INTRODUCTION:
Exocrine pancreatic function is a critical host factor in determining the intestinal microbiota composition. Diseases affecting the exocrine pancreas could therefore influence the gut microbiome. We investigated the changes in gut microbiota of patients with chronic pancreatitis (CP).
METHODS:
Patients with clinical and imaging evidence of CP (n = 51) were prospectively recruited and compared with twice the number of nonpancreatic disease controls matched for distribution in age, sex, body mass index, smoking, diabetes mellitus, and exocrine pancreatic function (stool elastase). From stool samples of these 153 subjects, DNA was extracted, and intestinal microbiota composition was determined by bacterial 16S ribosomal RNA gene sequencing.
RESULTS:
Patients with CP exhibited severely reduced microbial diversity (Shannon diversity index and Simpson diversity number, P < 0.001) with an increased abundance of facultative pathogenic organisms (P < 0.001) such as Enterococcus (q < 0.001), Streptococcus (q < 0.001), and Escherichia.Shigella (q = 0.002). The CP-associated changes were independent of exocrine pancreatic insufficiency. Short-chain fatty acid producers, considered protective for epithelia such as Faecalibacterium (q < 0.001), showed reduced abundance in patients with CP. Of 4 additional patients with CP previously treated with antibiotics (ceftriaxone and metronidazole), 3 patients were characterized by distinct Enterococcus overgrowth.
DISCUSSION:
CP is associated with marked gut microbiota dysbiosis, greatly reduced diversity, and increased abundance of opportunistic pathogens, specifically those previously isolated from infected pancreatic necrosis. Taxa with a potentially beneficial role in intestinal barrier function are depleted. These changes can increase the probability of complications from pancreatitis such as infected fluid collections or small intestinal bacterial overgrowth (see Graphical Abstract, Supplementary Digital Content 1, http://links.lww.com/CTG/A383).
INTRODUCTION
Chronic pancreatitis (CP) distinctly reduces patients' quality of life and is associated with early death (1). Known causes of CP include long time exposure to alcohol (2) and smoking, metabolic (3) and vascular diseases (4), autoimmune (5) or genetic disorders (6,7), and less common mechanisms (8,9). In a significant proportion of CP cases, the underlying cause remains obscure, and they are labelled as idiopathic or sporadic (3). In addition to the signs of exocrine pancreatic insufficiency, patients with CP frequently suffer from unspecific symptoms such as abdominal distension, bloating, or flatulence, which can be the result of small intestinal bacterial overgrowth (10), a condition that describes a state of microbial dysbiosis in the small bowel. Recently, we have shown that the exocrine pancreas represents one of the most important host factors regulating the gut microbiota composition in healthy individuals free of pancreatic disease (11). Whether this is because of an altered availability of substrates for microbial metabolism or a reduced secretion of pancreatic antimicrobial peptides, as suggested by a rodent model (12), is currently unclear. It seems plausible that the gut microbial dysbiosis in CP would not only affect the small bowel but also be reflected by a changed microbiota composition in the colon, the body's largest microbial reservoir (13). Data on the role of gut microbiota and its composition in patients with CP are still scarce and inconclusive (14,15). In the present study, we analyzed fecal microbiota profiles generated by 16S ribosomal RNA (rRNA) gene sequencing of patients with CP (n = 51) and compared them with a group of control individuals with a matched phenotype distribution (n = 102). This allowed us to determine whether intestinal microbiota changes occur and to what degree they depend on either the presence of CP or, alternatively, on the impairment of exocrine pancreatic function.
METHODS
Study participants
Patients with CP were prospectively recruited at the University Medicine Greifswald (Germany) in the period of November 2010–October 2017. Diagnosis was based on both, clinical symptoms consistent with CP (recurrent epigastric abdominal pain, endocrine and/or exocrine insufficiency) in combination with signs of CP on transsectional imaging (computed tomography, magnetic resonance imaging, and abdominal or endoscopic ultrasound) and required unequivocal evidence of calcifications, definitive ductal or parenchymal changes (Cambridge), pseudocysts, or pancreatic duct calculi. All participants provided written informed consent, and the study was approved by the ethics committee of the University Medicine Greifswald (III UV 91/03b). The etiology of CP was alcohol related (n = 35) in most cases, followed by idiopathic (n = 13) and CP associated with pancreatic secretory trypsin inhibitor (SPINK1) (16) mutation (n = 3). One patient who was suffering from CP associated with SPINK1 mutation also had pancreas divisum. None of the CP cases included had pancreatic carcinoma or intraductal papillary mucinous neoplasm. As controls, we selected control individuals with neither a history of nor evidence for pancreatic disease from a volunteer cohort who participated in the population-based Study of Health in Pomerania (11,17,18). These cases were selected to create a control group with a matched distribution for age, sex, body mass index (BMI), smoking, diabetes mellitus, and exocrine pancreatic function, as determined by stool elastase measurements. None of the included cases or controls was under antibiotic therapy at the time of sample collection.
Determination of fecal pancreatic elastase levels
Pancreatic elastase levels were determined in fecal samples using a monospecific pancreatic elastase ELISA (BIOSERV Diagnostics GmbH, Germany) that has a lower detection limit of 5.5 μg/g stool. All steps were performed according to the manufacturer's protocol. Pancreatic elastase concentrations (μg/g stool) were calculated photometrically (optical density 450 nm). A standard solution was also tested for comparison.
16S rRNA gene sequencing and taxonomic annotation
Sequencing was performed, as described before in detail (11). In brief, fecal samples were collected in a tube containing a stabilizing EDTA buffer, and DNA was isolated using the PSP Spin Stool DNA Kit (Stratec Biomedical AG, Birkenfeld, Germany). After amplification of the V1-V2 region of bacterial 16S rRNA genes, sequencing was performed on a MiSeq platform (Illumina, San Diego, CA). MiSeq Fast-Q files were created using CASAVA 1.8.2 (https://support.illumina.com/sequencing/sequencing_software/casava). The open-source software package DADA2 (v.1.10) (19) was used for amplicon-data processing. This software enables single-nucleotide resolution of amplicons (amplicon sequence variants). Data processing was performed according to the recommended procedure for large data sets (https://benjjneb.github.io/dada2/bigdata.html), adapted to the targeted V1-V2 region. In brief, 5 bases were truncated from the 5′ end of the sequence of both reads. Forward and reverse reads were truncated to a length of 200 and 150 bases, respectively. Read pairs were excluded if they contained ambiguous bases, had expected errors higher than 2, or when originating from PhiX spike-in. Error profiles were inferred based on 1 million reads of the respective sequencing run. Subsequently dereplication, error correction, and merging of forward and reverse reads were performed. A combination of amplicon sequence variant abundance of tables of all samples and identification and removal of chimeric amplicon sequences were conducted using the removeBimeraDenovo() function in a consensus mode. Taxonomic annotation was performed with a Bayesian classifier and the Ribosomal Database Project training set version 16.
