INTRODUCTION:
We investigated to compare the effect of empirical therapy vs clarithromycin resistance–guided tailored therapy (tailored therapy) for eradication of Helicobacter pylori.
METHODS:
In this prospective, single center, open-label randomized controlled trial, we enrolled 72 patients with H. pylori infection from January 2019 through June 2019 in Korea. The patients were randomly assigned to both groups received empirical (n = 36) or tailored therapy (n = 36). Empirical therapy was defined as triple therapy with esomeprazole, amoxicillin, and clarithromycin for 10 days irrespective of clarithromycin resistance. Tailored therapy was triple or quadruple therapy with esomeprazole, metronidazole, tetracycline, and bismuth for 10 days based on genotype markers of resistance determined by gastric biopsy. Resistance-associated mutations in 23S rRNA were confirmed by multiplex polymerase chain reaction. Eradication status was assessed by 13C-urea breath test, and the primary outcome was eradication rates.
RESULTS:
H. pylori was eradicated in 27 patients (75.0%), given empirical therapy and 32 patients (88.9%) treated with tailored therapy (P = 0.136) in intention-to-treat analysis. In per protocol analysis, the eradication rate was 97.0% and 81.8% in tailoredvs empirical groups (P = 0.046). Although clarithromycin-resistant H. pylori was eradicated in 3/9 (33.3%) with empirical therapy, it was treated in 11/12 (91.7%) with tailored therapy (P = 0.009). There was no difference in compliance between 2 groups. The rate of adverse events of the tailored group was higher than that of the empirical group (P = 0.036) because quadruple therapy had more side effects than those of triple therapy (P = 0.001).
DISCUSSION:
Tailored therapy based on polymerase chain reaction is a good alternative to increase eradication rates in a region of high prevalence of clarithromycin resistance (see Visual Abstract, Supplementary Digital Content 1, http://links.lww.com/CTG/A342).
INTRODUCTION
Helicobacter pylori is associated with upper gastrointestinal diseases, including gastritis, peptic ulcer disease, mucosa-associated lymphoid tissue (MALT) lymphoma, and gastric cancer (1). The indications for H. pylori treatment in the United States and Korea include peptic ulcer disease, low-grade MALT lymphoma, and the endoscopic resection of early gastric cancer (EGC), with H. pylori infection (2,3). In Japan, reversible early stage of preneoplastic change and Helicobacter-related gastritis were added to the indications for H. pylori treatment starting in February 2013 (4,5).
In the current Korean guidelines for the treatment of H. pylori eradication, published in 2013, triple therapy, including a proton-pump inhibitor (PPI), clarithromycin, and amoxicillin, has been recommended for primary eradication therapy (3). However, the Maastricht V guidelines recommended quadruple therapy because the primary treatment in areas with higher than 15% clarithromycin resistance (low level, grade of recommendation; strong), which was applied to the revised H. pylori Clinical Practice Guideline Amendment. One recent multicenter, randomized trial and a meta-analysis suggested that 10-day sequential therapy could represent a primary treatment option (6,7). However, the standard triple therapy treatment protocol remains the recommended primary treatment for H. pylori eradication in Korea (8).
Antibiotic resistance in H. pylori, especially clarithromycin resistance, is a major cause of eradication failure, and the clarithromycin-resistance rate in Korea has recently been reported to have increased to 17.8%, based on bacterial cultures (minimum inhibitory concentration [MIC] of >1 μg/mL) (9). The concordance between bacterial cultures and polymerase chain reaction (PCR)-based susceptibility findings was approximately 95% (10,11). Furthermore, based on the identification of 23S rRNA point mutations, the clarithromycin-resistance rate has increased drastically, with up to 32% of the Korean population presenting clarithromycin-resistance (12).
The reported eradication rate for standard triple therapy in Korea has been increasingly unsatisfactory, as the antibiotic resistance rate of H. pylori increases. The ideal eradication rate for H. pylori is approximately 90% (13); however, in a recently reported randomized, multicenter study examining primary eradication, the overall eradication rate was 63.9%, in the intention-to-treat (ITT) analysis, and 71.4%, in the per protocol (PP) analysis (6). In addition, one meta-analysis suggested that clarithromycin resistance reduces the efficacy of triple therapy by 66% (95% confidence interval: 58.2–74.2) (14). Another systemic review and meta-analysis reported that clarithromycin-susceptible strains showed a 90.2% eradication rate, whereas clarithromycin-resistant strains demonstrated a 22.2% eradication rate in response to triple therapy in the ITT analysis (15).
To detect bacterial resistance, bacterial H. pylori cultures are required for the agar dilution test or E-test. Performing antimicrobial susceptibility testing on H. pylori cultures is important for predicting antibiotic treatment outcomes and guiding clinicians in their choice of therapy. However, the cultivation of H. pylori to determine the MIC values of antimicrobial substances can be very difficult and time consuming (9,16) because of the characteristics of microaerophilic bacteria. In addition, the culture yields are generally low, and this method is quite costly. Recently, tailored treatments based on clarithromycin susceptibility have been proposed, using the results of a dual-priming oligonucleotide-based multiplex (DPO)-PCR test (17–20). This method uses PCR to verify the presence of mutations, especially A2142G and A2143G, which are 23S ribosomal RNA point mutations known to be highly related to clarithromycin resistance (20). This test can only be performed by gastric biopsy; however, the examination time can be as short as several hours. DPO-PCR to identify H. pylori was shown to have sensitivity, specificity, and concordance rates of over 87%, 83%, and 90%, respectively, compared with bacterial cultures or the 13C-urea breath tests (13C-UBTs) (10,21).
This study aimed to examine the effectiveness H. pylori infections treated using clarithromycin resistance–guided tailored therapy (tailored therapy) compared with empirical therapy in a randomized, controlled trial.
METHODS
Trial design and patients
This study was a single center, open-label, parallel, randomized trial. The Institutional Review Board of the Seoul National University Hospital and clinicaltrial.gov approved this study (IRB number: 1811-029-983, clinicaltrials.gov ID: NCT04006340). We performed the trial following the Declaration of Helsinki regarding human experimentation.
Patients with H. pylori who were expected to undergo endoscopic resection for gastric neoplasms or diagnosed with gastric MALT lymphoma were eligible. The gastric neoplasms included EGCs or gastric adenomas on endoscopy. Exclusion criteria were as follows: (i) history of gastrectomy, (ii) patients aged younger than 20 years or older than 80 years, (iii) history of H. pylori eradication therapies or other antibiotics therapy within a month, (iv) contraindication or previous allergic reaction to the treatment drugs (amoxicillin, clarithromycin, metronidazole, tetracycline, and esomeprazole), and (v) pregnant or lactating women. All patients had written informed consents before enrollment. Demographic data were obtained from interview or medical records, including age, sex, body mass index, comorbidity, history of cigarette smoking or alcohol drinking, and tumor findings; pathological data of gastric neoplasm were achieved from medical records (gross type, Lauren type, differentiation, depth of invasion, vertical location, size, en-bloc resection, and resection margin). Complete resection was defined as an en-bloc resection with tumor-negative margin in specimen after endoscopic resection.
