors, in addition to non‐selective accumulation of boron compounds in the tumor, these experiments failed. Owing to the toxicity and adverse effects, the United States stopped these clinical trials of BNCT in 1961.
In 1968, Hatanaka reported the results of clinical trials of BNCT in Japan with borocaptate sodium wherein the beam of neutron was aimed directly at the intracranial tumor bed. In this experiment a 5‐year survival rate of 58% was achieved [5]. This gave rise to renewed interest in BNCT clinical trials in the United States and Europe. The use of boronophenylalanine as a boron compound was first reported in 1987 in Japan by Mishima, who applied BNCT to treat malignant melanoma [6].
As a result of the efforts of different scientific research groups, today there are several BNCT clinics equipped with different types of charged particle accelerators and targets:
A Japanese company, Sumitomo Heavy Industries (Tokyo, Japan) manufactured and installed a cyclotron with an energy of 30 MeV and a current of 2 mA, with a beryllium target, in the Clinic of South Tohoku (Koriyama, Japan) [3]. On 12 March, 2020, Sumitomo Heavy Industries, Ltd. announced that they obtained approval from Japanese Ministry of Health, Labor and Welfare for manufacturing and selling the accelerator‐based BNCT system (NeuCure™ System) and the dose calculation program (NeuCure™ Dose Engine). It is worth noting that this approval is valid only for the treatment of unresectable head and neck carcinoma.
The University of Tsukuba together with the High Energy Accelerator Research Organization, Japan Atomic Energy Agency, Hokkaido University, Ibaraki Prefecture, and Mitsubishi Heavy Industry Co. have produced an 8‐MeV 5‐mA linac with a beryllium target for the BNCT clinic in Tokai (Tsukuba, Ibaraki, Japan) [7]. To date, a proton beam with a current of 2 mA has been obtained.
The third BCNT clinic is located at the National Cancer Center in Tokyo. Here, a 2.5 MeV linac with a current of 20 mA is used, which was installed by Cancer Intelligence Care Systems, Inc. [8]. To date, a proton beam with a current of 11 mA has been obtained.
The fourth BNCT clinic is being built at the Helsinki University Hospital (Helsinki, Finland). For this clinic, Neutron Therapeutics Inc. (Danvers, MA, USA) manufactured a 2.6 MeV, 30 mA direct‐acting electrostatic accelerator Hyperion™ with a rotating lithium target and began to assemble it in the fall of 2018. In July 2019, it was announced that Neutron Therapeutics Inc. has agreed with the Tokushukai Medical Group to install a nuBeam system for BNCT at Shonan Kamakura General Hospital in Kamakura, Japan. Clinical trials with involvement of patients with recurrent head and neck cancer will be initiated if the Finnish health authority gives an approval.
The fifth BNCT clinic is being built in Xiamen Humanity Hospital (Xiamen, Fujian, China). For this clinic, Neuboron Medtech Ltd. (Nanjing, Jiangsu, China), TAE Life Sciences (Foothill Ranch, CA, USA), and the Budker Institute of Nuclear Physics were commissioned to manufacture a 2.5 MeV, 10 mA tandem accelerator with vacuum insulation and a lithium target, prototypes of which were proposed and developed at the Budker Institute of Nuclear Physics (Novosibirsk, Russia). This facility was expected to enter operation by fall 2019.
The sixth clinic is being built in Osaka, Japan. Kansai BNCT Medical Center has a Sumitomo Heavy Industries cyclotron with an energy of 30 MeV and a current of 2 mA, with a beryllium target.
It should be noted that only three accelerators meet the requirements of the International Atomic Energy Agency (IAEA) in respect of current parameters and give a more monochromatic neutron spectrum [9]. The latter is necessary to reduce the adverse effects of γ radiation and thermal neutrons. Another important factor in minimizing the adverse effects of irradiation is the main characteristic of the neutron beam, namely the homogeneous distribution of thermal neutrons in the tumor, including the area around the tumor and areas suspected of harboring tumors. This is because the absorbed dose in a healthy tissue is less than that in a boron‐containing tumor tissue. Also, for the production of parallel beams of focused neutrons, a neutron collimator is required. The following accelerators are available: the linac, manufactured by Hitachi; the tandem accelerator with vacuum insulation, manufactured by the Budker Institute of Nuclear Physics; and the direct‐acting electrostatic accelerator Hyperion™, manufactured by Neutron Therapeutics. On the other hand, the requirements of IAEA are out of date and need to be revised in accordance with technological progress and development.
Accordingly, today we are witnessing a surge in the development and the clinical research and testing of BNCT based on the use of different types of accelerator. Clinical trials of several accelerators are undergoing: Phase II clinical trials are underway in Japan and trials are soon to be initiated in Finland [1]. The high‐tech companies are open to engaging in this process; for example, TAE Life Sciences is developing their own accelerator, which is compact and designed for optimal BNCT delivery. In case of successful clinical trials, BNCT undoubtedly has great prospects for development and implementation in routine practice.
As for its use on the territory of the Russian Federation, it is planned to build a clinic for BNCT in collaboration with the Budker Institute of Nuclear Physics using a tandem accelerator characterized by a proton beam current of 9 mA and an energy of 2.3 MeV [10]. To gain the necessary proton beam, a new type of particle accelerator has been proposed and built – a tandem accelerator with vacuum insulation. With this new accelerator, the values of current and energy required for BNCT have been obtained. A lithium target has been developed and used to generate neutrons. This target contains a thin layer of lithium evaporated over backing, which is being effectively cooled and is radiation‐resistant. Targets of this design with some variations are now in use within almost every project. The moderator and compound reflector have been used in the beam‐shaping assembly for the first time, thus increasing the quality of the resulting therapeutic neutron beam. Ideas realized in the charged particle accelerator, in the neutron‐generating target, and in the beam‐shaping assembly are protected by patents. Of course, the constant functioning of these accelerators is accelerating the identification and testing of new boron isotope delivery agents, and their entry into the market.
2.2. Physical bases
BNCT is based on nuclear capture and 10B(n, α)7Li fission reactions [11]. 10B atoms capture the thermal neutrons and instantly decay to produce an alpha particle (4He), a recoiled lithium nucleus (7Li), and low linear energy transfer (LET) γ radiation (Figure 1).
FIGURE 1.
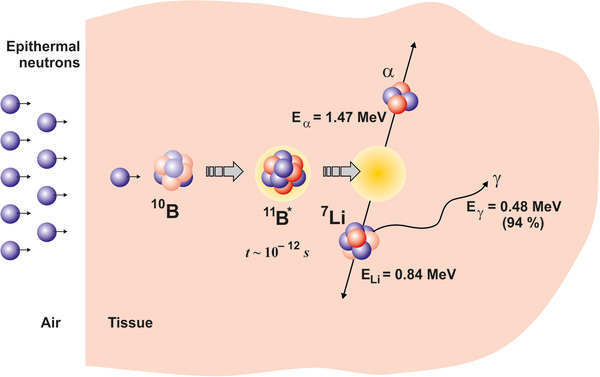
The principle of boron neutron capture therapy action on malignant cells
10B isotope atoms absorb low‐energy thermal neutrons (< 0.5 eV), resulting in the reaction: 10B + n → 7Li + 4He, wherein alpha particles acquire the high‐LET, ≈150 keV/μm, 7Li ion, ≈175 keV/μm [12]. A feature of these particles is that they provide a high energy along their very brief pathway (< 10 μm), which is comparable to the diameter of one cell. Also the effectiveness of BNCT depends on the intracellular localization of 10B. Thus, only cells containing 10B are destroyed by the use of these methods. It is assumed that any cells not containing 10B are spared from the high‐LET radiation. The minimum concentration of 10B required for BNCT (lethal damage) is about 1 × 109 10B atoms per cell or 20 μg/g of tissue [1]. To measure boron pools in vitro, equipment with subcellular scale resolution, for example, a secondary ion mass spectrometer (SIMS), is used [13]. The effectiveness of BNCT depends more on the delivery of 10B in the tumor than on the characteristics of beam. Consequently, the development of boron delivery agents is very active today, as discussed further below. For the administration of a boron compound, the intravenous infusion is used usually, followed by the irradiation with thermal neutrons. Because of the specificity of delivery agents of 10B to tumor cells, the neutron beams selectively destroy the boron‐containing tumor cells, wherein surrounding normal tissue stay intact. The γ radiation can penetrate tissue, contributing to the total absorbed dose, and therefore small collimator‐scattered proton beams are considered to be responsible for some adverse effects of BNCT [14, 15].
2.3. Delivery agents
The requirements necessary for boron delivery agents are listed below: low normal tissue uptake with high tumor tissue uptake, boron concentration in tumor should be ∼20 μg 10B/g tumor tissue, tumor‐normal tissue (T/N) and tumor‐blood (T/B) boron concentration ratios not less than 3, rapid clearance from blood and normal tissues, low systemic toxicity, and constant concentration in tumor during BNCT [16].
Three groups of boron delivery agents are distinguished on the basis of their specificity regarding tumor cells and toxicity [11]:
2.3.1. First‐generation boron compounds
Boric acid and its derivatives used in the 1950s and 1960s in the first clinical trials. These were elementary chemical compounds that were non‐discriminatory and had low specificity for tumor.
