Abstract
The cornea is a transparent avascular tissue on the anterior segment of the eye responsible for providing refractive power and forming a protective barrier against the external environment. Infectious and inflammatory conditions can compromise the structure of the cornea, leading to visual impairment and blindness. Galectins are a group of β-galactoside-binding proteins expressed by immune and non-immune cells that play pivotal roles in innate and adaptive immunity. In this brief review, we discuss how different members of this family of proteins affect both pro-inflammatory and anti-inflammatory responses in the cornea, particularly in the context of infection, transplantation and wound healing. We further describe recent research showing beneficial effects of galectin-targeted therapy in corneal diseases.
Keywords: Cornea, Galectins, Glycobiology, Infection, Inflammation
Introduction
The cornea is a transparent avascular tissue located on the anterior segment of the eye that allows the passage of light to the retina. It plays a vital role in visual function by providing most of the refractive power for the eye and forming a protective barrier against fluid loss and the external environment [1]. Three anatomical layers can be found in the cornea (Figure 1A). The outermost layer is composed of a nonkeratinized, stratified squamous epithelium of a very uniform thickness. The intermediate stroma is a specialized layer of connective tissue produced by keratocytes and constitutes most of the thickness of the human cornea. The endothelial layer lines the posterior surface and is composed of flattened hexagonal cells responsible for regulating corneal nutrition and hydration.
Figure 1. Galectins as regulators of corneal inflammation.
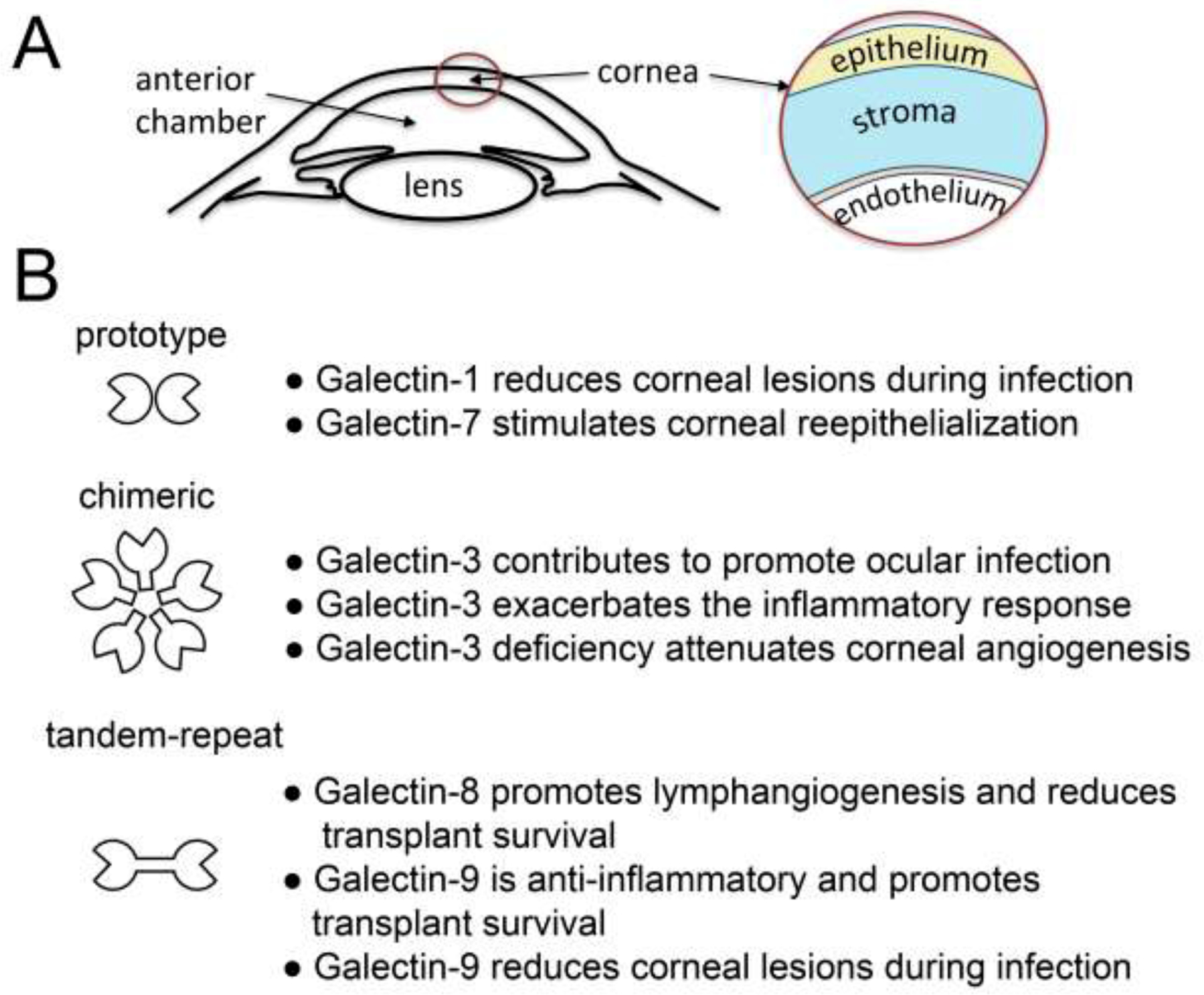
(A) Schematic representation showing the structure of the anterior segment of the eye. (B) Prototype, chimeric and tandem-repeat galectins differentially regulate inflammatory processes in the cornea.