Data analysis
All statistical analyses were performed using the software environment “R” (20). The alpha diversity scores—“Shannon diversity index” (H) and “Simpson diversity number” (N2)—were calculated using the diversity function of the R package “vegan” (21). For analysis of beta diversity, the Bray-Curtis dissimilarity using the “vegan” function vegdist was computed. Principal coordinate analysis was performed with the cmdscale function (“vegan”). To determine the contribution of the trait CP to the Bray-Curtis dissimilarity, permutational analysis of variance (PERMANOVA) was carried out (“vegan” function adonis; 10,000 permutations). For matching of controls, the function “matchit” (package “MatchIt”) was used to assign controls for CP cases (2:1 ratio) using the method = “optimal” (22). This procedure included calculation of a propensity score based on a logistic regression model (distance = “logit”). Matches were then selected by the algorithm, reducing the average distance across all matched pairs. The 2-tailed Mann-Whitney test (MW) was performed for the assessment of significance in case of continuous data. The Fisher exact test was used for categorical data. To identify differentially abundant taxa between CP patients and their controls, we analyzed all genera that were present in at least 25% of either CP cases or control samples and with a mean abundance of at least 0.1% (total group) using the MW test. P values for comparisons between different genera were adjusted for multiple testing after the Benjamini-Hochberg procedure and called “q-values.” P values and q values <0.05 were considered significant. All P and q values were rounded to 3 significant digits.
RESULTS
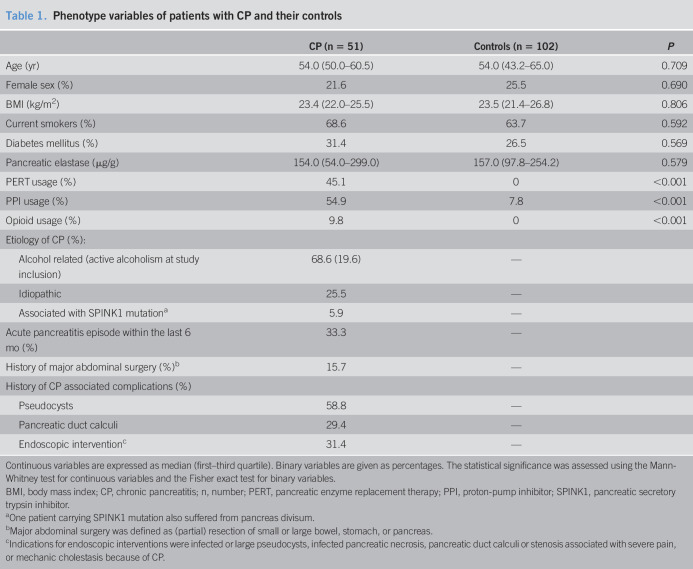
Patients with CP and controls exhibited similar distributions of age, sex, BMI, smoking, diabetes mellitus, and pancreatic elastase concentrations that are all known to be associated with changes in the gut microbiome (11,23), as shown in Table 1.
Table 1.
Phenotype variables of patients with CP and their controls
| CP (n = 51) | Controls (n = 102) | P | |
| Age (yr) | 54.0 (50.0–60.5) | 54.0 (43.2–65.0) | 0.709 |
| Female sex (%) | 21.6 | 25.5 | 0.690 |
| BMI (kg/m2) | 23.4 (22.0–25.5) | 23.5 (21.4–26.8) | 0.806 |
| Current smokers (%) | 68.6 | 63.7 | 0.592 |
| Diabetes mellitus (%) | 31.4 | 26.5 | 0.569 |
| Pancreatic elastase (μg/g) | 154.0 (54.0–299.0) | 157.0 (97.8–254.2) | 0.579 |
| PERT usage (%) | 45.1 | 0 | <0.001 |
| PPI usage (%) | 54.9 | 7.8 | <0.001 |
| Opioid usage (%) | 9.8 | 0 | <0.001 |
| Etiology of CP (%): | |||
| Alcohol related (active alcoholism at study inclusion) | 68.6 (19.6) | — | |
| Idiopathic | 25.5 | — | |
| Associated with SPINK1 mutationa | 5.9 | — | |
| Acute pancreatitis episode within the last 6 mo (%) | 33.3 | — | |
| History of major abdominal surgery (%)b | 15.7 | — | |
| History of CP associated complications (%) | |||
| Pseudocysts | 58.8 | — | |
| Pancreatic duct calculi | 29.4 | — | |
| Endoscopic interventionc | 31.4 | — |
Continuous variables are expressed as median (first–third quartile). Binary variables are given as percentages. The statistical significance was assessed using the Mann-Whitney test for continuous variables and the Fisher exact test for binary variables.
BMI, body mass index; CP, chronic pancreatitis; n, number; PERT, pancreatic enzyme replacement therapy; PPI, proton-pump inhibitor; SPINK1, pancreatic secretory trypsin inhibitor.
One patient carrying SPINK1 mutation also suffered from pancreas divisum.
Major abdominal surgery was defined as (partial) resection of small or large bowel, stomach, or pancreas.
Indications for endoscopic interventions were infected or large pseudocysts, infected pancreatic necrosis, pancreatic duct calculi or stenosis associated with severe pain, or mechanic cholestasis because of CP.
