Abstract
Introduction
Hypertensive disorder of pregnancy is the second commonest causes of maternal death globally. Different public health studies were conducted on hypertensive disorder of pregnancy which presented inconsistent result. Therefore, this systematic review and meta-analysis was commenced to summarize the findings conducted in several parts of the country and to generate the nationwide representative data on the prevalence and risk factors of hypertensive disorder of pregnancy in Ethiopia.
Methods and materials
Electronic databases such as PubMed, Scopus, Google Scholar, Hinari, and African Journals Online were searched for studies published in English up to March, 2020. Joanna Briggs Institute Meta-Analysis of Statistics Assessment and Review Instrument and Newcastle-Ottawa Scale were used for data extraction and quality assessment of the included studies. The meta- regression analysis was computed at 95% CI to present the pooled prevalence and risk factors of hypertensive disorder of pregnancy.
Results
Thirty four studies were included in this systematic review and meta-analysis. The pooled prevalence of hypertensive disorder of pregnancy and preeclampsia in Ethiopia were 6.82% (95% CI (5.90, 7.74)) and 4.74% (95% CI (3.99, 5.49)) respectively. Maternal age ≥35 years (Adjusted Odds Ratio (AOR): 2.91 (95% CI: 1.60, 5.26)), twin pregnancy (AOR: 3.04 (95% CI: 1.89, 4.87)), previous history of preeclampsia (AOR: 5.36 (95% CI: 3.37, 8.53)), family history of hypertension (AOR: 4.01 (95% CI: 2.65, 6.07)), family history of diabetes mellitus (AOR: 3.07 (95% CI: 1.66, 7.70)), body mass index ≥25 (AOR: 3.92 (95% CI: 1.82, 8.42)), alcohol consumption (AOR: 1.77 (95% CI: 1.11, 2.83)), urinary tract infection (AOR: 4.57 (95% CI: 3.47, 6.02)), lack of nutritional counseling during antenatal period (AOR: 4.87 (95% CI: 3.36, 7.06)), lack of fruits (AOR: 3.49 (95% CI: 2.29, 5.30)), and vegetables consumption (AOR: 2.94 (95% CI: 2.01, 4.31)) were the risk factors of hypertensive disorder of pregnancy in Ethiopia.
Conclusions
The pooled prevalence of hypertensive disorder of pregnancy is relatively higher compared with the previous reports. Maternal age ≥35 years, twin pregnancy, previous history of preeclampsia, family history of hypertension, family history of diabetes mellitus, body mass index ≥25, alcohol consumption, urinary tract infection, lack of fruits and vegetables during pregnancy were risk factors of hypertensive disorder of pregnancy. The governments and stakeholders should work to strengthen the antenatal care practice to include the possible risk factors of hypertensive disorders of pregnancy.
1. Introduction
Hypertensive disorders of pregnancy (HDP) include; preeclampsia, gestational hypertension, chronic hypertension, and chronic hypertension with superimposed preeclampsia [1]. HDP affects 5 to 10% of pregnant women worldwide and, resulted poor maternal and prenatal outcome [1, 2]. It is the second common cause of maternal death worldwide. HDP accounted about 76, 000 maternal and 500, 000 prenatal deaths globally per year [3]. According to World Health Organization (WHO) report in 2019, 295 000 maternal deaths was recorded globally due to pregnancy and child birth related causes in 2017. The risk of maternal death is 40 times higher in the least developed countries compared with European counties. Sub-Saharan African and Southern Asian countries accounted about 66% and 20% of the global maternal death, respectively [4].
Ethiopia is one of the highest estimated number of maternal deaths observed in the world that accounts about 4.8% (14, 000) of the global share in 2017 [4]. Based on the Ethiopian demographic health survey (EDHS) report in 2016, pregnancy related maternal deaths were 412 from 100, 000 live births [5]. In Ethiopia, nationwide cohort studies were not conducted that indicate the incidence and risk factors of HDP. However, different public health studies involving varied study design have been conducted on HDP and reported inconsistent results on the prevalence 1.2% [6] to 19.1% [7] and risk factors of HDP [8, 9].
Researchers identified different risk factors for HDP [9, 10]. Factors like; advanced maternal age, twin pregnancy, being primigravida, previous history of preeclampsia, family history of hypertension (HTN), family history of diabetes mellitus (DM) [11], being overweight or obesity or body mass index (BMI) ≥25 [9, 11], urinary tract infection (UTI) [12], alcohol consumption [13], lack of fruits and vegetables [14] during pregnancy increase the risk of HDP. In Ethiopia, only one systematic review and meta-analysis was published by Berhe and his colleges and reported 6.07% of HDP prevalence [15] which is lower than the prevalence of HDP in Africa which was 10% [16]. The previous review was including 17 (13 cross-sectional and 4 case-control) studies for the estimation of prevalence of HDP in Ethiopia. There is no published systematic review and Meta-analysis study that shows the risk factors of HDP in Ethiopia. Therefore, the current systematic review and meta-analysis was planned to assess the risk factors of HDP in Ethiopia. In addition, this review was planned to estimate the prevalence of HDP in Ethiopia by including more articles published since the previous review up-to March, 2020.
2. Methods and materials
2.1. Study design and search strategy
A systematic review and Meta-analysis of published and unpublished studies were conducted to assess the prevalence and risk factors of HDP in Ethiopia. We searched the following databases: Scopus, PubMed, Hinari, Google Scholar and African Journals Online (AJOL). The search was done by using the following Medical Subject Headings (MeSH) search terms; "Prevalence", "Risk Factors", "Hypertension", "Pregnancy", "Pregnancy induced hypertension", "Pre-Eclampsia" AND "Ethiopia" through using string term "AND" and "OR". A combination of MeSH terms and free terms were used for the searching strategy to conduct this systematic review and meta-analysis (S5 File). All published and unpublished studies up-to March, 2020 in Ethiopia were retrieved to be assessed for eligibility of inclusion in this review. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline was utilized to conduct this review [17].
2.2. Eligibility criteria
Inclusion and exclusion criteria;
Studies with cross-sectional, case-control and cohort designs were included.
Articles that report prevalence or risk factors of HDP in Ethiopia were included.
Published and unpublished articles written in English were included
Studies reporting HDP as an outcome variable were included.
Studies conducted in the community or in the health institution levels were included.
Conference papers, editorial notes and systematic reviews and meta-analyses were excluded from the study.
2.3. Study selection and screening
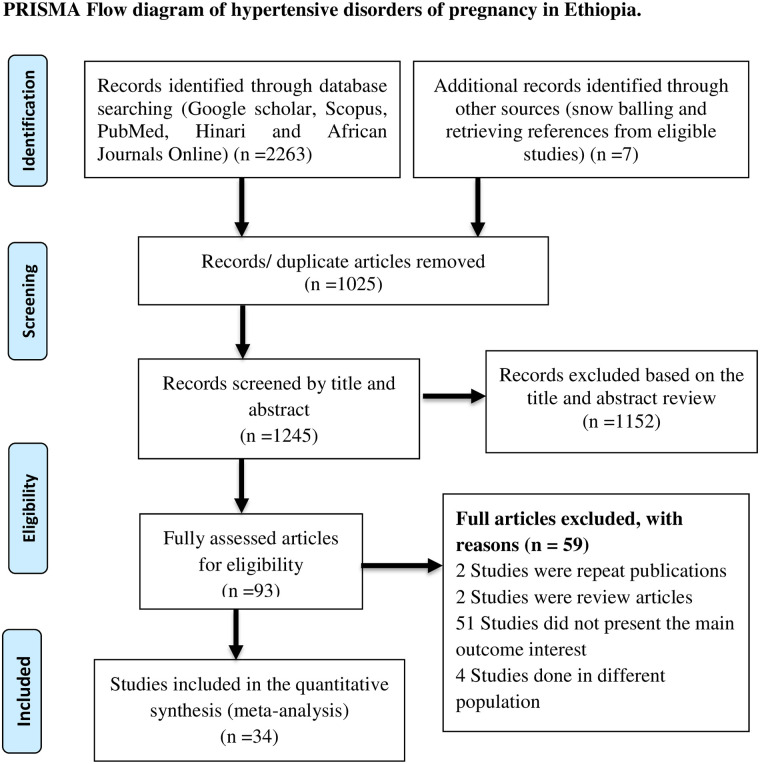
All citations identified by our search strategy were exported to EndNote -X9- and duplicate articles were removed. And then the titles and abstracts of the identified articles were screened by two independent reviewers, and eligible studies were included for further review. The full texts of selected articles were retrieved and read thoroughly to ascertain the suitability prior to data extraction. In case of disagreement between the two reviewers, discussion has been held to reach consensus and the third reviewer was consulted. The search process was presented in PRISMA flow chart that clearly shows the studies that were included and excluded with sound reasons of exclusion (Fig 1) [17].