2.3.2. Second‐generation boron compounds
These include sodium mercaptoundecahydro‐closo‐dodecaborate (Na2B12H11SH) or sodium borocaptate (BSH), and the boron‐containing amino acid (L)‐4‐dihydroxy‐borylphenylalanine, or boronophenylalanine (BPA), which were used in 1960s. It is noteworthy that these compounds are currently used in many research studies and clinical trials [17, 18], e.g., L‐BPA as a fructose complex (BPA‐F, 2‐fluoro‐4‐borono‐l‐phenylalanine), or the combination of L‐BPA and BSH, or (18)F‐BPA [where (18)F is used as positron emitters] to determine the uptake of BPA‐F by positron emission tomography (PET). L‐[(18)F]BPA/PET is essential for selecting the appropriate candidate for BNCT by evaluating the BPA accumulation in the tumor using T/N or T/B ratio. It has been already used in a routine clinical practice before BNCT. BPA enters tumor cells by selective uptake mediated by the system L‐amino acid transporters (LAT1). The expression of LAT1 is highly up‐regulated in various cancers, where it is presumed to contribute to tumor growth by increasing the amino acid supply. The functions of the different transporters (ATB0,+, LAT1, and LAT2) in BPA uptake has been evaluated by high‐performance liquid chromatography [19]. Furthermore, (18)F‐BPA is also a substrate of LAT1 and shows comparable transport ability with BPA into the tumors, and tissue boron concentration of BPA can be quantitatively estimated using L‐[(18)F]BPA/PET [20, 21, 22, 23].
BSH contains 12 boron atoms and is an anionic polyhedral borane icosahedron. It is considered that BSH uses passive diffusion through the plasma membrane to enter tumor cells [24]. BSH has a high T/N ratio with weak accumulation in tumor cells, while BPA accumulates well in tumor cells but has a low T/N ratio [25, 26]. At 2 h after the intravenous administration of BPA, the maximum T/N boron concentration ratios are observed [27]. In 1994, BPA‐fructose complex was used to treat glioblastoma [28]. It is interesting that BPA and boron‐containing unnatural amino acid, 1‐amino‐3‐borono‐cyclopentanecarboxylic acid (cis‐ABCPC) could deliver approximately 70% of the boron in the free or bound form to the nucleus and cytoplasm of human glioblastoma cells [13]. There is some balance between intracellular and extracellular boron concentrations. In the case of BPA, the presence of phenylalanine in the nutrient medium may affect the intracellular free boron. Accordingly, it would be better to give patients a low phenylalanine diet before BNCT [13]. In a non‐clinical experimental study, it has been shown that in the treatment of osteosarcoma, a high boron concentration in tumor is necessary prior to BNCT as a valid therapeutic option [21]. As regards the excretion route of BPA and its metabolites, a high concentration of boron is found in the kidneys [27, 29].
2.3.3. Third‐generation boron compounds
Since the first‐ and second‐generation compounds did not adequately satisfy the requirements for boron delivery compounds indicated above, third‐generation agents including one or more polyhedral anions of borane or carboranes have been investigated [30]. The stable boron delivery agents with low and high molecular weight connected with a tumor‐targeting component or moiety via a hydrolytically stable linkage (boron carriers) belong to this group of compounds (Figure 2). In particular, these third‐generation boron compounds include boronated DNA intercalators [30], boronated amino acids [31], boronated lipopeptides [32], boronated peptides [33], boronated folate receptor [34], boronated epidermal growth factor (EGF) or epidermal growth factor receptor (EGFR) monoclonal antibodies (MAbs) [35], boron‐containing immunoliposomes [36] and liposomes [37, 38], BSH‐loaded polymeric micelles [39], transferrin‐polyethylene glycol liposomes [40], maleimide‐functionalized closo‐dodecaborate albumin conjugates [41], carboranyl nucleosides [42], boronated porphyrins [43], boron‐containing nanoparticles [44] coupled with cationic microbubble‐assisted focused ultrasound (MB+FUS) treatment [45], pentanuclear porphyrazine complexes [46], boronated cyclic peptides [47], and boron nitride nanotubes [48].
FIGURE 2.
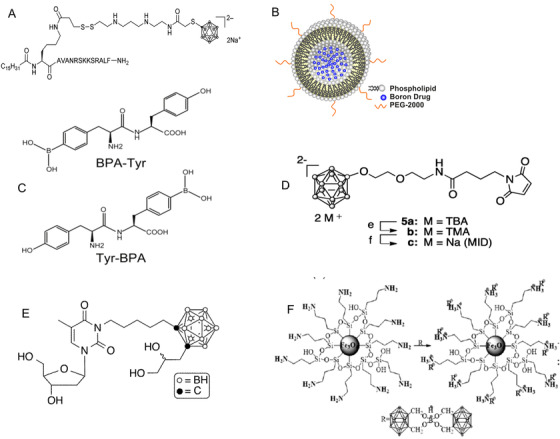
Chemical structures of several boron compounds, including third‐generation boron compounds. A: boronated lipopeptides [32]. B: boron‐containing liposomes [38]. C: boronated peptides [33]. D: maleimide‐functionalized closo‐dodecaborate albumin conjugates [41]. E: carboranyl nucleosides [42]. F: boron‐containing nanoparticles [44]
Promising results have been achieved using electroporation as well as FUS treatment and pulsed high‐intensity focused ultrasound (HIFU) as methods for the delivery of boronated compounds to tumor cells [43]. It has been shown that electroporation can significantly increase the absolute and relative tumor boron concentrations and assist in optimizing the qualitative micro distribution of boron, as well as the retention of boron in vitro and in vivo, for different tumor cells [49, 50]. To increase the efficiency of boron delivery to the tumor, combination of large boronated compounds with FUS treatment has been proposed since FUS facilitates the transport of such compounds across the blood‐brain barrier (BBB) into tumor [45].
One of the problems that nuclear doctors face when using neutron irradiation is the lack of a quantitative imaging method for evaluation of the boron concentration. To solve this problem and to achieve selective tumor accumulation and reduced toxicity, several approaches have been applied: (1) coating of boronated porphyrins with biocompatible poly(lactide‐co‐glycolide)‐monomethoxypoly(polyethylene‐glycol) (PLGA‐mPEG) micelles [43], functionalizing mesoporous silica nanoparticles [51], labeling MMT1242 with a nitrobenzoxadiazole frame, which emits green‐yellow fluorescence [52], and so on, in general, labeling vesicles by different fluorophores or molecules with fluorescence properties [53, 54]. Ion microscopy may also be used to evaluate the distribution of isotope 10B, as was shown in two brain tumor models, the 9L rat gliosarcoma and the F98 rat glioma [55].
Generally, these compounds show greater specificity for tumor cells, tumor cell nuclei, and tumor cell DNA, so the required concentration of boron compounds for effective BNCT can be reduced. All these compounds are currently under evaluation, but only BPA and BSH are used in the clinic. This is why, in parallel to the search for new boron delivery agents, there is a need to improve the dosing and delivery of BPA and BSH.
2.4. The role and impacts of the BBB in brain drug delivery
BNCT is aimed at treating difficult‐to‐treat tumors, particularly brain tumors, where surgery, chemotherapy, and radiotherapy would either be ineffective or lead to incapacity of the patient. Acceptable clinical results of pharmacotherapy for brain diseases are dependent upon safe drug delivery into the brain parenchyma. Brain capillary endothelial cells, supported by astrocytes and pericytes, form the BBB, which controls drug transport into the brain [56]. Most pharmaceuticals do not sufficiently penetrate into the central nervous system (CNS) and fail to yield the intended therapeutic outcomes, which is why the use of an in vitro BBB model for screening studies may be of value in the early safety assessment of new boron delivery drugs [19].
The boron delivery agents could interact with the solute carrier transporters which are involved in influx transport of substances through the BBB, and other potentially involved systems and transporters include the choline transport system, the nucleoside transport system, peptide transporters, the hexose transport system, the neutral amino acid transporter, the beta amino acid transporter, anionic amino acid transporters, cationic amino acid transporters, the monocarboxylic acid transport system, and the medium‐chain fatty acid transporter [56]. It is worthy of note that in glioblastoma there is disruption of the BBB by the primary tumor sites and that this altered BBB allows passive accumulation of drugs in the brain [57].
3. MECHANISMS OF BNCT‐INDUCED CELL DEATH
All ionizing radiation (X and γ rays, alpha and beta particles, neutrons, protons, and so on) has a pronounced biological effect [58]. In the process of converting ionizing radiation into chemical energy in the body, active centers of radiation‐chemical reactions are generated, and ionization and excitation of atoms and molecules occur [59]. Radiation breaks the bonds between atoms. Free radicals are formed in the case of paramagnetic breakage of bonds, while ionic fragments are formed in the case of diamagnetic breakage of bonds.
The primary cell target of direct ionizing radiation is DNA, but besides this it can damage different cellular macromolecules, resulting in the modulation of their functions [60]. Free radicals and ionic fragments produced by radiolysis of water (non‐direct effect) may damage such macromolecules as nucleic acids, proteins, lipids, and polysaccharides and particularly influence their stability or degradation in response to radiation stress [61]. It is well known that lipid peroxidation due to free radicals results in damage to unsaturated fatty acids in cell membranes, leading to disruption of active transport of substances through the cell membrane, reduction of ionic gradients in the cell, release of enzymes from their nuclear localization, and, as a result, disorganization of nuclear structures and cell death [62, 63]. In addition, there are important biological effects, namely irradiation‐induced bystander effects which are the result of interactions between irradiated and non‐irradiated cells [64, 65]. The following bystander effects are distinguished: (1) the abscopal effect (or long‐range bystander responses), which mediated through lymphatic or circulatory systems and gap junction; (2) short‐range bystander responses, which occur in neighboring non‐targeted cells. The type of bystander response depends on type of received radiations (γ radiation, α particle, neutron, and proton). The abscopal effect of BCNT was shown in the study [66], provided the proof of principle.