Beta diversity analysis of CP cases compared with controls
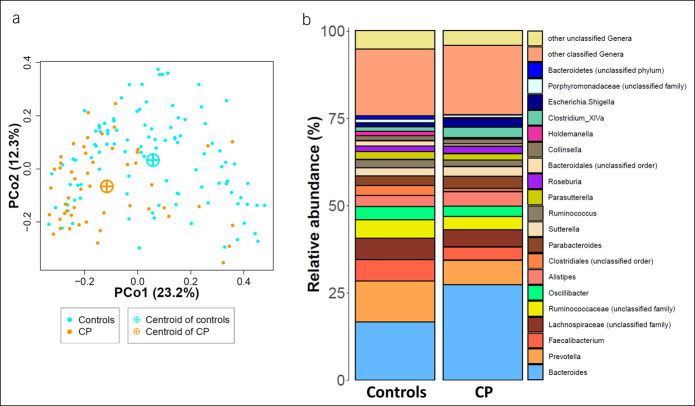
To explore structural differences in the gut microbiota community structure, we calculated the Bray-Curtis dissimilarity, which estimates the difference between the samples (beta diversity), and performed a principal coordinate analysis. Figure 1a shows a spatial segregation of CP cases compared with their control samples confirmed by a significant PERMANOVA (r2 = 0.05, P < 0.001). Including the aforementioned possible confounding factors, age, sex, BMI, smoking, diabetes mellitus, and pancreatic elastase as covariates into PERMANOVA neither reduced the explained variation nor the significance of the association of CP (r2 = 0.05, P < 0.001). This underlines the equal distribution of these phenotypic factors in CP cases and controls. Exploring the mean relative abundance at the genus level revealed Bacteroides to be the most abundant genus in both groups (Figure 1b).
Figure 1.
Gut microbiota structure in CP cases and controls. (a) Principal coordinate analysis (PCoA) based on Bray-Curtis dissimilarity. Orange and cyan dots represent samples from CP cases (CP, n = 51) and control individuals (n = 102), respectively. CP cases are clearly shifted from controls. (b) Average gut microbiota composition. Stacked bar plots show the mean relative abundances for CP cases and their controls. CP, chronic pancreatitis.
Alpha diversity analysis of CP cases compared with controls
To evaluate the within-sample variation of the gut microbial communities, we determined the alpha diversity scores—Simpson diversity number (N2) and Shannon diversity index (H). CP cases had median N2 and H scores of 26.4 (20.0–38.5, first–third quartile) and 4.1 (3.7–4.3) that were distinctly lower than in control samples with 40.1 (28.0–57.9, P < 0.001, MW) and 4.4 (4.1–4.7, P < 0.001, MW), respectively (Figure 2).
Figure 2.
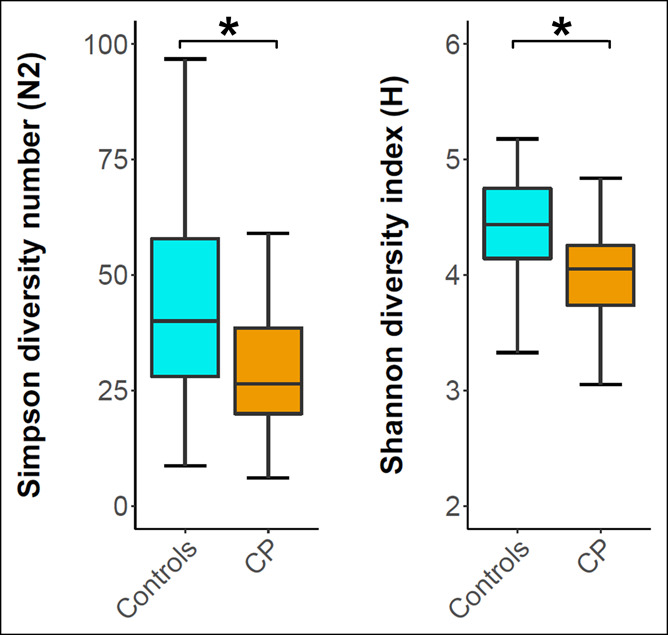
Alpha diversity analysis of CP cases and controls. Box plots show the distribution of the alpha diversity scores Simpson diversity number (N2) and the Shannon diversity index (H). Whiskers are drawn up to 1.5 times the interquartile range (Tukey). Outliers are not shown. * Indicates a significant result (P < 0.05) according to the Mann-Whitney test. CP, chronic pancreatitis.
Intestinal microbiota alterations in CP
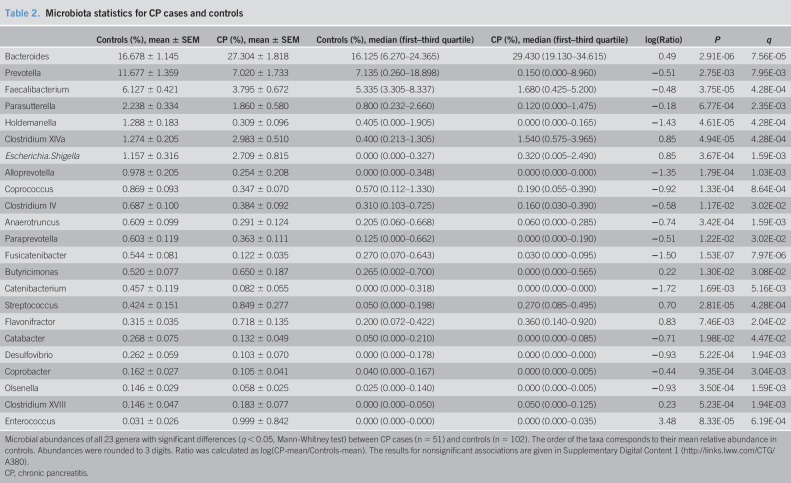
Comparison of relative abundance data between CP and controls using the MW test revealed 23 genera with differential abundance. These taxa accounted for 51.6% of the total microbial abundance in the group of CP. Fifteen of these taxa exhibited a negative association with CP, whereas only 8 increased in abundance (Table 2, Figure 3a, and Supplementary Digital Content 1, http://links.lww.com/CTG/A380) in line with the observation of decreased microbial alpha diversity in CP. The most distinct increase in abundance, percentagewise, was found for Enterococcus (q < 0.001). In absolute terms, Bacteroides showed the largest increase (mean 27.3% in CP compared with 16.7% in controls, q < 0.001). The largest absolute reduction in CP cases was found for Faecalibacterium (3.8% in CP and 6.1% in controls, q < 0.001) and Prevotella (7.0% in CP and 11.7% in controls, q = 0.008).