Fig 1. Flow diagram shows the included studies for the systematic review and meta-analysis on the prevalence and determinants of hypertensive disorders of pregnancy in Ethiopia.
2.4. Definition of outcome interest
The primary outcome of this study was the prevalence of HDP. HDP includes; preeclampsia, gestational hypertension, chronic hypertension, chronic hypertension with superimposed preeclampsia and eclampsia.
Hypertension: Systolic blood pressure ≥140mmHg and/or diastolic ≥90mmHg that is measured at least two times within four hours interval.
Proteinuria: urinary protein excretion of ≥300mg /24-h urine sample or ≥1+ on qualitative dipstick examination or a total protein: creatinine ratio ≥30 mg/ mmol (or ≥0.3 when both are measured in mg/dL).
Gestational hypertension: hypertension diagnosed after 20 weeks of gestation.
Preeclampsia: gestational hypertension plus proteinuria.
Chronic hypertension: hypertension occurs before pregnancy or diagnosed before 20 weeks of gestation for the first time.
Chronic hypertension with superimposed preeclampsia: Occurrence of proteinuria in women with chronic hypertension before 20 weeks of gestation.
Eclampsia: Seizures in women with hypertension that cannot be attributed to other causes [18].
The secondary outcomes of the current review were risk factors of HDP in Ethiopia (such as age, gravidity, previous history of preeclampsia, family history of hypertension and DM, BMI, nutritional counseling, alcohol drinking, UTI, eating of fruits and vegetables).
2.5. Quality assessment
For case-control and cohort studies, we used Newcastle-Ottawa Scale (NOS) to assess the quality of the included studies while for cross sectional studies the modified version of NOS was used to assess the quality of the studies for inclusion [19]. The NOS included 3 categorical criteria with a maximum score of 9 points. The quality of each study was rated using the following scoring algorithms: ≥7 points was considered as “Good quality”, 3 to 6 points was considered as “Fair quality”, and ≤3 point was considered as “Poor quality” (S2 File). Accordingly, in order to improve the validity of this systematic review result, we only included primary studies with fair to good quality [19].
2.6. Data extraction process
The data extraction was done using a tool developed by the 2014 Joanna Briggs Institute Reviewers’ Manual data extraction form [20]. The abstract and full-text were reviewed by the two independent reviewers. Data extraction includes: author’s name, publication year, study period, study design (cross-sectional, case–control and cohort), sample size, study area (region), age, gravidity, twin pregnancy, previous history of hypertensive disorders of pregnancy, family history of hypertension, family history of DM, obtaining nutritional counseling during antenatal period, BMI, consumption of alcohol, UTI, eating of fruits and vegetables and the prevalence of HDP were extracted from each article.
2.7. Result synthesis and statistical analysis
The data were entered into Microsoft Excel and the meta-analysis was conducted using Stata 14 software. Forest plots were used to show the prevalence and risk factors of HDP in Ethiopia at 95% CI. Subgroup analysis was done by the region (Amhara, Oromia, SNNPR, Addis Ababa, Tigray and Somali) and year of study (1991 to 2010, 2011 to 2014 and 2016 to 2020). Risk factors like; advanced maternal age, twin pregnancy, primigravidity, previous history of preeclampsia, family history of hypertension, family history of DM, BMI ≥25, alcohol consumption, UTI, eating of fruits and vegetables were assessed for their association with HDP at 95% CI.
2.8. Heterogeneity and publication bias
Statistical heterogeneity was estimated by using Cochrane Q, I2 statistic and P-value. If I2 statistic value < 25%, 25–50%, and ≥50% was used to declare the heterogeneity test as low, medium and high heterogeneity. In this review, random effect model was used for analysis. To cope with the reasons of heterogeneity subgroup analysis and sensitivity test were performed (S4 File) [21]. In addition, publication bias was assessed by using funnel plot asymmetry test and Newcastle-Ottawa quality assessment Scale.
3. Results
3.1. Characteristics of the included studies
A total of 2270 articles were retrieved through electronic search by using different search terms of which 1245 article were eligible for title and abstract assessment after removal of 1025 duplicate records. Out of 1245 articles screened for eligibility 1152 records were excluded by their title and abstract assessment. A total of 93 articles were undergo full- text assessment for eligibility, 59 studies were excluded due to different reasons (51 articles didn’t full fill the inclusion criteria, 4 articles were done on different population, 2 articles were repeated publications and 2 were review articles).
In this review a total of 34 studies were included. Twenty of them were cross-sectional, thirteen studies were case-control and one study was cohort. Published and unpublished studies done in Ethiopia up- to March, 2020 were included. Most regions of Ethiopia were represented in this systematic review and meta- analysis. Eleven studies were conducted in Amhara region, eight studies from Addis Ababa city, five studies from Oromia region, four studies were from Tigray region, four studies were from South Nations and Nationalities Peoples’ region (SNNPR), one study from Somali region and one was a nation based study. In the included studies the smallest sample size was 129 [22] and the maximum was 174, 561 [6]. Overall, this systematic review and meta- analysis included a total of 320,942 pregnant women in Ethiopia (Table 1).
Table 1. Summary of research articles included in the systematic review and meta-analysis of HDP in Ethiopia (N = 34).