Among the various forms of molecular damage, a special place is occupied by DNA radiation damage. If damage to other types of molecule can be compensated by the remaining intact protein molecules, polysaccharides, and other molecules, such a path in DNA is excluded. Ionizing radiation causes different DNA lesions, including single‐strand breaks (SSB), double‐strand breaks (DSB), base damage, interruption of the sugar‐phosphate backbone, and DNA‐DNA and DNA‐protein cross links [67]. Nearly 40 DSBs, 1000 SSBs, and 3000 damaged bases are induced by 1 Gy of X rays in one cell [68]. In general, BNCT causes lethal chromosome aberrations (dicentrics, rings, micronuclei, and large deletions) than conventional radiotherapy in tumor cells [69]. In the event of DNA damage, two alternative pathways, i.e., cycle arrest/DNA repair and apoptosis, are activated by the tumor suppressor p53, depending on the type of p53 modifications (e.g., acetylation, phosphorylation, methylation) [70]. It has been demonstrated that BNCT can increase apoptosis by the Bcl‐2/Bax pathway in glioma cells and by the mitochondria‐mediated pathway through inducing the release of cytochrome c and the activation of Caspase‐9 proteins [71]. The fast and thermalized neutrons are responsible for 50%‐65% of DNA strand breaks; although this is less than the value for X rays, it is still the majority of the DNA strand breaks [72]. With increasing10B isotope concentration, the amount of SSBs and DSBs could increase in thermalized neutrons.
Besides this, ionizing radiation causes endoplasmic reticulum stress and reactive oxygen species‐dependent and ‐independent damages in cells that involves macromolecular (basically, DNA) damage, and all these factors may induce autophagy [73]. The autophagy signaling begins with the inhibition of the Akt/mTOR pathway. At the same time, molecules associated with autophagy form another complex through the PI3K class 3 complex and are recruited over a double‐membrane structure to form autophagosomes, which merge with lysosomes and lead to degradation of the contents. It is worth noting that in cancer, myopathies, neurodegeneration, and heart, liver, and gastrointestinal disorders, autophagy is deregulated. Cancer development is closely associated with autophagy: in various cancers and neurodegenerative disorders, mutated genes such as Beclin1, PARK2, and PINK1 are found [74]. Malignant cells with increased autophagy appear to be highly resistant to various stresses and chemotherapy compared to their normal counterparts [75]. During BNCT, autophagy is induced by the factors described above, and in view of the fact that malignant cells already have an enhanced level of autophagy, it has been suggested that the efficacy of chemotherapy can be improved with autophagy inhibitors.
The types of induced DNA lesions, their distribution, and the mechanism of the repair mechanism depend on the type of radiation used during BNCT, and particularly on LET characteristics (each component of ionizing radiation has different LET characteristics). Low‐LET γ rays in the beam, as well as those resulting from the capture of thermal neutrons by hydrogen atoms [1H(n,γ)2H], high‐LET protons obtained as a result of scattering of fast neutrons, and high‐LET protons as a result of capture of thermal neutrons by nitrogen atoms [14N(n,p)14C] produce a non‐specific background dose. Previously, it was shown that the relative biological effectiveness (RBE) values for the fast neutron and 14N(n,p)14C reaction components of the total dose were too low [76, 77]. At the same time, the apparent RBE for the 10B(n,α)7Li reaction with BPA was higher than that with any other boron compound tested, namely the sulfhydryl dodecaborate monomer or dimer, or boric acid. In this connection, different effects could be received from different LET radiations because, in comparison with low‐LET radiation, high‐LET radiation induces DSBs, broken chromosomes, and complex chromosome rearrangements very efficiently [78]. SSBs and base damages are of minor relevance for cell survival insofar as the base excision repair mechanism efficiently repairs these lesions [79].
Two pathways, non‐homologous end joining (NHEJ) and homologous recombination (HR), participate in reparation of the majority of DSBs induced by low‐LET radiation [80, 81]. DSBs produced by high‐LET radiation mostly represent the complex type, little DSB repair occurs [67]. This has been explained by the fact that high‐LET radiation induces high RBE due to inefficient DNA repair, Ku‐dependent NHEJ, which in turn may be associated with ineffective binding of Ku proteins with DNA [82]. When cells are exposed to high‐LET radiation, traces of heavy ions do more damage to neighboring DNA components than traces of low‐LET radiation, and it generates short DNA fragments of less than < 40 bp. These short DNA fragments impact on Ku binding efficiency, which lead to inefficient NHEJ, more cell death, and high RBE. The MRN complex which contains MRE11, RAD50, and NBS1, can initiate HR repair; ATM and ATR are involved in the signaling and various phosphorylation downstream participants. The choice of NHEJ or HR is dependent on BRCA1, which counteracts TP53BP1 at DSB ends in the S and G2 phases, thereby impeding NHEJ and facilitating HR. Which of the pathways will be chosen for DNA repair depends on these mediators (53BP1 and BRCA1; Ku proteins and the MRN complex).
Less than 5% of DSBs produced by low‐LET radiation cannot be repaired due to plenty complexity. The cell will continue its cell cycle if the DNA damage can be completely and correctly repaired. Otherwise, there will be the initiation of cell cycle arrest in the G2/M phase, cell death by apoptosis, mitotic catastrophe, or senescence. It was shown for BNCT that in the in vitro experiments, glioma stem/progenitor cells were killed through cell cycle arrest and apoptosis [71].
For further development of BNCT, it is necessary to evaluate early and late markers of the cellular responses after BNCT. It has been shown that γH2AX (phosphorylated histone H2AX), TP53BP1, poly(ADP‐ribose), and high‐mobility group protein 1 (HMGB1) may be markers of value in monitoring the DNA damage induced by BNCT [18]. The persistent presence of γH2AX and PAR in the nuclei may serve as a late marker, whereas HMGB1 up‐regulation could be an early marker [83]. HMGB1 directly binds to a variety of bulky DNA lesions and allows them to participate in DNA repair pathways, including NER, base excision repair, DNA mismatch repair, and NHEJ [84, 85]. Respectively, lack of HMGB1 under oxidative stress, chemotherapy, and irradiation leads to DNA damage and decreases DNA repair.
In conclusion, it is worthy of note that the exact mechanism of cell death after BNCT is unknown. There are several reasons why the cell may die, relating in particular to the cell type, the cell cycle phase when irradiation occurs, the oxygen supply, and the radiation dose [86]. Hematopoietic, lymphoid, and leukemia cells are susceptible to rapid irradiation‐induced cell death by apoptosis. The apoptosis is more common among most solid tumors than mitotic cell death. However, when irradiated, most normal cells must follow the pathway of senescence.
4. BNCT IN CANCER TREATMENT
BNCT combines two fundamental approaches, i.e., chemotherapy and traditional radiotherapy. While the selective concentration of boron compounds in tumor cells can enhance the effect of neutron beam radiation [87], BNCT may fail because of non‐specificity of the boron delivery agents, the presence of boron delivery agents in a high concentration in the blood, and inadequate radiation dose [1].
Of course, this type of therapy is not yet a widely available cancer treatment, and indications for its use are limited. To date, BNCT has been applied to treat the following cancers: glioblastoma multiforme [88], head and neck cancer [89], multifocal hepatocellular carcinoma [90], recurrent lung cancer [91], squamous cell carcinomas, salivary gland carcinomas, sarcomas, recurrent malignant meningioma [92], and extramammary Paget's disease [93]. Experiences with BCNT for cancer treatment are summarized in Table 1. Due to the fact that BNCT is more often used to treat glioblastoma, melanoma, head and neck cancer, and malignant mesothelioma, we focus on these malignancies below.
TABLE 1.
Boron neutron capture therapy in cancer treatment
| Type of cancer | Number of patients | Country | Year | Reference |
|---|---|---|---|---|
| Recurrent head and neck cancer | 62 | Japan | 2001‐2007 | [87] |
| Recurrent glioblastoma multiforme | 22 | Finland | 2008 | [120] |
| Head and neck cancer | 26 (19 squamous cell carcinomas, 4 salivary gland carcinomas, and 3 sarcomas) | Japan | 2001‐2009 | [18] |
| Recurrent malignant meningioma | 19 | Japan | 2005‐2011 | [121] |
| Recurrent malignant meningioma | 30 | Finland | 2003‐2010 | [122] |
| Recurrent late stage head and neck cancer | 10 | China | 2003‐2004 | [123] |
| Recurrent head and neck malignancies | 6 | Japan | 2004 | [124] |
| Recurrent malignant gliomas | 7 | Japan | 2013‐2014 | [125] |
| Glioblastoma | 21 | Japan | 2002‐2007 | [126] |
| Glioblastoma multiforme | 9 | Czech Republic | 2000‐2002 | [127] |
| Glioblastoma multiforme | 53 | USA | 1994‐1999 | [28] |
| Brain tumors | 22 | USA | 1996‐1999 | [128] |
| Glioblastoma multiforme | 6 | USA | 2002‐2003 | [129] |
| Extensive squamous cell carcinoma | 1 | Japan | 2007 | [130] |
| Glioblastoma multiforme | 17 | Sweden | 2002 | [131] |
| Recurrent hepatic cancer | 1 | Japan | 2011 | [132] |
| Recurrent lung cancer | 1 | Japan | 2012 | [133] |
| Recurrent laryngeal cancer | 9 | Finland | 2006‐2012 | [112] |
| Extramammary Paget's disease | 2 | Japan | 2012 | [134] |
| Vulvar melanoma and genital extramammary Paget's disease | 7 | Japan | 2005‐2014 | [93] |
4.1. Brain tumor
Glioblastoma multiforme (GBM) or grade IV glioma, according to the World Health Organization (WHO) classification, is the most common malignant brain cancer among adults. It has a very poor prognosis, and there is urgent need to improve patient stratification and treatment. GBM are neuroepithelial tumors. It originates from the supporting glial cells of the CNS. Several histological types of glial tumor have been identified, i.e., astrocytoma, oligodendroglioma, mixed oligo‐astrocytic tumor, and mixed glioneuronal tumor, which originate from astrocytic, oligodendroglial, mixed oligoastrocytic, and neuronal‐glial cells, respectively. On the basis of histopathologic characteristics (cytological atypia, anaplasia, mitotic activity, microvascular proliferation, and necrosis), the WHO classification categorizes gliomas from grade 1 (lowest grade) to grade 4 (highest grade). It should also be mentioned that integrated diagnosis incorporating all the tissue‐based information and the molecular genetic information can assist in improving clinicopathologic predictions, interobserver reproducibility, and therapeutic planning [94].