Table 2.
Microbiota statistics for CP cases and controls
| Controls (%), mean ± SEM | CP (%), mean ± SEM | Controls (%), median (first–third quartile) | CP (%), median (first–third quartile) | log(Ratio) | P | q | |
| Bacteroides | 16.678 ± 1.145 | 27.304 ± 1.818 | 16.125 (6.270–24.365) | 29.430 (19.130–34.615) | 0.49 | 2.91E-06 | 7.56E-05 |
| Prevotella | 11.677 ± 1.359 | 7.020 ± 1.733 | 7.135 (0.260–18.898) | 0.150 (0.000–8.960) | −0.51 | 2.75E-03 | 7.95E-03 |
| Faecalibacterium | 6.127 ± 0.421 | 3.795 ± 0.672 | 5.335 (3.305–8.337) | 1.680 (0.425–5.200) | −0.48 | 3.75E-05 | 4.28E-04 |
| Parasutterella | 2.238 ± 0.334 | 1.860 ± 0.580 | 0.800 (0.232–2.660) | 0.120 (0.000–1.475) | −0.18 | 6.77E-04 | 2.35E-03 |
| Holdemanella | 1.288 ± 0.183 | 0.309 ± 0.096 | 0.405 (0.000–1.905) | 0.000 (0.000–0.165) | −1.43 | 4.61E-05 | 4.28E-04 |
| Clostridium XlVa | 1.274 ± 0.205 | 2.983 ± 0.510 | 0.400 (0.213–1.305) | 1.540 (0.575–3.965) | 0.85 | 4.94E-05 | 4.28E-04 |
| Escherichia.Shigella | 1.157 ± 0.316 | 2.709 ± 0.815 | 0.000 (0.000–0.327) | 0.320 (0.005–2.490) | 0.85 | 3.67E-04 | 1.59E-03 |
| Alloprevotella | 0.978 ± 0.205 | 0.254 ± 0.208 | 0.000 (0.000–0.348) | 0.000 (0.000–0.000) | −1.35 | 1.79E-04 | 1.03E-03 |
| Coprococcus | 0.869 ± 0.093 | 0.347 ± 0.070 | 0.570 (0.112–1.330) | 0.190 (0.055–0.390) | −0.92 | 1.33E-04 | 8.64E-04 |
| Clostridium IV | 0.687 ± 0.100 | 0.384 ± 0.092 | 0.310 (0.103–0.725) | 0.160 (0.030–0.390) | −0.58 | 1.17E-02 | 3.02E-02 |
| Anaerotruncus | 0.609 ± 0.099 | 0.291 ± 0.124 | 0.205 (0.060–0.668) | 0.060 (0.000–0.285) | −0.74 | 3.42E-04 | 1.59E-03 |
| Paraprevotella | 0.603 ± 0.119 | 0.363 ± 0.111 | 0.125 (0.000–0.662) | 0.000 (0.000–0.190) | −0.51 | 1.22E-02 | 3.02E-02 |
| Fusicatenibacter | 0.544 ± 0.081 | 0.122 ± 0.035 | 0.270 (0.070–0.643) | 0.030 (0.000–0.095) | −1.50 | 1.53E-07 | 7.97E-06 |
| Butyricimonas | 0.520 ± 0.077 | 0.650 ± 0.187 | 0.265 (0.002–0.700) | 0.000 (0.000–0.565) | 0.22 | 1.30E-02 | 3.08E-02 |
| Catenibacterium | 0.457 ± 0.119 | 0.082 ± 0.055 | 0.000 (0.000–0.318) | 0.000 (0.000–0.000) | −1.72 | 1.69E-03 | 5.16E-03 |
| Streptococcus | 0.424 ± 0.151 | 0.849 ± 0.277 | 0.050 (0.000–0.198) | 0.270 (0.085–0.495) | 0.70 | 2.81E-05 | 4.28E-04 |
| Flavonifractor | 0.315 ± 0.035 | 0.718 ± 0.135 | 0.200 (0.072–0.422) | 0.360 (0.140–0.920) | 0.83 | 7.46E-03 | 2.04E-02 |
| Catabacter | 0.268 ± 0.075 | 0.132 ± 0.049 | 0.050 (0.000–0.210) | 0.000 (0.000–0.085) | −0.71 | 1.98E-02 | 4.47E-02 |
| Desulfovibrio | 0.262 ± 0.059 | 0.103 ± 0.070 | 0.000 (0.000–0.178) | 0.000 (0.000–0.000) | −0.93 | 5.22E-04 | 1.94E-03 |
| Coprobacter | 0.162 ± 0.027 | 0.105 ± 0.041 | 0.040 (0.000–0.167) | 0.000 (0.000–0.005) | −0.44 | 9.35E-04 | 3.04E-03 |
| Olsenella | 0.146 ± 0.029 | 0.058 ± 0.025 | 0.025 (0.000–0.140) | 0.000 (0.000–0.005) | −0.93 | 3.50E-04 | 1.59E-03 |
| Clostridium XVIII | 0.146 ± 0.047 | 0.183 ± 0.077 | 0.000 (0.000–0.050) | 0.050 (0.000–0.125) | 0.23 | 5.23E-04 | 1.94E-03 |
| Enterococcus | 0.031 ± 0.026 | 0.999 ± 0.842 | 0.000 (0.000–0.000) | 0.000 (0.000–0.035) | 3.48 | 8.33E-05 | 6.19E-04 |
Microbial abundances of all 23 genera with significant differences (q < 0.05, Mann-Whitney test) between CP cases (n = 51) and controls (n = 102). The order of the taxa corresponds to their mean relative abundance in controls. Abundances were rounded to 3 digits. Ratio was calculated as log(CP-mean/Controls-mean). The results for nonsignificant associations are given in Supplementary Digital Content 1 (http://links.lww.com/CTG/A380).
CP, chronic pancreatitis.
