Abstract
Aflatoxin contamination of diets results in disease and death in humans and animals. The objective of the present paper was to review the development of innovative enterosorption strategies for the detoxification of aflatoxins. NovaSil clay (NS) has been shown to decrease exposures to aflatoxins and prevent aflatoxicosis in a variety of animals when included in their diets. Results have shown that NS clay binds aflatoxins with high affinity and high capacity in the gastrointestinal tract, resulting in a notable reduction in the bioavailability of these toxins without interfering with the utilization of vitamins and other micronutrients. This strategy is already being utilized as a potential remedy for acute aflatoxicosis in animals, and as a sustainable intervention via diet. Animal and human studies have confirmed the apparent safety of NS and refined NS clay (with uniform particle size). Studies in Ghanaians at high risk of aflatoxicosis have indicated that NS (at a dose level of 0.25% w/w) is effective at decreasing biomarkers of aflatoxin exposure and does not interfere with levels of serum vitamins A and E, or iron or zinc. A new spinoff of this strategy is the development and use of broad-acting sorbents for the mitigation of environmental chemicals and microbes during natural disasters and emergencies. In summary, enterosorption strategies/therapies based on NS clay are promising for the management of aflatoxins and as sustainable public health interventions. The NS clay remedy is novel, inexpensive, and easily disseminated.
Keywords: ACCS100, Aflatoxins, Aflatoxin binder, Aflatoxin enterosorbent, Aflatoxin Sequestrant, Calcium Montmorillonite, Geophagy, HSCAS, Interceptor Molecules, NovaSil Clay, UPSN
INTRODUCTION
The bioavailability of Aflatoxins (AF) (Figure 1) poses significant risks to human and animal health, so innovative strategies have been develop to diminish these risks by mitigating exposure through contaminated food and feed. Based on the extant scientific literature, some of these approaches are already in the stages of clinical intervention and translation. Studies describing materials that adsorb AF tightly onto internal and/or external surfaces interfering with toxin uptake and bioavailability have been reviewed recently (Kensler et al., 2004; Miller et al., 2014). Extensive studies with Camontmorillonite (NovaSil, or NS) and dietary chlorophyllin in humans and animals indicate that these interventions are approaching implementation, but still require further clinical evaluation in the field to delineate the effects of dose and time on efficacy and safety as well as acceptability (Phillips et al., 2002; Wild and Turner, 2002). Other AF-sequestering materials with limited evidence of efficacy will require preclinical trials in animals to confirm safety, followed by clinical intervention trials in humans prior to implementation. Before full-scale implementation, all of these products should be evaluated rigorously in vitro and in vivo, and should meet the following criteria: (1) favorable thermodynamic characteristics of aflatoxin sorption; (2) tolerable levels of potential hazardous contaminants; (3) safety and efficacy in multiple animal species; (4) safety and efficacy in long-term studies; and (5) negligible interactions with vitamins, iron and zinc, and other micronutrients. Based on these criteria, NS clay is one of the most thoroughly characterized sorbent materials and its production has led the only aflatoxin intervention trials in humans. The use of NS clay has demonstrated potential application for the mitigation of AF exposure in animals and humans, and this is the focus of the present review.
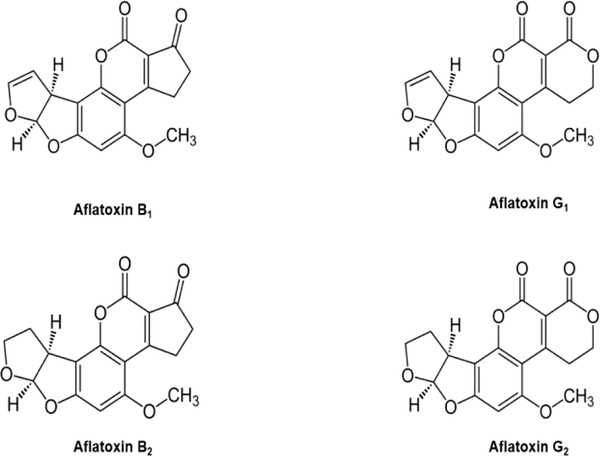
Figure 1.
Chemical structures of the four naturally occurring aflatoxins: B1, B2, G1, and G2.
Consumption of clay
The concept of eating clay falls under the scientific term geophagy and is practiced by humans and animals alike. For centuries, people have used clays in food preparation, for the treatment of diarrhea, for toxin removal, in condiments or spices, or in food during famine (Callahan, 2003). Clay consumption is also practiced during pregnancy, especially in sub-Saharan African populations (Callahan, 2003). Aflatoxin binding to NS and the reduction of toxin exposure from contaminated diets was discovered in pioneering work by T.D. Phillips (Phillips et al., 1987, 1988), in which the efficacy of NS to decrease the negative health effects of AF exposure in multiple animal species was reported. The observation that populations at high risk of exposure to AFs commonly engage in geophagy led to the investigation of the toxin-binding properties of clays. Furthermore, isothermal analyses, thermodynamics, and molecular modeling techniques have been employed to characterize and validate NS for the ‘enterosorption’ (tight binding in the stomach and intestines) of AF.
Clay minerals
Clay minerals are structurally and chemically diverse. Many are ineffective as sorbents and some could be hazardous, e.g. kaolinites containing dioxins. Research has demonstrated that NS clay has a notable preference and binding capacity for AFB1 (which is the most toxic and carcinogenic form of AF) due to the structural and chemical compositions of the NS and aflatoxin. Similar clays (including Na-montmorillonite and bentonite) can also bind aflatoxins, with variable affinities and capacities. The solid particles of soil are classified into three categories based on their size: sand (0.05–2 mm), silt (0.002–0.05 mm), and clay (<2 μm). The relative contribution of each type of particle to a particular soil determines its physical attributes (e.g. texture) and is used to name soil classes. The soil-mineral classes are divided based on the density of the dominant anionic group with silicates making up the largest class. Ca-montmorillonite falls under the phyllosilicate class. The functionality of this class of minerals is a result of the distinctive structural and chemical properties of the silicate layers containing both tetrahedral and octahedral sheets. The tetrahedral sheets are composed of SiO4 tetrahedra linked together, each sharing three O2− ions with adjacent tetrahedra. Together, this forms a plane of basal oxygens. The fourth O2− of each tetrahedron is referred to as the apical oxygen and is free to bind to other structural elements. The octahedral sheet consists of two planes of apical O2− (from the tetrahedral sheets) combined with OH− groups that form a hexagonal close-packing arrangement. In the case of montmorillonites (like NS), Al3+ fills two of every three octahedral sites to counter the negative charge of this structure and to produce a dioctahedral arrangement. With this structure, the apical oxygens from the tetrahedral sheet coordinate with Al3+ to link the octahedral and tetrahedral sheets in a 2:1 layer structure in which an octahedral sheet is bound on either side by a tetrahedral sheet. Frequently, cations in either the tetrahedral or octahedral sheets are missing or have been replaced through isomorphic substitution with another cation of lesser charge, resulting in a permanent negative charge. Thus, NS attracts Ca2+ (and other ions) into the region between the layers (i.e. the interlayer space) (Schulze et al., 1989).