The mortality of brain cancer patients remained stable from 2000 to 2014, while the incidence varied between 2009 and 2013 according to sex: among men there was a significant decrease in the incidence of brain cancer and other nervous system cancers over this period (brain tumors: P = 0.005), whereas among women the incidence remained unchanged [95]. Primary brain tumors are the second most common pediatric malignant tumors [96]. According to the data of National Cancer Institute, approximately 23,820 new cases of brain cancer and other nervous system cancers were diagnosed in the United States in 2019 [97]. Gliomas account for 32% of all primary CNS tumors, 17% of which are astrocytic tumors; 28% are glioblastomas in young adults (20‐34 years of age) [98]. The life expectancy of patients with GBM is approximately 12‐15 months, even when using the standard therapy, surgery and radiotherapy with the simultaneous administration of temozolomide. GBM is the second most common cause of cancer death in children under the age of 15. Despite the generally very poor prognosis, 2‐year overall survival rates of 25‐40% have been observed for high‐grade gliomas [99].
The different subtypes of GBM respond differently to treatments. There is a high heterogeneity between different types of cell line. It is associated with the expression and mutations in several key genes. Several genes (PDGFRA, IDH1, EGFR, and NF1) are considered as genetic markers to improve the classification of subtypes [100, 101].
Treatment of glioblastoma is associated with several difficulties, among which are the limited penetrability of the BBB, the resistance to conventional therapy, and low DNA repair capacity. Unfortunately, the clinical results obtained by different groups have not shown sufficient effectiveness of BNCT as a treatment modality for glioblastoma [102, 103, 104, 105]. According to the European Organization for the Research and Treatment of Cancer, the best treatment results are obtained using the combination of postoperative photon irradiation with the administration of temozolomide, and only effective and newly developed delivery drugs suitable for inclusion in clinical trials could change this paradigm.
4.2. Head and neck cancer
Head and neck squamous cell carcinoma (HNSCC), commonly named head and neck cancer, is a common malignant cancer with a high mortality and morbidity [106]. Worldwide, approximately 650,000 cases and 330,000 deaths due to HNSCC occur annually. Head and neck cancer is formed from the squamous cells that line the surface of the mucous membrane inside the head and neck (for example, inside the mouth, nose, and throat) and is also formed from the salivary gland in rare cases. In most cases (75%), these cancers are caused by tobacco and alcohol use [107]. Human papillomavirus type 16 and Epstein‐Barr virus infection can also be considered risk factors for some types of head and neck cancer, especially oropharyngeal and nasopharyngeal cancers.
Treatment of HNSCC includes surgery, radiotherapy, and platinum‐based chemotherapy. The only targeted therapy for HNSCC is cetuximab, which was approved by FDA, and represent a monoclonal antibody targeting EGFR, but it has displayed limited efficacy due to the emergence of resistance [108]. In addition, HNSCC is often radio‐ and chemoresistant. BNCT could be a solution to this problem in general. It was shown in vitro that BNCT inhibits oral SCC cells in p53‐dependent and ‐independent manners [109]. Oral SCC cells with p53 mutations are more resistant to BNCT than those with p53 wild‐type; in addition, the absence of G1 arrest and subsequent apoptosis could contribute to the resistance [110]. Recently, the efficacy of BNCT on HNSCC has been demonstrated in different clinical trials with an overall response rate of up to 90% [111, 112, 113]. In spite of this, due to non‐homogeneous distribution of BPA‐F in tumor, recurrence has often been observed [112].
4.3. Melanoma
Malignant melanoma is considered an aggressive cancer in humans and is responsible for almost 60% of all lethal skin tumors. Melanomas occur when unrepaired DNA damage to skin cells causes mutations that lead to their reproduction. There are several types of melanoma: nodular melanoma, acral lentiginous melanoma, lentigo maligna, and superficial spreading melanoma. Nodular melanoma is invasive from the outset, whereas the remaining three types begin in situ on the top layers of the skin and may become invasive. The occurrence of melanoma is due to the interaction between exogenous and endogenous risk factors. The incidence of melanoma has increased significantly in the past two decades among both men and women [95]. Age‐standardized incidence rates from 2000 to 2014 were 16.6 and 26.8 per 100,000 persons for females and males, respectively. It has been shown that melanoma is characterized by high genomic instability [114]. This is a feature uncommonly encountered in benign melanocytic lesions, including Spitz nevi.
The standard treatment of melanoma is surgical excision. However, when the tumor is at an advanced stage with metastases, the effectiveness of these therapies is poor. In 1987, BNCT was applied to treat malignant melanoma using BPA [6]. This was the first time that BNCT had been used outside the CNS. Patients with melanoma who are treated with the same BNCT protocols can show different clinical outcomes despite identical histopathologic diagnoses [29]. Because of the ability of melanomas to metastasize, both immunotherapy and BNCT, could be recommended for treatment of melanomas in difficult anatomic regions, such as the vulva [1].
4.4. Malignant mesothelioma
Malignant mesothelioma (MM) is a rare and aggressive tumor with a poor prognosis. The incidence and mortality of MM depend on the past level of exposure to asbestos, and MM may occur even after a latency period of 30‐50 years. The median survival since diagnosis is shorter than 9‐12 months [115]. While malignant cells are found in the pleura or the peritoneum, MM may also form in the heart or testicles, though this is rare [116]. The presence of several radiosensitive tissues decreases the effectiveness of conventional radiotherapy and imposes restrictions on the maximum dose [117]. An effective targeted therapy does not exist due to the lack of highly specific molecular markers, which also reduces the possibility of early diagnosis of MM [118]. BNCT is a promising treatment for inoperable patients due to their age or the presence of other illnesses [119].
5. CRITICAL PERSPECTIVE ON BNCT
Several obstacles stand in the way of development of BNCT. The transition from one technology involving a source of neutrons and reactors to another, accelerators, has required both time and financial investment, as well as the development of principles of its use. The latter was spelled out in the requirements of the IAEA [9]. This technical memorandum for the parameters of a neutron beam was developed in 2001. For BNCT implementation, it is necessary to have a beam of epithermal neutrons of high intensity (with energies from 0.5 eV to 10 keV). In this case, the neutron spectrum of the beam should be such that the maximum density of the thermal neutron flux is obtained at the location of the tumor. Over the past several decades, many projects involving accelerator neutron sources have been proposed for BNCT, but only a small number are approaching successful completion because of the complexity of the task. Neutron beam parameters of all accelerator‐based neutron sources meet the requirements of IAEA [9]: providing intensity of epithermal neutron flux more than 10˄(9)n × cm˄(‐2) × s˄(‐1); fast neutron and photon beam contamination less than 2 × 10˄(‐12)Gy × cm˄(2)/n. Three neutron sources (№№ 3, 4, 5), using low‐energy protons and lithium target, provide neutron flux with a narrower energy distribution and a smaller contribution of thermal and fast neutrons, which is important for the quality of therapy. For ongoing development and improvement of different accelerators, the IAEA technical memorandum needs to be revised periodically.
Regarding the development of new delivery agents, the limiting factor is the lack of a large number of accelerators in different countries for their routine testing and validation and to facilitate their entry into the market. The construction and launch of new BNCT clinics using accelerators will lend impetus to the search for third‐generation boron compounds. Indeed, we expect new accelerators to be commissioned in the near future. Another limiting factor is the availability of 10B, in terms of both its sale on the market and the procurement of raw materials for its manufacture. Currently, around the world a limited number of companies sell 10B, and a few produce delivery agents for BNCT; for example, in Russia there are no manufacturers of 10B or its derivatives.
The agents being developed should all meet the requirements, including low cytotoxicity; sufficient concentration of 10B isotope in cancer cells; a definite ratio between 10B isotope concentration in cancer cells, blood, and normal cells; chemical stability; water solubility; and preservation of a constant concentration during the procedure. The alternative to the discovery of new delivery agents is to change the dosage and methods of administration for already existing boron compounds, BSH and BPA (e.g., by increasing the dose of BPA and the infusion time), or to further develop delivery methods, in particular FUS treatment and electroporation.
6. CONCLUSIONS
Although several accelerators for BNCT are currently being evaluated in clinical trials, and the results will be announced in the near future, the need for a specific delivery agent for the 10B isotope with high tumor accumulation could obstruct the use of BNCT as a cancer treatment modality. Despite some problems with the direct source of neutrons and radiation dosimetry, the main challenges are the development of a new and more effective delivery vehicle or a new approach to delivery and better microdistribution of already existing delivery drugs (BPA and BSH). Pharmacological companies and research laboratories should have access to accelerators for large‐scale screening of new, more specific boron delivery agents. Only after the announcement of the results of clinical trials in which all the above challenges have been overcome will one convincingly be able to say that BNCT is a cancer treatment modality.
FUNDING
This study was supported by the Russian Science Foundation (project No. 19‐72‐30005) and the Russian State funded budget project (ICBFM SB RAS АААА‐А17‐117020210023‐1).
AUTHOR CONTRIBUTIONS
V.A. Richter had the idea for the article, M.A. Dymova performed the literature search and data analysis, and S.Y. Taskaev and E.V. Kuligina drafted and critically revised the work. All authors read and approved the final manuscript.
COMPETING INTERESTS
The authors declare that they have no conflict of interests.
