INTRODUCTION:
Anti–tumor necrosis factor (TNF) therapy is effective in inducing remission in Crohn's disease in 60% of patients. No serological biomarkers are available, which can predict response to anti-TNF. We aimed to investigate serological markers of collagen turnover reflecting tissue inflammation as predictors of response to anti-TNF.
METHODS:
In 2 retrospective observational cohorts, markers for matrix metalloproteinase–degraded type III and IV collagens (C3M and C4M, respectively) and for formation of type III and IV collagens (PRO-C3 and PRO-C4, respectively) were measured in serum and compared with standard C-reactive protein in patients with active Crohn's disease who started infliximab (IFX, n = 21) or adalimumab (ADA, n = 21). Disease activity was classified by the Harvey-Bradshaw index (active disease ≥5); response was defined as clinical remission.
RESULTS:
Seventeen patients (81%) treated with IFX were in remission at week 14; 15 patients (71%) treated with ADA were in remission at week 8. Serum C4M at baseline was increased in nonresponders compared with responders (IFX: 35.0 ± 2.4 vs 23.2 ± 2.6, P = 0.04, ADA: 53.0 ± 3.2 vs 34.1 ± 2.8, P = 0.006). C4M levels at baseline predicted response in both cohorts (IFX: odds ratio 39 [95% confidence interval, 2.4–523.9] P = 0.02, cutoff 35.2 nmol/L; ADA: odds ratio 26 [95% confidence interval, 1.8–332.5], P = 0.01, cutoff 46.9 nmol/L). C-reactive protein was not able to predict response to anti-TNF.
DISCUSSION:
Response to anti-TNF therapy within the first 14 weeks of treatment can be predicted based on baseline levels of basement membrane marker C4M. This marker could be used as biomarker for response to anti-TNF and could aid in early therapy decision making. Validation in larger well-defined cohorts is needed.
INTRODUCTION
Anti–tumor necrosis factor (anti-TNF) agents such as infliximab (IFX) and adalimumab (ADA) are effective in inducing and maintaining clinical and endoscopic remission in luminal and fistulizing Crohn's disease (CD) (1–6). Up to 30% of patients with CD who start anti-TNF have no or insufficient response after 14 weeks of treatment, and there are no available biomarkers, which can predict primary nonresponse (7). As new medical therapeutic options such as anti–integrin α4β7 (vedolizumab) or anti–interleukin (IL)-12 and IL-23 (ustekinumab) have become available for patients with CD, the need for biomarkers that predict treatment response increases (8). Also, increasing evidence suggests that early ileocecal resection in nonstricturing CD could be considered a reasonable alternative to IFX therapy (9). Biomarkers that predict the response to anti-TNF may help in the decision to switch class of therapy or to recommend surgery (9).
C-reactive protein (CRP), a valuable biomarker of disease activity in CD, can discriminate between responders and nonresponders to anti-TNF, but conflicting results are reported (10–13). CRP, which is almost exclusively excreted by hepatocytes as part of the acute-phase response on stimulation by IL-1 and IL-6 and by TNF originating from the site of inflammation, is a relatively indirect inflammatory marker as it is not produced in the organ in which the inflammation takes place (10,14). Instead, biomarkers that are produced at the site of inflammation may be superior in predicting response to anti-TNF.
A disturbed balance in the remodeling of extracellular matrix (ECM) contributes to the pathophysiology of CD (15). Matrix metalloproteinases (MMPs) are collagenases, which are upregulated on inflammation in active CD (16). These enzymes are produced at the site of inflammation by inflammatory cells such as macrophages and T cells (15). As shown previously, levels of MMP-9–degraded type III collagen (C3M) are elevated in patients with CD with active inflammation (defined as CPR ≥5) compared to CD without active inflammation (defined as CRP <5) and to healthy controls (16,17). Type IV collagen is the most abundant collagen of the basement membrane. Epithelial damage on inflammation leads to an increase in permeability of the intestinal basement membrane, which is largely restored on IFX therapy (18,19). Markers of MMP-mediated degradation of type III (C3M) (20) and IV (C4M) collagens (21) might be superior in predicting response to anti-TNF compared with CRP.
Several studies reported increased mucosal and submucosal fibrosis in ileocecal resection specimens from patients with CD who required surgery following IFX treatment failure compared with patients who were IFX naive and underwent primary ileocecal resection (22,23). Increased messenger RNA (mRNA) expression of procollagen peptidases has been associated with primary IFX nonresponse (23). Therefore, formation products of type III (PRO-C3) (24) and type IV (PRO-C4) (25) collagens might also be suitable biomarkers for early detection of patients who do not respond to anti-TNF.