| No | Authors | Publication Year | Study Site (Region) | Study Design | Sample Size | Prevalence of HDP (% CI) | SE | Quality assessment |
|---|---|---|---|---|---|---|---|---|
| 1 | Hinkosa et al., 2020 [9] | 2020 | Oromia | Case Control | 597 | 3.57 [2.08, 5.06] | 0.759 | 7 points |
| 2 | Walle & Azagew, 2019 [23] | 2019 | Amhara | Cross -Sectional | 422 | 16.8 [13.23, 20.37] | 1.820 | 7 points |
| 3 | Belay and Wudad, 2019 [22] | 2019 | Oromia | Cross- Sectional | 129 | 12.4 [6.71, 18.09] | 2.902 | 7 points |
| 4 | Gudeta and Regassa, 2019 [24] | 2019 | SNNPR | Cross- Sectional | 422 | 7.9 [5.33, 10.47] | 1.313 | 5 points |
| 5 | Legesse et al., 2019 [25] | 2019 | Tigray | Cross- Sectional | 8, 502 | 5.08 [4.61, 5.55] | 0.238 | 4 points |
| 6 | Mekonnen et al., 2018 [7] | 2018 | Somali | Cross- Sectional | 408 | 19.1 [15.29, 22.91] | 1.946 | 6 points |
| 7 | Gudeta et al., 2018 [26] | 2018 | Oromia | Cross- Sectional | 356 | 10.3 [7.14, 13.46] | 1.611 | 5 points |
| 8 | Kahsay et al., 2018 [27] | 2018 | Tigray | Cross-Sectional | 45, 329 | 2.98 [2.94, 3.26] | 0.081 | 4 points |
| 9 | Wodajo and Reddy, 2016 [28] | 2016 | Amhara | Cross-Sectional | 320 | 8.8 [5.70, 11.90] | 1.584 | 7 points |
| 10 | Wagnew et al., 2016 [29] | 2016 | Addis Ababa | Cross-Sectional | 42,963 | 4.2 [4.01, 4.39] | 0.097 | 6 points |
| 11 | Shegaze et al., 2016 [30] | 2016 | SNNPR | Cross-Sectional | 422 | 18.25 [14.57, 21.94] | 1.880 | 7 points |
| 12 | Terefe et al., 2015 [31] | 2015 | Amhara | Cross-Sectional | 8, 626 | 3.9 [3.49, 4.31] | 0.208 | 5 points |
| 13 | Tessema et al., 2015 [32] | 2015 | Amhara | Cross-Sectional | 490 | 8.4 [5.94, 10.86] | 1.253 | 5 points |
| 14 | Vata et al.,2015 [33] | 2015 | SNNPR | Cross-Sectional | 7,702 | 2.23 [1.90, 2.56] | 0.168 | 5 points |
| 15 | Seyom et al., 2015 [34] | 2015 | Oromia | Cross-Sectional | 5, 415 | 2.4 [1.99, 2.81] | 0.208 | 6 points |
| 16 | Selamawit and Sisay, 2015 [35] | 2015 | Addis Ababa | Cross-Sectional | 3,488 | 7.2 [6.34, 8.06] | 0.438 | 4 points |
| 17 | Mariamawit and Shiferaw, 2014 [36] | 2014 | Addis Ababa | Cross- Sectional | 3,351 | 7.8 [6.89, 8.71] | 0.463 | 4 points |
| 18 | Wolde et al., 2011 [37] | 2011 | Oromia | Cross-Sectional | 1, 863 | 8.48 [7.22, 9.75] | 0.645 | 5 points |
| 19 | Gaym et al., 2011 [6] | 2011 | Nation Based | Cross -Sectional | 174, 561 | 1.2 [1.15, 1.25] | 0.026 | 4 points |
| 20 | Teklu and Gaym, 2006 [38] | 2006 | Addis Ababa | Cohort | 3, 424 | 5.3 [4.55, 6.05] | 0.383 | 4 points |
| 21 | Mekbebe and Ketsela, 1991 [39] | 1991 | Addis Ababa | Cross-Sectional | 6, 766 | 5.14 [4.61, 5.67] | 0.268 | 5 points |
| 22 | Hailu and Kebede, 1991 [40] | 1991 | Amhara | Cross-Sectional | 567 | 12.2 [9.51, 14.89] | 1.374 | 6 points |
| 23 | Mekie et al., 2020 [41] | 2020 | Amhara | Case Control | 330 | --- | --- | 7 points |
| 24 | Fantahun and Berhane, 2019 [42] | 2019 | Amhara | Case Control | 291 | --- | --- | 5 points |
| 25 | Girum et al., 2018 [43] | 2018 | Addis Ababa | Case Control | 243 | --- | --- | 7 points |
| 26 | Kahsay et al., 2018 [44] | 2018 | Tigray | Case Control | 330 | --- | --- | 6 points |
| 27 | Grum et al., 2017 [13] | 2017 | Addis Ababa | Case Control | 291 | --- | --- | 7 points |
| 28 | Temesgen, 2017 [45] | 2017 | Amhara | Case Control | 470 | --- | --- | 5 points |
| 29 | Mohammed et al., 2017 [46] | 2017 | Addis Ababa | Case Control | 261 | --- | --- | 7 points |
| 30 | Aklilu et al., 2016 [47] | 2016 | Amhara | Case Control | 831 | --- | --- | 4 points |
| 31 | Ayele et al., 2016 [8] | 2016 | SNNPR | Case Control | 466 | --- | --- | 5 points |
| 32 | Tesfay et al., 2016 [48] | 2016 | Tigray | Case Control | 400 | --- | --- | 5 points |
| 33 | Endeshaw et al., 2016 [49] | 2016 | Amhara | Case Control | 453 | --- | --- | 7 points |
| 34 | Endeshaw et al., 2015 [14] | 2015 | Amhara | Case Control | 453 | --- | --- | 7 points |
3.2. Prevalence of HDP in Ethiopia
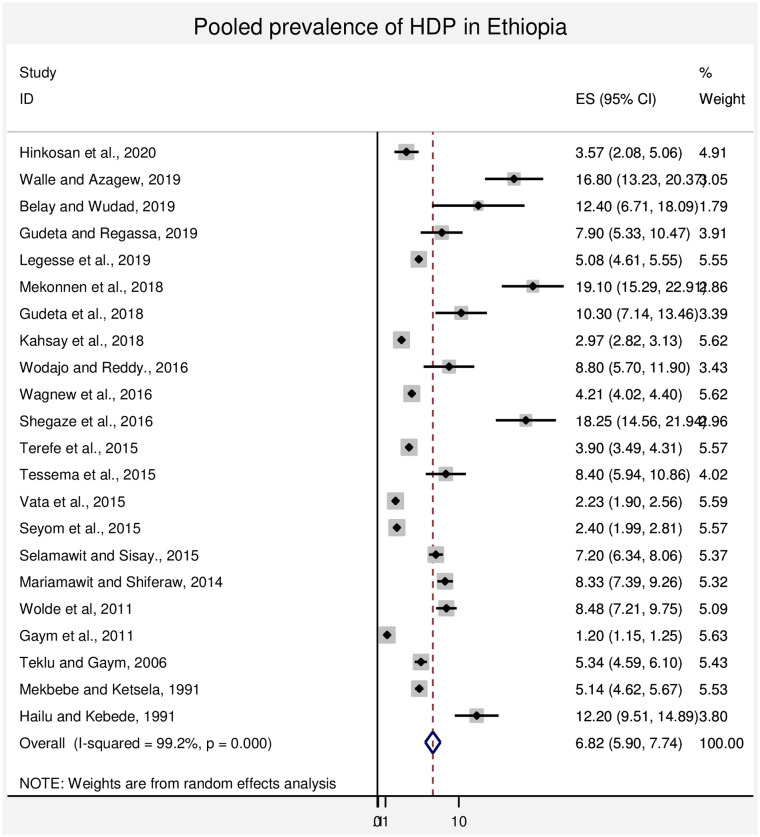
A wider difference in the prevalence of HDP was observed in the studies included in this systematic review and meta-analysis. A lower prevalence (1.2%) of HDP was reported in the nation based study [6] and the higher prevalence (19.1%) of HDP was observed in the study conducted in Somali region [7] The overall pooled prevalence of HDP in Ethiopia was 6.82% (95% CI: (5.90%, 7.74%)) and summarized in Fig 2. Twenty two articles are included for the estimation of the pooled prevalence of HDP. Out of twenty two articles twenty articles were further utilized for the assessment of pooled prevalence of preeclampsia 4.74% (3.99%, 5.49%) at 95% CI.
Fig 2. Forest plot of the pooled prevalence of HDP in Ethiopia.
3.3. Subgroup analysis of HDP prevalence in Ethiopia
The subgroup analysis of HDP prevalence by region showed the highest pooled prevalence 9.88% (5.12, 14.64)) at 95% CI in Amhara region followed by 9.29% (1.01, 17.56) at 95% CI SNNPR region. The lowest prevalence 4.02% (1.95, 6.08) at 95% CI of HDP was observed in Tigray region. The subgroup analysis of HDP by the year of study had shown the highest pooled prevalence 7.82 (6.68, 8.96) at 95% CI in the studies conducted from 2016 to 2020. This indicates that the current trend of HDP has increased compared with the studies conducted from 2006 to 2015 (Table 2).
Table 2. Subgroup analysis of HDP by region and year of study in Ethiopia.
| Subgroup | No of studies | Pooled prevalence (95% CI) | Heterogeneity test (I2) | P-value |
|---|---|---|---|---|
| Amhara | 5 | 9.88 (5.12, 14.64) | 96.1% | <0.001 |
| SNNPR | 3 | 9.29 (1.01, 17.56) | 97.8% | <0.001 |
| Oromia | 5 | 6.91 (3.51, 10.31) | 96.4% | <0.001 |
| Addis Ababa | 5 | 6.00 (4.65, 7.35) | 96.7% | <0.001 |
| Tigray | 2 | 4.02 (1.95, 6.08) | 98.6% | <0.001 |
| Somali | 1 | 19.10 (15.29, 22.91) | -- | -- |
| Nation based | 1 | 1.20 (1.15, 1.25) | -- | -- |
| Total pooled | 22 | 6.82 (5.90, 7.74) | 99.2% | <0.001 |
| 2016–2020 | 11 | 7.82 (6.68, 8.96) | 97.3% | <0.001 |
| 2006–2015 | 8 | 5.09 (3.62, 6.57) | 99.1% | <0.001 |
| 1990–2005 | 3 | 6.81 (4.89, 8.73) | 92.1% | <0.001 |
| Total pooled | 22 | 6.82 (5.90, 7.74) | 99.2% | <0.001 |
3.4. Risk factors of hypertensive disorders of pregnancy in Ethiopia
In this systematic review and meta- analysis different risk factors like; age, gravidity, previous history of preeclampsia, twin pregnancy, family history of hypertension, family history of DM, BMI, alcohol consumption, urinary tract infection, obtaining nutritional counseling during antenatal care (ANC) period, fruits and vegetables consumption were evaluated for their association with hypertensive disorders of pregnancy.