Determination of H. pylori infection and clarithromycin resistance
Histologic evaluation was conducted by gastric biopsy specimen from the antrum and body and stained with hematoxylin and eosin and Giemsa stains. H. pylori status was assessed by the Sydney system. The rapid urease test (RUT) (CLOtest; Delta West, Bentley, Australia) was also conducted by biopsy samples at gastric antrum. Because the eligible patients in our study had gastric neoplasm or gastric MALT lymphoma, 2 endoscopies were needed at the time of diagnosis and further examination (endoscopic ultrasound) or treatment (endoscopic submucosal dissection). Therefore, we evaluated the status of H. pylori infection by both histology and RUT at the first endoscopy, and the clarithromycin resistance by DPO-PCR test at the second endoscopy.
DPO-PCR test (Seeplex ClaR-H. pylori ACE Detection; Seegene Institute of Life Science, Seoul, Korea) was performed on gastric biopsy specimens if histology or RUT was positive. Eligible H. pylori status for enrollment was judged as positive if DPO-PCR test was positive. DPO-PCR tests were conducted to identify genetic point mutations according to the manufacturer's recommendations. An initial incubation was performed at 94 °C for 15 minutes, and the next processes allowed 40 amplification cycles in a GeneAmp PCR system 9700 (Applied Biosystems, Foster City, CA) at 94 °C for 30 seconds, 65 °C for 30 seconds, and 72 °C for 1 minute. The last extension was proceeded at 72 °C for 10 minutes.
The amplified DNA products were identified using an ultraviolet transilluminator in electrophoresis. The detection kit including 3 primer pairs with a DPO structure was used for the amplification of the H. pylori 23S rDNA (621 bp). A single 621-bp DNA product was considered belonging to wild-type H. pylori. The presence of the A2142G and A2143G mutations resulted in DNA bands at 475-bp and 194-bp, respectively. The kit includes a primer pair for internal control.
Randomization and intervention
Eligible patients were randomly assigned into one of the following treatment groups: (A) empirical therapy or (B) clarithromycin resistance–guided tailored therapy using a computer-generated list that had block randomization with a block size of 4, 6 in a 1:1 ratio (https://www.e-ciencecentral.org/articles/pubreader/SC000026629#_fn_header_idm5766816, openepi.com).
The empirical therapy group (empirical group) received the standard triple regimen and clarithromycin resistance–guided tailored therapy group (tailored group) taken the triple therapy or quadruple therapy according to clarithromycin resistance determined by the DPO-PCR test. Patients were treated with triple therapy including esomeprazole 40 mg, amoxicillin 1 g, and clarithromycin 500 mg twice daily for 10 days, or quadruple therapy containing esomeprazole 40 mg and bismuth 300 mg twice daily, tetracycline 500 mg 4 times daily, and metronidazole 500 mg 3 times daily for 10 days.
Outcome assessment
The eradication status of H. pylori was identified by 13C-UBT at least 4 weeks after therapy. Before 13C-UBT, study patients discontinued histamine 2 blocker or PPI during at least 2 weeks. We asked for all subjects to report any adverse event throughout this trial. The adverse effects and compliance at the completion of treatment were investigated by a standardized interview. Poor compliance was defined as the case where the pill taken below 90%. Patients who did not have 13C-UBT after the end of therapy were excluded from the PP analysis. Incremental cost-effectiveness ratios were calculated for the tailored group, and the outcome was measured as the eradication rate. The average costs were defined as the combined costs of endoscopy, RUT, biopsy/histology, medication, 13C-UBT, and/or DPO-PCR test. The incremental cost-effectiveness ratio value was defined as the difference between the average total costs for the tailored group and those for the empirical group divided by the average difference in the eradication rate (22):
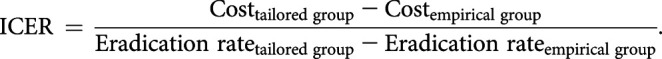 |
Statistical analysis
The eradication rate of clarithromycin resistance–guided tailored group was assumed to be 95.7% based on our pilot study. The calculated sample size was at least 36 in each group to detect a 30% difference to give a statistical power of 80% at a 5% significance level on a 2-sided test with a follow-up loss rate of 10% (Fleiss JL. Statistical Methods for Rates and Proportions: John Wiley & Sons; 1981). All randomized patients were included in the intention-to-treat (ITT) analysis. The primary end point was the eradication rates according to ITT and PP analyses. The secondary end points were the compliance and the adverse events.
Clarithromycin resistance–guided tailored therapy vs empirical therapy groups were compared for demographic and clinicopathological data using the Pearson χ2 tests, Fisher exact tests, Mann-Whitney U tests, Student t-tests, and logistic regression model. Pearson χ2 tests and Fisher exact tests were used for the analysis of categorical variables, whereas Mann-Whitney U tests and Student t-tests for the analysis of continuous variables. Factors affecting the eradication rates were analyzed by the logistic regression model and subgroup analysis. A P value of <0.05 was considered statistically significant. All statistics were analyzed using the Statistical Package for the Social Sciences, version 19.0 (SPSS, Chicago, IL).
RESULTS
Study population and baseline characteristics
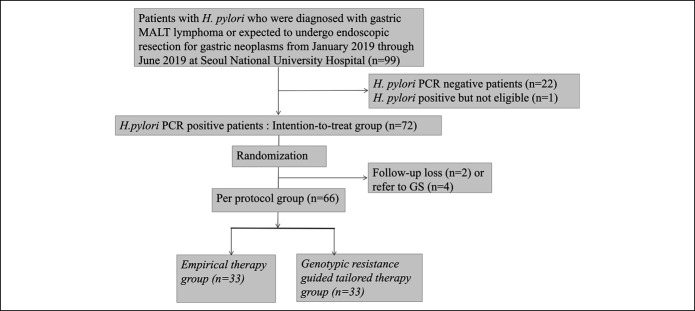
A total of 72 patients underwent randomization of the 99 patients who were screened from January 2019 through June 2019 (Figure 1). Of these patients, 6 patients were excluded because 4 subjects were referred to surgery without endoscopic resection and 2 cases did not receive a trial medication. Finally, 66 were included in the PP population: 33 in the empirical therapy group and 33 in the clarithromycin resistance–guided tailored therapy group. Most of the demographic and tumor characteristics were similar between both groups; empirical group had more obese patients and bigger size of neoplasm (Table 1).
Figure 1.
Enrollment, randomization, and follow-up. GS, general surgery; MALT, mucosa-associated lymphoid tissue; PCR, polymerase chain reaction.
Table 1.