In this pilot study, we aimed to provide a proof of concept by showing that biomarkers of ECM turnover can predict response to anti-TNF within the first 8 to 14 weeks of anti-TNF treatment.
MATERIALS AND METHODS
Study design and population IFX (Groningen) and ADA (Aarhus) cohort
This retrospective observational pilot study was designed to compare serum levels of collagen formation and degradation markers between responders vs nonresponders to anti-TNF in 2 independent cohorts of patients with CD, starting IFX (Groningen, the Netherlands) and ADA (Aarhus, Denmark) remission induction therapy. Sera from patients with biopsy-confirmed CD who started IFX induction therapy between November 2009 and March 2016 (Remicade, Janssen Biologics B.V., Leiden, Holland; intravenous infusions of 5 mg/kg body weight) were collected from the database of the inflammatory bowel disease (IBD) center of the University Medical Center Groningen, the Netherlands (UMCG, single center, Table 1). Blood samples were collected before patients received the infusion at baseline and 2, 6, and 14 weeks after treatment was initiated. After retrieval, samples were centrifuged for 10 minutes (2000g at room temperature), pipetted in 0.5 mL cryovials, and stored at −80 °C until further analysis. Harvey-Bradshaw index (HBI) scores were collected from baseline and at week 14. Sera from patients with biopsy-confirmed CD who received ADA induction therapy between January 2009 and October 2012 (Humira; Abbott, Chicago, IL; subcutaneous injections of 160 mg at baseline, 80 mg at week 2, and then 40 mg every week or every other week) were collected at the Department of Hepatology and Gastroenterology at Aarhus University Hospital, Denmark (single center, Table 1) (26). Blood samples were collected before patients received the infusion at baseline and 1 and 8 weeks after treatment was initiated. After retrieval, samples were centrifuged for 10 minutes (300g at 20 °C), pipetted in 1.5 mL cryovials, and stored at −80 °C until further analysis. HBI scores were documented at baseline and at week 8.
Table 1.
Demographics separated by cohort
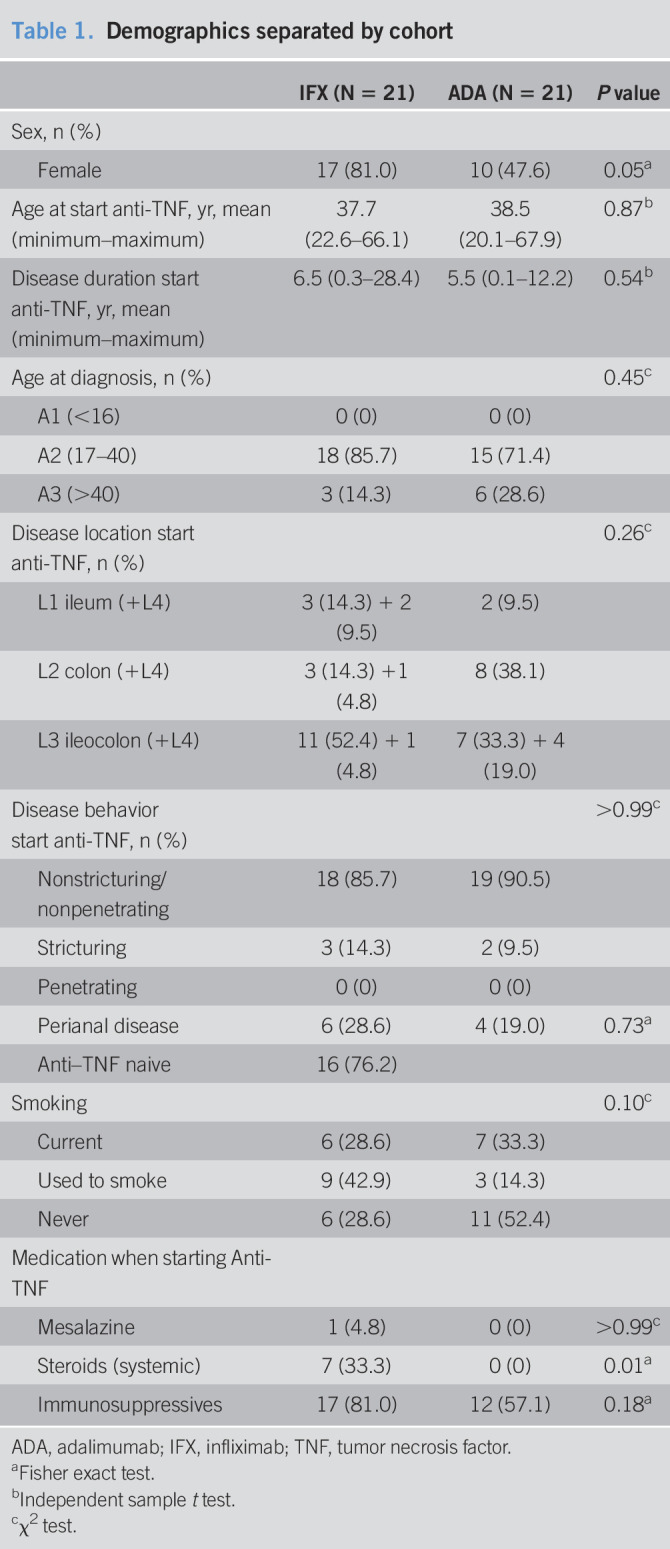