3.4.1. Association between maternal age and HDP
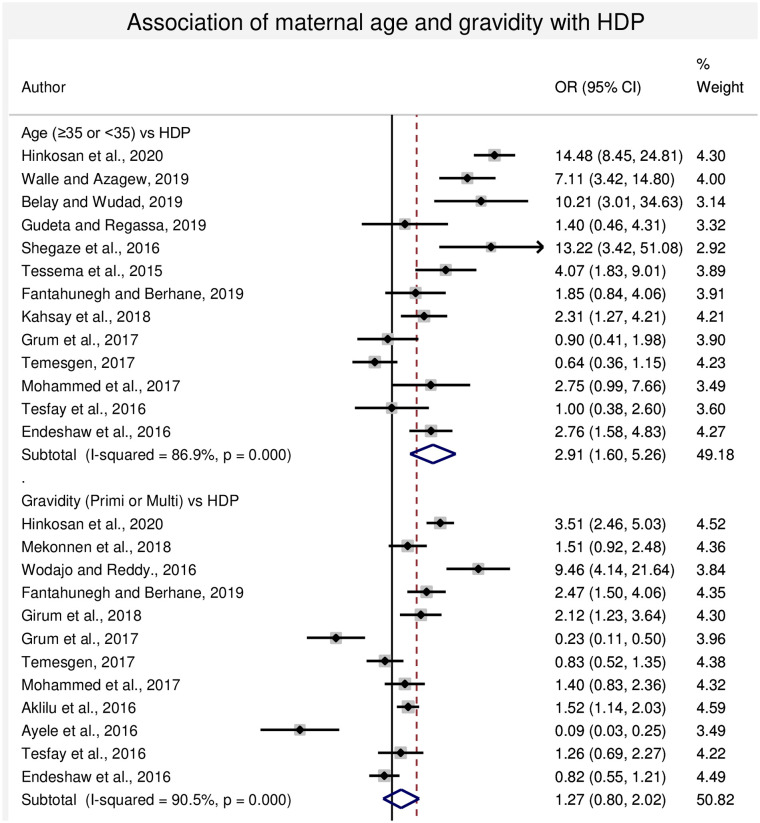
In this sub-categorical analysis, thirteen studies were included for the assessment of age as a risk factor for HDP [9, 13, 22–24, 30, 32, 42, 44–46, 48, 50]. Seven studies had shown statistical significant association between increased maternal age with HDP [9, 22, 23, 30, 32, 44, 49]. Whereas, two studies [13, 45] had shown lower risk of HDP as maternal age increases whereas four studies [24, 42, 46, 48] reported non-significant difference between younger and older mothers. The pooled meta-regression analysis showed that there is statistical significant association in the occurrence of HDP in the maternal age ≥35 years compared with the maternal age <35 years with the odds of 2.91 (95% CI: 1.60, 5.26) (Fig 3).
Fig 3. Forest plot of odds ratio for the association of maternal age and gravidity with HDP in Ethiopia.
3.4.2. Association between gravidity and HDP
In this sub-categorical analysis, twelve studies were included for the assessment gravidity as a risk factor for HDP [7–9, 13, 28, 42, 43, 45–49]. Five of the included studies had shown statistical significant association with primigravida and HDP [9, 28, 42, 43, 47]. Whereas, four studies [8, 13, 45, 49] showed lower risk of HDP among primigravida women and three studies [7, 46, 48] showed non-significant difference. The pooled meta-regression analysis showed that there is no statistical significant difference in the occurrence of hypertensive disorders of pregnancy in the primigravida and multigravida women, OR = 1.27 (95% CI: 0.78, 2.41) (Fig 3).
3.4.3. Association between twin pregnancy and HDP
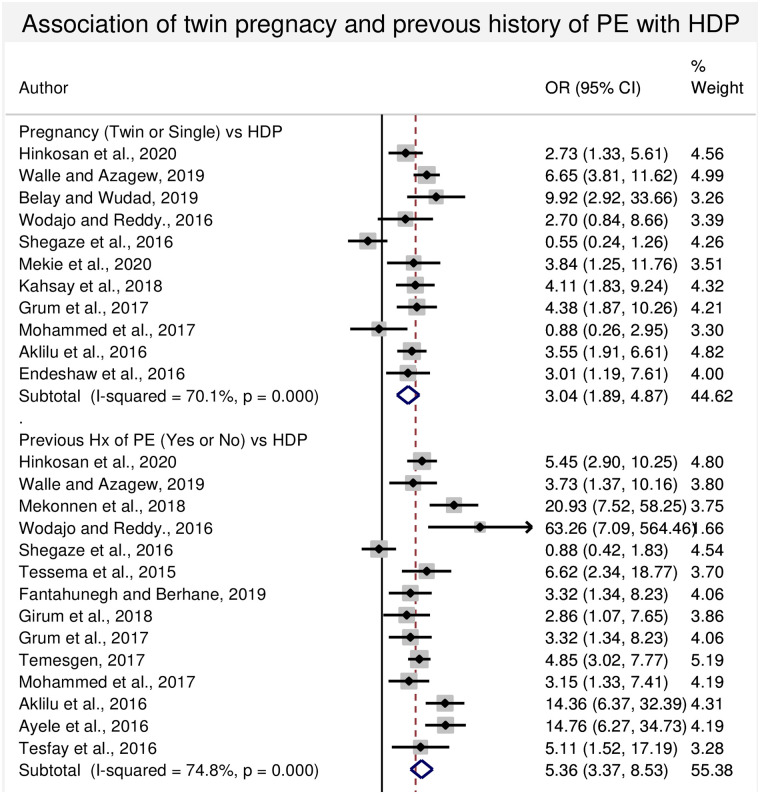
In this sub-categorical analysis, eleven studies were included for the assessment of twin pregnancy as a risk factor for HDP [9, 13, 22, 23, 28, 30, 41, 44, 46, 47, 49]. Eight of the included studies had shown statistical significant association among twin pregnancy and HDP [9, 13, 22, 23, 41, 44, 47, 49]. Although, two studies [30, 46] showed a lower risk of HDP among twin pregnancy, another one study [28] showed non-significant difference between the groups. The pooled meta-regression analysis showed that there is a statistical significant association between the occurrence of HDP and twin pregnancy, the odds being 3.04 (95% CI: 1.89, 4.87) times higher than that of singleton pregnancy (Fig 4).
Fig 4. Forest plot of odds ratio for the association of twin pregnancy and previous history of preeclampsia and HDP in Ethiopia.
3.4.4. Association between Previous history of preeclampsia and HDP
In this sub-categorical analysis, fourteen studies were included for the assessment of previous history of preeclampsia as a risk factor for HDP [7–9, 13, 23, 28, 30, 32, 42, 43, 45–48]. Thirteen of the included studies had shown a statistical significant association between previous history of preeclampsia and HDP [7–9, 13, 23, 28, 32, 42, 43, 45–48]. However, one study showed a lower risk of HDP among previous history of preeclampsia or HDP [30]. The pooled meta-regression analysis showed that there is a statistical significant association in the occurrence of HDP and previous history of preeclampsia with the odds of 5.36 (95% CI: 3.37, 8.53) (Fig 4).