Baseline characteristics of the patients in the intention-to-treat population
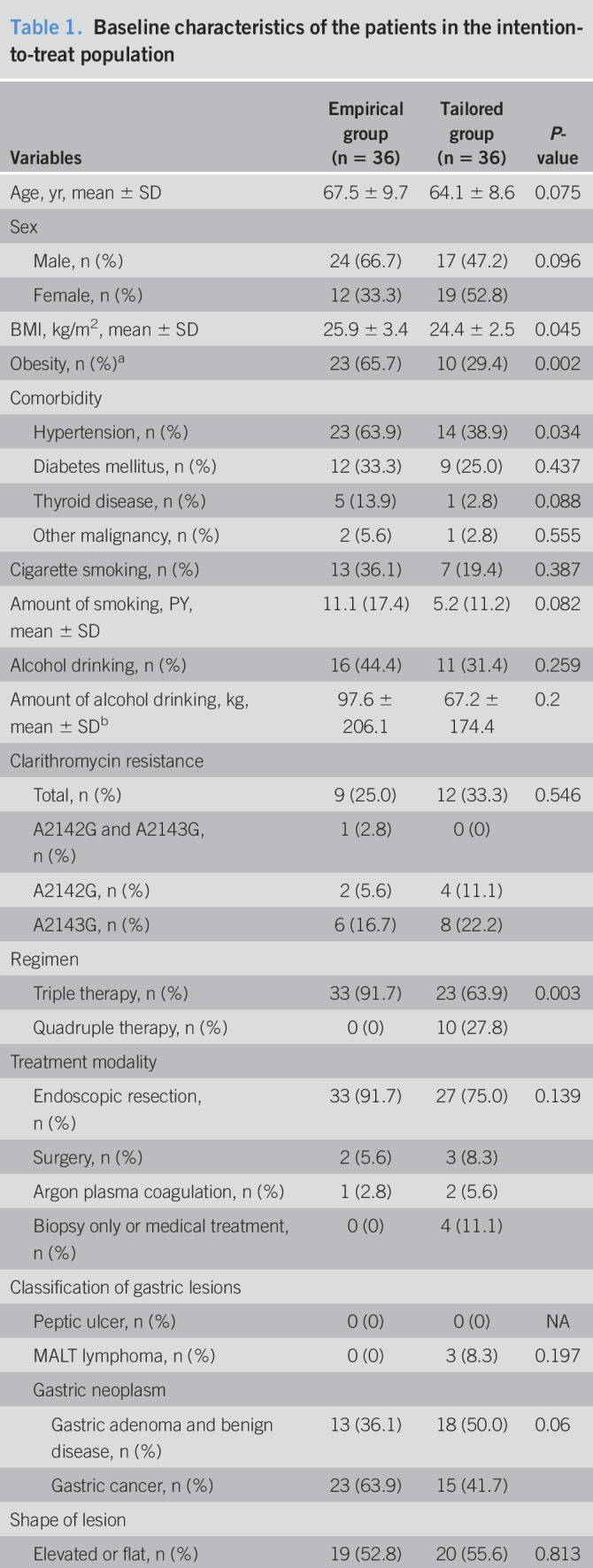
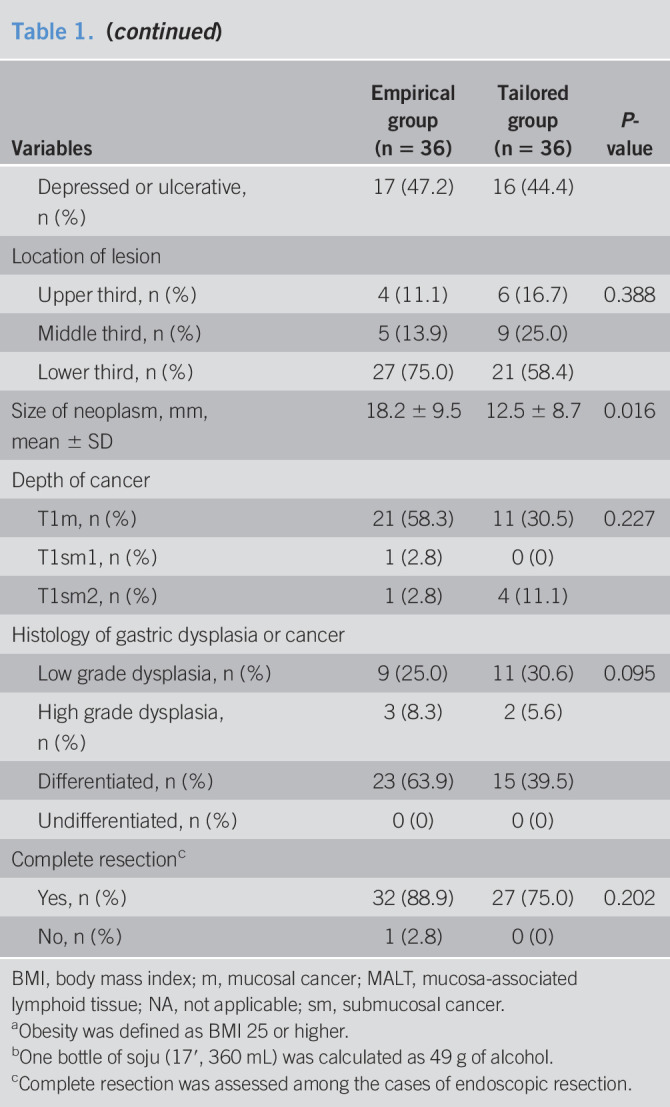
| Variables | Empirical group (n = 36) | Tailored group (n = 36) | P-value |
| Age, yr, mean ± SD | 67.5 ± 9.7 | 64.1 ± 8.6 | 0.075 |
| Sex | |||
| Male, n (%) | 24 (66.7) | 17 (47.2) | 0.096 |
| Female, n (%) | 12 (33.3) | 19 (52.8) | |
| BMI, kg/m2, mean ± SD | 25.9 ± 3.4 | 24.4 ± 2.5 | 0.045 |
| Obesity, n (%)a | 23 (65.7) | 10 (29.4) | 0.002 |
| Comorbidity | |||
| Hypertension, n (%) | 23 (63.9) | 14 (38.9) | 0.034 |
| Diabetes mellitus, n (%) | 12 (33.3) | 9 (25.0) | 0.437 |
| Thyroid disease, n (%) | 5 (13.9) | 1 (2.8) | 0.088 |
| Other malignancy, n (%) | 2 (5.6) | 1 (2.8) | 0.555 |
| Cigarette smoking, n (%) | 13 (36.1) | 7 (19.4) | 0.387 |
| Amount of smoking, PY, mean ± SD | 11.1 (17.4) | 5.2 (11.2) | 0.082 |
| Alcohol drinking, n (%) | 16 (44.4) | 11 (31.4) | 0.259 |
| Amount of alcohol drinking, kg, mean ± SDb | 97.6 ± 206.1 | 67.2 ± 174.4 | 0.2 |
| Clarithromycin resistance | |||
| Total, n (%) | 9 (25.0) | 12 (33.3) | 0.546 |
| A2142G and A2143G, n (%) | 1 (2.8) | 0 (0) | |
| A2142G, n (%) | 2 (5.6) | 4 (11.1) | |
| A2143G, n (%) | 6 (16.7) | 8 (22.2) | |
| Regimen | |||
| Triple therapy, n (%) | 33 (91.7) | 23 (63.9) | 0.003 |
| Quadruple therapy, n (%) | 0 (0) | 10 (27.8) | |
| Treatment modality | |||
| Endoscopic resection, n (%) | 33 (91.7) | 27 (75.0) | 0.139 |
| Surgery, n (%) | 2 (5.6) | 3 (8.3) | |
| Argon plasma coagulation, n (%) | 1 (2.8) | 2 (5.6) | |
| Biopsy only or medical treatment, n (%) | 0 (0) | 4 (11.1) | |
| Classification of gastric lesions | |||
| Peptic ulcer, n (%) | 0 (0) | 0 (0) | NA |
| MALT lymphoma, n (%) | 0 (0) | 3 (8.3) | 0.197 |
| Gastric neoplasm | |||
| Gastric adenoma and benign disease, n (%) | 13 (36.1) | 18 (50.0) | 0.06 |
| Gastric cancer, n (%) | 23 (63.9) | 15 (41.7) | |
| Shape of lesion | |||
| Elevated or flat, n (%) | 19 (52.8) | 20 (55.6) | 0.813 |
| Depressed or ulcerative, n (%) | 17 (47.2) | 16 (44.4) | |
| Location of lesion | |||
| Upper third, n (%) | 4 (11.1) | 6 (16.7) | 0.388 |
| Middle third, n (%) | 5 (13.9) | 9 (25.0) | |
| Lower third, n (%) | 27 (75.0) | 21 (58.4) | |
| Size of neoplasm, mm, mean ± SD | 18.2 ± 9.5 | 12.5 ± 8.7 | 0.016 |
| Depth of cancer | |||
| T1m, n (%) | 21 (58.3) | 11 (30.5) | 0.227 |
| T1sm1, n (%) | 1 (2.8) | 0 (0) | |
| T1sm2, n (%) | 1 (2.8) | 4 (11.1) | |
| Histology of gastric dysplasia or cancer | |||
| Low grade dysplasia, n (%) | 9 (25.0) | 11 (30.6) | 0.095 |
| High grade dysplasia, n (%) | 3 (8.3) | 2 (5.6) | |
| Differentiated, n (%) | 23 (63.9) | 15 (39.5) | |
| Undifferentiated, n (%) | 0 (0) | 0 (0) | |
| Complete resectionc | |||
| Yes, n (%) | 32 (88.9) | 27 (75.0) | 0.202 |
| No, n (%) | 1 (2.8) | 0 (0) |
BMI, body mass index; m, mucosal cancer; MALT, mucosa-associated lymphoid tissue; NA, not applicable; sm, submucosal cancer.