| IFX (N = 21) | ADA (N = 21) | P value | |
| Sex, n (%) | |||
| Female | 17 (81.0) | 10 (47.6) | 0.05a |
| Age at start anti-TNF, yr, mean (minimum–maximum) | 37.7 (22.6–66.1) | 38.5 (20.1–67.9) | 0.87b |
| Disease duration start anti-TNF, yr, mean (minimum–maximum) | 6.5 (0.3–28.4) | 5.5 (0.1–12.2) | 0.54b |
| Age at diagnosis, n (%) | 0.45c | ||
| A1 (<16) | 0 (0) | 0 (0) | |
| A2 (17–40) | 18 (85.7) | 15 (71.4) | |
| A3 (>40) | 3 (14.3) | 6 (28.6) | |
| Disease location start anti-TNF, n (%) | 0.26c | ||
| L1 ileum (+L4) | 3 (14.3) + 2 (9.5) | 2 (9.5) | |
| L2 colon (+L4) | 3 (14.3) +1 (4.8) | 8 (38.1) | |
| L3 ileocolon (+L4) | 11 (52.4) + 1 (4.8) | 7 (33.3) + 4 (19.0) | |
| Disease behavior start anti-TNF, n (%) | >0.99c | ||
| Nonstricturing/nonpenetrating | 18 (85.7) | 19 (90.5) | |
| Stricturing | 3 (14.3) | 2 (9.5) | |
| Penetrating | 0 (0) | 0 (0) | |
| Perianal disease | 6 (28.6) | 4 (19.0) | 0.73a |
| Anti–TNF naive | 16 (76.2) | ||
| Smoking | 0.10c | ||
| Current | 6 (28.6) | 7 (33.3) | |
| Used to smoke | 9 (42.9) | 3 (14.3) | |
| Never | 6 (28.6) | 11 (52.4) | |
| Medication when starting Anti-TNF | |||
| Mesalazine | 1 (4.8) | 0 (0) | >0.99c |
| Steroids (systemic) | 7 (33.3) | 0 (0) | 0.01a |
| Immunosuppressives | 17 (81.0) | 12 (57.1) | 0.18a |
ADA, adalimumab; IFX, infliximab; TNF, tumor necrosis factor.
Fisher exact test.
Independent sample t test.
χ2 test.
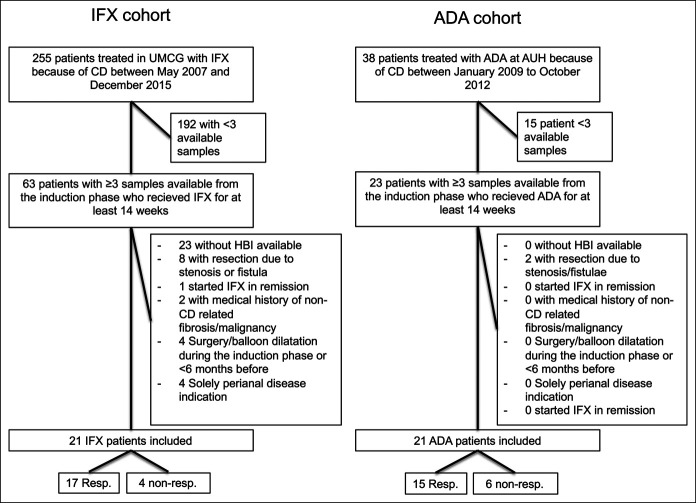
Inclusion criteria were patients with active disease (HBI ≥5) at baseline receiving either IFX or ADA for at least 14 weeks (27). Exclusion criteria were (i) no HBI available, (ii) resection due to intra-abdominal stenosis or fistulae, (iii) autoimmune and/or fibrotic diseases not (related to) CD, (iv) any kind of cancer, except skin cancer or hematologic cancer, (v) any kind of (also non–CD related) surgery or balloon dilatation within 6 months before a serum sample was taken or during the induction phase, and (vi) solely perianal disease indication for starting IFX (Figure 1). Disease activity was based on HBI (active disease: HBI ≥5). Clinical response was defined as an HBI <5 (remission) at week 8 (ADA cohort) or at week 14 (IFX cohort).