3.4.5. Association between family history of hypertension and HDP
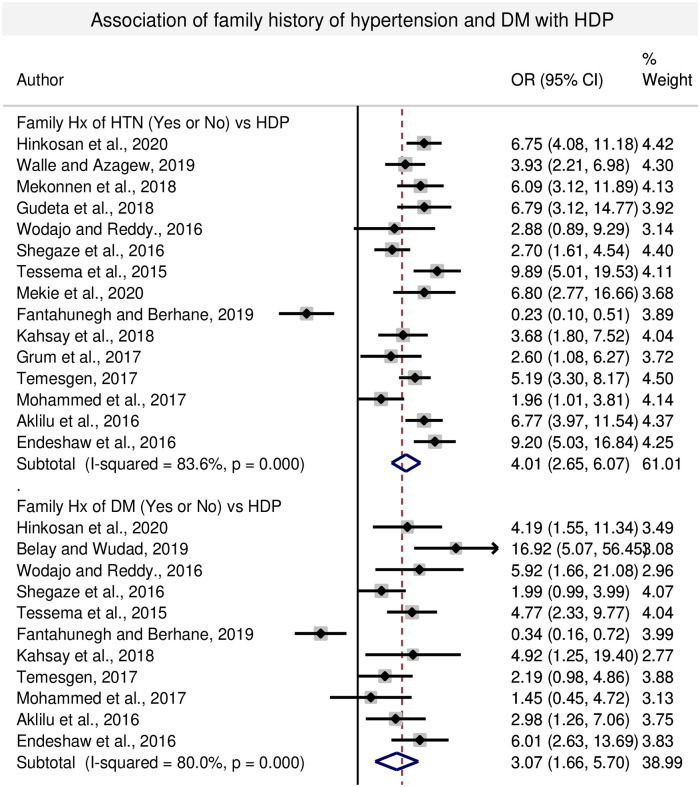
In this sub-categorical analysis, fifteen studies were included for the assessment of family history of hypertension (HTN) as a risk factor for HDP [7, 9, 13, 23, 26, 28, 30, 32, 41, 42, 44–47, 49]. Thirteen of the included studies had shown statistical significant association of family history of HTN with HDP [7, 9, 13, 23, 26, 30, 32, 41, 44–47, 49]. Whereas, two studies [28, 42] showed lower risk and non-significant association with the women having family history of HTN and HDP. The pooled meta-regression analysis had shown statistical significant association between HDP and family history of hypertension with the odds of 4.01 (95% CI: 2.65, 6.07) compared to the women with no family history of hypertension (Fig 5).
Fig 5. Forest plot of odds ratio for the association of family history of DM and HDP in Ethiopia.
3.4.6. Association between family history of DM and HDP
In this sub-categorical analysis, eleven studies were included for the assessment of family history of diabetes mellitus as a risk factor for HDP [9, 22, 28, 30, 32, 42, 44–47, 49]. Seven of the included studies showed a statistically significant association of family history of diabetes mellitus with HDP [9, 22, 28, 32, 44, 47, 49]. While, one study [42] showed lower risk of HDP among pregnant women having family history of diabetes mellitus and three studies did not show significant association with HDP [30, 45, 46]. The pooled meta-regression analysis showed that there is a statistical significant association between the occurrence of HDP and women having family history of DM, with the odds of 3.07 (95% CI: 1.66, 5.70) (Fig 5).
3.4.7. Association of BMI, nutritional counseling, UTI alcohol consumption, fruits and vegetables ingestion with HDP
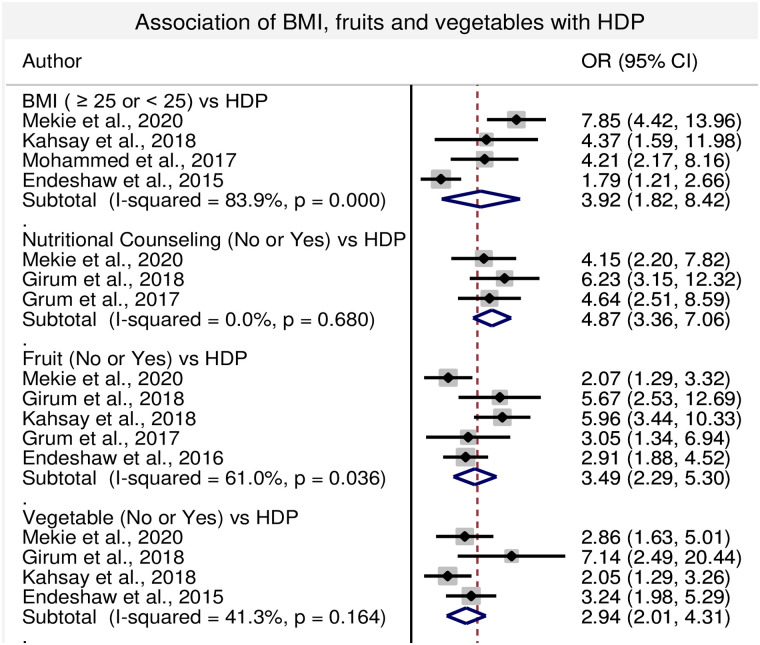
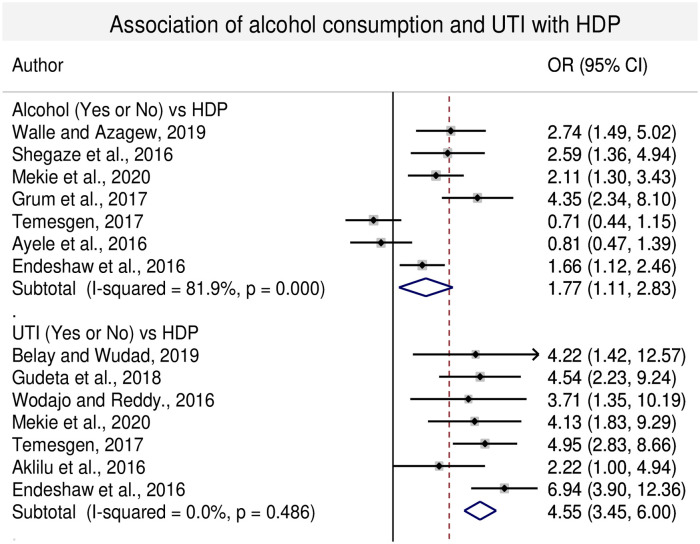
In this sub-category analysis obtaining nutritional counseling during antenatal period, consumption of fruits and vegetables reduce the risk of HDP. Lack of nutritional counseling during ANC period, lack of fruits and vegetables consumption during pregnancy increase the risk of HDP with the odds of 4.87 (95% CI: 3.36, 7.06), 3.49 (95% CI: 2.29, 5.30) and 2.94 (95%: 2.01, 4.31) time, respectively. Similarly, BMI of ≥25kg/m2, alcohol consumption and presence of UTI during pregnancy increased the risk of HDP with the odds of 3.92 (95% CI: 1.82, 8.42), 1.77 (95% CI: 1.11, 2.83) and 4.57 (95% CI: 3.47, 6.02) times, respectively compared with the women whose BMI< 25, and women having no history of alcohol consumption and UTI during pregnancy (Figs 6 and 7).
Fig 6. Forest plot of odds ratio for the association of BMI, nutritional counseling, fruits and vegetables consumption with HDP in Ethiopia.
Fig 7. Forest plot of odds ratio for the association of alcohol consumption and UTI with HDP in Ethiopia.
3.5. Risk of publication bias
The results of this systematic review and meta-analysis were heterogeneous. To understand the cause of heterogeneity publication bias was assessed. The funnel plot figure shows the presence of possible publication bias (S3 File). This bias might be due to missing of unpublished articles in the country. The other causes might be due to the inclusion of articles with different methodology and different outcome of interest for the assessment of risk factors of HDP. Subgroup analysis and sensitivity test were done to further explore the causes of heterogeneity (S4 and S6 Files).
4. Discussion
This review was conducted to determine the pooled prevalence and risk factors of hypertensive disorders of pregnancy in Ethiopia. In this meta- analysis the pooled prevalence of all forms of HDP and preeclampsia were 6.82% (95% CI: (5.90%, 7.74%)) and 4.74% (95% CI (3.99, 5.49)) respectively. This is slightly higher to the previous meta- analysis report done by Berhe and his colleagues which was 6.07% (95% CI: 4.83%, 7.31%) [15]. This result found in the range of the global prevalence of HDP which is 5.2–8.2% (51). However, in this review the pooled prevalence of HDP was relatively lower than the meta-analysis done in Africa [16]. This discrepancy might be due to the number of included studies and study setting. Additionally, in this review there are retrospective cross-sectional studies that might under report the prevalence of HDP due to poor secondary data storage system [27]. In this meta-analysis the pooled prevalence of preeclampsia was 4.74% (95% CI: 3.99%, 5.49%) that is found in the range of the global report and slightly lower than the meta- analysis finding in African continent level [16, 51]. In the subgroup analysis, higher prevalence of HDP was observed in Amhara region and articles published from 2016–2020 with the prevalence of 9.88% (5.12, 14.64)) and 7.82 (6.68, 8.96), respectively (p-value <0.001) (S6 File). This difference might be due to different methodology and different outcome of interest for the assessment of risk factors of HDP in Ethiopia.