Obesity was defined as BMI 25 or higher.
One bottle of soju (17′, 360 mL) was calculated as 49 g of alcohol.
Complete resection was assessed among the cases of endoscopic resection.
Eradication rates of H. pylori
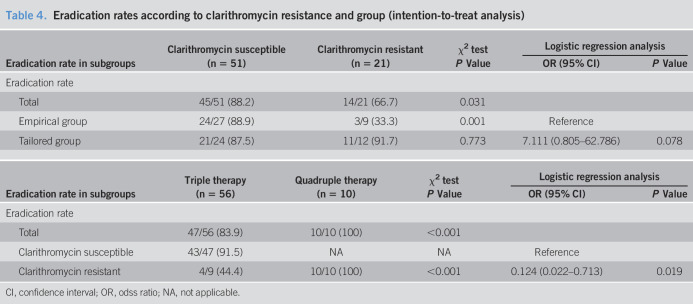
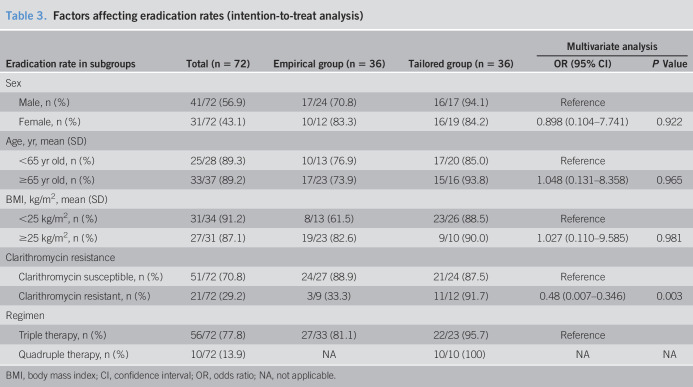
The eradication rates of intention-to-treat analysis were 27 of 36 (75.0%) in the empirical therapy group and 32 of 36 (88.9%) in the tailored therapy group (P = 0.136). In PP analysis, the eradication rates of the tailored therapy and empirical therapy groups were 32 of 33 (97.0%) and 27 of 33 (81.8%), respectively (P = 0.046, Table 2). The factor affecting eradication failure was clarithromycin resistance (odds ratio, 0.48; 95% confidence interval: 0.007–0.346; P = 0.003) when adjusted for other variables including age, sex, body mass index, and regimen (Table 3). In the subgroup analysis for those with clarithromycin-resistant H. pylori, the eradication rate was much higher in the tailored group than that in the empirical group (91.7% vs 33.3%; P = 0.009). After the eradication rate of the tailored group was similar irrespective of clarithromycin resistance, that of the empirical group was significantly different the clarithromycin susceptible and resistant groups (P = 0.001, 88.9% vs 33.3%, Table 4). Although clarithromycin-resistant cases that were treated with quadruple therapy achieved total eradication in 10 of 10 patients (100%), eradication was achieved in 4/9 patients (44.4%) treated with triple therapy (P < 0.001). In addition, we analyzed the eradication rate according to specific disease, there were no difference among MALT lymphoma and gastric adenoma including benign disease and gastric cancer groups (P = 0.457, 100.0%, 90.3%, and 73.7%, respectively). Although H. pylori is one of etiologic factors of gastric cancer, gastric adenoma, and MALT lymphoma, these underlying diseases are not associated with the eradication rate of H. pylori.
Table 2.
Eradication rate, adverse effects, and compliance
| Variables | Empirical group (n = 36) | Tailored group (n = 36) | P Value |
| Eradication rate | |||
| Eradication rate (ITT analysis), n (%) | 27/36 (75.0) | 32/36 (88.9) | 0.136 |
| Eradication rate (PP analysis), n (%) | 27/33 (81.8) | 32/33 (97.0) | 0.046 |
| Adverse effects and compliance | |||
| Any adverse effect, n (%) | 1 (3.1) | 6 (20.0) | 0.036 |
| Skin rash, n (%) | 0 (0) | 1 (3.1) | |
| Taste distortion, n (%) | 1 (3.1) | 0 (0) | |
| Bloating, n (%) | 0 (0) | 0 (0) | |
| Abdominal pain, n (%) | 0 (0) | 1 (3.1) | |
| Nausea, n (%) | 0 (0) | 1 (3.1) | |
| Vomiting, n (%) | 0 (0) | 0 (0) | |
| Diarrhea, n (%) | 0 (0) | 1 (3.1) | |
| Constipation, n (%) | 0 (0) | 1 (3.1) | |
| Cardiac arrhythmia, n (%) | 0 (0) | 1 (3.1) | |
| Poor compliance, n (%) | 0 (0) | 0 (0) | NA |
| Variables | Triple group (n = 56) | Quadruple group (n = 10) | P Value |
| Adverse effects | |||
| Any adverse effect, n (%) | 3 (5.4) | 4 (40.0) | 0.001 |
| Skin rash, n (%) | 1 (1.8) | 0 (0) | |
| Taste distortion, n (%) | 1 (1.8) | 0 (0) | |
| Bloating, n (%) | 0 (0) | 0 (0) | |
| Abdominal pain, n (%) | 0 (0) | 1 (10.0) | |
| Nausea, n (%) | 0 (0) | 1 (10.0) | |
| Vomiting, n (%) | 0 (0) | 0 (0) | |
| Diarrhea, n (%) | 1 (1.8) | 0 (0) | |
| Constipation, n (%) | 0 (0) | 1 (10.0) | |
| Cardiac arrhythmia, n (%) | 0 (0) | 1 (10.0) |
ITT, intention-to-treat; NA, not applicable; PP, per-protocol.