Figure 1.
Inclusion flowchart of patients in the IFX and ADA cohorts. ADA, adalimumab; IFX, infliximab.
Biomarker assays
Neoepitope fragments of ECM synthesis and degradation were assessed by solid-phase competitive enzyme-linked immunosorbent assays (ELISAs). The markers included in this study are markers for MMP-degraded type III and IV collagens (C3M and C4M, respectively) and formation of type III and IV collagens (PRO-C3 and PRO-C4, respectively).
Ninety-six-well plates precoated with streptavidin (Roche Diagnostic cat. No. 11940279, Hvidovre, Denmark) were coated with a biotinylated antigen against the biomarker for 30 minutes at room temperature. All samples were diluted in incubation buffer containing 1% bovine serum albumin (Sigma-Aldrich, cat. No. a-7906, ≥98 purity). Samples and controls were incubated in the antigen and streptavidin precoated plates with horseradish peroxidase–conjugated monoclonal antibodies for 1–3 hours at 4 °C/20 °C or for 20 hours at 4 °C with agitation at 300 rpm, according to the manufacturer's protocols. Subsequently, tetramethylbenzidine (Kem-En-Tec cat. No. 438OH, Taastrup, Denmark) was added (100 µL/well), and plates were incubated for 15 minutes at room temperature and agitated at 300 rpm. Stopping buffer (1% H2SO4) was added to stop the tetramethylbenzidine reaction. After each incubation step, wells were washed with washing buffer (25 mM TRIZMA, 50 mM NaCl, 0.036% Bronidox L5, 0.1% Tween 20) using a standardized ELISA plate washing machine (BioTek Instruments, Microplate washer, ELx405 Select CW, Winooski, VT). An ELISA reader (VersaMAX; Molecular Devices, Wokingham Berkshire, UK) was used to read optical densities at 450 and 650 nm. A standard curve was plotted using a 4-parametric mathematical fit model.
Statistical analysis
All data were considered nonparametric. Categorical data from the IFX vs the ADA cohort and from responders vs nonresponders were compared using a Pearson χ2 test or Fisher exact test where needed, whereas continuous data were compared using a Mann-Whitney U test (Tables 2 and 3). Differences between responders and nonresponders in CRP, marker levels, and ratios were compared using a Mann-Whitney U test with post hoc Bonferroni correction for multiple comparisons. Longitudinal differences between marker levels/ratios from baseline vs week x were compared using the Friedman test with the post hoc Wilcoxon signed-rank test with Bonferroni correction for multiple comparisons. Marker levels are presented as mean and SE of the mean. Spearman rank correlation coefficient was used to determine correlation between the markers/ratios and HBI/CRP (see Tables 1 and 2, Supplementary Digital Content 1, http://links.lww.com/CTG/A372). Receiver operating characteristic curves were calculated using MedCalc for Windows (MedCalc Software, Ostend, Belgium, version 14.8.1) for markers, which were different between responders and nonresponses. Optimal cutoff concentrations for each cohort were determined by MedCalc aiming at a combination of high sensitivity and specificity. Cutoff concentrations were used to determine odds ratios (ORs, with 95% confidence interval [95% CI]) and sensitivity/specificity using Fisher exact contingency analysis. GraphPad Prism version 6.0 (La Jolla, CA) was used to design figures and for Fisher exact contingency analysis. Analysis of patient characteristics and markers compared with CRP/HBI was performed using Statistical Package for Social Sciences 23.0 (SPSS, Chicago, IL). P values of <0.05 were considered significant.
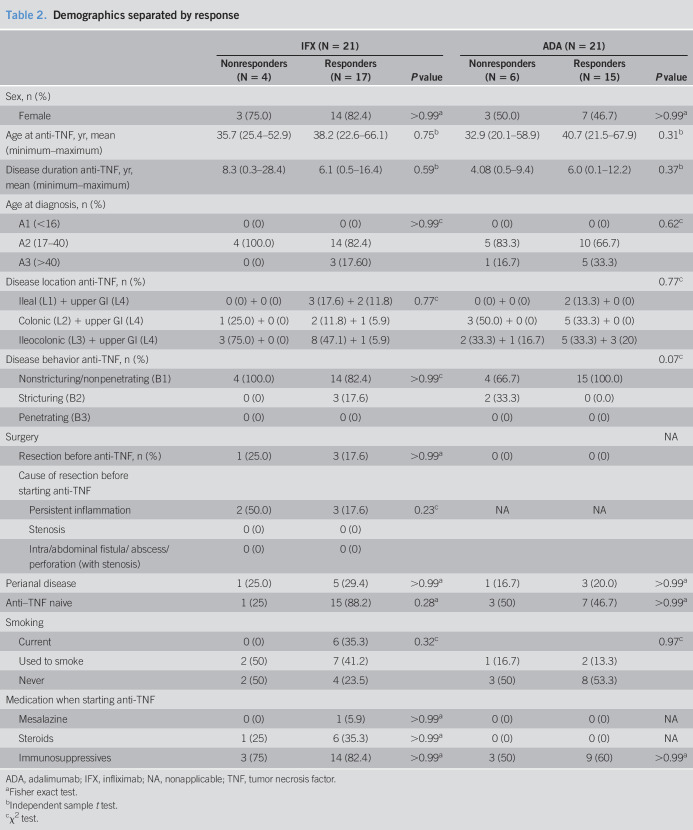
Table 2.