Mechanisms
Due to the overall negative charges on NS clay layers, compounds with positive charges can be attracted to these areas. The most toxic and carcinogenic congener of the aflatoxins is AFB1. The dicarbonyl system and the planarity of the AFB1 ring (with the exception of the terminal furan) have been shown to be essential in the adsorption process (Figure 2). Data suggest that AFB1/NS binding in the interlayer of the NS is probably the result of a chemisorptive mechanism with high enthalpy (Grant, 1998; Phillips, 1999; Phillips et al., 2002; Deng, 2010). Early work demonstrated the importance of spatial orientation of AFB1 on NS surfaces. In isothermal adsorption studies, data were fitted to multiple equations (Kinniburgh, 1986; Grant and Phillips, 1988). The shapes of the plots were given classifications that describe the types of binding that occur (Giles et al., 1960, 1974; Giles et al., 1974). More specifically, the isotherm of AFB1 adsorption onto NS is categorized as an L2 plot that is reaching a plateau of adsorption, suggesting a saturable binding site on the clay. The maximum amount of AFB1 adsorbed onto NS was 0.336 mol/kg, which equates to 72.9% of the binding capacity (Qmax) derived from fitting the Langmuir model to the data. The Langmuir model was also used to estimate the Qmax at various temperatures and to calculate individual Kd values for the calculation of enthalpy of adsorption. These results confirmed the presence of multiple sites with different thermodynamic properties. The interlayer surfaces of NS were involved in a chemisorption mechanism because the enthalpy was −40 kJ/mol. The isothermal evidence combined with molecular modeling suggested that AF may react at multiple sites on NS clay particles with the interlayer region being the major site of chemisorption of AFB1 (Grant and Phillips, 1998). The importance of the interlayer space in the sorption of AF was further demonstrated by the decreased binding after heat-collapsing the clay and performing isothermal analyses. Results indicated that stereochemical differences in AF analogs affected significantly the tightness of binding; therefore, the adsorption of AFB1 onto NS may favor the furan alignment away from the surface. Based on the correlation between the magnitude of partial positive charges on carbons C11 and C1 of the AF dicarbonyl system and the strength of adsorption of planar analogs and derivatives of AFB1, an electron donor acceptor mechanism was postulated for the AFB1 sorption mechanism. Different humidity and exchange cations shifted adsorbed aflatoxin infrared bands, suggesting that aflatoxins were adsorbed through direct ion-dipole interactions and coordination between exchange cations and the carbonyl oxygens at low humidity and H-bonding at high humidity (Deng et al., 2010). Another hypothesis on binding of AF to clay is an electron donor-acceptor mechanism; others are possible. Recent characterizations have indicated similar binding capacity (Qmax) and affinity (Kd) of a refined form of NS, marketed as Uniform Particle Size NovaSil (UPSN) (Marroquin-Cardona, 2011).
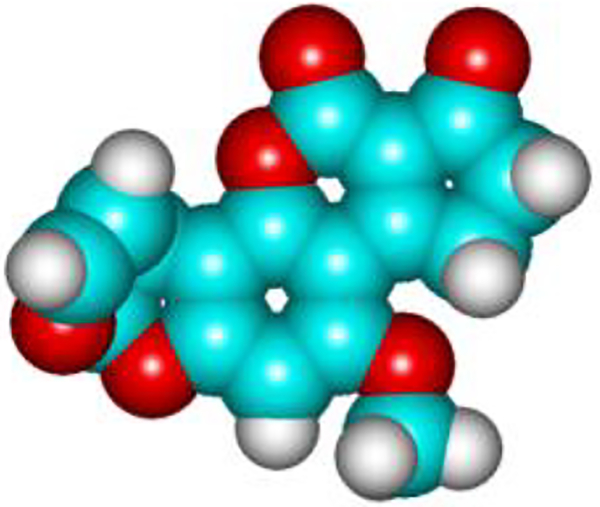
Figure 2.
Spatial model of the aflatoxin B1 showing the furan rings connected to a coumarin ring with a cyclopentenone ring to the right. The outer furan ring is kinked in the cis configuration away from the planar structure.
Animal studies
In vivo studies in animals have confirmed that NS clay successfully binds AFB1 and protects animals against exposure to toxic levels. Importantly, the clay does not interfere with the utilization of essential vitamins and micronutrients in the diets. Initially, NS was sold as an anticaking additive for animal feeds and was identified as a mitigating agent due to its ‘GRAS’ (Generally Recognized as Safe) classification. Previous radiolabeled studies using [14C]AFB1 in chicks demonstrated markedly diminished radioactivity in the blood and hepatic tissues of animals dosed with either 0.1 or 0.5% NS w/w (weight of clay/weight of feed), suggesting that NS decreased AF bioavailability in vivo (Davidson et al., 1987). Furthermore, the addition of 0.5% NS in the diet rescued broiler and leghorn chicks from the toxic effects of 7.5 ppm AF (Phillips et al., 1988, 2006). Though the levels in these early USDA studies were exceedingly high, they suggested the possibility of NS being used during seasonal drought, disasters, and acute-outbreak emergencies (Phillips et al., 1988). Following these initial studies, the efficacy of NS for AF protection has been confirmed in multiple animal species including pregnant rodents (Mayura et al., 1998), chickens (Phillips et al., 1988; Kubena et al., 1990; Pimpukdee et al., 2004), turkeys (Kubena et al., 1991), swine (Lindemann et al., 1993), and lambs (Harvey et al., 1991). These studies show that NS is a preferential enterosorbent for AF when included in the diet from 0.25 to 0.5% (w/w) in animals (Phillips et al., 2002). More recently, a study in which Sprague-Dawley rats ingested NS clay at dietary concentrations as high as 2% throughout pregnancy showed neither maternal nor fetal toxicity, and showed no significant trace-metal bioavailability in a variety of tissues (Wiles et al., 2004). A large volume of scientific literature indicates that dietary inclusion of NS clay is effective for reducing AF exposure. Also, NS rescued chicks with diminished levels of vitamin A after AFB1 exposure (Pimpukdee et al., 2004), and reduced the effects of AFB1 on serum concentrations of cholesterol, albumin, triglycerides, calcium, glucose, and total protein (Kubena et al., 1990; Kubena et al., 1993; Abo-Norag et al., 1995). No observable adverse effects were reported following ingestion of NS clay in any of these short-term animal studies (Phillips et al., 2002). Importantly, the minimal effective dose (MED) was determined to reduce significantly aflatoxicosis to 0.25% w/w (Phillips et al., 1990, 1995). In the early 1990s, urinary and milk AFM1 biomarkers were employed in safety and efficacy studies in cows. AFM1 is a hydroxylated metabolite of AFB1 that can be produced in milk and urine, facilitating its use as a short-term biomarker of aflatoxin exposure. These studies demonstrated reduced bioavailability of AFM1 when aflatoxin was added to the ration for cows. Inclusion of 1% NS clay reduced excretion of AFM1 in the milk of dairy cows and goats by 44% and 51.9%, respectively (Harvey et al., 1991; Smith et al., 1994; Maki et al., 2016, 2017). Urinary AFM1 measurements revealed reductions of 48.4% in dogs (Bingham, 2004) and of >90% in rats (Sarr et al., 1995).
Long-term exposure in rodents (pre-clinical trial)
To determine the potential toxicity of long-term dietary exposure to NS, 5–6-week-old male and female Sprague Dawley rats were fed rations containing 0, 0.25, 0.5, 1.0, or 2.0% (w/w) levels of refined NS for 28 weeks (Afriyie-Gyawu et al., 2005). Uniform particle size NS (UPSN) was produced by Texas EnteroSorbents, Inc., to increase the overall uniformity of the product and to make the product more palatable and reproducible. The parameters measured during the study included body-weight gain, feed-conversion efficiency, relative organ weights, gross and histological appearance of major organs, hematological and serum biochemistry parameters, and essential nutrient levels, including vitamins A and E, and Zn. Very few statistically significant differences were noted between rats consuming treated vs. untreated diets, with most differences unrelated to NS consumption and dose-independent. Overall, the study concluded that ingestion of up to 2% NS was safe in a sub-chronic protocol. Notably, serum and hepatic vitamins A and E levels were slightly increased in the 1% NS-females compared to untreated female rats. In addition, dioxin and furan levels in NS were measured and showed negligible levels below the PTDI (Provisional Tolerable Daily Intake). In another study in rodents dosed for 3 months, no overall toxicity was observed for UPSN (Marroquin-Cardona et al., 2011). No changes were observed for most of the blood and serum biochemical parameters; increased serum Na, Ca, vitamin E, and Na/K ratio and the reduction of serum K and Zn were reported in males with all parameters within the normal clinical ranges for rats and no trends of dose dependency. The authors have concluded that the ingestion of low levels of UPSN does not present a health risk.