Table 4.
Eradication rates according to clarithromycin resistance and group (intention-to-treat analysis)
| Eradication rate in subgroups | Clarithromycin susceptible (n = 51) | Clarithromycin resistant (n = 21) | χ2 test | Logistic regression analysis | |
| P Value | OR (95% CI) | P Value | |||
| Eradication rate | |||||
| Total | 45/51 (88.2) | 14/21 (66.7) | 0.031 | ||
| Empirical group | 24/27 (88.9) | 3/9 (33.3) | 0.001 | Reference | |
| Tailored group | 21/24 (87.5) | 11/12 (91.7) | 0.773 | 7.111 (0.805–62.786) | 0.078 |
| Eradication rate in subgroups | Triple therapy (n = 56) | Quadruple therapy (n = 10) | χ2 test | Logistic regression analysis | |
| P Value | OR (95% CI) | P Value | |||
| Eradication rate | |||||
| Total | 47/56 (83.9) | 10/10 (100) | <0.001 | ||
| Clarithromycin susceptible | 43/47 (91.5) | NA | NA | Reference | |
| Clarithromycin resistant | 4/9 (44.4) | 10/10 (100) | <0.001 | 0.124 (0.022–0.713) | 0.019 |
CI, confidence interval; OR, odss ratio; NA, not applicable.
Table 3.
Factors affecting eradication rates (intention-to-treat analysis)
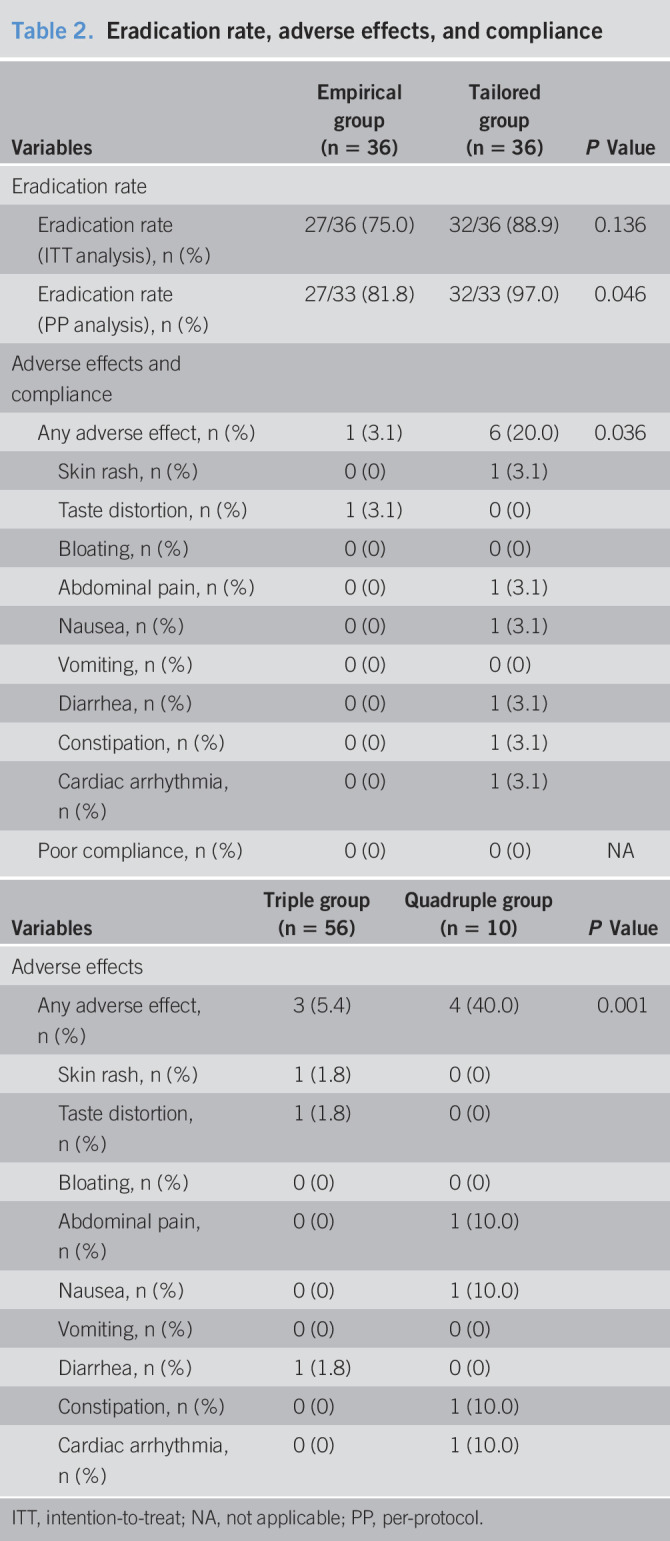
| Eradication rate in subgroups | Total (n = 72) | Empirical group (n = 36) | Tailored group (n = 36) | Multivariate analysis | |
| OR (95% CI) | P Value | ||||
| Sex | |||||
| Male, n (%) | 41/72 (56.9) | 17/24 (70.8) | 16/17 (94.1) | Reference | |
| Female, n (%) | 31/72 (43.1) | 10/12 (83.3) | 16/19 (84.2) | 0.898 (0.104–7.741) | 0.922 |
| Age, yr, mean (SD) | |||||
| <65 yr old, n (%) | 25/28 (89.3) | 10/13 (76.9) | 17/20 (85.0) | Reference | |
| ≥65 yr old, n (%) | 33/37 (89.2) | 17/23 (73.9) | 15/16 (93.8) | 1.048 (0.131–8.358) | 0.965 |
| BMI, kg/m2, mean (SD) | |||||
| <25 kg/m2, n (%) | 31/34 (91.2) | 8/13 (61.5) | 23/26 (88.5) | Reference | |
| ≥25 kg/m2, n (%) | 27/31 (87.1) | 19/23 (82.6) | 9/10 (90.0) | 1.027 (0.110–9.585) | 0.981 |
| Clarithromycin resistance | |||||
| Clarithromycin susceptible, n (%) | 51/72 (70.8) | 24/27 (88.9) | 21/24 (87.5) | Reference | |
| Clarithromycin resistant, n (%) | 21/72 (29.2) | 3/9 (33.3) | 11/12 (91.7) | 0.48 (0.007–0.346) | 0.003 |
| Regimen | |||||
| Triple therapy, n (%) | 56/72 (77.8) | 27/33 (81.1) | 22/23 (95.7) | Reference | |
| Quadruple therapy, n (%) | 10/72 (13.9) | NA | 10/10 (100) | NA | NA |
BMI, body mass index; CI, confidence interval; OR, odds ratio; NA, not applicable.
Adverse effect and compliance
Compliance in both groups was similar and relatively good (Table 2). The rate of adverse effects of the tailored group was higher than that of the empirical group (P = 0.036) because the quadruple therapy group had more side effects than those of the triple therapy group (P = 0.001). One patient of the quadruple therapy group experienced paroxysmal supraventricular tachycardia that was less likely to be related to quadruple therapy. The patient was followed up with medication as an outpatient after being diagnosed at the emergency room.