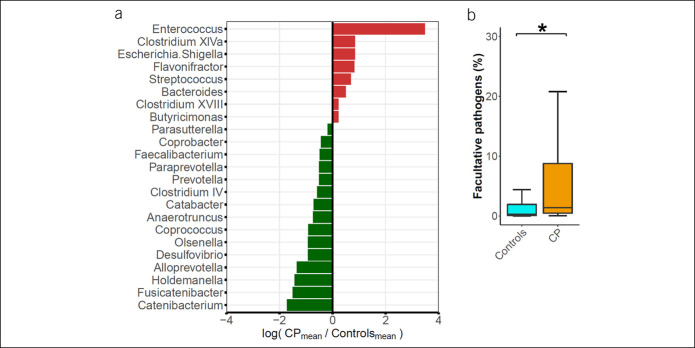
Figure 3.
Intestinal microbiota alterations in CP cases. (a) Shown are all genera with significant differential abundance between CP cases and controls according to the Mann-Whitney test (q < 0.05). Abundance changes are depicted as log-fold change of mean abundance ratio (CP/controls). (b) Boxplot shows the distribution of important facultative pathogenic bacteria (summarized Citrobacter, Enterobacter, Enterococcus, Enterobacteriaceae, Escherichia. Shigella, Klebsiella, Pseudomonas, Proteus, Staphylococcus, and Streptococcus counts) in CP cases compared with controls. * Indicates a significant difference (P < 0.05). CP, chronic pancreatitis.
Analysis of important facultative pathogenic bacteria that has previously been found in isolates of pancreatic necrosis or peripancreatic fluid collections in patients with acute pancreatitis or CP (24,25), namely Citrobacter, Enterobacter, Enterococcus, Enterobacteriaceae, Escherichia.Shigella, Klebsiella, Pseudomonas, Proteus, Staphylococcus, and Streptococcus revealed a distinct 2.8-fold and 5.0-fold increase of the mean and median, respectively, in CP samples when compared with controls (P < 0.001, MW) (Figure 3b). Next, we calculated a linear regression model of log-transformed abundance values (zeros treated as not available, n = 13) of important facultative pathogenic bacteria within the combined group of CP cases and controls. As covariates, we included age, sex, BMI, smoking, diabetes mellitus, pancreatic elastase levels, and CP status (present or absent). Within this model, there was no significant contribution of sex, BMI, smoking, diabetes mellitus, or pancreatic elastase. Positive associations were found for age (estimate = 0.03, P = 0.022) and particularly for CP disease status (estimate = 1.06, P = 0.002) with the amount of facultative pathogens.
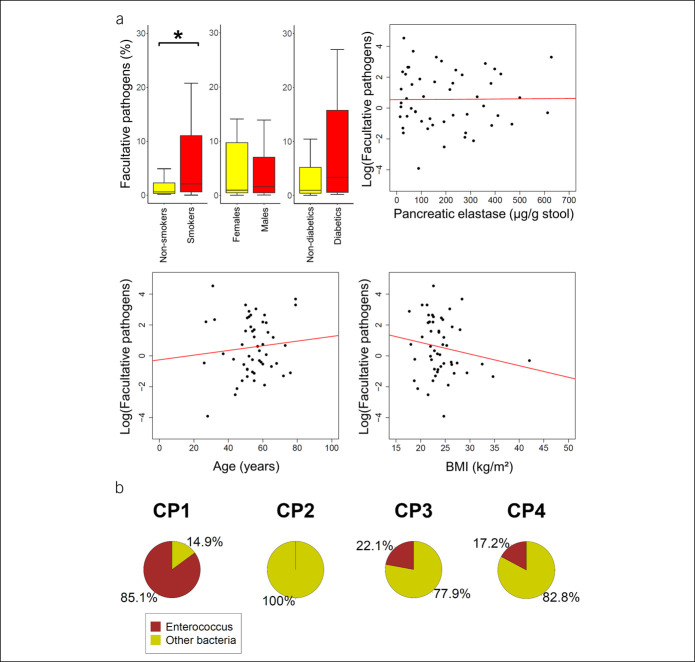
When investigating the factors contributing to the level of facultative pathogens only within the group of CP cases, we found a higher median facultative pathogen abundance of 2.1% (0.6–11.1, first–third quartiles) in smokers compared with non-smokers with 0.6% (0.3–2.3) (P = 0.040, MW). Other phenotypic factors such as age, sex, BMI, diabetes mellitus, or pancreatic elastase levels did not exhibit a significant correlation with the amount of facultative pathogenic bacteria in patients with CP (Figure 4a).
Figure 4.
Factors contributing to increase in facultative pathogens in patients with CP. (a) Boxplots display the level of facultative pathogenic bacteria depending on smoking status, sex, or presence of diabetes mellitus. Whiskers are drawn up to 1.5 times the interquartile range (Tukey). Outliers are not shown. Scatter plots show the association of age, BMI, and pancreatic elastase with facultative pathogens (displayed as log[abundance]). Only smoking status exhibited a significant correlation. * Indicates a significant result (P < 0.05). (b) Enterococcus overgrowth under antibiotic therapy. Pie charts show the proportion of Enterococcus reads (brown) in fecal samples of 4 patients with CP (CP1-4). All patients were under treatment with ceftriaxone and metronidazole at the time of sample collection. BMI, body mass index; CP, chronic pancreatitis.
CP cases and controls differed in medication usage such as pancreatic enzyme replacement therapy, proton-pump inhibitor, and opioid usage. To investigate, whether these factors may have affected the findings of increased facultative pathogens in CP, we compared those CP patients with or without intake of the respective medication. Levels of facultative pathogens for pancreatic enzyme replacement therapy, proton-pump inhibitor, and opioid users were 1.1% (0.3–9.7), 2.0% (0.5–6.2), and 1.4% (0.2–12.5), respectively, that were not significantly different than the corresponding groups without medication with 1.7% (0.6–5.7), 1.1% (0.5–10.4), and 1.5% (0.5–8.1). As comparison, the levels of facultative pathogens in control individuals without CP were 0.3% (0.1–1.9).