Dymova MA,Taskaev SY, Richter VA, Kuligina EV. Boron neutron capture therapy: Current status and future perspectives. Cancer Communications. 2020;40:406–421. 10.1002/cac2.12089
REFERENCES
- 1. Barth RF, Zhang Z, Liu T. A realistic appraisal of boron neutron capture therapy as a cancer treatment modality. Cancer Commun (London, England). 2018;38:36 10.1186/s40880-018-0280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chadwick J. The Existence of a Neutron. PRSL. 1932. 10.1098/rspa.1932.0112. [DOI] [Google Scholar]
- 3. Locher G. Biological effects and therapeutic possibilities of neutrons. Am J Roentgenol Radium Ther. 1936;36 (1):1‐13. https://ci.nii.ac.jp/naid/10026692840/. Accessed 20 Feb 2018. [Google Scholar]
- 4. Farr LE, Sweet WH, Locksley HB RJ. Neutron capture therapy of gliomas using boron‐10. Trans Am Neurol Assoc. 1954;79:110‐3. [PubMed] [Google Scholar]
- 5. Hatanaka H. Clinical results of boron neutron capture therapy. Basic Life Sci. 1990;54:15‐21. http://www.ncbi.nlm.nih.gov/pubmed/2268236. Accessed 20 Feb 2018. [DOI] [PubMed] [Google Scholar]
- 6. Mishima Y, Ichihashi M, Hatta S, Honda C, Yamamura K, Nakagawa T, et al. First human clinical trial of melanoma neutron capture. Diagnosis and therapy. Strahlenther Onkol. 1989;165:251‐4. http://www.ncbi.nlm.nih.gov/pubmed/2494743. Accessed 20 Feb 2018. [PubMed] [Google Scholar]
- 7. Kumada H, Matsumura A, Sakurai H, Sakae T, Yoshioka M, Kobayashi H, et al. Project for the development of the linac based NCT facility in University of Tsukuba. Appl Radiat Isot. 2014;88:211‐5. 10.1016/J.APRADISO.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 8. Yoshioka M. Review of accelerator‐based boron neutron capture therapy machines. 2016. http://accelconf.web.cern.ch/AccelConf/ipac2016/papers/thxb01.pdf. Accessed 9 Aug 2019.
- 9. Current status of neutron capture therapy . 2001. https://www-pub.iaea.org/MTCD/publications/PDF/te_1223_prn.pdf. Accessed 9 Aug 2019.
- 10. Taskaev SY. Accelerator based epithermal neutron source. Phys Part Nucl. 2015;46:956‐90. 10.1134/S1063779615060064. [DOI] [Google Scholar]
- 11. Nedunchezhian K. Boron Neutron Capture Therapy ‐ A Literature Review. J Clin Diagnostic Res. 2016;10:1‐4. 10.7860/JCDR/2016/19890.9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suzuki M. Boron neutron capture therapy (BNCT): a unique role in radiotherapy with a view to entering the accelerator‐based BNCT era. Int J Clin Oncol. 2019:1‐8. 10.1007/s10147-019-01480-4. [DOI] [PubMed] [Google Scholar]
- 13. Chandra S, Ahmad T, Barth RF, Kabalka GW. Quantitative evaluation of boron neutron capture therapy (BNCT) drugs for boron delivery and retention at subcellular‐scale resolution in human glioblastoma cells with imaging secondary ion mass spectrometry (SIMS). J Microsc. 2014;254:146‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carlsson J, Forssell‐Aronsson E, Glimelius B, Swedish Cancer Society Investigation Group . Radiation therapy through activation of stable nuclides. Acta Oncol. 2002;41:629‐34. http://www.ncbi.nlm.nih.gov/pubmed/14651206. Accessed 4 Jun 2018. [DOI] [PubMed] [Google Scholar]
- 15. Taskaev SY. VITA Means Life. Boron Neutron Capture Therapy of Cancer. Sci First Hand. 2012;32 http://scfh.ru/en/papers/vita-means-life-boron-neutron-capture-therapy-of-cancer/. Accessed 3 May 2019. [Google Scholar]
- 16. Barth RF, Mi P, Yang W. Boron delivery agents for neutron capture therapy of cancer. Cancer Commun. 2018;38:35 10.1186/s40880-018-0299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barth RF, H Vicente MG, Harling OK, Kiger W, Riley KJ, Binns PJ, et al. Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Radiat Oncol. 2012;7:146 10.1186/1748-717X-7-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kato I, Fujita Y, Maruhashi A, Kumada H, Ohmae M, Kirihata M, et al. Effectiveness of boron neutron capture therapy for recurrent head and neck malignancies. Appl Radiat Isot. 2009;67:S37‐42. 10.1016/j.apradiso.2009.03.103. [DOI] [PubMed] [Google Scholar]
- 19. Wongthai P, Hagiwara K, Miyoshi Y, Wiriyasermkul P, Wei L, Ohgaki R, et al. Boronophenylalanine, a boron delivery agent for boron neutron capture therapy, is transported by ATB 0,+ , LAT1 and LAT2. Cancer Sci. 2015;106:279‐86. 10.1111/cas.12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hanaoka K, Watabe T, Naka S, Kanai Y, Ikeda H, Horitsugi G, et al. FBPA PET in boron neutron capture therapy for cancer: Prediction of 10B concentration in the tumor and normal tissue in a rat xenograft model. EJNMMI Res. 2014;4:70 10.1186/s13550-014-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Watabe T, Hanaoka K, Naka S, Kanai Y, Ikeda H, Aoki M, et al. Practical calculation method to estimate the absolute boron concentration in tissues using 18F‐FBPA PET. Ann Nucl Med. 2017;31:481‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yoshimoto M, Honda N, Kurihara H, Hiroi K, Nakamura S, Ito M, et al. Non‐invasive estimation of 10 B‐4‐borono‐L‐phenylalanine‐derived boron concentration in tumors by PET using 4‐borono‐2‐18 F‐fluoro‐phenylalanine. Cancer Sci. 2018;109:1617‐26. 10.1111/cas.13553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yoshimoto M, Kurihara H, Honda N, Kawai K, Ohe K, Fujii H, et al. Predominant contribution of L‐type amino acid transporter to 4‐borono‐2‐18F‐fluoro‐phenylalanine uptake in human glioblastoma cells. Nucl Med Biol. 2013;40:625‐9. 10.1016/j.nucmedbio.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 24. Farhood B, Samadian H, Ghorbani M, Zakariaee SS, Knaup C. Physical, dosimetric and clinical aspects and delivery systems in neutron capture therapy. Reports Pract Oncol Radiother. 2018;23:462‐73. 10.1016/j.rpor.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goodman JH, Yang W, Barth RF, Gao Z, Boesel CP, Staubus AE, et al. Boron neutron capture therapy of brain tumors: biodistribution, pharmacokinetics, and radiation dosimetry sodium borocaptate in patients with gliomas. Neurosurgery. 2000;47:608‐21; discussion 621‐2. 10.1097/00006123-200009000-00016. [DOI] [PubMed] [Google Scholar]
- 26. Koivunoro H, Hippeläinen E, Auterinen I, Kankaanranta L, Kulvik M, Laakso J, et al. Biokinetic analysis of tissue boron (10B) concentrations of glioma patients treated with BNCT in Finland. Appl Radiat Isot. 2015;106:189‐94. 10.1016/j.apradiso.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 27. Ferrari C, Zonta C, Cansolino L, Clerici AM, Gaspari A, Altieri S, et al. Selective uptake of p‐boronophenylalanine by osteosarcoma cells for boron neutron capture therapy. Appl Radiat Isot. 2009;67:S341‐4. 10.1016/j.apradiso.2009.03.059. [DOI] [PubMed] [Google Scholar]
- 28. Diaz AZ. Assessment of the results from the phase I/II boron neutron capture therapy trials at the Brookhaven National Laboratory from a clinician's point of view. J Neurooncol. 2003;62:101‐9. [DOI] [PubMed] [Google Scholar]
- 29. Carpano M, Perona M, Rodriguez C, Nievas S, Olivera M, Santa Cruz GA, et al. Experimental Studies of Boronophenylalanine (10BPA) Biodistribution for the Individual Application of Boron Neutron Capture Therapy (BNCT) for Malignant Melanoma Treatment. Int J Radiat Oncol. 2015;93:344‐52. 10.1016/j.ijrobp.2015.05.039. [DOI] [PubMed] [Google Scholar]
- 30. Fuentes I, García‐Mendiola T, Sato S, Pita M, Nakamura H, Lorenzo E, et al. Metallacarboranes on the Road to Anticancer Therapies: Cellular Uptake, DNA Interaction, and Biological Evaluation of Cobaltabisdicarbollide [COSAN] −. Chem ‐ A Eur J. 2018;24:17239‐54. [DOI] [PubMed] [Google Scholar]
- 31. He T, Chittur SV, Musah RA. Impact on Glioblastoma U87 Cell Gene Expression of a Carborane Cluster‐Bearing Amino Acid: Implications for Carborane Toxicity in Mammalian Cells. ACS Chem Neurosci. 2019;10:1524‐34. [DOI] [PubMed] [Google Scholar]
- 32. Isono A, Tsuji M, Sanada Y, Matsushita A, Masunaga S, Hirayama T, et al. Design, Synthesis, and Evaluation of Lipopeptide Conjugates of Mercaptoundecahydrododecaborate for Boron Neutron Capture Therapy. ChemMedChem. 2019:823‐32. [DOI] [PubMed] [Google Scholar]
- 33. Miyabe J, Ohgaki R, Saito K, Wei L, Quan L, Jin C, et al. Boron delivery for boron neutron capture therapy targeting a cancer‐upregulated oligopeptide transporter. J Pharmacol Sci. 2019;139:215‐22. 10.1016/j.jphs.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 34. Kanemitsu T, Kawabata S, Fukumura M, Futamura G, Hiramatsu R, Nonoguchi N, et al. Folate receptor‐targeted novel boron compound for boron neutron capture therapy on F98 glioma‐bearing rats. Radiat Environ Biophys. 2019;58:59‐67. 10.1007/s00411-018-0765-2. [DOI] [PubMed] [Google Scholar]