Initial NS dosimetry study in human participants
As a result of the extensive safety data in animal models, it was hypothesized that NS may be safe and beneficial to humans. A randomized and double-blinded phase I clinical trial was conducted to evaluate the safety and tolerance of NS and to establish dosimetry protocols for long-term efficacy studies (Wang et al., 2005). The doses used for this study were extrapolated from dosimetry data in animal models (Phillips, 1999; Phillips et al., 2002). The high dose (3 g/day) was selected based on findings that no toxic effects were demonstrated in animals dosed at levels approximately ten times greater (Afriyie-Gyawu et al., 2005). The low dose (1.5 g/day) was equivalent to the minimal effective concentration (minimal effective dose; MED) that reduced the effects of AF in animals. The NS clay used was tested for levels of environmental contaminants, including dioxins and heavy metals, in order to comply with federal (US) and international standards. The NS capsules were manufactured in the same color and size under sterile conditions using FDA-regulated (Food & Drug Administration, USA) Good Manufacturing Practices (Texas EnteroSorbents, Inc., Bastrop, Texas, USA). Following the treatment of 50 healthy adult volunteers for 2 weeks, no significant differences in or adverse effects related to hematology, liver and kidney function, electrolytes, vitamins A and E, and minerals were observed between the two randomized dosage groups. The only symptoms reported were gastrointestinal in nature and included abdominal pain (6%, 3/50), bloating (4%, 2/50), constipation (2%, 1/50), diarrhea (2%, 1/50), and flatulence (8%, 4/50). The results from this study demonstrated the relative safety of NS clay in human subjects and served as a basis for long-term human trials in populations at high risk for aflatoxicosis.
Phase II study in Ghana (delivery of clay in capsules)
The NS was then investigated for safety, tolerance, and aflatoxin-sorption efficacy in a 3-month double-blind and placebo-controlled, phase IIa clinical trial in the Ejura-Sekyedumase district of the Ashanti region of Ghana (Afriyie-Gyawu et al., 2008; Wang et al., 2008). This region was chosen as the intervention study site based on a report that AFB1-alb adducts and AFM1 metabolites were detected in 100% of 140 sera samples and in 91.2% of 91 urine samples collected from study participants in the area (Jolly et al., 2006), consistent with reports of 75–100% incidence of exposure in people of East and West Africa (Wild et al., 1992; Wild and Turner, 2002). The NS dosimetry protocol was the same as reported by Wang et al. (2005). Individuals who qualified as study subjects met the following criteria: healthy status based on physical examination results, age 18–58 years, intake of corn and/or groundnut-based foods at least four times per week, blood AFB1-alb adduct levels >0.5 pmol AFB1 per mg of alb adducts (Figure 3), no history of chronic disease(s), no use of prescribed medications for chronic or acute illness, non-pregnant and/or non-breastfeeding females, normal ranges of hematological parameters, liver and renal function indicators (blood and urine parameters), and they submitted a signed consent form. The subjects who met the recruitment criteria were divided randomly into three study groups with 60 per group: high-dose (HD), low-dose (LD), and placebo-control (PL) based on serum AFB1-alb adduct levels to avoid selection bias. Importantly, this study employed the use of well-trained study monitors who delivered the capsules daily, witnessed ingestions, and recorded any symptoms that subjects might have experienced; NS was delivered before meals via capsule. Urine and blood samples from each participant were collected at the baseline and after 1, 2, and 3 months of treatment followed by a treatment follow-up sample at month 4. Overall, 92% of participants completed the study and compliance was >97%. Similar to the safety study, adverse events were minimal and no significant differences were shown in hematology, liver and kidney function, or electrolytes in the three treatment groups, nor did treatment interfere with the levels of serum vitamins A and E, Fe, or Zn (Afriyie-Gyawu et al., 2008). Importantly, levels of AFB1-alb adduct were decreased significantly (>40% reduction) in the HD and LD groups by month 3. Similarly, levels of AFM1 in urine samples were decreased by up to 58% in the median level of AFM1 in samples collected at 3 months in the HD group as compared to the PL group. The study demonstrated that NS clay capsules can be used effectively to reduce the bioavailability of dietary AF, thus confirming earlier work in animal models. Samples from the study were later analyzed to evaluate the ability of NS clay to reduce urinary FB1 (Fumonisin B1). 56% of the samples had detectable levels of FB1 and >90% of the median urinary FB1 was decreased significantly in the high-dose NS group (2% w/w) (Robinson et al., 2012). This same study demonstrated a significant decrease in FB1 after treatment with 2% clay by 20% at 24 h post-gavage and 50% at 48 h post-gavage.
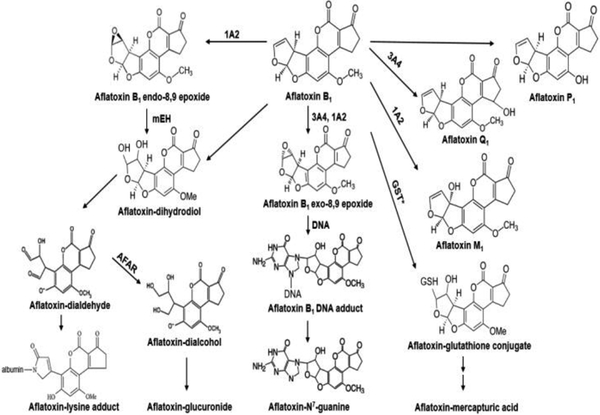
Figure 3.
Metabolism of aflatoxin B1 by phase I and phase II enzymes. Phase I enzymes include CYP3A4 and 1A2. Biomarkers are highlighted in blood (white box) and urine (gray box) (after Wild and Turner, 2002).
Crossover trials in Ghana (clay added to food)
Implementation of refined NS as a food additive was investigated in a 2010 human crossover trial in the same region of Ghana. In that study, either UPSN or placebo was included in the prepared foods at 0.25% (w/w) for 2 weeks (Mitchell et al., 2013). Participants exhibited significantly decreased levels of urinary AFM1 compared to placebo groups (55% reduction) and reported no adverse reactions. This study indicated that UPSN can reduce AF exposure safely and effectively when included in food. Utilization of the clay as a food additive could allow for reduced cost of production, decreased impact on subjects’ daily lives (i.e. eliminate the routine of taking pills), and improved sustainability.
Children’s clinical trial (clay added to food)
The results of the phase I and II clinical trials, in addition to the extensive safety testing in animals, demonstrate that ingestion of NS up to 3 g/day in adults is safe for up to 3 months. Based on these detailed studies, ingestion of UPSN at levels efficacious for reducing AFB1 biomarkers was determined to be safe in children. A phase I clinical intervention in children ages 3–9 was completed in the Ejura-Sykedumase district of Ghana. The study followed a double-blind, placebo-controlled trial design for 2 weeks (Mitchell et al., 2014a). The three treatment arms consisted of: a placebo group, which received 0.75 g of calcium carbonate twice daily; a low-dose group which received 0.375 g of UPSN twice daily; and a high-dose group, which received 0.75 g of UPSN twice daily. (The high dose was twice that of the low dose.) The results indicated a significant reduction of AFM1 biomarkers, with serum biochemical and hematological parameters within the normal range for all groups. The study demonstrated, for the first time, that UPSN is a safe and effective product in children.
Phase II study in San Antonio, Texas (delivery of clay by capsules)
South Texas currently has the highest incidence of hepatocellular carcinoma (HCC) in the United States, a disease that disproportionately affects Latino populations in the region. AFB1 has been detected in a variety of foods in the United States, including corn and corn products. Importantly, it is a dietary risk factor contributing to a greater incidence of HCC in populations which consume AFB1-contaminated diets frequently. In a randomized double-blind placebo controlled trial, the effects of a 3-month administration of ACCS100 (purified UPSN) were evaluated. Serum AFB1-lysine adduct (AFB-Lys) level and serum biochemistry were tested in 234 healthy men and women residing in Bexar and Medina counties, Texas. Participants recruited from 2012 to 2014 received either a placebo, 1.5 g, or 3 g ACCS100 each day for 3 months, and no treatment during the fourth month. Adverse event rates were similar across treatment groups and no significant differences were observed for serum biochemistry or haematology parameters. Differences in levels of AFB-Lys at 1, 3, and 4 months were compared between placebo and active treatment groups. Although serum AFB-Lys levels were decreased by month 3 for both treatment groups, the low dose was the only treatment with significant reduction (p = 0.0005). Possible reasons for this finding may include: (1) overall lower AF exposures in the US, making detection of substantial reductions difficult over the course of the study; (2) limitations in recruitment methods for large communities; (3) uneven distribution of participants at randomization and completion of the study; and (4) sub-optimal subject adherence. In conclusion, the observed effect in the low-dose treatment group suggests that the use of ACCS100 may be a viable strategy to reduce dietary AFB1 bioavailability during aflatoxin outbreaks and potentially in populations chronically exposed to this carcinogen.