Demographics separated by response
| IFX (N = 21) | ADA (N = 21) | |||||
| Nonresponders (N = 4) | Responders (N = 17) | P value | Nonresponders (N = 6) | Responders (N = 15) | P value | |
| Sex, n (%) | ||||||
| Female | 3 (75.0) | 14 (82.4) | >0.99a | 3 (50.0) | 7 (46.7) | >0.99a |
| Age at anti-TNF, yr, mean (minimum–maximum) | 35.7 (25.4–52.9) | 38.2 (22.6–66.1) | 0.75b | 32.9 (20.1–58.9) | 40.7 (21.5–67.9) | 0.31b |
| Disease duration anti-TNF, yr, mean (minimum–maximum) | 8.3 (0.3–28.4) | 6.1 (0.5–16.4) | 0.59b | 4.08 (0.5–9.4) | 6.0 (0.1–12.2) | 0.37b |
| Age at diagnosis, n (%) | ||||||
| A1 (<16) | 0 (0) | 0 (0) | >0.99c | 0 (0) | 0 (0) | 0.62c |
| A2 (17–40) | 4 (100.0) | 14 (82.4) | 5 (83.3) | 10 (66.7) | ||
| A3 (>40) | 0 (0) | 3 (17.60) | 1 (16.7) | 5 (33.3) | ||
| Disease location anti-TNF, n (%) | 0.77c | |||||
| Ileal (L1) + upper GI (L4) | 0 (0) + 0 (0) | 3 (17.6) + 2 (11.8) | 0.77c | 0 (0) + 0 (0) | 2 (13.3) + 0 (0) | |
| Colonic (L2) + upper GI (L4) | 1 (25.0) + 0 (0) | 2 (11.8) + 1 (5.9) | 3 (50.0) + 0 (0) | 5 (33.3) + 0 (0) | ||
| Ileocolonic (L3) + upper GI (L4) | 3 (75.0) + 0 (0) | 8 (47.1) + 1 (5.9) | 2 (33.3) + 1 (16.7) | 5 (33.3) + 3 (20) | ||
| Disease behavior anti-TNF, n (%) | 0.07c | |||||
| Nonstricturing/nonpenetrating (B1) | 4 (100.0) | 14 (82.4) | >0.99c | 4 (66.7) | 15 (100.0) | |
| Stricturing (B2) | 0 (0) | 3 (17.6) | 2 (33.3) | 0 (0.0) | ||
| Penetrating (B3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Surgery | NA | |||||
| Resection before anti-TNF, n (%) | 1 (25.0) | 3 (17.6) | >0.99a | 0 (0) | 0 (0) | |
| Cause of resection before starting anti-TNF | ||||||
| Persistent inflammation | 2 (50.0) | 3 (17.6) | 0.23c | NA | NA | |
| Stenosis | 0 (0) | 0 (0) | ||||
| Intra/abdominal fistula/ abscess/perforation (with stenosis) | 0 (0) | 0 (0) | ||||
| Perianal disease | 1 (25.0) | 5 (29.4) | >0.99a | 1 (16.7) | 3 (20.0) | >0.99a |
| Anti–TNF naive | 1 (25) | 15 (88.2) | 0.28a | 3 (50) | 7 (46.7) | >0.99a |
| Smoking | ||||||
| Current | 0 (0) | 6 (35.3) | 0.32c | 0.97c | ||
| Used to smoke | 2 (50) | 7 (41.2) | 1 (16.7) | 2 (13.3) | ||
| Never | 2 (50) | 4 (23.5) | 3 (50) | 8 (53.3) | ||
| Medication when starting anti-TNF | ||||||
| Mesalazine | 0 (0) | 1 (5.9) | >0.99a | 0 (0) | 0 (0) | NA |
| Steroids | 1 (25) | 6 (35.3) | >0.99a | 0 (0) | 0 (0) | NA |
| Immunosuppressives | 3 (75) | 14 (82.4) | >0.99a | 3 (50) | 9 (60) | >0.99a |
ADA, adalimumab; IFX, infliximab; NA, nonapplicable; TNF, tumor necrosis factor.