Because of the success rate of corneal transplantation, the cornea has been defined as an immune privilege site that resists inflammation through inhibition of innate and adaptive immune responses [2]. The absence of lymphatic vessels and the presence of cell surface proteins that promote apoptosis of inflammatory cells, such as Fas ligand and programmed death ligand-1, are critical to prevent immunological attack. However, the immune privilege of the cornea can be abolished in the setting of inflammation, neovascularization or trauma [3]. Microbial infection of the cornea also activates the innate response by engaging Toll-like receptors and triggering the synthesis of pro-inflammatory cytokines. These insults can lead to corneal inflammation or keratitis, a pathological state that, if not adequately treated, results in tissue scarring and vision loss.
Galectins are a family of small soluble proteins defined by their affinity towards β-galactose-containing glycoconjugates and the presence of carbohydrate-binding domains (CRDs) that appear to have evolved from a common ancestral gene [4]. In this context it is important to note that each galectin CRD has unique affinity binding to different types of glycan structures. Galectins are produced by multiple cell types, including epithelial, stromal, endothelial and immune cells, and regulate many biological functions, such as those driving the immune response, cell migration and fibrosis [5]. They have been classified into three major groups according to structure; (i) prototypical, with a single CRD that may associate to form homodimers, (ii) chimeric, with a single CRD and a large amino-terminal domain that contributes to self-aggregation and, (iii) tandem-repeat, with at least two CRDs covalently connected by a linker peptide [6]. In this review we focus on the functions of these groups of proteins in the regulation of corneal inflammation.
Prototype galectins
The prototype galectins comprise the largest group of galectins and include galectin-1, −2, −5, −7, −10, −11, −13, −14 and −15. Galectin-1 was the first galectin identified by its hemagglutination properties [7]. It is produced by a wide range of immune cells such as T and B cells, macrophages and dendritic cells, and is known to act as a proresolving mediator by repressing innate and adaptive immune responses [8]. In the cornea, it is expressed in low levels within the cytoplasm of epithelial and stromal cells [9]. The few studies available on prototype galectins in the cornea have focused on the effects of galectin-1 in the course of a microbial infection.
Viruses and bacteria can induce T cell responses and inflammatory lesions in the cornea. Some of the most frequent and pathogenic organisms include herpes simplex virus type 1 (HSV-1) and Pseudomonas aeruginosa. Infection with these pathogens involves a rapid inflammatory response in the eye, defined by CD4+ Th1 and Th17 cells, which orchestrate the migration and activation of neutrophils, and the development of keratitis [10–12]. Administration of recombinant galectin-1 has been shown to reduce the severity of corneal lesions in animal models of HSV-1 and P. aeruginosa infection through multiple mechanisms. These include a reduction in the Th17 cell response, the diminished recruitment of tissue-damaging neutrophils and the promotion of anti-inflammatory IL-10 [13, 14].
Efficient healing of corneal wounds requires a proper inflammatory response and its resolution. While there is limited information on how prototype galectins contribute to the immune response after wounding, results from the Panjwani laboratory have identified galectin-7 as one of the genes differentially upregulated in healing mouse corneas [15]. These findings have been associated with the ability of galectin-7 to stimulate corneal epithelial cell migration and reepithelialization [16, 17].