Crossover study in Kenya (clay added to water)
Aflatoxicosis fatality rates have been documented to be as high as 40% in Kenya. The inclusion in the diet of calcium silicate 100 (ACCS100) reduced aflatoxin bioavailability, thus potentially decreasing the risk of aflatoxicosis. That study investigated the efficacy, acceptability, and palatability of ACCS100 in a population in Kenya with recurring aflatoxicosis outbreaks. Healthy adult participants were enrolled in a double-blinded, crossover clinical trial in 2014. Following informed consent, participants (n = 50) were randomized to receive either ACCS100 (3 g/day) or placebo (3 g/day) for 7 days. Treatments were switched following a 5-day washout period. Urine samples were collected on a daily basis and assessed for urinary aflatoxin M1 (AFM1). Blood samples were collected at the beginning and end of the trial and assessed for aflatoxin B1-lysine adducts from serum albumin (AFB1-lys). AFM1 concentrations in urine were reduced significantly while taking ACCS100 compared with the calcium carbonate placebo (β = 0.49, 95% confidence limit = 0.32–0.75). The 20-day interval included both the placebo and ACCS100 treatments as well as a washout period. No statistically significant differences were shown in reported taste, aftertaste, appearance, color, or texture by treatment. No statistically significant differences were shown in self-reported adverse events by treatment. Most participants were reported to be willing to take ACCS100 (98%) and to give it to their children (98%). ACCS100 was effective, acceptable, and palatable. More work is needed to test ACCS100 among vulnerable populations and to determine if it remains effective at the levels of aflatoxin exposure that induce aflatoxicosis.
SUMMARY
Based on multiple animal and human studies, NovaSil and refined clays have been confirmed to be safe for animal and human consumption and to be effective at aflatoxin adsorption, with significant binding capacity, affinity, and enthalpy. Recent spinoffs from these studies have also resulted in the development of field-practical and cost-effective sorbents for the mitigation of environmental chemicals and microbes. People and animals can be exposed unintentionally to mixtures of environmental chemicals and microbes following natural and man-made disasters through contaminated water and food. The Phillips Laboratory has worked at amending and functionalizing NS clays with natural products to change the clay surfaces. In addition, a new sorbent has been developed from parent NS using patentable techniques. Based on in vitro isothermal analyses along with in vivo assays, these newly developed sorbents have significantly increased binding capacity, affinity, and enthalpy for environmental chemicals (e.g. pentachlorophenol, benzo[a]pyrene, lindane, diazinon, aldicarb, and linuron) and microbes (E. coli) compared to parent clays. Besides increased adsorption of individual toxins, these sorbents also have shown protection against complex chemical mixtures in contaminated water samples collected after Hurricane Harvey in Houston (Texas, USA), for example. The extensive work with NovaSil and processed and amended clays by Phillips will facilitate the global translation of clay-based therapies for aflatoxins (and other mycotoxins) and decrease toxin exposures to humans and animals from contaminated water and food.
Table 1.
Animal and human studies with NS and similar clays: 1988–2018.
| Treatment group | Mycotoxin | Clay treatment (and duration) | Major effects of clay reported in the study | References |
|---|---|---|---|---|
| Chickens | Aflatoxins | 0.5% (28 days) | Growth inhibition diminished; gross hepatic changes prevented. | Phillips et al. (1988) |
| Chickens | Aflatoxins | 0.5% (28 days) | Growth inhibition diminished; decreased mortality. | Kubena et al. (1990) |
| Chickens | Aflatoxins | 0.1%; 0.5% (24 h) | Reduced bioavailability of aflatoxin to the liver and blood in a dose-dependent manner. | Davidson et al. (1987) |
| Chickens | Aflatoxins | 0.5%; 1.0% (21 days) | Growth inhibitory effects reduced. | Araba and Wyatt (1991) |
| Chickens | Aflatoxins | 0%–1.0% (21 days) | Feed conversions improved; growth inhibition diminished. | Doerr (1989) |
| Chickens | Aflatoxins | 1.0% (21 days) | Growth inhibition completely prevented. | Ledoux et al. (1999) |
| Chickens | Afl/Ochratoxin A | 0.5% (21 days) | Decreased growth inhibitory effects; no effect against ochratoxin. | Huff et al. (1992) |
| Chickens | Afl/Trichothecenes | 0.5% (21 days) | Diminished growth inhibition; no effect against trichothecenes. | Kubena et al. (1990) |
| Chickens | None | 0.5%; 1.0% (14 days) | NS did not impair phytate or inorganic phosphorous utilization. | Chung and Baker (1990) |
| Chickens | None | 0.5%; 1.0% (14 days) | NS did not impair utilization of riboflavin, vitamin A, or Mn; slight reduction of Zn. | Chung et al. (1990) |
| Chickens | Aflatoxins | 0.1%; 0.2% | 0.2% significantly reduced toxicity in the liver, 0.1% was not able to prevent toxicity. | Jayaprakash et al. (1992) |
| Chickens | Afl/Trichothecenes | 0.25%; 0.37%; 0.8% (21 days) | Diminished growth inhibition; no effect against trichothecenes. | Kubena et al. (1993) |
| Chickens | Aflatoxins | 0.125%; 0.25%; 0.5% (21 days) | Protected against vitamin A depletion in the livers of chicks exposed to aflatoxins. | Pimpukdee et al. (2004) |
| Chickens | None (def. diets) | 0.5% (19 days) | Did not affect growth performance or tibial mineral concentrations of chicks. | Southern et al. (1994) |
| Chickens | Aflatoxins | 0.5 HCSAS; 0.5 HCSAS + 16.5 mg VM/Kg (28 days) | HSCAS and HSCAS+VM (virginiamycin) counteracted some of the toxic effects of AF in growing broiler chicks. | Abo-Norag et al. (1995) |
| Chickens | Cyclopiazonic acid | 1.0% (21 days) | Clay did not significantly prevent the adverse effects of clyclopiazonic acid. | Dwyer et al. (1997) |
| Chickens | Aflatoxins | 0.5%; 0.5% + 0.5 TMP (3 wks) | Improved feed intake and weight gain. Alleviated the adverse effects of AFB1 on some serum chemistry. | Gowda et al. (2008) |
| Chickens | Aflatoxins | 0.1%; 0.2% (21 days) | Clay effectively alleviated the negative effect of AFB1 on growth performance and liver damage. | Zhao et al. (2010) |
| Chickens | Aflatoxin, Ochratoxin, T-2 toxin | 0.2% (42 days) | Increased feed intake and apparent retention of phosphorus. Prevented adverse effects to mycotoxins. | Liu et al. (2011) |
| Turkeys | Aflatoxins | 0.5% (21 days) | Decreased mortality. | Kubena et al. (1991) |
| Turkeys | Aflatoxins | 0.5% (21 days) | Decreased urinary excretion of aflatoxin M1. | Edrington et al. (1996) |
| Pigs | Aflatoxins | 0.5% (35 days) | Clay prevented hepatocellular changes normally associated with aflatoxin consumption. | Colvin et al. (1989) |
| Pigs | Aflatoxins | 0.5% | Decreased DNA adducts in the liver and reduced tissue residues of total aflatoxins. | Beaver et al. (1990) |
| Pigs | Aflatoxins | 0.5% (42 days) | Diminished growth inhibition. | Lindemann et al. (1993) |
| Pigs | Aflatoxins | 0.5%; 2.0% (28 days) | Decreased growth inhibition; prevention of serum effects and hepatic lesions. | Harvey et al. (1994) |
| Pigs | Aflatoxins | 0.5%; 2.0% (28 days) | Diminished growth inhibition, hepatic lesions and immunosuppression. | Harvey et al. (1998) |