Fisher exact test.
Independent sample t test.
χ2 test.
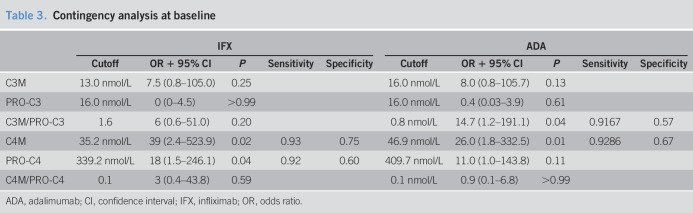
Table 3.
Contingency analysis at baseline
| IFX | ADA | |||||||||
| Cutoff | OR + 95% CI | P | Sensitivity | Specificity | Cutoff | OR + 95% CI | P | Sensitivity | Specificity | |
| C3M | 13.0 nmol/L | 7.5 (0.8–105.0) | 0.25 | 16.0 nmol/L | 8.0 (0.8–105.7) | 0.13 | ||||
| PRO-C3 | 16.0 nmol/L | 0 (0–4.5) | >0.99 | 16.0 nmol/L | 0.4 (0.03–3.9) | 0.61 | ||||
| C3M/PRO-C3 | 1.6 | 6 (0.6–51.0) | 0.20 | 0.8 nmol/L | 14.7 (1.2–191.1) | 0.04 | 0.9167 | 0.57 | ||
| C4M | 35.2 nmol/L | 39 (2.4–523.9) | 0.02 | 0.93 | 0.75 | 46.9 nmol/L | 26.0 (1.8–332.5) | 0.01 | 0.9286 | 0.67 |
| PRO-C4 | 339.2 nmol/L | 18 (1.5–246.1) | 0.04 | 0.92 | 0.60 | 409.7 nmol/L | 11.0 (1.0–143.8) | 0.11 | ||
| C4M/PRO-C4 | 0.1 | 3 (0.4–43.8) | 0.59 | 0.1 nmol/L | 0.9 (0.1–6.8) | >0.99 | ||||
ADA, adalimumab; CI, confidence interval; IFX, infliximab; OR, odds ratio.
Ethical considerations
All patients from the Aarhus cohort provided written informed consent, and the study protocol was approved by the Central Denmark Region Committees on Biomedical Research Ethics {journal no. 20080092, journal no. 20060197 [registered at ClinicalTrials.gov (NCT00955123)], and journal no. 20040150}. All patients from the Groningen cohort gave written informed consent for the use of patient data and serum (approved by the Institutional Review Board UMCG, IRB no. 08/279) from the UMCG IBD database and biobank.
RESULTS
Cohort characteristics
Twenty-one patients were included in the IFX cohort, and 21 patients were included in the ADA cohort. At baseline, patients in the IFX cohort used significantly more systemic steroids compared with the patients in the ADA cohort (Table 1). A trend for more female patients and for patients with ileal disease in the IFX cohort in contrast to more male patients and patients with colonic disease in the ADA cohort was observed (Table 1). Baseline biomarker levels were equal between IFX and ADA for CRP, C3M/PRO-C3, and C4M/PRO-C4. Baseline levels of PRO-C3 (11.4 ± 1.1 vs 16.1 ± 0.8, P = 0.001), C3M (12.2 ± 1.1 vs 17.4 ± 2.2, P = 0.04), PRO-C4 (274.1 ± 36.3 vs 510.5 ± 99.6, P = 0.04), and C4M (25.8 ± 2.4 vs 38.8 ± 2.9, P = 0.001) were higher in the ADA cohort compared with the IFX cohort. Seventeen patients (81%) responded in the IFX cohort, and 15 patients (71%) responded in the ADA cohort (see Tables 1 and 2, Supplementary Digital Content 1, http://links.lww.com/CTG/A372). No differences in baseline characteristics were observed between responders and nonresponders in the IFX or the ADA cohort. Especially, steroid use was not different between responders and nonresponders in the IFX cohort or the ADA cohort.