Chimera-type galectin-3
Galectin-3 is the only known species of chimeric galectins present in vertebrates. It is produced by virtually all types of immune cells and also by epithelial and stromal cells of the human cornea [9, 18]. In the epithelium, multimeric galectin-3 contributes to maintain barrier function and support innate defense through binding to transmembrane mucins on the apical glycocalyx [19, 20]. Both HSV-1 and P. aeruginosa lipopolysaccharide recognize galectin-3 expressed in human corneal epithelial cells, an interaction that contributes to promote ocular infection [21, 22]. However, the highly glycosylated mucins provide steric hindrance and act as high affinity counter-receptors for galectin-3, thereby reducing the efficiency by which pathogens bind to, and infect, host cells [22].
Pathological alterations of the epithelial glycocalyx can have detrimental effects on the cornea, promoting new galectin-3 interactions that favor the establishment of a proinflammatory environment. Ocular autoimmune disease and high levels of TNFα have been associated with changes in the expression of N-glycan–processing enzymes responsible for the biosynthesis of galectin-3 ligands, particularly on mucins [23, 24]. The disruption of mucin glycosylation, in conjunction with an increased presence of immune cells at the ocular surface, might explain the elevated amounts of galectin-3 found in the tear fluid of patients with ocular disorders [25, 26]. It is under these conditions that galectin-3 could contribute to exacerbate the inflammatory response. Studies using corneal epithelial cells have sown that exogenous galectin-3 bind the matrix metalloprotease inducer CD147 to trigger expression of MMP9, a known mediator of wound healing and inflammation, and amplify the IL- 1β- mediated inflammatory response [27, 28].
Galectin-3 also appears to actively participate in regulating the inflammatory microenvironment following the establishment of heterotypic cell-cell interactions in wounded corneas. Multiple studies have shown that it is overexpressed at the epithelial–stromal junction in healing corneal epithelium [17, 29–31]. Injuries that lead to the rupture of the basement membrane allow epithelial cells to come into close contact with the underlying stroma. It is here, in the absence of basement membrane, that epithelial cells expose galectin-3 directly to individual fibroblasts in the underlying stroma, promoting the paracrine action of secreted IL-1β and the initiation of matrix remodeling processes [32]. Stimulation of corneal fibroblasts with soluble galectin-3 also results in increased expression of additional mediators of the inflammatory response, including CCL5 and CXCL10, which could play important roles in the chemotactic recruitment of leukocytes.
The expression of galectin-3 in the corneal stroma significantly increases in sterile and nonsterile inflammation [33, 34]. These results have led to important research exploring the therapeutic potential of inhibiting galectin-3 in the context of pathological neovascularization and fibrosis. Studies using the cornea as a model system in combination with knockout mice have shown that galectin-3 is a critical modulator of the VEGF/VEGFR-2 signaling pathway and that its deficiency attenuates inflammatory angiogenesis [35, 36]. Galectin-3 is also required for TGF-β–mediated myofibroblast activation, a key event in fibrotic disease [37]. Treatment with a small molecular weight inhibitor of galectin-3 has been effective in reducing corneal angiogenesis and opacification, as well as in decreasing the amounts of the fibrosis-related protein α-SMA [38], results that highlight the potential of using these inhibitors for drug development applications in the cornea.
Lastly, galectin-3 has been shown to bind clusterin in corneal epithelial cells to facilitate barrier function [39]. It is possible that this interaction also has a relevance to corneal inflammation and autoimmune disease. Clusterin is a molecular chaperon important in extracellular protein folding quality control, and it is induced in response to a wide variety of tissue injuries [40]. Clusterin potentiates the phagocytosis of late apoptotic cells by macrophages and prevents apoptotic cell-induced autoimmune responses [41]. The implications of galectin-3 binding to the immune-mediated functions of clusterin deserve further investigation.
Tandem-repeat type galectins
Members of the tandem-repeat type galectins include galectin-4, −6, −8, −9, and −12. Similar to other types of galectins, these galectins are ubiquitously expressed in mammalian tissues and can influence both innate and adaptive immune responses [42]. In the cornea, galectin-8 and −9 can be found primarily in epithelium and stroma [9]. However, infiltrating immune cells, injured epithelium, activated tissue-resident macrophages and angiogenic endothelial cells can contribute to increase their levels in the stroma following injury [33].