- 35. Yang W, Barth RF, Wu G, Huo T, Tjarks W, Ciesielski M, et al. Convection enhanced delivery of boronated EGF as a molecular targeting agent for neutron capture therapy of brain tumors. J Neurooncol. 2009;95:355‐65. 10.1007/s11060-009-9945-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gadan MA, González SJ, Batalla M, Olivera MS, Policastro L, Sztejnberg ML. Reprint of Application of BNCT to the treatment of HER2+ breast cancer recurrences: Research and developments in Argentina. Appl Radiat Isot. 2015;106:260‐4. 10.1016/j.apradiso.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 37. Olusanya TOB, Calabrese G, Fatouros DG, Tsibouklis J, Smith JR. Liposome formulations of o‐carborane for the boron neutron capture therapy of cancer. Biophys Chem. 2019;247:25‐33. 10.1016/j.bpc.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 38. Luderer MJ, Muz B, Alhallak K, Sun J, Wasden K, Guenthner N, et al. Thermal Sensitive Liposomes Improve Delivery of Boronated Agents for Boron Neutron Capture Therapy. Pharm Res. 2019;36:144 10.1007/s11095-019-2670-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu J, Ai X, Zhang H, Zhuo W, Mi P. Polymeric micelles with endosome escape and redox‐responsive functions for enhanced intracellular drug delivery. J Biomed Nanotechnol. 2019;15:373‐81. [DOI] [PubMed] [Google Scholar]
- 40. Sonali, Singh RP , Singh N, Sharma G, Vijayakumar MR, Koch B, et al. Transferrin liposomes of docetaxel for brain‐targeted cancer applications: formulation and brain theranostics. Drug Deliv. 2016;23:1261‐71. 10.3109/10717544.2016.1162878. [DOI] [PubMed] [Google Scholar]
- 41. Kikuchi S, Kanoh D, Sato S, Sakurai Y, Suzuki M, Nakamura H. Maleimide‐functionalized closo‐dodecaborate albumin conjugates (MID‐AC): Unique ligation at cysteine and lysine residues enables efficient boron delivery to tumor for neutron capture therapy. J Control Release. 2016;237:160‐7. 10.1016/j.jconrel.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 42. Barth RF, Yang W, Nakkula RJ, Byun Y, Tjarks W, Chu Wu L, et al. Evaluation of TK1 targeting carboranyl thymidine analogs as potential delivery agents for neutron capture therapy of brain tumors. Appl Radiat Isot. 2015;106:251‐5. 10.1016/j.apradiso.2015.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shi Y, Li J, Zhang Z, Duan D, Zhang Z, Liu H, et al. Tracing Boron with Fluorescence and Positron Emission Tomography Imaging of Boronated Porphyrin Nanocomplex for Imaging‐Guided Boron Neutron Capture Therapy. ACS Appl Mater Interfaces. 2018;10:43387‐95. 10.1021/acsami.8b14682. [DOI] [PubMed] [Google Scholar]
- 44. Dukenbayev K, Korolkov IV, Tishkevich DI, Kozlovskiy AL, Trukhanov SV, Gorin YG, et al. Fe3O4 nanoparticles for complex targeted delivery and boron neutron capture therapy. Nanomaterials. 2019;9:1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45. Fan CH, Wang TW, Hsieh YK, Wang CF, Gao Z, Kim A, et al. Enhancing Boron Uptake in Brain Glioma by a Boron‐Polymer/Microbubble Complex with Focused Ultrasound. ACS Appl Mater Interfaces. 2019;11:11144‐56. [DOI] [PubMed] [Google Scholar]
- 46. Viola E, Donzello MP, Testani S, Luccisano G, Astolfi ML, Rizzoli C, et al. Tetra‐2,3‐pyrazinoporphyrazines with Peripherally Appended Pyridine Rings. 19. Pentanuclear Octa(2‐pyridyl)tetrapyrazinoporphyrazines Carrying Externally Carboranthiolate Groups: Physicochemical Properties and Potentialities as Anticancer Drugs. Inorg Chem. 2019;58:1120‐33. 10.1021/acs.inorgchem.8b02269. [DOI] [PubMed] [Google Scholar]
- 47. Kang W, Svirskis D, Sarojini V, McGregor AL, Bevitt J, Wu Z. Cyclic‐RGDyC functionalized liposomes for dual‐targeting of tumor vasculature and cancer cells in glioblastoma: An in vitro boron neutron capture therapy study. Oncotarget. 2017;8:36614‐27. 10.18632/oncotarget.16625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ferreira T, Miranda M, Rocha Z, Leal A, Gomes D, Sousa E. An Assessment of the Potential Use of BNNTs for Boron Neutron Capture Therapy. Nanomaterials. 2017;7:82 10.3390/nano7040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Garabalino MA, Olaiz N, Portu A, Saint Martin G, Thorp SI, Pozzi ECC, et al. Electroporation optimizes the uptake of boron‐10 by tumor for boron neutron capture therapy (BNCT) mediated by GB‐10: a boron biodistribution study in the hamster cheek pouch oral cancer model. Radiat Environ Biophys. 2019;58:455‐67. 10.1007/s00411-019-00796-z. [DOI] [PubMed] [Google Scholar]
- 50. Wu C‐Y, Chan P‐C, Chou L‐S, Chang C‐W, Yang F‐Y, Liu R‐S, et al. Pulsed‐focused ultrasound enhances boron drug accumulation in a human head and neck cancer xenograft‐bearing mouse model. Mol imaging Biol. 2014;16:95‐101. 10.1007/s11307-013-0675-2. [DOI] [PubMed] [Google Scholar]
- 51. Vares G, Jallet V, Matsumoto Y, Rentier C, Takayama K, Sasaki T, et al. Functionalized mesoporous silica nanoparticles for innovative boron‐neutron capture therapy of resistant cancers. Nanomedicine Nanotechnology, Biol Med. 2020;27:102195 10.1016/J.NANO.2020.102195. [DOI] [PubMed] [Google Scholar]
- 52. Tsurubuchi T, Shirakawa M, Kurosawa W, Matsumoto K, Ubagai R, Umishio H, et al. Evaluation of a Novel Boron‐Containing α‐d‐Mannopyranoside for BNCT. Cells. 2020;9:1277 10.3390/cells9051277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang D, Meng Y, Wang X, Xia G, Zhang Q. The Endocytic Mechanism and Cytotoxicity of Boron‐containing Vesicles. Chem Pharm Bull (Tokyo). 2020. 10.1248/cpb.c19-00971. [DOI] [PubMed] [Google Scholar]
- 54. Qi P, Chen Q, Tu D, Yao S, Zhang Y, Wang J, et al. The potential role of borophene as a radiosensitizer in boron neutron capture therapy (BNCT) and particle therapy (PT). Biomater Sci. 2020;8:2778‐85. 10.1039/d0bm00318b. [DOI] [PubMed] [Google Scholar]
- 55. Smith DR, Chandra S, Barth RF, Yang W, Joel DD, Coderre JA. Quantitative imaging and microlocalization of boron‐10 in brain tumors and infiltrating tumor cells by SIMS ion microscopy: relevance to neutron capture therapy. Cancer Res. 2001;61:8179‐87. http://www.ncbi.nlm.nih.gov/pubmed/11719448. Accessed 25 Sep 2019. [PubMed] [Google Scholar]
- 56. Barar J, Rafi MA, Pourseif MM, Omidi Y. Blood‐brain barrier transport machineries and targeted therapy of brain diseases. BioImpacts. 2016;6:225‐48. 10.15171/bi.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kim S‐S, Harford JB, Pirollo KF, Chang EH. Effective treatment of glioblastoma requires crossing the blood‐brain barrier and targeting tumors including cancer stem cells: The promise of nanomedicine. Biochem Biophys Res Commun. 2015;468:485‐9. 10.1016/j.bbrc.2015.06.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Donya M, Radford M, ElGuindy A, Firmin D, Yacoub MH. Radiation in medicine: Origins, risks and aspirations. Glob Cardiol Sci Pract. 2014;2014:437‐48. 10.5339/gcsp.2014.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Çalişkan B, Çalişkan AC. Interaction with Matter of Ionizing Radiation and Radiation Damages (Radicals) In: Ionizing Radiation Effects and Applications. InTech; 2018. 10.5772/intechopen.74691. [DOI] [Google Scholar]
- 60. Desouky O, Ding N, Zhou G. Targeted and non‐targeted effects of ionizing radiation. J Radiat Res Appl Sci. 2015;8:247‐54. 10.1016/J.JRRAS.2015.03.003. [DOI] [Google Scholar]
- 61. Isozaki T, Fujita M, Yamada S, Imadome K, Shoji Y, Yasuda T, et al. Effects of carbon ion irradiation and X‐ray irradiation on the ubiquitylated protein accumulation. Int J Oncol. 2016;49:144‐52. 10.3892/ijo.2016.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276‐85. 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 63. Gaschler MM, Stockwell BR. Lipid peroxidation in cell death. Biochem Biophys Res Commun. 2017;482:419‐25. 10.1016/j.bbrc.2016.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Little JB. Genomic instability and bystander effects: a historical perspective. Oncogene. 2003;22:6978‐87. 10.1038/sj.onc.1206988. [DOI] [PubMed] [Google Scholar]
- 65. Verma N, Tiku AB. Significance and nature of bystander responses induced by various agents. Mutat Res Mutat Res. 2017;773:104‐21. 10.1016/j.mrrev.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 66. Trivillin VA, Pozzi ECC, Colombo LL, Thorp SI, Garabalino MA, Monti Hughes A, et al. Abscopal effect of boron neutron capture therapy (BNCT): proof of principle in an experimental model of colon cancer. Radiat Environ Biophys. 2017;56:365‐75. 10.1007/s00411-017-0704-7. [DOI] [PubMed] [Google Scholar]