In this review, different risk factors were assessed with their association to HDP in Ethiopia. Age is an important predictor for HDP and assessed for its association with HDP by classifying maternal age ≥35 and maternal age <35 years. Maternal age of ≥35 years is almost three times more likely to develop HDP compared with the maternal age <35 years and the association were statistically significant. Similar findings were reported in the studies conducted in Kenya, Asian, China, Latin American and Caribbean women among older maternal age group with HDP compared with maternal age <35 years [11, 52–54].
The frequency of gravidity as a risk factor for HDP was assessed in this systematic review and meta-analysis. The odds of developing HDP in primigravida were 1.27 times compared with multigravida pregnant women. There was not statistical significant difference in the occurrence of HDP between primigravida and multigravida pregnant women in Ethiopia, although studies conducted in Kenya, China and Latin America had shown than women in primigravida were 2.1, 1.5 and 2.38 times more likely of developing HDP compared to multigravida pregnant women, respectively [11, 53, 54]. This difference might be attributed to the heterogeneity of the articles included in this review in respect to a particular variable. In this regard, without considering one article published by Ayele et al. (2016) in the analysis the odds of primigravidity to be associated with HDP 1.5 times compared to multigravidity and was statistically significant [8].
In this meta-analysis, twin pregnancy increased the risk of developing HDP three times more compared to singleton pregnancy and the association was statistically significant. This is similar with the studies conducted in China multiple pregnancy where they have shown a 3.68-fold higher risk of HDP compared with singleton pregnancy [55]. Similar report was observed in other multicenter trials were the odds of developing preeclampsia were 2.62 times higher compared with singleton pregnancy [56]. Multiple pregnancy causes an increased placental mass or placental hypoxia that possibly leads to the secretion of placental circulating antiangiogenic factors like;- soluble fms-like tyrosine kinase 1(sFlt1) and soluble endoglin (sEng) which antagonize the placental growth factors and vascular endothelial growth factors results in hypertension, protein and maternal syndromes [57].
Previous history of preeclampsia is an important risk factor for HDP. In this meta-analysis, women having previous history of preeclampsia were shown to develop HDP and the likelihood of its occurrence could be increased by five times as compared with HDP in those women having no previous history of preeclampsia and the association was statistically significant. Supporting evidence of our current finding was reported in the study conducted in China [11]. Similarly, family history of HTN could also increase the risk of developing HDP by four- fold compared with women having no family history of HTN. Likewise, the women having family history of DM had an increased risk of developing HDP by three-fold compared to women having no family history of DM. Thus, family history of HTN and family history of DM have shown statistical significant association with HDP. This report is consistent with the studies conducted in Swedish medical center, China and US hospitals [55, 58, 59].
Obesity is one of an important predictor for HDP or preeclampsia. In the current meta- analysis, women having BMI ≥25 had 3.9 times more risk of developing HDP compared with the women having BMI< 25 and the association was statistically significant. Supporting evidence has been found in the systematic review and meta- analysis conducted by Wang and his colleagues [60]. The exact mechanism how obesity and overweight are associated with HDP or preeclampsia is not well elucidated but obesity and overweight associated with hyperinsulinism, insulin resistance and maternal systemic inflammation. This is one of the proposed mechanisms of endothelial dysfunction, hypertension, proteinuria and multi-organ damage that occur in HDP and preeclampsia [61].
In this review, women obtained nutritional counseling during antenatal period, women consuming fruits and vegetables had lower risk of developing HDP compared with their counter- parts and the association is statistically significant. Similar report was observed in the prospective cohort study done by Timmermans and his colleagues [62]. Experimental studies in animal model proved that higher fiber and lower fat diet improve fetal development and growth through improving the antioxidant defense mechanisms that is one of the proposed pathways in the pathogenesis of HDP or preeclampsia [63]. Similar report also observed in the studies conducted in U.S.A on pregnant women in which taking higher dietary fiber was shown lower risk of preeclampsia [64].
Drinking of alcohol during the second and third trimesters of pregnancy increases the risk of preterm birth and results in different adverse consequences on the fetal development [65]. In the current review, alcohol consumption during pregnancy had shown the odds of developing HDP to be 1.77 times more compared with the women did not drink alcohol (p<0.05). The same evidence has been reported in China and Japan [55, 66]. Similarly, UTI increases the risk of developing HDP by 4.55 times more compared with those women who did not have history of UTI and the association was statistically significant. Similar results were reported by Easter et al. (2016) and Yan et al. (2018) [12, 67].
5. Strength and limitation
Strength
This systematic review and meta-analysis showed the national pooled image on the risk factors of hypertensive disorders of pregnancy in Ethiopia. In addition, it produced an updated data on the prevalence of hypertensive disorders of pregnancy in Ethiopia via including more articles from the previously published reviews.
Limitation
The search strategy may miss unpublished articles; publication bias likely high. In addition, high statistical heterogeneity was observed in this review because the inclusion of articles with different methodology and different outcome of interest for the assessment of risk factors of HDP. The included studies lack consistency to include more articles for a particular variable of risk factor that leads to small study effect. These together reduce the quality of the generated evidence.
6. Conclusion
The pooled prevalence of hypertensive disorders of pregnancy was found to relatively higher than what was reported previously in Ethiopia. In the subgroup analysis, the highest prevalence was observed in Amhara region and in the studies conducted between 2016 to 2020. Maternal age ≥35 years, twin pregnancy, previous history of preeclampsia, family history of hypertension, family history of diabetes mellitus, body mass index ≥25, alcohol consumption and urinary tract infection during pregnancy were significantly increased the risk of developing hypertensive disorders of pregnancy. Conversely, pregnant women obtaining nutritional counseling during antenatal period, fruit and vegetable consumption during pregnancy significantly reduce the risk of developing hypertensive disorders of pregnancy. During patient diagnosis and management clinicians will conduct detail patient evaluation to identify the risk factors of hypertensive disorders of pregnancy and to develop better treatment protocol. The governments and stakeholders should work to broaden and strengthen the antenatal care practice by involving all possible risk factors of hypertensive disorders of pregnancy in the ANC follow up guidelines. Additionally, large-scale prospective cohort studies should be needed to identify risk factors of hypertensive disorders of pregnancy in Ethiopia.
Supporting information
(DOC)
(XLSX)
(XLSX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Abbreviations
- ANC
Antenatal care
- BMI
Body mass index
- CI
Confidence interval
- DM
Diabetes mellitus
- HDP
Hypertensive disorders of pregnancy
- HTN
Hypertension
- JBI-MAStARI
Joanna Briggs Institute Meta-Analysis of Statistics Assessment and Review Instrument
- NOS
Newcastle-Ottawa Scale
- OR
Odds ratio
- SNNPR
South Nations and Nationalities Peoples’ region
- U.S.A
United states of America
- UTI
Urinary tract infection
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best practice & research Clinical obstetrics & gynaecology. 2011;25(4):391–403. [DOI] [PubMed] [Google Scholar]
- 2.Sánchez-Aranguren LC, Prada CE, Riaño-Medina CE, Lopez M. Endothelial dysfunction and preeclampsia: role of oxidative stress. Frontiers in physiology. 2014;5:1–11. 10.3389/fphys.2014.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salam RA, Das JK, Ali A, Bhaumik S, Lassi ZS. Diagnosis and management of preeclampsia in community settings in low and middle-income countries. J Family Med Prim Care. 2015;4(4):501–6. 10.4103/2249-4863.174265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO U, UNFPA, World Bank Group and the United Nations Population Division. Trends in Maternal Mortality 2000 to 2017. 2019.