Cost analysis
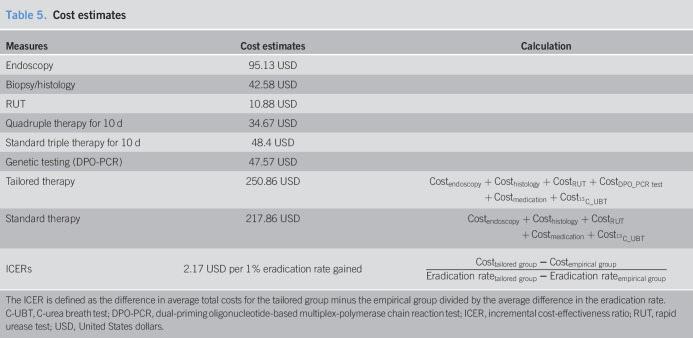
We compared the mean direct costs associated with empirical therapy with those for tailored therapy. The cost of 10-day standard triple therapy was calculated as 48.40 US Dollars (USD), whereas the cost of 10-day quadruple therapy was 34.67 USD, and the cost of genetic testing using DPO-PCR was 47.57 USD. A patient in the tailored therapy group paid an average of 250.86 USD, whereas a patient in the empirical therapy group paid an average of 217.86 USD. We included the prices of endoscopy, biopsy/histology, RUT, medication, and 13C-UBT in the cost estimation. The costs for the tailored group were only 33 USD higher than those for the empirical group, and the cost per additional percentage increase of eradication rate was 2.17 USD (Table 5).
Table 5.
Cost estimates
| Measures | Cost estimates | Calculation |
| Endoscopy | 95.13 USD | |
| Biopsy/histology | 42.58 USD | |
| RUT | 10.88 USD | |
| Quadruple therapy for 10 d | 34.67 USD | |
| Standard triple therapy for 10 d | 48.4 USD | |
| Genetic testing (DPO-PCR) | 47.57 USD | |
| Tailored therapy | 250.86 USD | |
| Standard therapy | 217.86 USD | |
| ICERs | 2.17 USD per 1% eradication rate gained |
The ICER is defined as the difference in average total costs for the tailored group minus the empirical group divided by the average difference in the eradication rate.
C-UBT, C-urea breath test; DPO-PCR, dual-priming oligonucleotide-based multiplex-polymerase chain reaction test; ICER, incremental cost-effectiveness ratio; RUT, rapid urease test; USD, United States dollars.
DISCUSSION
This study represents the first randomized, controlled trial to compare empirical triple therapy outcomes with those of tailored therapy based on the results of the DPO-PCR test. The present study demonstrated that the DPO-PCR–based eradication strategy could represent a promising alternative to increase eradication efficacy compared with the outcomes associated with empirical therapy. Clarithromycin resistance was a major factor affecting eradication failure.
The World Health Organization classified H. pylori as a group I carcinogen for gastric cancer in 1994 (23). The concept of “point of no return” suggested that new gastric cancers with advanced histologic changes could develop, even after the eradication of H. pylori (24,25). However, some randomized, controlled trials have suggested that H. pylori treatment improves gastric preneoplastic lesions and reduces the incidence of metachronous gastric cancers after the endoscopic resection of EGC (26–28). Because the necessity of H. pylori eradication has become clinically accepted, the national health insurance in South Korea has been amended to allow the treatment of all H. pylori-positive patients who wish to receive eradication treatment, starting in 2018.
In a meta-analysis of randomized, controlled trials, the cure rate for triple therapy was reported to be the unacceptable level of 68.9% in the ITT analysis (29). A recent Korean, randomized, multicenter analysis reported that the overall primary triple eradication rate was 63.9% in the ITT analysis, and 71.4% in the PP analysis (6). Compared with the ideal eradication rate, which is generally accepted as above 90% and preferably above 95% (30), the total eradication rate of clarithromycin-guided tailored therapy in the same study was 97.0% in the PP analysis and 88.9% in the ITT analysis, which approached the ideal rate.
Although our pilot study demonstrated a 30% difference between the eradication rates for the empirical group and the tailored group, this study reported only a 15.2% difference in the eradication rates because the eradication rate for the empirical group was higher than expected. The clarithromycin mutation rate was lower and the compliance rate was higher than expected. Although 47.8% of the empirical group and 45.8% of the tailored group presented with clarithromycin resistance in the pilot study, the ratio of clarithromycin resistance in this study was 25.0% for the empirical group and 33.3% for the tailored group. In addition, because compliance was extremely high, above 90%, the eradication rate may have increased in the present trial. These factors all contributed to the higher eradication rate for the control group than we expected.
Although this study did not include the prevalence of amoxicillin and metronidazole resistance, a recent multicenter study (9) showed that the resistance rates against clarithromycin, amoxicillin, and metronidazole, based on bacterial culture analysis, were 17.8%, 29.5%, and 9.5%, retrospectively (9). However, clarithromycin resistance was a more impactful factor for the eradication rate than resistance to the other antibiotics (12,31), which was consistent with the results of this study. As the clarithromycin-resistance rate of H. pylori increases, the eradication rate of primary triple therapy in Korea decreases. In this trial, the clarithromycin-resistance rate was relatively high, at 29.2%, which was similar to the 32% resistance rate reported in a previous study (12). The cure rate for clarithromycin-susceptible strains was significantly higher than that for clarithromycin-resistant strains (88.2% vs 66.7%, P = 0.031, in the ITT analysis; 95.7% vs 73.7%, P = 0.008, in the PP analysis). The eradication rate for triple therapy was 91.5% in clarithromycin-susceptible patients and 44.4% in clarithromycin-resistant patients. Therefore, the development of improved treatments for clarithromycin-resistant patients remains important for increasing the eradication rate.
The Maastricht V guideline does not recommend standard triple therapy without previous susceptibility testing in regions where the clarithromycin-resistance rate is above 15% (8). Standard culture studies, based on MIC, can be difficult to perform in a clinical setting because they are time consuming, inconvenient, and not standardized. Recently, DPO-based multiplex PCR was introduced for the detection of H. pylori and clarithromycin susceptibility (11). DPO-based multiplex PCR is a strategy for the accurate detection of the most common point mutations that occur in the 23S rRNA gene of H. pylori, based on gastric biopsy samples, and the results can be obtained within a few hours. DPO-PCR has been reported to have a sensitivity of 97.7% and a specificity of 83.1% when using the MIC cultures as the reference test, and the concordance with phenotypic susceptibility testing was 95.3% (10). When compared with the 13C-UBT, H. pylori detection by DPO-PCR had a sensitivity of 87.5%, a specificity of 91.3%, and an accuracy of 90.0% (21). In the present study, the eradication rate of the tailored group was superior to that of the empirical group (97%, in the PP analysis, P = 0.046 vs 88.9%, in the ITT, P = 0.136). Therefore, the assessment of clarithromycin resistance using the DPO-based multiplex PCR method in advance of treatment is recommended, as is the administration of quadruple therapy to patients with demonstrated clarithromycin resistance.