Gut microbiota in CP under antibiotic therapy
A commonly used antibiotic regimen to treat infections of suspected abdominal origin is the combination of ceftriaxone and metronidazole. Ceftriaxone, however, has no relevant activity against Enterococcus (26). This may lead to Enterococcus overgrowth in patients who already have a high abundance of this bacterium in their microbiome, as shown above for CP. We therefore evaluated the proportion of Enterococcus in 4 patients who were excluded from the main data set because of the intake of ceftriaxone and metronidazole at the time of sample collection. This additional data set comprised 2 men and 2 women aged from 41 to 70 years (mean 52.5). The etiology of CP was alcohol related in 3 and idiopathic in 1 case. Indications for antibiotic treatment were cholangitis, pancreatic abscess or suspected infection of pancreatic pseudocyst, or necrosis. In this data set, we found a very high abundance of Enterococcus in 3 of 4 patients (Figure 4b).
DISCUSSION
We investigated changes in the intestinal microbial community structure of individuals suffering from CP. It was characterized by a distinct decrease in gut microbiota diversity, which was independent of exocrine pancreatic function, as far as can be determined by matching for stool pancreatic elastase concentrations. Low diversity is generally considered an indicator of an unhealthy gut microbiome because it is assumed that low diversity reduces the resilience of an ecosystem against introduction of foreign species by exposing uncovered niches (27). These niches can subsequently be occupied by microbial pathogens. In line with these assumptions, reduced gut microbiota diversity was previously reported in a range of intestinal disorders such as Crohn's disease, ulcerative colitis, or recurrent Clostridium difficile-associated diarrhea (28–30). The latter is characterized by overgrowth of the normal gut flora by C. difficile, which is facilitated by a reduction of gut microbial diversity and perturbation of the resident flora after antibiotic therapy (30). The reduced microbial diversity found in patients with CP is therefore likely to increase the susceptibility toward overgrowth by microbial pathogens. Accordingly, we found a marked increase of the opportunistic pathogen Enterococcus in CP in comparison to controls. In a recent rodent model, intestinal overgrowth of Enterococcus was triggered by antibiotic therapy that resulted in systemic dissemination of the bacteria (31). Therefore, the finding of increased Enterococcus abundance in the gut lumen of patients with CP may increase the risk of systemic infections with these facultative pathogens. This may also explain the high rate of Enterococci found in aspirates of pancreatic pseudocysts (24). Interestingly, in an additional data set comprising 4 patients with CP who were under treatment with ceftriaxone and metronidazole at the time of sample collection, we found extremely high rates of Enterococcus in the gut microbiome. The combination of the aforementioned antibiotics offers a broad-spectrum antimicrobial coverage including Gram-negative organisms and anaerobic bacteria. However, ceftriaxone is inherently nonactive against Enterococcus as a monotherapy (26). Therefore, the anaerobic growth suppression mediated by metronidazole in combination with the Gram-negative activity of ceftriaxone seems to have created an open niche for intestinal Enterococcus overgrowth in 3 of the 4 individuals. This, again, may substantially increase the risk for subsequent Enterococcus septicemia. An investigation in immunocompromised patients undergoing stem cell transplantation showed that gut microbiota domination by Enterococcus, which could be triggered by administration of metronidazole, increased the risk for subsequent Vancomycin-resistant Enterococcus bacteremia 9-fold (32). Whether the bacterial overgrowth in patients with CP also translates into increased rates of subsequent Enterococcus-related infections cannot be answered at this point and warrants further investigations.
In the present study, we also found a gut microbiota enrichment of other facultative pathogens such as Streptococcus and Escherichia.Shigella in patients with CP. These bacteria have also been detected in infected pancreatic pseudocysts or pancreatic necrotic tissue (24,25). When comparing the combined relative abundance of only those bacteria previously isolated from pancreatic necrosis or fluid collections between patients and controls, we found a 2.8-fold increase of these pathogens in patients with CP. This represents a significant amplification of the bacterial pathogenic reservoir in the intestine and one with specific relevance to pancreatitis because the source of pathogens in infected pancreatic necrosis has long been suspected to be the gut. Of note, CP cases who were smokers exhibited higher levels of facultative pathogens compared with non-smokers, underlining the need for smoking cessation in patients suffering from CP. We can only speculate about the explanation for this observation. However, smoking can induce inflammation and fibrosis, as previously shown in an animal model (33), leading to progressive destruction of the pancreas and impairment of its important physiological role in regulating the gut microbiome.
Another group has recently published data from a CP cohort finding similarly reduced microbial diversity and an increase in the facultative pathogen Escherichia.Shigella (15). In that study, however, control samples were not matched regarding the presence of impaired exocrine pancreatic function, which distinctly alters the gut microbiome even in the absence of CP (11). Our study shows that the dysbiotic microbiome in patients with CP cannot merely be explained by impaired exocrine pancreatic function but rather represents an additional burden of the disease.
In accordance with another previous study (34), we also found a reduction of Faecalibacterium in patients with CP. These groups are considered to have positive health effects because they participate in the production of short-chain fatty acids (SCFAs) such as butyrate (35). SCFAs are an energy source for colonic epithelia (36) and modulate the immune response through affecting colonic regulatory T-cell function that has been reported to alleviate experimental colitis in a rodent model (37). Another potentially beneficial taxon that was reduced in patients with CP was Fusicatenibacter that also participates in the production of SCFA and produces lactic acid (38). As a result, Fusicatenibacter may exhibit anti-inflammatory properties, as shown for other lactic acid producing bacteria (39). It should be noted that the anti-inflammatory effect of lactate has experimentally been shown to be clearly beneficial in pancreatitis (40). Because an intact intestinal barrier is a critical component for the defense against the translocation of gut-borne pathogens into areas of pancreatic necrosis or fluid collections, a depletion of these species would facilitate the development of the most feared complications of pancreatitis.
We hypothesize that the changes seen in the gut microbiota of patients with CP are largely the result, rather than the cause, of the disease and probably mediated via pathways of chronic inflammation or an altered composition of the intestinal chyme with resulting changes in the availability of substrates for microbial metabolism. Because this is the first study to correct for exocrine pancreatic function (stool elastase), the changes in intestinal microbiota composition and diversity are more likely to be directly related to CP and its complications rather than merely to the impaired secretion of digestive enzymes.