| Pigs | Aflatoxins | 0.5% (35 days) | Growth inhibitory effects reduced. | Schell et al. (1993) |
| Pigs | Ochratoxins | 1.0% | No significant effect. | Bauer (1994) |
| Pigs | Trichothecenes | 0.5%; 1.0% (7–13 days) | No significant effect. | Patterson and Young (1993) |
| Dogs | Aflatoxins | 0.5% (48 h) | Significantly reduced the bioavailability of aflatoxins and excretion of M1 in urine. | Bingham et al. (2004) |
| Lambs | Aflatoxins | 2.0% (42 days) | Diminished growth inhibition and immunosuppression. | Harvey et al. (1991) |
| Mink | Aflatoxins | 0.5% (77 days) | Mortality was prevented. | Bonna et al. (1991) |
| Mink | Zearalenone | 0.5% (24 days) | Clay did not appreciably alter the hyperestrogenic effects. | Bursian et al. (1992) |
| Dairy Cows | Aflatoxins | 0.5%; 1.0% (28 days) | Reduction of aflatoxin M1 in milk. | Harvey et al. (1991) |
| Dairy Goats | Aflatoxins | 1.0%; 2.0%; 4.0% (12 days) | Reduction of aflatoxin M1 in milk. | Smith et al. (1994) |
| Mice | Zearalenone | 400 mg/kg bw; 5 g/kg bw (48 h) | Prevented the general toxicity of ZEN. | Abbès et al. (2006) |
| Mice | Zearalenone | 400, 600 or 800 mg/kg bw (48 h) | Decreased chromosomal aberrations and increased the number of polychromatic erythrocytes in bone-marrow cells. | Abbès et al. (2007) |
| Rats (and Sheep) | Ergotamine | Rats: 2.0% (28 days) Sheep: 20% (17 days) | HSCAS did not significantly protect rats or sheep from fescue toxicosis. | Chestnut et al. (1992) |
| Rats | Aflatoxins | 0.1%; 1.0%(8wks) | Partial protection against lesions in the liver. | Voss et al. (1993) |
| Rats | Aflatoxins | 0.5% (21 days) | Prevention of maternal/developmental toxicity. | Mayura et al. (1998) |
| Rats | Aflatoxins | 0.5% (21 days) | Decreased growth inhibition in pregnant rats. | Abdel-Wahhab et al. (1998) |
| Rats | Aflatoxins | 0.5% (48 h) | Decreased urinary excretion of aflatoxin metabolites (M1 and P1). | Sarr et al. (1995) |
| Rats | None | 2.0% (16 days) | In pregnant rats, Rb was reduced in groups with clay. Neither NSP nor SWY-2 influenced mineral intake. | Wiles et al. (2004) |
| Rats | None | 0.25%; 0.5%; 1.0%; 2.0% (6 mo) | No adverse effects including vitamin utilization. | Afriyie-Gyawu et al. (2005) |
| Rats | Aflatoxins | 5 g TM/kg; 5g HSCAS/kg (30 days) | Prevented deleterious effects of aflatoxins. | Abbes et al. (2010) |
| Rats | None | 0.25%; 2.0% (3 months) | Increased serum Ca, Na, Vit. E. Reduced Zn in males at 2% clay. Reduced serum K in males of clay groups. | Marroquin-Cardona et al. (2011) |
| Rats (and Humans) | Afl/Fumonisins | 2.0%, 1.5 g/d; 3 g/d (3 mo) | Reduction of urinary FB1 in rats and humans. | Robinson et al. (2012) |
| Rats | Afl/Fumonisins | 0.25%; 2.0% (1 week) | Reduced bioavailability of AFB1 and FB1 individually and in combination. | Mitchell et al. (2013) |
| Humans | None | 1.5 g; 3 g (2 weeks) | Mild GI effects. No difference in hematology, electrolytes, liver and kidney function. | Wang et al. (2005) |
| Humans | None | 1.5 g/day; 3 g/day (3 months) | Moderate effects, though not significant. No significant difference in hematology, electrolytes, liver and kidney function. | Afriyie-Gyawu et al. (2008) |
| Humans | N/A | N/A | Review Article. NS was shown to reduce biomarkers of aflatoxin exposure from urine and serum in humans. | Phillips et al. (2008) |
| Humans | N/A | In capsules: 1.5 g/day;3 g/day (3 months) | Significantly reduced AFM1 biomarker in urine and AFB1-albumin biomarker in serum. | Wang et al. (2008) |
| Humans | N/A | 1.5 g/day; 3 g/day (3 mo) | No significant effects in vitamins A and E and micronutrients, except for strontium. | Afriyie-Gyawu et al. (2008) |
| Humans | N/A | N/A | Review Article. NS is effective in binding aflatoxin from food that is highly contaminated. | Wu et al. (2010) |
| Hydra | N/A | 0.1%; 0.3%; 0.5% (92 hr) | No toxicity from NS. | Marroquin-Cardona et al. (2009) |
| Hydra | Afl/Fumonisins | 0.01%; 0.7%; 1.4%; 2.0% (92 h) | Protection from AFB1, FB1, and co-exposure to AFB1/FB1. | Brown et al. (2014) |
| Humans | N/A | 1.5 g/day; 3 g/day (3 months) | FB1 was detected in the urine of participants and were decreased by > 90% in the high dose of NS. | Robinson et al. (2012) |
| Red Drum | Aflatoxin | 0–5 ppm in the diet with 0, 1% or 2% NS for 7 weeks | NS inclusion improved weight gain, feed efficiency, muscle somatic index and intraperitoneal fat ratios. | Zychowski et al. (2013) |
| Children (3–9 years) | N/A | 0.75 g/day; 1.5 g/day (2 weeks) | Significantly reduced AFM1 in urine with no adverse events from treatment. | Mitchell et al. (2014) |
| Humans | N/A | 3 g/day (in breakfast and dinner); 2 week crossover study | A reduction up to 55% in median AFM1 levels was observed within 5 days of treatment. All participants said they would eat the food again. No adverse events were associated with UPSN consumption. | Mitchell et al. (2013) |
| Children | N/A | 6 g/day; 12 g/day (3 days) | Significantly reduced stool output in children with acute watery diarrhea | Dupont et al. (2009) |
| Human | Aflatoxin | 1.5 g/day | Sustainable reduction of aflatoxins. | Elmore et al. (2014) |
| Human | Aflatoxin | 1.5 g/day; 3 g/day (3 months) | Reduction of aflatoxin serum biomarker at low dose. | Pollock et al. (2016) |
| Human | Aflatoxin | 3 g/day (7 days) | Reduction in urinary metabolite (AFM1). | Awuor et al. (2016) |
| Rats | Afl/Fumonisins | 0.125 mg AF, or 25 mg FB (singly and in combination); 72 h | UPSN significantly reduced the bioavailability of both AF and FB and the combination of toxins. | Mitchell et al. (2014) |
| Mice | N/A | 4% w/w diet for 4 week trial. | NS mitigated the effects of TNBS-induced colitis based on reduction in systemic markers of inflammation, significant improvement in weight gain and intestinal microbial profile. | Zychowski et al. (2015) |
| Dairy Cows | Aflatoxins | Latin-Square, 5 14-d periods (day 1–7 data; day 8–14 washout); 100 ppb aflatoxins. TX | NSP reduced the transfer and excretion of AFM1 in milk with no negative effects on dry matter intake, milk production, milk quality and composition. | Maki et al. (2016) |
| Dairy Cows | Aflatoxins | Latin-Square, 14-day periods; 100 ppb aflatoxins. GA | NSP reduced the transfer and excretion of AFM1 in milk without interfering with milk quality or composition. | Maki et al. (2016) |
| Dairy Cows | Aflatoxins | Latin-Square, 5 10-day periods; NS at 0.125 and 0.25% w/w | Compared to all studies, NSP resulted in a linear decrease in AFM1 ranging from 17% to 71% without interfering with milk quality and composition. | Maki et al. (2017) |
| Rats | Afl/Fumonisins | 150 ug/kg AF for 14 days; 250 mg/kg FB for 21 days | Sequential exposure to AF + FB synergistically increased the numbers of liver GTP-P+ foci by 7.3 and 12.9 fold. | Qian et al. (2016) |
| Rats | Afl/Fumonisins | 150 ug/kg AF for 14 days; 250 mg/kg FB for 21 days; 0.5 and 1.0% USPN clay | UPSN clay at a dose up to 0.5% in the diet was shown to be effective in modulating the toxicity and carcinogenicity of co-exposure to AFB1 and FB1 | Xue et al. (2018) |
Acknowledgments
The present work was supported partially by funding through the National Institute of Health (NIH) 1RO1MD005819–01, and the Superfund Hazardous Substance Research and Training Program (NIEHS) P42 ES0277704.