The Maastricht V guideline also recommended quadruple therapy as an alternative primary treatment method in areas with high levels of clarithromycin resistance (8). The efficacy rates between tailored therapy and quadruple therapy are predicted to be similar in regions with a high prevalence of clarithromycin resistance. However, the cure rate of clarithromycin-susceptible strains treated with triple therapy reached the ideal value of 91.5% in this trial and in previous studies (91%–96%) (12,31). Although clarithromycin-susceptible patients are likely to achieve the ideal treatment outcome, regardless of whether they receive triple or quadruple therapy as the first-line treatment, adverse events were reported more frequently for the quadruple therapy treatment group than for the triple therapy group in our study (40% vs 5.4%, P = 0.001). Therefore, triple therapy continues to be advantageous for clarithromycin-susceptible patients. However, clarithromycin-resistant strains require the use of quadruple therapy as the primary treatment because triple therapy in these strains only achieved an eradication rate of 44.4%, whereas quadruple therapy in these patients achieved a cure rate of 100%.
In a recent, randomized, multicenter study, 10-day sequential therapy was recommended as an alternative primary treatment method for H. pylori infections in a country with clarithromycin resistance higher than 15% (6). The eradication rate of the 10-day sequential therapy was superior to that of 7-day triple therapy. The eradication rates were 76.3% in the ITT analyses and 85.0% in the PP analyses. One meta-analysis suggested that sequential therapy was able to eradicate 72.8% of strains resistant to clarithromycin (7). When compared with triple therapy that lasted 7 and 10 days, the 10-day sequential therapy had better eradication rates of 86.5% and 84.3%, respectively. However, the present study showed that tailored therapy achieved superior results compared with those for sequential therapy, achieving eradication rates of 88.9% in the ITT analysis and 97.0% in the PP analysis.
Previous studies (32) have examined tailored therapy, using various treatment regimens and have reported various eradication rates. The efficacy of the tailored treatment varies. The efficacies (eradication rates of tailored therapy [in the ITT analysis]) have been reported as 71.9%, 72%, 80%, 86%, 91%, 94.5%, 94.6%, and 96%. However, these population results are not transferable to other geographical areas with different patterns of resistance. The most important strength of our study was the development of a method that was able to determine whether to apply tailored therapy to specific patients in a region with a high incidence of clarithromycin resistance and an intermediate risk of metronidazole resistance.
Many regimens associated with tailored therapies that have been reported in previous studies included PPI, amoxicillin, and metronidazole when clarithromycin-resistant strains were detected. A meta-analysis (33) showed that the eradication rate of triple therapy including PPI, amoxicillin, and metronidazole was lower than that for triple therapy including PPI, amoxicillin, and clarithromycin. In settings with high levels of clarithromycin resistance, the choice of therapy should be based on the frequency of metronidazole and dual clarithromycin and metronidazole resistance. In geographical areas where metronidazole resistance is almost negligible (e.g., Japan), replacing clarithromycin with metronidazole during triple therapy (i.e., PPI-metronidazole-amoxicillin) continues to demonstrate excellent cure rates (34). Metronidazole resistance in Korea has been reported to range from 9% to 30.3% (9,35). The Maastricht V/Florence Consensus guideline (8) recommended that in areas with high dual clarithromycin and metronidazole resistance, bismuth quadruple therapy is the recommended first-line treatment. Therefore, based on the presence of clarithromycin resistance, choosing regimens consisting of PPI, amoxicillin, and metronidazole is not reasonable in Korea. Many previous studies have adopted the combination of PPI, amoxicillin, and metronidazole for tailored regimens, and various eradication rates have been reported for tailored therapy groups; however, this combination is not a good choice in Korea. Our study was a prospective, randomized, controlled trial, in that we chose the therapeutic medications for the tailored group in advance, based on clarithromycin sensitivity, and investigated eradication rates according to clarithromycin susceptibility. Tailored therapy using a bismuth quadruple therapy regimen to treat the presence of H. pylori in Korea is a reasonable option to adopt in the future.
Tailored therapy based on DPO-PCR results also showed cost benefits when the eradication rate of the 14-day empirical therapy declined below 80% (19). When we analyzed the mean direct prices of both types of therapy, a patient in tailored therapy using DPO-PCR would pay approximately 250.86 USD, including costs associated with endoscopy, biopsy/histology, RUT, DPO-PCR test, medication, and 13C-UBT. Most patients in the tailored therapy group were successfully treated with one remedy (97.0% in our PP analysis). By contrast, a patient treated with empirical triple therapy would pay 217.86 USD, including the costs associated with endoscopy, biopsy/histology, RUT, medication, and 13C-UBT. Considering the cost per additional percentage increase of eradication rate of 2.17 USD, the expenses associated with the use of second-line medications, additional 13C-UBT evaluations, outpatient clinic registration, and revisits because of treatment failure, the costs of tailored therapy using DPO-PCR are unlikely to be higher than those for empirical therapy. Therefore, clarithromycin-guided tailored therapy allows physicians to choose triple or quadruple therapy to treat H. pylori infection in regions with a high prevalence of clarithromycin resistance.
This study has several limitations. First, it was conducted in a tertiary single center because the eligible patients were referred for the treatment of gastric MALT lymphoma, gastric adenoma, and EGC. However, we attempted to maintain consistency during the diagnostic procedures, the establishment of a treatment plan, and treatment because endoscopic resections are sophisticated and technically demanding procedures. Second, ethical issues may apply to our trial design because of the use of an empirical group. However, in South Korea, standard triple therapy has generally remained the primary therapy option in clinical practice and was able to eradicate H. pylori in up to 75% of the empirical group population in this study. Our study suggests a practical method for increasing the eradication rate of H. pylori infections in South Korea.
In conclusion, clarithromycin resistance–guided tailored therapy, based on DPO-multiplex PCR, was superior to empirical therapy for first-line eradication treatment in a region with a high prevalence of clarithromycin resistance.
CONFLICTS OF INTEREST
Guarantor of the article: Soo-Jeong Cho, MD, PhD.
Specific author contributions: J.L.K., S.-J.C.: planning and/or conducting the study. J.L.K., S.-J.C., S.J.C., A.L., J.C., H.C.: collecting and/or interpreting data. J.L.K., S.-J.C., S.G.K.: drafting/revision of the manuscript. J.L.K., S.-J.C., S.J.C., A.L., J.C., H.C., S.G.K.: approval of the final draft of the submitted manuscript.
Financial support: This study was supported by a grant from the National Research Foundation of Korea (#NRF-2019R1A2C1009923) and the Korean Gastroenterology Fund for Future Development (2018). The work was independently conducted of the funding source. The funding source played no role in the report of this trial.
Potential competing interests: None to report.
Clinical trial registration: NCT04006340.
Study Highlights.
WHAT IS KNOWN
✓ Eradication rate of triple therapy is unsatisfactory as antibiotic resistance rate of H. pylori increases.
✓ Clarithromycin resistance of H. pylori based on 23S rRNA point mutations is a major cause of eradication failure.
✓ DPO-PCR can detect H. pylori, which had a good sensitivity, specificity, and concordance rate.
WHAT IS NEW HERE
✓ DPO-PCR based eradication strategy had superior eradication efficacy to empirical therapy in a region of high prevalence of clarithromycin resistance.
✓ Tailored group had more side effects, which were mild degree not to affect compliance.
TRANSLATIONAL IMPACT
✓ PCR-based tailored therapy increases eradication rate of H. pylori infection.