- 67. Maier P, Hartmann L, Wenz F, Herskind C, Maier P, Hartmann L, et al. Cellular Pathways in Response to Ionizing Radiation and Their Targetability for Tumor Radiosensitization. Int J Mol Sci. 2016;17:102 10.3390/ijms17010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hall EJ, Giaccia AJ. Radiobiology for the radiologist. Seventh edition. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012. https://trove.nla.gov.au/work/9970501?selectedversion=NBD46579610. Accessed 15 Mar 2019. [Google Scholar]
- 69. Ballarini F, Bakeine J, Bortolussi S, Bruschi P, Cansolino L, Clerici AM, et al. Cell death following BNCT: A theoretical approach based on Monte Carlo simulations. Appl Radiat Isot. 2011;69:1745‐7. 10.1016/J.APRADISO.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 70. Masunaga S, Kobayashi J, Tano K, Sanada Y, Suzuki M, Ono K. The Effect of p53 Status on Radio‐Sensitivity of Quiescent Tumor Cell Population Irradiated With γ‐Rays at Various Dose Rates. J Clin Med Res. 2018;10:815‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sun T, Zhang Z, Li B, Chen G, Xie X, Wei Y, et al. Boron neutron capture therapy induces cell cycle arrest and cell apoptosis of glioma stem/progenitor cells in vitro. Radiat Oncol. 2013;8:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jamsranjav E, Ito A, Kato Y, Tatebe Y, Takase N, Yoshida S. DNA Strand Breaks Induced by Fast and Thermal Neutrons from YAYOI Research Reactor in the Presence and Absence of Boric Acid. Radiat Res. 2019;191:483 10.1667/RR15249.1. [DOI] [PubMed] [Google Scholar]
- 73. Chaurasia M, Bhatt AN, Das A, Dwarakanath BS, Sharma K. Radiation‐induced autophagy: mechanisms and consequences. Free Radic Res. 2016;50:273‐90. 10.3109/10715762.2015.1129534. [DOI] [PubMed] [Google Scholar]
- 74. Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17:528‐42. 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bhutia SK, Mukhopadhyay S, Sinha N, Das DN, Panda PK, Patra SK, et al. Autophagy: cancer's friend or foe? Adv Cancer Res. 2013;118:61‐95. 10.1016/B978-0-12-407173-5.00003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Coderre JA, Makar MS, Micca PL, Nawrocky MM, Liu HB, Joel DD, et al. Derivations of relative biological effectiveness for the high‐let radiations produced during boron neutron capture irradiations of the 9l rat gliosarcoma in vitro and in vivo. Int J Radiat Oncol. 1993;27:1121‐9. 10.1016/0360-3016(93)90533-2. [DOI] [PubMed] [Google Scholar]
- 77. Sato T, Masunaga SI, Kumada H, Hamada N. Depth distributions of rbe‐weighted dose and photon‐isoeffective dose for boron neutron capture therapy. Radiat Prot Dosimetry. 2019;183:247‐50. [DOI] [PubMed] [Google Scholar]
- 78. Savage JRK. Radiation‐Induced Chromosome Damage in Man. Int J Radiat Biol Relat Stud Physics, Chem Med. 1984;46:654–654. 10.1080/09553008414551861. [DOI] [Google Scholar]
- 79. Prasad R, Beard WA, Batra VK, Liu Y, Shock DD, Wilson SH. A review of recent experiments on step‐to‐step "hand‐off" of the DNA intermediates in mammalian base excision repair pathways. Mol Biol (Mosk). 2011;45:586‐600. http://www.ncbi.nlm.nih.gov/pubmed/21954590. Accessed 6 Mar 2019. [PMC free article] [PubMed] [Google Scholar]
- 80. Chapman JR, Taylor MRG, Boulton SJ. Playing the end game: DNA double‐strand break repair pathway choice. Mol Cell. 2012;47:497‐510. 10.1016/j.molcel.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 81. Kondo N, Sakurai Y, Hirota Y, Tanaka H, Watanabe T, Nakagawa Y, et al. DNA damage induced by boron neutron capture therapy is partially repaired by DNA ligase IV. Radiat Environ Biophys. 2016;55:89‐94. 10.1007/s00411-015-0625-2. [DOI] [PubMed] [Google Scholar]
- 82. Wang H, Zhang X, Wang P, Yu X, Essers J, Chen D, et al. Characteristics of DNA‐binding proteins determine the biological sensitivity to high‐linear energy transfer radiation. Nucleic Acids Res. 2010;38:3245‐51. 10.1093/nar/gkq069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Masutani M, Baiseitov D, Itoh T, Hirai T, Berikkhanova K, Murakami Y, et al. Histological and biochemical analysis of DNA damage after BNCT in rat model. Appl Radiat Isot. 2014;88:104‐8. 10.1016/j.apradiso.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 84. Lange SS, Vasquez KM. HMGB1: the jack‐of‐all‐trades protein is a master DNA repair mechanic. Mol Carcinog. 2009;48:571‐80. 10.1002/mc.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kang R, Chen R, Zhang Q, Hou W, Wu S, Cao L, et al. HMGB1 in health and disease. Mol Aspects Med. 2014;40:1‐116. 10.1016/j.mam.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486‐541. 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Suzuki M, Kato I, Aihara T, Hiratsuka J, Yoshimura K, Niimi M, et al. Boron neutron capture therapy outcomes for advanced or recurrent head and neck cancer. J Radiat Res. 2014;55:146‐53. 10.1093/jrr/rrt098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Miyatake S‐I, Kawabata S, Hiramatsu R, Kuroiwa T, Suzuki M, Ono K. Boron Neutron Capture Therapy of Malignant Gliomas. Progress in Neurological Surgery. 2018: 48‐56. 10.1159/000469679. [DOI] [PubMed] [Google Scholar]
- 89. Wang L‐W, Liu Y‐WH, Chou F‐I, Jiang S‐H. Clinical trials for treating recurrent head and neck cancer with boron neutron capture therapy using the Tsing‐Hua Open Pool Reactor. Cancer Commun. 2018;38:37 10.1186/s40880-018-0295-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yanagie H, Higashi S, Seguchi K, Ikushima I, Fujihara M, Nonaka Y, et al. Pilot clinical study of boron neutron capture therapy for recurrent hepatic cancer involving the intra‐arterial injection of a 10BSH‐containing WOW emulsion. Appl Radiat Isot. 2014;88:32‐7. 10.1016/j.apradiso.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 91. Suzuki M, Suzuki O, Sakurai Y, Tanaka H, Kondo N, Kinashi Y, et al. Reirradiation for locally recurrent lung cancer in the chest wall with boron neutron capture therapy (BNCT). Int Cancer Conf J. 2012;1:235‐8. 10.1007/s13691-012-0048-8. [DOI] [Google Scholar]
- 92. Koivunoro H, Kankaanranta L, Seppälä T, Haapaniemi A, Mäkitie A, Joensuu H. Boron neutron capture therapy for locally recurrent head and neck squamous cell carcinoma: An analysis of dose response and survival. Radiother Oncol. 2019;137:153‐8. 10.1016/j.radonc.2019.04.033. [DOI] [PubMed] [Google Scholar]
- 93. Hiratsuka J, Kamitani N, Tanaka R, Yoden E, Tokiya R, Suzuki M, et al. Boron neutron capture therapy for vulvar melanoma and genital extramammary Paget's disease with curative responses. Cancer Commun (London, England). 2018;38:38 10.1186/s40880-018-0297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Komori T. The 2016 WHO Classification of Tumours of the Central Nervous System: The Major Points of Revision. Neurol Med Chir. 2017;57:301‐11. 10.2176/nmc.ra.2017-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson AB, et al. Annual Report to the Nation on the Status of Cancer, 1975‐2014, Featuring Survival. JNCI J Natl Cancer Inst. 2017;109 10.1093/jnci/djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson AB, et al. Annual Report to the Nation on the Status of Cancer, 1975‐2014, Featuring Survival. JNCI J Natl Cancer Inst. 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7‐34. 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 98. Forst DA, Nahed B V, Loeffler JS, Batchelor TT. Low‐grade gliomas. Oncologist. 2014;19:403‐13. 10.1634/theoncologist.2013-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gupta P, Jalali R. Long‐term Survivors of Childhood Brain Tumors: Impact on General Health and Quality of Life. Curr Neurol Neurosci Rep. 2017;17:99 10.1007/s11910-017-0808-0. [DOI] [PubMed] [Google Scholar]
- 100. Verhaak RGW, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98‐110. 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Cuperlovic‐Culf M, Ferguson D, Culf A, Morin P, Touaibia M. 1H NMR metabolomics analysis of glioblastoma subtypes: Correlation between metabolomics and gene expression characteristics. J Biol Chem. 2012;287:20164‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Miyatake S‐I, Kawabata S, Kajimoto Y, Aoki A, Yokoyama K, Yamada M, et al. Modified boron neutron capture therapy for malignant gliomas performed using epithermal neutron and two boron compounds with different accumulation mechanisms: an efficacy study based on findings on neuroimages. J Neurosurg. 2005:1000‐9. 10.3171/jns.2005.103.6.1000. [DOI] [PubMed] [Google Scholar]