However, because here we report an association without specific intervention, we cannot prove or rule out that the gut microbiota composition, in turn, has direct effects on the progression and severity of CP. In another pancreatic disease, namely pancreatic cancer, recent evidence from rodent models points toward a contribution of gut microbiota to the oncogenesis and progression of pancreatic cancer by affecting the tumor microenvironment (41,42). Ablation of the microbiota by broad-spectrum therapy with multiple antibiotics resulted in reduced tumor growth. This indicates a causal contribution of microbiota to the pathogenesis or progression of pancreatic cancer. There may be similar mechanisms in CP promoting inflammation and subsequent fibrosis of the pancreas. Because most of the mechanistic work that proposes an effect of microbiota on pancreatic disease progression has relied on rodent models, there remains a need for validation in human studies. Because we used 16S rRNA gene sequencing to characterize changes in gut microbiota composition, we cannot accurately determine at this point whether every strain among the facultative pathogenic bacteria detected has clear pathogenic capabilities. However, the overall increase in opportunistic, potential pathogens, and the simultaneous depletion of potentially protective taxa make it likely that the observed changes in gut microbiota have a negative effect on disease progression and complications of pancreatitis.
In the present study, we accounted for the confounding factors, such as age, sex, BMI, smoking, diabetes mellitus, and, most importantly, exocrine pancreatic function. Because we did not collect comparable data on dietary habits for CP cases and controls, we cannot rule out that differences in food preference partially contributed to the presented findings. Similarly, no comparable data for the possible confounder alcohol consumption (11) were available. However, because only 10 CP cases were active alcoholics, differences in alcohol consumption are unlikely to explain the distinct microbiota changes observed in patients with CP.
In summary, we have studied the intestinal microbiota composition and diversity in patients with CP and found a significant microbiota dysbiosis, overgrowth by opportunistic, facultative pathogens such as Enterococcus, and depletion of short-chain fatty acid and lactate producers such as Faecalibacterium or Fusicatenibacter. These changes can facilitate the translocation of pathogens into areas of necrosis, if and when CP recurs with an acute episode, or allow the development of small intestinal bacterial overgrowth when pancreatitis remains chronic. Certain antibiotic regimens such as the combination of ceftriaxone and metronidazole may further amplify the unfavorable microbiota composition of patients with CP.
CONFLICTS OF INTEREST
Guarantor of the article: Markus M. Lerch, MD.
Specific author contributions: M.M.L., H.V., J.M., U.V., and A.F.: planning and concept of study. F.F., T.K., M.R., C.B., M.S., and F.U.W.: acquisition of data. F.F., T.K., and M.R.: statistical analysis. FF, F.U.W., M.S., T.K., G.H., H.V., G.L., U.V., A.F., M.R., C.B., A.A.A., J.M., and M.M.L.: data interpretation and manuscript revision. F.F. and M.M.L.: writing committee.
Financial support: This work was supported by the RESPONSE-project (BMBF grant number 03ZZ0921E), the EnErGie/P2 Project (ESF/14-BM-A55-0008/18), and the PePPP-project (ESF/14-BM-A55_0045/16). Study of Health in Pomerania is part of the Research Network Community Medicine of the University Medicine Greifswald, which is supported by the German Federal State of Mecklenburg-West Pomerania.
Potential competing interests: None to report.
Ethics approval: Local institutional review board, University Medicine Greifswald, Germany, registration number III UV 91/03b.
Study Highlights.
WHAT IS KNOWN
✓ CP severely affects patients' quality of life and survival.
✓ Exocrine pancreatic function is one of the most important host regulators of intestinal microbiome composition in adults.
WHAT IS NEW HERE
✓ CP is associated with a high degree of gut microbial dysbiosis, the degree of which is independent of exocrine pancreatic insufficiency.
✓ Abundance of epithelial-protective short-chain fatty acid and lactate producers is reduced in CP.
✓ Opportunistic pathogens, previously identified in infected pancreatic necrosis, such as Enterococcus are greatly increased.
TRANSLATIONAL IMPACT
✓ Gut microbiota changes in CP may, if and when pancreatitis recurs with an acute episode, facilitate the translocation of pathogens into necrotic collections or, when it remains chronic, permit the development of small intestinal bacterial overgrowth.
Supplementary Material
ACKNOWLEDGMENTS
We thank Anja Wiechert, Susanne Wiche, and Doris Jordan for technical assistance.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A380
REFERENCES
- 1.Bang UC, Benfield T, Hyldstrup L, et al. Mortality, cancer, and comorbidities associated with chronic pancreatitis: A Danish nationwide matched-cohort study. Gastroenterology 2014;146(4):989–94. [DOI] [PubMed] [Google Scholar]
- 2.Maléth J, Balázs A, Pallagi P, et al. Alcohol disrupts levels and function of the cystic fibrosis transmembrane conductance regulator to promote development of pancreatitis. Gastroenterology 2015;148(2):427–39.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmeister A, Mayerle J, Beglinger C, et al. English language version of the S3-consensus guidelines on chronic pancreatitis: Definition, aetiology, diagnostic examinations, medical, endoscopic and surgical management of chronic pancreatitis. Z Gastroenterol 2015;53(12):1447–95. [DOI] [PubMed] [Google Scholar]
- 4.Weidenbach H, Lerch MM, Gress TM, et al. Vasoactive mediators and the progression from oedematous to necrotising experimental acute pancreatitis. Gut 1995;37(3):434–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimosegawa T, Chari ST, Frulloni L, et al. International consensus diagnostic criteria for autoimmune pancreatitis: Guidelines of the International Association of Pancreatology. Pancreas 2011;40(3):352–8. [DOI] [PubMed] [Google Scholar]
- 6.Kereszturi E, Szmola R, Kukor Z, et al. Hereditary pancreatitis caused by mutation-induced misfolding of human cationic trypsinogen: A novel disease mechanism. Hum Mutat 2009;30(4):575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitcomb DC, LaRusch J, Krasinskas AM, et al. Common genetic variants in the CLDN2 and PRSS1-PRSS2 loci alter risk for alcohol-related and sporadic pancreatitis. Nat Genet 2012;44(12):1349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wartmann T, Mayerle J, Kähne T, et al. Cathepsin L inactivates human trypsinogen, whereas cathepsin L-deletion reduces the severity of pancreatitis in mice. Gastroenterology 2010;138(2):726–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lerch MM, Saluja AK, Dawra R, et al. The effect of chloroquine administration on two experimental models of acute pancreatitis. Gastroenterology 1993;104(6):1768–79. [DOI] [PubMed] [Google Scholar]