- 5.CSA. demographic and health survey 2016: key indicators report. Report. 2016;363:249–51. [Google Scholar]
- 6.Gaym A, Bailey P, Pearson L, Admasu K, Gebrehiwot Y, Ethiopian National Em ONCAT. Disease burden due to pre-eclampsia/eclampsia and the Ethiopian health system’s response. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2011;115(1):112–6. [DOI] [PubMed] [Google Scholar]
- 7.Mekonen L, Shiferaw Z, Wubshet Ea, Haile S. Pregnancy Induced Hypertension and Associated Factors among Pregnant Women in Karamara Hospital, Jijiga, Eastern Ethiopia, 2015. Journal of Pregnancy and Child Health. 2018;5(3). [Google Scholar]
- 8.Ayele G, Lemma S, Agedew E. Factors Associated with Hypertension during Pregnancy in Derashie Woreda South Ethiopia, Case Control. Quality in Primary Care. 2016;24(5):207–13. [Google Scholar]
- 9.Hinkosa L, Tamene A, Gebeyehu N. Risk factors associated with hypertensive disorders in pregnancy in Nekemte referral hospital, from July 2015 to June 2017, Ethiopia: case-control study. BMC Pregnancy Childbirth. 2020;20(16):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galaviz-Hernandez C, Sosa-Macias M, Teran E, Garcia-Ortiz JE, Lazalde-Ramos BP. Paternal Determinants in Preeclampsia. Front Physiol. 2018;9:1870 10.3389/fphys.2018.01870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye C, Ruan Y, Zou L, Li G, Li C, Chen Y, et al. The 2011 Survey on Hypertensive Disorders of Pregnancy (HDP) in China: Prevalence, Risk Factors, Complications, Pregnancy and Perinatal Outcomes. PloS one. 2011;9(6):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan L, Jin Y, Hang H, Yan B. The association between urinary tract infection during pregnancy and preeclampsia: A meta-analysis. Medicine (Baltimore). 2018;97(36):e12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grum T, Seifu A, Abay M, Angesom T, Tsegay L. Determinants of pre-eclampsia/Eclampsia among women attending delivery Services in Selected Public Hospitals of Addis Ababa, Ethiopia: a case control study. BMC Pregnancy Childbirth. 2017;17(1):307 10.1186/s12884-017-1507-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Endeshaw M, Abebe F, Bedimo M, Asart A. Diet and Pre-eclampsia: A Prospective Multicentre Case-Control Study in Ethiopia. Midwifery. 2015;31(6):617–24. 10.1016/j.midw.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 15.Berhe AK, Kassa GM, Fekadu GA, Muche AA. Prevalence of hypertensive disorders of pregnancy in Ethiopia: a systemic review and meta-analysis. BMC Pregnancy Childbirth. 2018;18(1):34 10.1186/s12884-018-1667-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noubiap JJ, Bigna JJ, Nyaga UF, Jingi AM, Kaze AD, Nansseu JR, et al. The burden of hypertensive disorders of pregnancy in Africa: A systematic review and meta-analysis. Journal of clinical hypertension (Greenwich, Conn). 2019;21(4):479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nature reviews Nephrology. 2014;10(8):466–80. 10.1038/nrneph.2014.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartling L, Milne A, Hamm MP, Vandermeer B, Ansari M, Tsertsvadze A, et al. Testing the Newcastle Ottawa Scale showed low reliability between individual reviewers. Journal of clinical epidemiology. 2013;66(9):982–93. 10.1016/j.jclinepi.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 20.Institute JB. Joanna Briggs Institute Reviewers’ Manual: 2014 edition / Supplement. Joanna Briggs Institute. 2014.
- 21.Higgins J, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed). 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belay AS, Wudad T. Prevalence and associated factors of pre-eclampsia among pregnant women attending anti-natal care at Mettu Karl referal hospital, Ethiopia: cross-sectional study. Clinical hypertension. 2019;25:14 10.1186/s40885-019-0120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walle TA, Azagew AW. Hypertensive disorder of pregnancy prevalence and associated factors among pregnant women attending ante natal care at Gondar town health Institutions, North West Ethiopia 2017. Pregnancy hypertension. 2019;16:79–84. 10.1016/j.preghy.2019.03.007 [DOI] [PubMed] [Google Scholar]
- 24.Gudeta TA, Regassa TM. Pregnancy Induced Hypertension and Associated Factors among Women Attending Delivery Service at Mizan-Tepi University Teaching Hospital, Tepi General Hospital and Gebretsadik Shawo Hospital, Southwest, Ethiopia. Ethiopian journal of health sciences. 2019;29(1):831–40. 10.4314/ejhs.v29i1.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Legesse AY, Yibrah. B, Mohammednur SA, Teka Hale a, Goba G. Prevalence and Determinants of Maternal and Perinatal outcome of Preeclampsia ata a teritiary Hospital in Ethiopia. Ethiopian Journal of Reproductive Health. 2019;11(4):1–8. [Google Scholar]
- 26.Gudeta TA. Pregnancy Induced Hypertension and Associated Factors among Pregnant Women Receiving Antenatal Care Service at Jimma Town Public Health Facilities, South West Ethiopia. Journal of Gynecology and Womens Health. 2018;10(4). [Google Scholar]
- 27.Kahsay HB, Gashe FE, Ayele WM. Patterns of Hypertensive Disorders of Pregnancy in Selected Hospitals of Tigray, Ethiopia. Journal of Advances in Medicine and Medical Research. 2018;27(12):1–14. [Google Scholar]
- 28.Wodajo S, Reddy S. Hypertensive Disorders of Pregnancy and Associated Factors among Admitted Pregnant cases in Dessie Town Referral Hospital, Northeast Ethiopia, 2015. Med Res Chron. 2016;3(3):297–306. [Google Scholar]
- 29.Wagnew M, Dessalegn M, Worku A, Nyagero J. Trends of preeclampsia/eclampsia and maternal and neonatal outcomes among women delivering in addis ababa selected government hospitals, Ethiopia: a retrospective cross-sectional study. The Pan African medical journal. 2016;25(Suppl 2):12 10.11604/pamj.supp.2016.25.2.9716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shegaze M, Markos Y, Estifaons W, Taye I. Magnitude and Associated Factors of Preeclampsia Among Pregnant Women who Attend Antenatal Care Service in Public Health Institutions in Arba Minch Town, Southern Ethiopia, 2016. Gynecology & Obstetrics. 2016;6(12). [Google Scholar]
- 31.Terefe W, Getachew Y, Hiruye A, Derbew M, Haile Mariam D, Mammo D, et al. Patterns of hypertensive disorders of pregnancy and associated factors in Debre Berhan Referral Hospital, North Shoa, Amhara Region. Ethiop Med J. 2015;2015:57–65. [PubMed] [Google Scholar]
- 32.Tessema GA, Tekeste A, Ayele TA. Preeclampsia and associated factors among pregnant women attending antenatal care in Dessie referral hospital, Northeast Ethiopia: a hospital-based study. BMC Pregnancy Childbirth. 2015;15(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vata PK, Chauhan NM, Nallathambi A, Hussein F. Assessment of prevalence of preeclampsia from Dilla region of Ethiopia. BMC research notes. 2015;8(1):816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seyom E, Abera M, Tesfaye M, Fentahun N. Maternal and fetal outcome of pregnancy related hypertension in Mettu Karl Referral Hospital, Ethiopia. J Ovarian Res. 2015;8(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wayima SD, Teklu S. Maternal and Perinatal outcomes of Pregnancy complicated by Preeclampsia /Eclampsia at Zewditu Memorial Hospital. Unpublished 2015.
- 36.Asfaw M, Negash S. Severe Preeclampsia: Effect Of Timing Of Termination Of Pregnancy On Maternal And Perinatal Outcome. Unpublished. 2014.