REFERENCES
- 1.Choi JM, Kim SG. Diagnosis and treatment of Helicobacter pylori infection: Korean and overseas guidelines. Korean J Med 2015;89:157–68. [Google Scholar]
- 2.Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: Treatment of Helicobacter pylori infection. Am J Gastroenterol 2017;112:212–39. [DOI] [PubMed] [Google Scholar]
- 3.Kim SG, Jung HK, Lee HL, et al. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. J Gastroenterol Hepatol 2014;29:1371–86. [DOI] [PubMed] [Google Scholar]
- 4.Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015;64:1353–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asaka M. A new approach for elimination of gastric cancer deaths in Japan. Int J Cancer 2013;132:1272–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim BJ, Lee H, Lee YC, et al. Ten-day concomitant, 10-day sequential, and 7-day triple therapy as first-line treatment for Helicobacter pylori infection: A nationwide randomized trial in Korea. Gut Liver 2019;13:531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gatta L, Vakil N, Vaira D, et al. Global eradication rates for Helicobacter pylori infection: Systematic review and meta-analysis of sequential therapy. BMJ 2013;347:f4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut 2017;66:6–30. [DOI] [PubMed] [Google Scholar]
- 9.Lee JH, Ahn JY, Choi KD, et al. Nationwide antibiotic resistance mapping of Helicobacter pylori in Korea: A prospective multicenter study. Helicobacter 2019;24:e12592. [DOI] [PubMed] [Google Scholar]
- 10.Lehours P, Siffré E, Mégraud F. DPO multiplex PCR as an alternative to culture and susceptibility testing to detect Helicobacter pylori and its resistance to clarithromycin. BMC Gastroenterol 2011;11:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woo HY, Park DI, Park H, et al. Dual-priming oligonucleotide-based multiplex PCR for the detection of Helicobacter pylori and determination of clarithromycin resistance with gastric biopsy specimens. Helicobacter 2009;14:22–8. [DOI] [PubMed] [Google Scholar]
- 12.Hwang TJ, Kim N, Kim HB, et al. Change in antibiotic resistance of Helicobacter pylori strains and the effect of A2143G point mutation of 23S rRNA on the eradication of H. pylori in a single center of Korea. J Clin Gastroenterol 2010;44:536–43. [DOI] [PubMed] [Google Scholar]
- 13.Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Rev Gastroenterol Hepatol 2008;5:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischbach L, Evans EL. Meta-analysis: The effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment Pharmacol Ther 2007;26:343–57. [DOI] [PubMed] [Google Scholar]
- 15.Luther J, Higgins PD, Schoenfeld PS, et al. Empiric quadruple vs. triple therapy for primary treatment of Helicobacter pylori infection: Systematic review and meta-analysis of efficacy and tolerability. Am J Gastroenterol 2010;105:65–73. [DOI] [PubMed] [Google Scholar]
- 16.Lee JH, Park J, Park MR, et al. A comparative study of Helicobacter pylori growth on different agar-based media. Korean J Helicobacter Upper Gastrointest Res 2017;17:208–12. [Google Scholar]
- 17.Lee JY, Kim N. Future trends of Helicobacter pylori eradication therapy in Korea. Korean J Gastroenterol 2014;63:158. [DOI] [PubMed] [Google Scholar]
- 18.Liou JM, Chen PY, Luo JC, et al. Efficacies of genotypic resistance-guided vs empirical therapy for refractory Helicobacter pylori infection. Gastroenterology 2018;155:1109–19. [DOI] [PubMed] [Google Scholar]
- 19.Gweon TG, Kim JS, Kim BW. An economic modeling study of Helicobacter pylori eradication: Comparison of dual priming oligonucleotide-based multiplex polymerase chain reaction and empirical treatment. Gut Liver 2018;12:648–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung YD, Kim Y-J, Chung WC. A pilot study of Helicobacter pylori eradication using a polymerase chain reaction-based test for clarithromycin resistance. Korean J Helicobacter Upper Gastrointest Res 2017;17:200. [Google Scholar]
- 21.Lee HJ, Kim JI, Cheung DY, et al. Eradication of Helicobacter pylori according to 23S ribosomal RNA point mutations associated with clarithromycin resistance. J Infect Dis 2013;208:1123–30. [DOI] [PubMed] [Google Scholar]
- 22.Bang H, Zhao H. Median-based incremental cost-effectiveness ratio (ICER). J Stat Theory Pract 2012;6:428–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Humans Iwgoteocrt. Schistosomes, Liver Flukes and Helicobacter pylori. International Agency for Research on Cancer: Lyon, 1994. [Google Scholar]
- 24.Correa P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol 1995;19:S37–43. [PubMed] [Google Scholar]
- 25.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med 2002;347:1175–86. [DOI] [PubMed] [Google Scholar]
- 26.Choi IJ, Kook MC, Kim YI, et al. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N Engl J Med 2018;378:1085–95. [DOI] [PubMed] [Google Scholar]
- 27.Choi JM, Kim SG, Choi J, et al. Effects of Helicobacter pylori eradication for metachronous gastric cancer prevention: A randomized controlled trial. Gastrointest Endosc 2018;88:475–85. [DOI] [PubMed] [Google Scholar]
- 28.Cho SJ, Choi IJ, Kook MC, et al. Randomised clinical trial: The effects of Helicobacter pylori eradication on glandular atrophy and intestinal metaplasia after subtotal gastrectomy for gastric cancer. Aliment Pharmacol Ther 2013;38:477–89. [DOI] [PubMed] [Google Scholar]
- 29.Venerito M, Krieger T, Ecker T, et al. Meta-analysis of bismuth quadruple therapy versus clarithromycin triple therapy for empiric primary treatment of Helicobacter pylori infection. Digestion 2013;88:33–45. [DOI] [PubMed] [Google Scholar]
- 30.Rimbara E, Fischbach LA, Graham DY. Optimal therapy for Helicobacter pylori infections. Nat Rev Gastroenterol Hepatol 2011;8:79. [DOI] [PubMed] [Google Scholar]
- 31.Kim N, Kim JM, Kim CH, et al. Institutional difference of antibiotic resistance of Helicobacter pylori strains in Korea. J Clin Gastroenterol 2006;40:683–7. [DOI] [PubMed] [Google Scholar]
- 32.Chen H, Dang Y, Zhou X, et al. Tailored therapy versus empiric chosen treatment for Helicobacter pylori eradication: A meta-analysis. Medicine (Baltimore) 2016;95:e2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puig I, Baylina M, Sanchez-Delgado J, et al. Systematic review and meta-analysis: Triple therapy combining a proton-pump inhibitor, amoxicillin and metronidazole for Helicobacter pylori first-line treatment. J Antimicrob Chemother 2016;71(10):2740–53. [DOI] [PubMed] [Google Scholar]
- 34.Nishizawa T, Maekawa T, Watanabe N, et al. Clarithromycin versus metronidazole as first-line Helicobacter pylori eradication: A multicenter, prospective, randomized controlled study in Japan. J Clin Gastroenterol 2015;49:468–71. [DOI] [PubMed] [Google Scholar]
- 35.Chung JW, Lee GH, Jeong JY, et al. Resistance of Helicobacter pylori strains to antibiotics in Korea with a focus on fluoroquinolone resistance. J Gastroenterol Hepatol 2012;27(3):493–7. [DOI] [PubMed] [Google Scholar]