The available data suggests that galectin-8 expression may promote inflammation in the cornea. Increased galectin-8 expression has been observed in rejected murine corneal allografts compared to accepted grafts, supporting the hypothesis that expression of galectin-8 plays a detrimental role in the cornea by reducing transplant survival [34]. Use of knockout mice indicates that the mechanism of rejection is associated with the ability of galectin-8 to promote lymphangiogenesis. Galectin-8-mediated interactions with lymphangiogenic integrins (α1β1/α5β1) and the mucin-type glycoprotein podoplanin are sufficient to activate the integrins and trigger the formation of new lymphatic vessels in the cornea [43]. The formation of this complex also potentiates the VEGF-C/VEGFR-3 signaling pathway, a major inducer of lymphangiogenesis.
As opposed to galectin-8, galectin-9 appears to exert an anti-inflammatory function in the cornea. Reduced levels of galectin-9 expression have been associated with rejection of corneal allografts, leading to the hypothesis that galectin-9 contributes to graft survival and the immune-privileged status of the cornea [34]. This hypothesis is supported by data indicating that galectin-9 binds to the T-cell immunoglobulin and mucin domain (Tim)-3, a regulatory molecule for T-cell function, and protects corneal endothelial cells from destruction by allo-reactive T cells [44]. The interaction between galectin-9 and Tim-3 is also critical to suppress corneal lesions caused by infection with HSV-1. The mechanisms by which galectin-9 functions in this context are multiple and include impairment of the CD8+ T cell response, induction of apoptosis of pathogenic effector Th1 cells and expansion of Treg cell activity [45, 46].
The immune modulatory functions of tandem-repeat galectins can certainly be exploited therapeutically for the treatment of corneal disease. Administration of the MAbT25 agonistic antibody, targeting the tumor necrosis factor receptor superfamily member 25 (TNFRSF25), before or at the time of HSV-1 infection, has been successfully used to expand Treg cells and to reduce the severity of stromal keratitis [47]. However, later administration of the antibody results in the expansion of proinflammatory effector T cells, which also express TNFRSF25. The Rouse laboratory tested a combination therapy consisting of MAbT25 and galectin-9 to overcome this limitation. This approach was more effective than the individual treatments as it allowed expansion of Tregs expressing CD103 needed to access inflammatory sites, and reduced the number of IFN-γ-producing CD4+ T cells, resulting in better control of the inflammatory process responsible for tissue damage [47].
Conclusions
Galectins play important roles in regulating the innate and adaptive immune response in the cornea (Figure 1B). They are constitutively expressed during homeostatic conditions but their pathological alteration can trigger both pro-inflammatory and anti-inflammatory responses. A persistent imbalance in galectin expression that favors pro-inflammatory pathways can dictate the ultimate response to the initial injury and prevent its resolution. Current efforts to develop highly specific inhibitors of individual galectins is leading to a wide range of novel strategies to treat inflammatory diseases of the cornea. Despite these advances, there are still many fundamental gaps in our knowledge. One of them is to understand how galectin signatures as a whole interact with the enormous structural complexity of glycans on cell surfaces. Similarly, it is necessary to further decipher how changes in cell surface glycosylation, known to be dynamic and highly dependent on the microenvironment, alters the relative affinity of galectins towards counter-receptors and their corresponding signal transduction pathways. These efforts will be facilitated by the development of new research tools and strategies in the field of glycobiology.
Acknowledgements
The author would like to express his deep appreciation to Dr. Noorjahan Panjwani for introducing him to the field of galectins and her continuous support.