Degradation fragments of type III and type IV collagens are elevated in anti-TNF nonresponders
Increased C4M and PRO-C4 concentrations at baseline were observed in the IFX and in the ADA cohort in nonresponders compared with responders. Type IV collagen formation marker C4M was increased in nonresponders in both cohorts compared with responders (IFX: 35.0 ± 2.4 vs 23.2 ± 2.6, P = 0.04, ADA: 53.0 ± 3.2 vs 34.1 ± 2.8, P = 0.006). Baseline levels of the type IV collagen formation marker PRO-C4 were also increased in nonresponders to anti-TNF (IFX: 465.8 ± 90.5 vs 219.4 ± 25.3, P = 0.011, ADA: 867.4 ± 324.2 vs 391.5 ± 62.3, P = 0.05) compared with responders. Baseline C3M/PRO-C3 ratios were not elevated in nonresponders at baseline in the IFX cohort (1.9 ± 0.8 vs 1.1 ± 0.1, P = 0.26), whereas they were increased in nonresponders in the ADA cohort (1.7 ± 0.5 vs 0.9 ± 0.1, P = 0.013). CRP levels were equal at baseline in both cohorts.
Anti-TNF therapy differently alters the turnover of type III and IV collagens in responders vs nonresponders
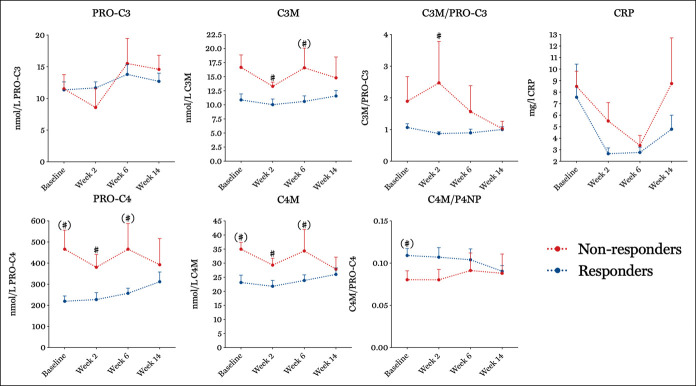
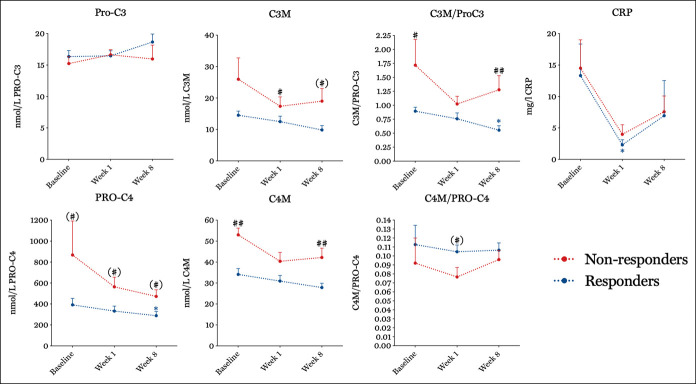
After initiation of therapy, levels of PRO-C4 remained increased in nonresponders to anti-TNF in both the IFX (466.0 ± 121.5 vs 257.1 ± 24.2, P = 0.05 [Figure 2]) and the ADA cohort at weeks 6 and 8, respectively (472.6 ± 63.2 vs 288.3 ± 39.7, P = 0.03 [Figure 3]) compared with responders. C4M levels were increased in nonresponders at week 2 in the IFX cohort (29.3 ± 2.5 vs 21.8 ± 2.1, P = 0.01) and at week 8 in the ADA cohort (42.2 ± 4.4 vs 27.9 ± 2.1, P = 0.01). In line with this, C3M levels remained increased in nonresponders in the IFX (16.6 ± 3.5 vs 10.6 ± 1.0, P = 0.01) and ADA (19.0 ± 4.1 vs 9.9 ± 1.4, P = 0.03) cohort at weeks 6 and 8, respectively.
Figure 2.
Serum biomarker levels and ratios responders (blue) vs nonresponders (red) to anti-TNF in the IFX cohort. Asterisks indicate the level of significance, *P < 0.05 at different time points compared with the baseline. Hashtags (#) indicate the level of significance #P < 0.05, ##P < 0.01 between responders and nonresponders at a given time point. Parentheses with a hashtag (#) indicate non-Bonferroni-corrected significant differences between responders and nonresponders at a given time point. Marker levels are presented as mean and SE of the mean. IFX, infliximab; TNF, tumor necrosis factor.
Figure 3.
Serum biomarker levels and ratios responders (blue) vs nonresponders (red) to anti-TNF in the ADA cohort. Asterisks indicate the level of significance, *P < 0.05 at different time points compared with the baseline. Hashtags (#) indicate the level of significance #P < 0.05, ##P < 0.01 between responders and nonresponders at a given time point. Parentheses with a hashtag (#) indicate non-Bonferroni-corrected significant differences between responders and nonresponders at a given time point. Marker levels are presented as mean and SE of the mean. ADA, adalimumab; TNF, tumor necrosis factor.