- 10.El Kurdi B, Babar S, El Iskandarani M, et al. Factors that affect prevalence of small intestinal bacterial overgrowth in chronic pancreatitis: A systematic review, meta-analysis, and meta-regression. Clin Transl Gastroenterol 2019;10(9):e00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frost F, Kacprowski T, Rühlemann M, et al. Impaired exocrine pancreatic function associates with changes in intestinal microbiota composition and diversity. Gastroenterology 2019;156(4):1010–5. [DOI] [PubMed] [Google Scholar]
- 12.Ahuja M, Schwartz DM, Tandon M, et al. Orai1-Mediated antimicrobial secretion from pancreatic acini shapes the gut microbiome and regulates gut innate immunity. Cell Metab 2017;25(3):635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitman WB, Coleman DC, Wiebe WJ. Prokaryotes: The unseen majority. Proc Natl Acad Sci USA 1998;95(12):6578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akshintala VS, Talukdar R, Singh VK, et al. The gut microbiome in pancreatic disease. Clin Gastroenterol Hepatol 2019;17(2):290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou CH, Meng YT, Xu JJ, et al. Altered diversity and composition of gut microbiota in Chinese patients with chronic pancreatitis. Pancreatology 2020;20(1):16–24. [DOI] [PubMed] [Google Scholar]
- 16.Threadgold J, Greenhalf W, Ellis I, et al. The N34S mutation of SPINK1 (PSTI) is associated with a familial pattern of idiopathic chronic pancreatitis but does not cause the disease. Gut 2002;50(5):675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Völzke H, Alte D, Schmidt CO, et al. Cohort profile: The study of health in Pomerania. Int J Epidemiol 2011;40(2):294–307. [DOI] [PubMed] [Google Scholar]
- 18.Frost F, Kacprowski T, Rühlemann MC, et al. Functional abdominal pain and discomfort (IBS) is not associated with faecal microbiota composition in the general population. Gut 2019;68(6):1131–3. [DOI] [PubMed] [Google Scholar]
- 19.Callahan BJ, McMurdie PJ, Rosen MJ, et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 2016;13(7):581–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2020. (https://www.R-project.org/). Accessed May 25, 2020. [Google Scholar]
- 21.Oksanen J, Blanchet FG, Friendly M, et al. vegan: Community Ecology Package: R Package Version 2.5-6. R Foundation for Statistical Computing: Vienna, Austria, 2019. (https://CRAN.R-project.org/package=vegan). Accessed May 25, 2020. [Google Scholar]
- 22.Ho DE, Imai K, King G, et al. MatchIt: Nonparametric preprocessing for parametric causal inference. J Stat Soft 2011;42(8). [Google Scholar]
- 23.Frost F, Storck LJ, Kacprowski T, et al. A structured weight loss program increases gut microbiota phylogenetic diversity and reduces levels of Collinsella in obese type 2 diabetics: A pilot study. PLoS One 2019;14(7):e0219489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Negm AA, Poos H, Kruck E, et al. Microbiologic analysis of peri-pancreatic fluid collected during EUS in patients with pancreatitis: Impact on antibiotic therapy. Gastrointest Endosc 2013;78(2):303–11. [DOI] [PubMed] [Google Scholar]
- 25.Rau B, Uhl W, Buchler MW, et al. Surgical treatment of infected necrosis. World J Surg 1997;21(2):155–61. [DOI] [PubMed] [Google Scholar]
- 26.Cleeland R, Squires E. Antimicrobial activity of ceftriaxone: A review. Am J Med 1984;77(4C):3–11. [PubMed] [Google Scholar]
- 27.Levine JM, D'Antonio CM. Elton revisited: A review of evidence linking diversity and invasibility. Oikos 1999;87(1):15. [Google Scholar]
- 28.Boland K, Turpin W, Mohammadi A, et al. Microbiome composition is altered in patients with IBD independent of endoscopic activity. Gastroenterology 2017;152(5):S991. [Google Scholar]
- 29.Manichanh C, Rigottier-Gois L, Bonnaud E, et al. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut 2006;55(2):205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang JY, Antonopoulos DA, Kalra A, et al. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis 2008;197(3):435–8. [DOI] [PubMed] [Google Scholar]
- 31.Archambaud C, Derré-Bobillot A, Lapaque N, et al. Intestinal translocation of enterococci requires a threshold level of enterococcal overgrowth in the lumen. Sci Rep 2019;9(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taur Y, Xavier JB, Lipuma L, et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 2012;55(7):905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xue J, Zhao Q, Sharma V, et al. Aryl hydrocarbon receptor ligands in cigarette smoke induce production of interleukin-22 to promote pancreatic fibrosis in models of chronic pancreatitis. Gastroenterology 2016;151(6):1206–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jandhyala SM, Madhulika A, Deepika G, et al. Altered intestinal microbiota in patients with chronic pancreatitis: Implications in diabetes and metabolic abnormalities. Sci Rep 2017;7:43640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polansky O, Sekelova Z, Faldynova M, et al. Important metabolic pathways and biological processes expressed by chicken cecal microbiota. Appl Environ Microbiol 2015;82(5):1569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol Rev 2001;81(3):1031–64. [DOI] [PubMed] [Google Scholar]
- 37.Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013;341(6145):569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takada T, Kurakawa T, Tsuji H, et al. Fusicatenibacter saccharivorans gen. nov., sp. nov., isolated from human faeces. Int J Syst Evol Microbiol 2013;63(Pt 10):3691–6. [DOI] [PubMed] [Google Scholar]
- 39.Ménard S, Candalh C, Bambou JC, et al. Lactic acid bacteria secrete metabolites retaining anti-inflammatory properties after intestinal transport. Gut 2004;53(6):821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoque R, Farooq A, Ghani A, et al. Lactate reduces liver and pancreatic injury in Toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology 2014;146(7):1763–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pushalkar S, Hundeyin M, Daley D, et al. The pancreatic cancer microbiome promotes oncogenesis by Induction of innate and adaptive immune suppression. Cancer Discov 2018;8(4):403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sethi V, Kurtom S, Tarique M, et al. Gut microbiota promotes tumor growth in mice by modulating immune response. Gastroenterology 2018;155(1):33–7.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.