- 37.Wolde Z, Segni H, Woldie M. Hypertensive Disorders of Pregnancy in Jimma University Speciallized Hospital. Ethiop J Health Sci. 2011;21(3):147–54. [PMC free article] [PubMed] [Google Scholar]
- 38.Teklu S, Gaym A. Prevalence and clinical correlates of the hypertensive disorders of pregnancy at Tikur Anbessa Hospital, Addis Ababa, Ethiopia. Ethiop Med J. 2006;44(1):17–26. [PubMed] [Google Scholar]
- 39.Mekbeb T, Ketsela K. Pre-eclampsia/eclampsia at Yekatit 12 Hospital, Addis Ababa, Ethiopia (1987–1989). East Afr Med J. 1991;68(11):893–9. [PubMed] [Google Scholar]
- 40.Hailu A, Kebede D. Determinants of Preeclampsia and Gestational Hypertension. EthiopJHealth Dev. 1991;5(1):24–34. [Google Scholar]
- 41.Mekie M, Mekonnen W, Assegid M. Cohabitation duration, obstetric, behavioral and nutritional factors predict preeclampsia among nulliparous women in West Amhara Zones of Ethiopia: Age matched case control study. PloS one. 2020;15(1):e0228127 10.1371/journal.pone.0228127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fantahunegn T, Berhane K. Determinants of Preeclampsia among women attending Delivery Services in Debre Berhan Referral Hospital, Debere Berhan, Ethiopia:(A case control studey). unpublished. 2019.
- 43.Grum T, Hintsa S, Hagos G. Dietary factors associated with preeclampsia or eclampsia among women in delivery care services in Addis Ababa, Ethiopia: a case control study. BMC research notes. 2018;11(1):683 10.1186/s13104-018-3793-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kahsay HB, Gashe FE, Ayele WM. Risk factors for hypertensive disorders of pregnancy among mothers in Tigray region, Ethiopia: matched case-control study. BMC Pregnancy Childbirth. 2018;18(1):482 10.1186/s12884-018-2106-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Temesgen MA. Factors Associated with Hypertensive Disorder of Pregnancy in Kombolcha. Clinics in Mother and Child Health. 2017;14(4). [Google Scholar]
- 46.Mohammed E, Agero G, Ali E. Preeclampsia risk factors among pregnant women attending in four Public Health Facilities of Addis Ababa City. Ethiopian Journal of Reproductive Health (EJRH). 2017;9(1):1–16. [Google Scholar]
- 47.Aklilu A, Abeje G, Azage M, Asmer S. Does anemia risk for pre-eclampsia? A multicenter, Case control study in Amhara region, Ethiopia. unpublished. 2019.
- 48.Tesfay Y, Berhe S, Aregay A. Risk factors of Pregnancy Related Hypertension among Women Attending Maternal Health Care Service in Selected Public Hospitals of Tigray, Ethiopia. International Journal of DEVELOPMENT RESEARCH. 2016;6(8):8904–11. [Google Scholar]
- 49.Endeshaw M, Abebe F, Bedimo M, Asrat A, Gebeyehu A, Keno A. Family history of hypertension increases risk of preeclampsia in pregnant women: a case-control study. Universa Medicina. 2016;35(3). [Google Scholar]
- 50.Endeshaw M, Abebe F, Worku S, Menber L, Assress M, Assefa M. Obesity in young age is a risk factor for preeclampsia: a facility based case-control study, northwest Ethiopia. BMC Pregnancy Childbirth. 2016;16:237 10.1186/s12884-016-1029-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Umesawa M, Kobashi G. Epidemiology of hypertensive disorders in pregnancy: prevalence, risk factors, predictors and prognosis. Hypertens Res. 2017;40(3):213–20. 10.1038/hr.2016.126 [DOI] [PubMed] [Google Scholar]
- 52.Conde-Agudelo A, Belizán JM. Risk factors for pre-eclampsia in a large cohort of Latin American and Caribbean women. BJOG: an international journal of obstetrics and gynaecology. 2000;107(1):75–83. [DOI] [PubMed] [Google Scholar]
- 53.Conde-Agudelo A, Belizh JM. Risk factors for pre-eclampsia in a large cohort of Latin American and Caribbean women. BJOG: an international journal of obstetrics and gynaecology. 2000;107(1):75–83. [DOI] [PubMed] [Google Scholar]
- 54.Logan GG, Njoroge PK, Nyabola LO, Mweu MM. Determinants of preeclampsia and eclampsia among women delivering in county hospitals in Nairobi, Kenya. F1000Research. 2020;9. [Google Scholar]
- 55.Ye C, Ruan Y, Zou L, Li G, Li C, Chen Y, et al. The 2011 survey on hypertensive disorders of pregnancy (HDP) in China: prevalence, risk factors, complications, pregnancy and perinatal outcomes. PloS one. 2014;9(6):e100180 10.1371/journal.pone.0100180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sibai BM, Hauth J, Caritis S, Lindheimer MD, MacPherson C, Klebanoff M, et al. Hypertensive disorders in twin versus singleton gestations. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. 2000;182(4):938–42. 10.1016/s0002-9378(00)70350-4 [DOI] [PubMed] [Google Scholar]
- 57.Bdolah Y, Lam C, Rajakumar A, Shivalingappa V, Mutter W, Sachs BP, et al. Twin pregnancy and the risk of preeclampsia: bigger placenta or relative ischemia? Am J Obstet Gynecol. 2008;198(4):428 e1–6. [DOI] [PubMed] [Google Scholar]
- 58.Qiu C, Williams MA, Leisenring WM, Sorensen TK, Frederick IO, Dempsey JC, et al. Family history of hypertension and type 2 diabetes in relation to preeclampsia risk. Hypertension. 2003;41(3):408–13. 10.1161/01.HYP.0000056996.25503.F5 [DOI] [PubMed] [Google Scholar]
- 59.Zhang J, Troendle JF, Levine RJ. Risks of hypertensive disorders in the second pregnancy. Paediatric and Perinatal Epidemiology. 2001;15:226–31. 10.1046/j.1365-3016.2001.00347.x [DOI] [PubMed] [Google Scholar]
- 60.Wang Z, Wang P, Liu H, He X, Zhang J, Yan H, et al. Maternal adiposity as an independent risk factor for pre-eclampsia: a meta-analysis of prospective cohort studies. Obes Rev. 2013;14(6):508–21. 10.1111/obr.12025 [DOI] [PubMed] [Google Scholar]
- 61.Lopez-Jaramillo P, Barajas J, Rueda-Quijano SM, Lopez-Lopez C, Felix C. Obesity and Preeclampsia: Common Pathophysiological Mechanisms. Front Physiol. 2018;9:1838 10.3389/fphys.2018.01838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Timmermans S, Steegers-Theunissen RP, Vujkovic M, Bakker R, den Breeijen H, Raat H, et al. Major dietary patterns and blood pressure patterns during pregnancy: the Generation R Study. Am J Obstet Gynecol. 2011;205(4):337 e1–12. [DOI] [PubMed] [Google Scholar]
- 63.Lin Y, Han XF, Fang ZF, Che LQ, Wu D, Wu XQ, et al. The beneficial effect of fiber supplementation in high- or low-fat diets on fetal development and antioxidant defense capacity in the rat. Eur J Nutr. 2012;51(1):19–27. 10.1007/s00394-011-0185-4 [DOI] [PubMed] [Google Scholar]
- 64.Qiu C, Coughlin KB, Frederick IO, Sorensen TK, Williams MA. Dietary fiber intake in early pregnancy and risk of subsequent preeclampsia. Am J Hypertens. 2008;21(8):903–9. 10.1038/ajh.2008.209 [DOI] [PubMed] [Google Scholar]
- 65.Sun SV, Manuck TA. Alcohol in pregnancy: not recommended at any gestational age. BJOG: an international journal of obstetrics and gynaecology. 2019;126(12):1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iwama N, Metoki H, Nishigori H, Mizuno S, Takahashi F, Tanaka K, et al. Association between alcohol consumption during pregnancy and hypertensive disorders of pregnancy in Japan: the Japan Environment and Children’s Study. Hypertens Res. 2019;42(1):85–94. 10.1038/s41440-018-0124-3 [DOI] [PubMed] [Google Scholar]
- 67.Easter SR, Cantonwine DE, Zera CA, Lim KH, Parry SI, McElrath TF. Urinary tract infection during pregnancy, angiogenic factor profiles, and risk of preeclampsia. Am J Obstet Gynecol. 2016;214(3):387 e1–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(XLSX)
(XLSX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.