References
- Abbes S, Ouanes Z, Salah-Abbes JB, Houas Z, Oueslati R Bacha H, and Othman O (2006) The protective effect of hydrated sodium calcium aluminosilicate against haematological, biochemical and pathological changes induced by Zearalenone in mice. Toxicon, 47(5), 567–574. [DOI] [PubMed] [Google Scholar]
- Abdel-Wahhab MA, nada SA, Farag IM, Abbas NF, and Amra HA (1998) Potential Protective Effect of HSCAS and Bentonite Against Dietary Aflatoxicosis in Rat: with Special Reference to Chromosomal Aberrations. Natural Toxins, 6, 211–218. [DOI] [PubMed] [Google Scholar]
- Abo-Norag M, Edrington T, Kubena LF, Harvey RB, and Phillips TD (1995) Influence of a hydrated sodium calcium aluminosilicate and virginiamycin on aflatoxicosis in broiler chicks. Poultry Science, 74(4), 626–632. [DOI] [PubMed] [Google Scholar]
- Afriyie-Gyawu E, Ankrah NA, Huebner HJ, Ofosuhene M, Kumi J, Johnson NM, Tang L, Xue L, Jolly PE, Ellis WO, Ofori-Adjei D, Willianms JH, Wang JS, and Phillips TD (2007) NovaSil clay intervention in Ghanaians at high risk for aflatoxicosis. I. Study design and clinical outcomes. Journal of Food Additives & Contaminants, 25(1), 76–87. [DOI] [PubMed] [Google Scholar]
- Afriyie-Gyawu E, Mackie J, Dash B, Wiles M, Taylor J, Huebner H, Tang L, Guan H, Wang JS, and Phillips TD (2007) Chronic toxicological evaluation of dietary NovaSil Clay in Sprague-Dawley rats. Journal of Food Additives & Contaminants, 22(3), 259–269. [DOI] [PubMed] [Google Scholar]
- Afriyie-Gyawu E, Wang Z,. Ankrah NA, Xu L, Johnson NM, Tang L, Guan H, Huebner HJ, Jolly PE, Ellis WO, Taylor R, Brattin B, Ofori-Adjei D, Williams JH, Wang JS, and Phillips TD (2008) NovaSil clay does not affect the concentrations of vitamins A and E and nutrient minerals in serum samples from Ghanaians at high risk for aflatoxicosis. Journal of Food Additives & Contaminants, 25(7), 872–884. [DOI] [PubMed] [Google Scholar]
- Araba M and Wyatt R (1991) Effects of sodium bentonite, hydrated sodium calcium aluminosilicate NovaSil™, and ethacal on aflatoxicosis in broiler chickens. Poultry Science, 70(6). [Google Scholar]
- Awuor AO, Yard E, Daniel JH, Martin C, Bii C, Romoser A, Oyugi E, Elmore S, Amwayi S, Vulule J, Zitomer NC, Rybak ME, Phillips TD, Montgomery JM, and Lewis LS (2016) Evaluation of the efficacy, acceptability and palatability of calcium montmorillonite clay used to reduce aflatoxin B1 dietary exposure in a crossover study in Kenya. Journal of Food Additives and Contaminants, 34(1), 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J (1994) Möglichkeiten zur Entgiftung mykotoxinhaltiger Futtermittel. Monatsh.Veterinarmed, 49, 175–181. [Google Scholar]
- Beaver RW, Wilson DM, James MA, Haydon KD, Colvin BM, Sangster LT, Pikul AH, and Groopman JD (1990) Distribution of aflatoxin in tissues of growing pigs fed an aflatoxin-contaminated diet amended with a high affinity aluminosilicate sorbent. Journal of American Medical Association, 32(1), 16–18. [PubMed] [Google Scholar]
- Binghan AK, Huebner HJ, Phillips TD, and Bauer JE (2004) Identification and reduction of urinary aflatoxin metabolites in dogs. Food and Chemical Toxicology, 42(11), 1851–1858. [DOI] [PubMed] [Google Scholar]
- Bonna RJ, Aulerich RJ, Bursian SJ Poppenga RH, Braselton WE, and Watson GL (1990) Efficacy of hydrated sodium calcium aluminosilicate and activated charcoal in reducing the toxicity of dietary aflatoxin to mink. Archives of Environmental Contamination and Toxicology, 20(3), 441–447. [DOI] [PubMed] [Google Scholar]
- Brown K, Mays T, Romoser A, Marroquin-Cardona A, Mitchell N, Elmore S, and Phillips T (2014) Modified hydra bioassay to evaluate the toxicity of multiple mycotoxins and predict the detoxification efficacy of a clay-based sorbent. Journal of Applied Toxicology, 34(1), 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan GN (2003) Eating dirt. Emerging Infectious Diseases, 9(8), 1016–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chestnt AB, Anderson PD, Cochran MA, Fribourg HA, and Gwinn KD (1992) Effects of hydrated sodium calcium aluminosilicate on fescue toxicosis and mineral absorption. Journal of Animal Science, 70(9), 2838–2846. [DOI] [PubMed] [Google Scholar]
- Chung TK, Ekdman JW, and Baker DH (1990) Hydrated Sodium Calcium Aluminosilicate: Effects on Zinc, Manganese, Vitamin A, and Riboflavin Utilization. Poultry Science, 69(8), 1364–1370. [DOI] [PubMed] [Google Scholar]
- Chung TK, Funk MA, and Baker DH (1990) L-2-Oxothiazolidine-4-Carboxylate as a Cysteine Precursor: Efficacy for Growth and Hepatic Glutathione Synthesis in Chicks and Rats. Journal of Nutrition, 120(2), 158–165. [DOI] [PubMed] [Google Scholar]
- Davidson JN, Babish JG, Delaney KA, Taylor DR, and Phillips TD (1987) Hydrated sodium calcium aluminosilicate decrease the bioavailability of aflatoxin in the chicken. Poultry Science, 66(Suppl. 1), 89. [Google Scholar]
- Deng Y, Velazquez ALB, Billes F, and Dixon JB (2010) Bonding mechanisms between aflatoxin B1 and smectite. Applied Clay Science, 50(1), 92–98. [Google Scholar]
- Dixon JB and Weed SB (1989) Minerals in soil environments. Soil Science Society of America, 1–34. [Google Scholar]
- Doerr JA (1989) Effect of an aluminosilicate on broiler chickens during aflatoxicosis. Poultry Science, 68(45). [Google Scholar]
- Dupont C Foo JLK, Garnier P, Moore N, Mathiex-Fortunet H, and Salazar-Lindo E (2009) Oral Diosmectite Reduces Stool Output and Diarrhea Duration in Children With Acute Watery Diarrhea. Clinical Gastroenterology and Hepatology, 7(4), 456–462. [DOI] [PubMed] [Google Scholar]
- Dwyer MR, Kubena LF, Harvey RB, Mayura K, Sarr AB, Buckley S, Bailey RH, and Phillips TD (1997) Effects of inorganic adsorbents and cyclopiazonic acid in broiler chickens. Poultry Science, 76(8), 1141–1149. [DOI] [PubMed] [Google Scholar]
- Edrington TS, Sarr AB, Kubena LF, Harvey RB, and Phillips TD (1996) Hydrated sodium calcium aluminosilicate (HSCAS), acidic HSCAS, and activated charcoal reduce urinary excretion of aflatoxin M1 in turkey poults. Lack of effect by activated charcoal on aflatoxicosis. Toxicology Letter, 89(2),115–122. [DOI] [PubMed] [Google Scholar]
- Elmore SE, Mitchell N, Mays T, Brown K, Marroquin-Cardona A, Romoser A, and Phillips TD (2014) Common African cooking processes do not affect the aflatoxin binding efficacy of refined calcium montmorillonite clay. Food Control, 37, 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles CH, Macewan TH, Nakhwa SN, and Smith D (1960) Studies in Adsorption. Part XI.* A System of Classification of Solution Adsorption Isotherms, and its Use in Diagnosis of Adsorption Mechanisms and in Measurement of Specific Surface Areas of Solids. Journal of the Society of Dyers and Colourists, 3973–3993. [Google Scholar]