Financial support
This work was supported by the National Institutes of Health, NEI Grants R01EY026147 and R01EY030928.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
● of special interest
●● of outstanding interest
- [1].Gonzalez-Andrades M, Argueso P, Gipson IK, Corneal Anatomy, in: Alio JL, Alio del Barrio JL, Arnalich-Montiel F (Eds.), Corneal Regeneration: Therapy and Surgery, Springer Nature Switzerland AG, Basel, Switzerland, 2019, pp. 3–12. [Google Scholar]
- [2].Niederkorn JY, Cornea: Window to Ocular Immunology, Curr Immunol Rev 7(3) (2011) 328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Niederkorn JY, Larkin DF, Immune privilege of corneal allografts, Ocul Immunol Inflamm 18(3) (2010) 162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Houzelstein D, Goncalves IR, Fadden AJ, Sidhu SS, Cooper DN, Drickamer K, Leffler H, Poirier F, Phylogenetic analysis of the vertebrate galectin family, Mol Biol Evol 21(7) (2004) 1177–87. [DOI] [PubMed] [Google Scholar]
- [5].Johannes L, Jacob R, Leffler H, Galectins at a glance, J Cell Sci 131(9) (2018) 1–9. [DOI] [PubMed] [Google Scholar]
- [6].Cummings RD, Liu FT, Galectins, in: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME (Eds.), Essentials of Glycobiology, Cold Spring Harbor; (NY: ), 2009, pp. 475–83. [PubMed] [Google Scholar]
- [7].Teichberg VI, Silman I, Beitsch DD, Resheff G, A beta-D-galactoside binding protein from electric organ tissue of Electrophorus electricus, Proc Natl Acad Sci U S A 72(4) (1975) 1383–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sundblad V, Morosi LG, Geffner JR, Rabinovich GA, Galectin-1: A Jack-of-All-Trades in the Resolution of Acute and Chronic Inflammation, J Immunol 199(11) (2017) 3721–30. [DOI] [PubMed] [Google Scholar]
- [9].Schlotzer-Schrehardt U, Andre S, Janko C, Kaltner H, Kopitz J, Gabius HJ, Herrmann M, Adhesion/growth-regulatory galectins in the human eye: localization profiles and tissue reactivities as a standard to detect disease-associated alterations, Graefes Arch Clin Exp Ophthalmol 250(8) (2012) 1169–80. [DOI] [PubMed] [Google Scholar]
- [10].Hazlett LD, Corneal response to Pseudomonas aeruginosa infection, Prog Retin Eye Res 23(1) (2004) 1–30. [DOI] [PubMed] [Google Scholar]
- [11].McKenna KC, Vicetti Miguel RD, Adaptive immune system and the eye: T-cell mediated immunity, in: Dartt DA, Dana R, D’Amore P, Niederkorn J (Eds.), Immunology, Inflammation and Diseases of the Eye, Academic Press2011, pp. 11–7. [Google Scholar]
- [12].Koganti R, Yadavalli T, Shukla D, Current and Emerging Therapies for Ocular Herpes Simplex Virus Type-1 Infections, Microorganisms 7(10) (2019) 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rajasagi NK, Suryawanshi A, Sehrawat S, Reddy PB, Mulik S, Hirashima M, Rouse BT, Galectin-1 reduces the severity of herpes simplex virus-induced ocular immunopathological lesions, J Immunol 188(9) (2012) 4631–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Suryawanshi A, Cao Z, Thitiprasert T, Zaidi TS, Panjwani N, Galectin-1-mediated suppression of Pseudomonas aeruginosa-induced corneal immunopathology, J Immunol 190(12) (2013) 6397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cao Z, Wu HK, Bruce A, Wollenberg K, Panjwani N, Detection of differentially expressed genes in healing mouse corneas, using cDNA microarrays, Invest Ophthalmol Vis Sci 43(9) (2002) 2897–904. [PubMed] [Google Scholar]
- [16].Cao Z, Said N, Wu HK, Kuwabara I, Liu FT, Panjwani N, Galectin-7 as a potential mediator of corneal epithelial cell migration, Arch Ophthalmol 121(1) (2003) 82–6. [DOI] [PubMed] [Google Scholar]
- [17].Cao Z, Said N, Amin S, Wu HK, Bruce A, Garate M, Hsu DK, Kuwabara I, Liu FT, Panjwani N, Galectins-3 and −7, but not galectin-1, play a role in re-epithelialization of wounds, J Biol Chem 277(44) (2002) 42299–305. [DOI] [PubMed] [Google Scholar]
- [18].Liu FT, Hsu DK, The role of galectin-3 in promotion of the inflammatory response, Drug News Perspect 20(7) (2007) 455–60. [DOI] [PubMed] [Google Scholar]
- [19].Argueso P, Guzman-Aranguez A, Mantelli F, Cao Z, Ricciuto J, Panjwani N, Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier, J Biol Chem 284(34) (2009) 23037–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mauris J, Mantelli F, Woodward AM, Cao Z, Bertozzi CR, Panjwani N, Godula K, Argueso P, Modulation of ocular surface glycocalyx barrier function by a galectin-3 N-terminal deletion mutant and membrane-anchored synthetic glycopolymers, PLoS One 8(8) (2013) e72304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gupta SK, Masinick S, Garrett M, Hazlett LD, Pseudomonas aeruginosa lipopolysaccharide binds galectin-3 and other human corneal epithelial proteins, Infect Immun 65(7) (1997) 2747–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Woodward AM, Mauris J, Argueso P, Binding of transmembrane mucins to galectin-3 limits herpesvirus 1 infection of human corneal keratinocytes, J Virol 87(10) (2013) 5841–7. [DOI] [PMC free article] [PubMed] [Google Scholar]; ● High affinity interactions between transmembrane mucins and galectin-3 on the epithelial glycocalyx constitute an innate barrier that limits the interaction between HSV-1 and host cells.