- 103. Miyatake S‐I, Kawabata S, Yokoyama K, Kuroiwa T, Michiue H, Sakurai Y, et al. Survival benefit of Boron neutron capture therapy for recurrent malignant gliomas. J Neurooncol. 2009;91:199‐206. 10.1007/s11060-008-9699-x. [DOI] [PubMed] [Google Scholar]
- 104. Kawabata S, Miyatake S‐I, Kuroiwa T, Yokoyama K, Doi A, et al. Boron Neutron Capture Therapy for Newly Diagnosed Glioblastoma. J Radiat Res. 2009;50:51‐60. 10.1269/jrr.08043. [DOI] [PubMed] [Google Scholar]
- 105. Kankaanranta L, Seppälä T, Koivunoro H, Välimäki P, Beule A, Collan J, et al. l‐Boronophenylalanine‐Mediated Boron Neutron Capture Therapy for Malignant Glioma Progressing After External Beam Radiation Therapy: A Phase I Study. Int J Radiat Oncol. 2011;80:369‐76. 10.1016/j.ijrobp.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 106. Jou A, Hess J. Epidemiology and Molecular Biology of Head and Neck Cancer. Oncol Res Treat. 2017;40:328‐32. 10.1159/000477127. [DOI] [PubMed] [Google Scholar]
- 107. Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, Preston‐Martin S, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282‐7. http://www.ncbi.nlm.nih.gov/pubmed/3365707. Accessed 10 Apr 2019. [PubMed] [Google Scholar]
- 108. Leonard B, Brand TM, O'Keefe RA, Lee ED, Zeng Y, Kemmer JD, et al. BET Inhibition Overcomes Receptor Tyrosine Kinase–Mediated Cetuximab Resistance in HNSCC. Cancer Res. 2018;78:4331‐43. 10.1158/0008-5472.CAN-18-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Fujita Y, Kato I, Iwai S, Ono K, Suzuki M, Sakurai Y, et al. Role of p53 mutation in the effect of boron neutron capture therapy on oral squamous cell carcinoma. Radiat Oncol. 2009;4:63 10.1186/1748-717X-4-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zhou G, Liu Z, Myers JN. TP53 Mutations in Head and Neck Squamous Cell Carcinoma and Their Impact on Disease Progression and Treatment Response. J Cell Biochem. 2016;117:2682‐92. 10.1002/jcb.25592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Aihara T, Morita N, Kamitani N, Ono K, Hiratsuka J, Harada T. BNCT for advanced or recurrent head and neck cancer. Appl Radiat Isot. 2014;88:12‐5. 10.1016/J.APRADISO.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 112. Haapaniemi A, Kankaanranta L, Saat R, Koivunoro H, Saarilahti K, Mäkitie A, et al. Boron Neutron Capture Therapy in the Treatment of Recurrent Laryngeal Cancer. Int J Radiat Oncol. 2016;95:404‐10. 10.1016/j.ijrobp.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 113. Kankaanranta L, Seppälä T, Koivunoro H, Saarilahti K, Atula T, Collan J, et al. Boron Neutron Capture Therapy in the Treatment of Locally Recurred Head‐and‐Neck Cancer: Final Analysis of a Phase I/II Trial. Int J Radiat Oncol. 2012;82:e67‐75. 10.1016/j.ijrobp.2010.09.057. [DOI] [PubMed] [Google Scholar]
- 114. Martincorena I. and Campbell P. J.. Erratum for the Review "Somatic mutation in cancer and normal cells" by I. Martincorena and P. J. Campbell. Science. 2016;351:aaf5401 10.1126/science.aaf5401. [DOI] [PubMed] [Google Scholar]
- 115. Alberti D, Deagostino A, Toppino A, Protti N, Bortolussi S, Altieri S, et al. An innovative therapeutic approach for malignant mesothelioma treatment based on the use of Gd/boron multimodal probes for MRI guided BNCT. J Control Release. 2018;280:31‐8. 10.1016/J.JCONREL.2018.04.043. [DOI] [PubMed] [Google Scholar]
- 116. van Meerbeeck JP, Scherpereel A, Surmont VF, Baas P. Malignant pleural mesothelioma: the standard of care and challenges for future management. Crit Rev Oncol Hematol. 2011;78:92‐111. 10.1016/j.critrevonc.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 117. Røe OD, Stella GM. Malignant pleural mesothelioma: history, controversy and future of a manmade epidemic. Eur Respir Rev. 2015;24:115‐31. 10.1183/09059180.00007014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lagniau S, Lamote K, van Meerbeeck JP, Vermaelen KY. Biomarkers for early diagnosis of malignant mesothelioma: Do we need another moonshot? Oncotarget. 2017;8:53751‐62. 10.18632/oncotarget.17910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Alberti D, Deagostino A, Toppino A, Protti N, Bortolussi S, Altieri S, et al. An innovative therapeutic approach for malignant mesothelioma treatment based on the use of Gd/boron multimodal probes for MRI guided BNCT. J Control Release. 2018;280:31‐8. 10.1016/j.jconrel.2018.04.043. [DOI] [PubMed] [Google Scholar]
- 120. Kankaanranta L, Kiovunoro H, Seppälä T et al. Outcome of the first twelve patients with locally recurred inoperable head and neck cancer treated in the Finnish head and neck cancer BNCT trial In: Proceedings of the13th International Congress on Neutron Capture Therapy Florence, Italy, 2012; November 2‐7. 2008. pp. 21‐25. [Google Scholar]
- 121. Kawabata S, Hiramatsu R, Kuroiwa T, Ono K, Miyatake S‐I. Boron neutron capture therapy for recurrent high‐grade meningiomas. J Neurosurg. 2013;119:837‐44. 10.3171/2013.5.JNS122204. [DOI] [PubMed] [Google Scholar]
- 122. Kankaanranta L, Saarilahti K, Mäkitie A, Välimäki P, Tenhunen M, Joensuu H. Boron neutron capture therapy (BNCT) followed by intensity modulated chemoradiotherapy as primary treatment of large head and neck cancer with intracranial involvement. Radiother Oncol. 2011;99:98‐9. 10.1016/j.radonc.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 123. Liu Y‐WH, Huang TT, Jiang SH, Liu HM. Renovation of epithermal neutron beam for BNCT at THOR. Appl Radiat Isot. 2004;61:1039‐43. 10.1016/j.apradiso.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 124. Kato I, Ono K, Sakurai Y, Ohmae M, Maruhashi A, Imahori Y, et al. Effectiveness of BNCT for recurrent head and neck malignancies. Appl Radiat Isot. 2004;61:1069‐73. 10.1016/j.apradiso.2004.05.059. [DOI] [PubMed] [Google Scholar]
- 125. Shiba H, Takeuchi K, Hiramatsu R, Furuse M, Nonoguchi N, KAWABATA S, et al. Boron Neutron Capture Therapy Combined with Early Successive Bevacizumab Treatments for Recurrent Malignant Gliomas – A Pilot Study. Neurol Med Chir (Tokyo). 2018;58:487‐94. 10.2176/nmc.oa.2018-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kawabata S, Miyatake S‐I, Nonoguchi N, Hiramatsu R, Iida K, Miyata S, et al. Survival benefit from boron neutron capture therapy for the newly diagnosed glioblastoma patients. Appl Radiat Isot. 2009;67:S15‐8. 10.1016/j.apradiso.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 127. Burian J, Marek M, Rataj J, Flibor S, Rejchrt J, Viererbl L, et al. Report on the First Patient Group of the Phase I BNCT Trial at the LVR‐15 Reactor. 2004. 10.1016/S0531-5131(03)01515-2. [DOI]
- 128. Palmer MR, Goorley JT, Kiger WS, Busse PM, Riley KJ, Harling OK, et al. Treatment planning and dosimetry for the Harvard‐MIT Phase I clinical trial of cranial neutron capture therapy. Int J Radiat Oncol Biol Phys. 2002;53:1361‐79. http://www.ncbi.nlm.nih.gov/pubmed/12128139. Accessed 3 May 2019. [DOI] [PubMed] [Google Scholar]
- 129. Kiger W., Lu X., Harling O., Riley K., Binns P., Kaplan J, et al. Preliminary Treatment Planning and Dosimetry for a Clinical Trial of Neutron Capture Therapy using a Fission Converter Epithermal Neutron Beam. Appl Radiat Isot. 2004;61:1075‐81. 10.1016/j.apradiso.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 130. Haginomori S‐I, Miyatake S‐I, Inui T, Araki M, Kawabata S, Takamaki A, et al. Planned fractionated boron neutron capture therapy using epithermal neutrons for a patient with recurrent squamous cell carcinoma in the temporal bone: a case report. Head Neck. 2009;31:412‐8. 10.1002/hed.20895. [DOI] [PubMed] [Google Scholar]
- 131. Capala J, Stenstam BH, Sköld K, Munck af Rosenschöld P, Giusti V, Persson C, et al. Boron neutron capture therapy for glioblastoma multiforme: clinical studies in Sweden. J Neurooncol. 2003;62:135‐44. http://www.ncbi.nlm.nih.gov/pubmed/12749709. Accessed 3 May 2019. [DOI] [PubMed] [Google Scholar]
- 132. Yanagie H, Higashi S, Seguchi K, Ikushima I, Oyama K, Nonaka Y et al. Pilot clinical study of boron neutron capture therapy for recurrent hepatic cancer and gastric cancer In: Presented at the 15th international congress on neutron capture therapy. Tsukuba, Japan; 2012. [Google Scholar]
- 133. Suzuki M. Reirradiation for locally recurrent lung cancer in the chest wall with boron neutron capture therapy (BNCT): A case report In: Presented at the15th international congress on neutron capture therapy. Tsukuba, Japan; 2012. [Google Scholar]
- 134. Sasaoka S. The first clinical trial of boron neutron captures therapy using 10B‐para‐boronophenylalanine for treating extra‐mammary Paget's disease In: Presented at the 15th International Congress on Neutron Capture Therapy. Tsukuba, Japan; 2012. [Google Scholar]