- Giles CH, D’Silva AP, and Easton IA (1974) A general treatment and classification of the solute adsorption isotherm part. II. Experimental interpretation. Journal of Colloid and Interface Science, 47(3), 766–778. [Google Scholar]
- Giles CH, Smith D, and Huitson A (1974) A general treatment and classification of the solute adsorption isotherm. I. Theoretical. Journal of Colloid and Interface Science, 47(3), 755–765. [Google Scholar]
- Gowda NKS, Ledoux DR, Rottinghaus GE, Bermudez AJ, and Chen YC (2008) Efficacy of Turmeric (Curcuma longa), Containing a Known Level of Curcumin, and a Hydrated Sodium Calcium Aluminosilicate to Ameliorate the Adverse Effects of Aflatoxin in Broiler Chicks. Poultry Science, 87(6), 1125–1130. [DOI] [PubMed] [Google Scholar]
- Grant PJ and Phillips TP (1998) Isothermal Adsorption of Aflatoxin B1 on HSCAS Clay. Journal of Agricultural and Food Chemistry, 46(2), 599–605. [DOI] [PubMed] [Google Scholar]
- Harvey RB, Huff WE, Kubena LF, Corrier DE, and Phillips TD (1988) Progression of aflatoxicosis in growing barrows. American Journal of Veterinary Research, 49(4), 482–487. [PubMed] [Google Scholar]
- Harvey RB, Kubena LF, Phillips TD, Corrier DE, Elissalde MH, and Huff WE (1991) Diminution of aflatoxin toxicity to growing lambs by dietary supplementation with hydrated sodium calcium aluminosilicate. American Journal of Veterinary Research, 52(1), 152–156. [PubMed] [Google Scholar]
- Harvey RB, Phillips TD, Ellis JA, Kubena LF, Huff WE, and Petersen HD (1991) Effects on aflatoxin M1 residues in milk by addition of hydrated sodium calcium aluminosilicate to aflatoxin-contaminated diets of dairy cows. American Journal of Veterinary Research, 52(9), 1556–1559. [PubMed] [Google Scholar]
- Harvey RB, Kubena LF, Elissalde MH, Corrier DE, and Phillips TD (1994) Comparison of 2 Hydrated Sodium-Calcium Aluminosilicate Compounds to Experimentally Protect Growing Barrows from Aflatoxicosis. Journal of Veterinary Diagnostic Investigation, 6(1), 88–92. [DOI] [PubMed] [Google Scholar]
- Huff WE, Kubena LF, Harvey RB, and Phillips TD (1992) Efficacy of Hydrated Sodium Calcium Aluminosilicate to Reduce the Individual and Combined Toxicity of Aflatoxin and Ochratoxin A. Poultry Science, 71(1), 64–69. [DOI] [PubMed] [Google Scholar]
- Jayaprakash M, Gowda RNS, Vijayasarathi SK, and Seshadri SJ (1992) Adsorbent efficacy of hydrated sodium calcium aluminosilicate in induced aflatoxicosis in broilers. Indian Journal of Veterinary Pathology, 16, 102–105. [Google Scholar]
- Jolly P, Jiang Y, Ellis W, Awuah R, Nnedu O, Phillips T, Wang J Afriyie-Gyawu E, Tang L, Person S, Williams J, and Jolly C (2006) Determinants of aflatoxin levels in Ghanaians: Sociodemographic factors, knowledge of aflatoxin and food handling and consumption practices. International Journal of Hygiene and Environmental Health, 209(4), 345–358. [DOI] [PubMed] [Google Scholar]
- Klnnlburgh DG (1986) General Purpose Adsorption Isotherms. Environmental Science and Technology, 20, 895–904. [DOI] [PubMed] [Google Scholar]
- Kubena LF, Harvey RB, Huff WE, Corrier DE, and Phillips TD (1990) Efficacy of a Hydrated Sodium Calcium Aluminosilicate to Reduce the Toxicity of Aflatoxin and T-2 Toxin. Poultry Science, 69(7), 1078–1086. [DOI] [PubMed] [Google Scholar]
- Kubena LF, Harvey RB, Phillips TD Corrier DE, and Huff WE. (1990) Diminution of aflatoxicosis in growing chickens by the dietary addition of a hydrated, sodium calcium aluminosilicate. Poultry Science, 69(5), 727–735. [DOI] [PubMed] [Google Scholar]
- Kubena LF, Huff WE, Harvey RB, Yersin AG, Elissalde MH, Witzel DA, Giroir LE, Phillips TD, and Petersen HD (1991) Effects of a hydrated sodium calcium aluminosilicate on growing turkey poults during aflatoxicosis. Poultry Science, 70(8), 1823–1830. [DOI] [PubMed] [Google Scholar]
- Kubena LF, Harvey RB, Huff WE, Elissalde MH, Yersin AG, Phillips TD, and Rottinghaus GE (1993) Efficacy of a Hydrated Sodium Calcium Aluminosilicate to Reduce the Toxicity of Aflatoxin and Diacetoxyscirpenol. Poultry Science, 72(1), 51–59. [DOI] [PubMed] [Google Scholar]
- Ledoux DR, Rottinghaus GE, Bermudez AJ, and Alonso-Debolt M (1999) Efficacy of a hydrated sodium calcium aluminosilicate to ameliorate the toxic effects of aflatoxin in broiler chicks. Poultry Science, 78(21), 204–210. [DOI] [PubMed] [Google Scholar]
- Lindemann MD, Blodgett DJ, Kornegay ET, and Schurig GG (1993) Potential ameliorators of aflatoxicosis in weanling/growing swine. Journal of Animal Science, 71(1), 171–178. [DOI] [PubMed] [Google Scholar]
- Liu YL, Meng GQ, Wang HR, Zhu HL, Hou YQ, Wang WJ, and Ding BY (2010) Effect of three mycotoxin adsorbents on growth performance, nutrient retention and meat quality in broilers fed on mould-contaminated feed. Journal of British Poultry Science, 52(2), 255–263. [DOI] [PubMed] [Google Scholar]
- Maki CR, Monteiro APA, Elmore SE, Tao S, Bernard JK, Harvey RB, Romoser AA, and Phillips TD (2016) Calcium montmorillonite clay in dairy feed reduces aflatoxin concentrations in milk without interfering with milk quality, composition or yield. Animal Feed Science and Technology, 214, 130–135. [Google Scholar]
- Maki CR, Thomas AD, Elmore SE, Romoser AA, Harvey RB, Ramirez-Ramirez HA, and Phillips TD (2016) Effects of calcium montmorillonite clay and aflatoxin exposure on dry matter intake, milk production, and milk composition. Journal of Dairy Science, 99(2), 1039–1046. [DOI] [PubMed] [Google Scholar]
- Maki CR, Allen S, Wang M Ward SH, Rude BJ, Bailey HR, Harvey RB, and Phillips TD. (2017) Calcium Montmorillonite Clay for the Reduction of Aflatoxin Residues in Milk and Dairy Products. Journal of Diary and Veterinary Scicences, 2(3). [Google Scholar]
- Marroquin-Cardona A, Deng Y, Garcia-Mazcorro JF, Johnson NM, Mitchell NJ, Tang L, Robinson A, Taylor JF, Wang JS, and Phillips TD (2011) Characterization and safety of uniform particle size NovaSil clay as a potential aflatoxin enterosorbent. Applied Clay Science, 54(3–4), 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marroquin-Cardona A, Deng Y, Taylor JF, Hallmark CT, Johnson NM, and Phillips TD (2008) In vitro and in vivo characterization of mycotoxin-binding additives used for animal feeds in Mexico. Journal of Food Additives and Contaminants, 26(5), 733–743. [DOI] [PubMed] [Google Scholar]
- Mayura K, Abdel-Wahhab MA, McKenzie KS, Sarr AB, Edwards JF, Naguib K, and Phillips TD (1998) Prevention of maternal and developmental toxicity in rats via dietary inclusion of common aflatoxin sorbents: potential for hidden risks. Toxicolgical Sciences, 41(2), 175–182. [DOI] [PubMed] [Google Scholar]
- Miller JD, Schaafsma AW, Bhatnagar D, Bondy G, Carbone I, Harris LJ, Harrison G, Munkvold GP, Oswald IP, Pestka JJ, Sharpe L, Sumarah MW, Tittlemier SA, and Zhou T (2014) Mycotoxins that affect the North American agri-food sector: state of the art and directions for the future. World Mycotoxin Journal, 7(1), 63–82. [Google Scholar]