- [23].Woodward AM, Lehoux S, Mantelli F, Di Zazzo A, Brockhausen I, Bonini S, Argueso P, Inflammatory stress causes N-glycan processing deficiency in ocular autoimmune disease, Am J Pathol 189(2) (2019) 283–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Taniguchi T, Woodward AM, Magnelli P, McColgan NM, Lehoux S, Jacobo SMP, Mauris J, Argueso P, N-Glycosylation affects the stability and barrier function of the MUC16 mucin, J Biol Chem 292(26) (2017) 11079–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hrdlickova-Cela E, Plzak J, Smetana K Jr., Melkova Z, Kaltner H, Filipec M, Liu FT, Gabius HJ, Detection of galectin-3 in tear fluid at disease states and immunohistochemical and lectin histochemical analysis in human corneal and conjunctival epithelium, Br J Ophthalmol 85(11) (2001) 1336–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Uchino Y, Mauris J, Woodward AM, Dieckow J, Amparo F, Dana R, Mantelli F, Argueso P, Alteration of galectin-3 in tears of patients with dry eye disease, Am J Ophthalmol 159(6) (2015) 1027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mauris J, Woodward AM, Cao Z, Panjwani N, Argueso P, Molecular basis for MMP9 induction and disruption of epithelial cell-cell contacts by galectin-3, J Cell Sci 127(Pt 14) (2014) 3141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Uchino Y, Woodward AM, Mauris J, Peterson K, Verma P, Nilsson UJ, Rajaiya J, Argueso P, Galectin-3 is an amplifier of the interleukin-1beta-mediated inflammatory response in corneal keratinocytes, Immunology 154(3) (2018) 490–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fujii A, Shearer TR, Azuma M, Galectin-3 enhances extracellular matrix associations and wound healing in monkey corneal epithelium, Exp Eye Res 137 (2015) 71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cruzat A, Gonzalez-Andrades M, Mauris J, AbuSamra DB, Chidambaram P, Kenyon KR, Chodosh J, Dohlman CH, Argueso P, Colocalization of Galectin-3 With CD147 Is Associated With Increased Gelatinolytic Activity in Ulcerating Human Corneas, Invest Ophthalmol Vis Sci 59(1) (2018) 223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Saravanan C, Liu FT, Gipson IK, Panjwani N, Galectin-3 promotes lamellipodia formation in epithelial cells by interacting with complex N-glycans on alpha3beta1 integrin, J Cell Sci 122(Pt 20) (2009) 3684–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].AbuSamra DB, Mauris J, Argueso P, Galectin-3 initiates epithelial-stromal paracrine signaling to shape the proteolytic microenvironment during corneal repair, Sci Signal 12(590) (2019) eaaw7095. [DOI] [PMC free article] [PubMed] [Google Scholar]; ●● This study exposes a direct role for galectin-3 in regulating the proteolytic microenvironment in epithelial-stromal interactions by promoting the paracrine action of secreted IL-1β.
- [33].Chen WS, Cao Z, Truong L, Sugaya S, Panjwani N, Fingerprinting of galectins in normal, P. aeruginosa-infected, and chemically burned mouse corneas, Invest Ophthalmol Vis Sci 56(1) (2015) 515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sugaya S, Chen WS, Cao Z, Kenyon KR, Yamaguchi T, Omoto M, Hamrah P, Panjwani N, Comparison of galectin expression signatures in rejected and accepted murine corneal allografts, Cornea 34(6) (2015) 675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]; ● Seminal study in the field of ophthalmology comparing the galectin expression pattern in accepted and rejected murine corneal allografts.