- Mitchell NJ, Kumi J, Johnson NM, Dotse E, Marroquin-Cardona A, Wang JS, Jolly PE, Ankrah NA, and Phillips TD (2013) Reduction in the urinary aflatoxin M-1 biomarker as an early indicator of the efficacy of dietary interventions to reduce exposure to aflatoxins. Biomarkers, 18, 391–398. [DOI] [PubMed] [Google Scholar]
- Mitchell NJ, Xue KS, Lin S, Marroquin-Cardona A, Brown KA, Elmore SE, Tang L, Romoser A, Gelderblom W, Wang JS, and Phillips TD (2013) Calcium montmorillonite clay reduces AFB1 and FB1 biomarkers in rats exposed to single and co-exposures of aflatoxin and fumonisin. Journal of Applied Toxicology, 34(7), 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell NJ, Kumi J, Aleser M, Elmore SE, Rychlik KA, Zychowski KE, Romoser AA, Phillips TD, and Ankrah N (2014) Short-Term Safety and Efficacy of Calcium Montmorillonite Clay (UPSN) in Children. The American Journal of Tropical Medicine and Hygiene, 91(4), 777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R and Yong LG (1993) Efficacy of hydrated sodium calcium aluminosilicate, screening and dilution in reducing the effects of mold contaminated corn in pigs. Canadian Journal of Animal Science, 73(3), 615–624. [Google Scholar]
- Phillips TD, Kubena LF, Harvey RB, Taylor DS, and Heidelbaugh ND (1987) Mycotoxin hazards in agriculture: New approach to control. Journal of the American Veterinary Medical Association, 190:1617 (Abstract). [Google Scholar]
- Phillips TD, Kubena LF Harvey RB, Taylor DS, and Heidelbaugh ND. (1988) Hydrated Sodium Calcium Aluminosilicate: A High Affinity Sorbent for Aflatoxin. Poultry Science, 67(2), 243–247. [DOI] [PubMed] [Google Scholar]
- Phillips TD, Clement BA, Kubena LF, and Harvey RB (1990) Detection and detoxification of aflatoxins: prevention of aflatoxicosis and aflatoxin residues with hydrated sodium calcium aluminosilicate. Strengthening Journalism in Europe,1(Suppl_15–19). [PubMed] [Google Scholar]
- Phillips TD, Sarr AB, and Grant PG. (1995) Selective chemisorption and detoxification of aflatoxins by phyllosilicate clay. Natrual Toxins, 3, 204–213. [DOI] [PubMed] [Google Scholar]
- Phillips TD (1999) Dietary clay in the chemoprevention of aflatoxin-induced disease. Toxicological Sciences, 52(suppl_1), 118–126. [DOI] [PubMed] [Google Scholar]
- Phillips TD, Lemke SL, and Grant PG (2002) Characterization of Clay-Based Enterosorbents for the Prevention of Aflatoxicosis. Mycotoxins and Food Safety, 504, 157–171. [DOI] [PubMed] [Google Scholar]
- Pimpukdee K, Kubena LF, Bailey CA, Huebner HJ, Afriyie-Gyawu E, and Phillips TD (2004) Aflatoxin-induced toxicity and depletion of hepatic vitamin A in young broiler chicks: protection of chicks in the presence of low levels of NovaSil PLUS in the diet. Poultry Science, 83(5), 737–744. [DOI] [PubMed] [Google Scholar]
- Pollock BH, Elmore S, Romoser A, Tang L, Kang M, Xue K, Rodriguez M, Dierschke N, Hayes HG, Hansen HA, Guerra F, Wang JS, and Phillips TD (2016) Intervention trial with calcium montmorillonite clay in a south Texas population exposed to aflatoxin. Journal of Food Additives and Contaminants, 33(8), 1346–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A, Johnson NM, Strey A, Taylor JF, Marroquin-Cardona A, Mitchell NJ, Afriyie-Gyawu E, Ankrah NA, Williams JH, Wang JS, Jolly PE, Nachman RJ, and Phillips TD (2012) Calcium montmorillonite clay reduces urinary biomarkers of fumonisin B1 exposure in rats and humans. Journal of Food Additives and Contaminants, 29(5), 809–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarr AB, Mayura K, Kubena LF, Harvey RB, and Phillips TD (1995) Effects of phyllosilicate clay on the metabolic profile of aflatoxin B1 in Fischer-344 rats. Toxicology Letter, 75(1–3), 145–151. [DOI] [PubMed] [Google Scholar]
- Schell TC, Lindemann MD, Kornegay ET, Blodgett DJ, and Doerr JA (1993) Effectiveness of different types of clay for reducing the detrimental effects of aflatoxin-contaminated diets on performance and serum profiles of weanling pigs. Journal of Animal Science, 71(5), 1226–1231. [DOI] [PubMed] [Google Scholar]
- Smith EE, Phillips TD, Ellis JA, Harvey RB, Kubena LF, Thompson J, and Newton G (1994) Dietary hydrated sodium calcium aluminosilicate reduction of aflatoxin M1 residue in dairy goat milk and effects on milk production and components. Journal of animal science, 72(3), 677–682. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Egner PA, Wang J, Zhu Y, Zhang B, Lu P, Chen J, Qian G, Kuang S, Jackson PE, Gangge SJ, Jacobson LP, Munoz A, and Groopman JD (2004) Chemoprevention of Hepatocellular Carcinoma in Aflatoxin Endemic Areas. Gastroenterology, 127, 310–318. [DOI] [PubMed] [Google Scholar]
- Voss KA, Dorner JW, and Cole RJ (1993) Melioration of Aflatoxicosis in Rats by Volclay NF-BC, Microfine Bentonite. Journal of Food Protection, 56(7), 595–598. [DOI] [PubMed] [Google Scholar]
- Wang JS, Luo H, Billam M, Wang Z, Guan H, Tang L, Goldston T, Afriyie-Gyawu E, Lovett C, Griswold J, Brattin B, Taylor RJ, Huebner HJ, and Phillips TD (2005) Short-term safety evaluation of processed calcium montmorillonite clay (NovaSil) in humans. Food Additives Contaminants, 22(3), 270–279. [DOI] [PubMed] [Google Scholar]
- Wang P, Afriyie-Gyawu E, Tang Y, Johnson NM, Xu L, Tang L, Huebner HJ, Ankrah NA, Ofori-Adjei D, Ellis W, Jolly PE, Williams JH, Wang JS, and Phillips TD (2008) NovaSil clay intervention in Ghanaians at high risk for aflatoxicosis: II. Reduction in biomarkers of aflatoxin exposure in blood and urine. Food Additives and Contaminants, 25(5), 622–634. [DOI] [PubMed] [Google Scholar]
- Wild CP and Turner PC (2002) The toxicology of aflatoxins as a basis for public health decisions. Mutagenesis, 17(6), 471–481. [DOI] [PubMed] [Google Scholar]
- Wild CP, Hudson GJ, Sabbioni G, Chapot B, Hall AJ, Wogan GN, Whittle H, Montesano R, and Groopman J (1992) Dietary intake of aflatoxins and the level of albumin-bound aflatoxin in peripheral blood in The Gambia, West Africa. Cancer Epidemiology, Biomarkers & Prevention, 1(3). [PubMed] [Google Scholar]
- Wiles M, Huebner H, Afriyie-Gyawu E, Taylor R, Bratton G, and Phillips T (2004) Toxicological evaluation and metal bioavailability in pregnant rats following exposure to clay minerals in the diet. Journal of Toxicology and Environmental Health A, 67(11), 863–874. [DOI] [PubMed] [Google Scholar]
- Zhao J, Shirley RB, Dibner JD, Uraizee F, Offier M, Kitchell M, Vazaquez-Anon M, and Knight CD (2010) Comparison of hydrated sodium calcium aluminosilicate and yeast cell wall on counteracting aflatoxicosis in broiler chicks. Poultry Science, 89(10), 2147–2156. [DOI] [PubMed] [Google Scholar]
- Zychowski KE, Elmore SE, Rychlik KA, Ly HJ, Pierezan F, Isaiah A, Suchodolski JS, Hoffmann AR, Romoser AA, and Phillips TD (2015) Mitigation of Colitis with NovaSil Clay Therapy. Digestive Diseases and Sciences, 60(2), 382–392. [DOI] [PubMed] [Google Scholar]