- [35].Markowska AI, Jefferies KC, Panjwani N, Galectin-3 protein modulates cell surface expression and activation of vascular endothelial growth factor receptor 2 in human endothelial cells, J Biol Chem 286(34) (2011) 29913–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Markowska AI, Liu FT, Panjwani N, Galectin-3 is an important mediator of VEGF- and bFGF-mediated angiogenic response, J Exp Med 207(9) (2010) 1981–93. [DOI] [PMC free article] [PubMed] [Google Scholar]; ●● Galectin-3 modulates VEGF- and bFGF-mediated angiogenesis by binding to N-glycans in αvβ3-integrin and subsequently activating the signaling pathways that promote the growth of new blood vessels.
- [37].Mackinnon AC, Gibbons MA, Farnworth SL, Leffler H, Nilsson UJ, Delaine T, Simpson AJ, Forbes SJ, Hirani N, Gauldie J, Sethi T, Regulation of transforming growth factor-beta1-driven lung fibrosis by galectin-3, Am J Respir Crit Care Med 185(5) (2012) 537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chen WS, Cao Z, Leffler H, Nilsson UJ, Panjwani N, Galectin-3 Inhibition by a Small-Molecule Inhibitor Reduces Both Pathological Corneal Neovascularization and Fibrosis, Invest Ophthalmol Vis Sci 58(1) (2017) 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bauskar A, Mack WJ, Mauris J, Argueso P, Heur M, Nagel BA, Kolar GR, Gleave ME, Nakamura T, Kinoshita S, Moradian-Oldak J, Panjwani N, Pflugfelder SC, Wilson MR, Fini ME, Jeong S, Clusterin Seals the Ocular Surface Barrier in Mouse Dry Eye, PLoS One 10(9) (2015) e0138958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wyatt AR, Yerbury JJ, Wilson MR, Structural characterization of clusterin-chaperone client protein complexes, J Biol Chem 284(33) (2009) 21920–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cunin P, Beauvillain C, Miot C, Augusto JF, Preisser L, Blanchard S, Pignon P, Scotet M, Garo E, Fremaux I, Chevailler A, Subra JF, Blanco P, Wilson MR, Jeannin P, Delneste Y, Clusterin facilitates apoptotic cell clearance and prevents apoptotic cell-induced autoimmune responses, Cell Death Dis 7 (2016) e2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Di Lella S, Sundblad V, Cerliani JP, Guardia CM, Estrin DA, Vasta GR, Rabinovich GA, When galectins recognize glycans: from biochemistry to physiology and back again, Biochemistry 50(37) (2011) 7842–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Chen WS, Cao Z, Sugaya S, Lopez MJ, Sendra VG, Laver N, Leffler H, Nilsson UJ, Fu J, Song J, Xia L, Hamrah P, Panjwani N, Pathological lymphangiogenesis is modulated by galectin-8-dependent crosstalk between podoplanin and integrin-associated VEGFR-3, Nat Commun 7 (2016) 11302. [DOI] [PMC free article] [PubMed] [Google Scholar]; ●● This study provides compelling evidence demonstrating that galectin-8 modulates VEGF-C-mediated lymphangiogenesis.
- [44].Shimmura-Tomita M, Wang M, Taniguchi H, Akiba H, Yagita H, Hori J, Galectin-9-mediated protection from allo-specific T cells as a mechanism of immune privilege of corneal allografts, PLoS One 8(5) (2013) e63620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sehrawat S, Suryawanshi A, Hirashima M, Rouse BT, Role of Tim-3/galectin-9 inhibitory interaction in viral-induced immunopathology: shifting the balance toward regulators, J Immunol 182(5) (2009) 3191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sehrawat S, Reddy PB, Rajasagi N, Suryawanshi A, Hirashima M, Rouse BT, Galectin-9/TIM-3 interaction regulates virus-specific primary and memory CD8 T cell response, PLoS Pathog 6(5) (2010) e1000882. [DOI] [PMC free article] [PubMed] [Google Scholar]; ●● This study demonstrates that manipulation of galectin-9 signaling can be used to regulate CD8+ T cell responses during the course of HSV-1 infection.
- [47].PB JR, Schreiber TH, Rajasagi NK, Suryawanshi A, Mulik S, Veiga-Parga T, Niki T, Hirashima M, Podack ER, Rouse BT, TNFRSF25 agonistic antibody and galectin-9 combination therapy controls herpes simplex virus-induced immunoinflammatory lesions, J Virol 86(19) (2012) 10606–20. [DOI] [PMC free article] [PubMed] [Google Scholar]