Type IV collagen degradation levels at baseline predict response to anti-TNF
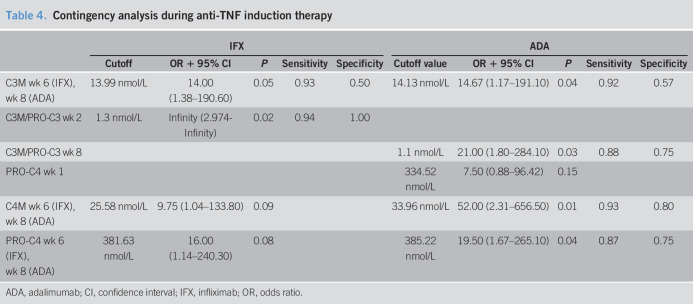
In both cohorts, degradation marker C4M at baseline was able to predict response to anti-TNF (IFX: C4M cutoff concentration: 35.2 nmol/L, OR 39 [95% CI, 2.41–523.90], P = 0.02, sensitivity: 0.93, specificity: 0.75; ADA: C4M cutoff concentration: 46.9 nmol/L, OR 26 [95% CI, 1.8–332.5], P = 0.01, sensitivity: 0.93, specificity: 0.67, Table 3). In contrast, CRP levels in neither the IFX nor the ADA cohort were able to predict response. Although not significant in both cohorts, similar trends were observed in both cohorts for the type IV collagen formation marker at baseline (IFX: PRO-C4 cutoff concentration: 339.2 nmol/L, OR 18 [95% CI, 1.53–246.1], P = 0.04, sensitivity: 0.92, specificity: 0.60; ADA: PRO-C4 cutoff concentration: 409.7 nmol/L, OR 11.0 [95% CI, 0.98–143.80], P = 0.11, Table 3). During the induction phase, C3M levels at weeks 6–8 were able to predict response to anti-TNF in both cohorts (IFX C3M cutoff value: 14.0 nmol/L, OR: 14.00 [95% CI, 1.38–190.60], P = 0.05, sensitivity: 0.93, specificity: 0.50; ADA C3M cutoff concentration: 14.1 nmol/L, OR: 14.7 [95% CI, 1.2–191.1], P = 0.04, sensitivity: 0.92, specificity: 0.57, Table 4).
Table 4.
Contingency analysis during anti-TNF induction therapy
| IFX | ADA | |||||||||
| Cutoff | OR + 95% CI | P | Sensitivity | Specificity | Cutoff value | OR + 95% CI | P | Sensitivity | Specificity | |
| C3M wk 6 (IFX), wk 8 (ADA) | 13.99 nmol/L | 14.00 (1.38–190.60) | 0.05 | 0.93 | 0.50 | 14.13 nmol/L | 14.67 (1.17–191.10) | 0.04 | 0.92 | 0.57 |
| C3M/PRO-C3 wk 2 | 1.3 nmol/L | Infinity (2.974-Infinity) | 0.02 | 0.94 | 1.00 | |||||
| C3M/PRO-C3 wk 8 | 1.1 nmol/L | 21.00 (1.80–284.10) | 0.03 | 0.88 | 0.75 | |||||
| PRO-C4 wk 1 | 334.52 nmol/L | 7.50 (0.88–96.42) | 0.15 | |||||||
| C4M wk 6 (IFX), wk 8 (ADA) | 25.58 nmol/L | 9.75 (1.04–133.80) | 0.09 | 33.96 nmol/L | 52.00 (2.31–656.50) | 0.01 | 0.93 | 0.80 | ||
| PRO-C4 wk 6 (IFX), wk 8 (ADA) | 381.63 nmol/L | 16.00 (1.14–240.30) | 0.08 | 385.22 nmol/L | 19.50 (1.67–265.10) | 0.04 | 0.87 | 0.75 | ||
ADA, adalimumab; CI, confidence interval; IFX, infliximab; OR, odds ratio.
Markers correlate with CRP but not with HBI
Both the IFX and in the ADA cohort, no correlation was found between the HBI vs markers, their ratios, and CRP (see Tables 1 and 2, Supplementary Digital Content 1, http://links.lww.com/CTG/A372). Negative correlation was found between CRP and PRO-C3 in the IFX cohort at week 14 (r: −0.60, P = 0.01) and in the ADA cohort at week 8 (r: −0.52, P = 0.03). Furthermore, (nonsignificant) correlation was found in between CRP and PRO-C4 in both cohorts (IFX: r: −0.79, P < 0.001; ADA: r: −0.054, P = 0.8).
DISCUSSION
In the present pilot study, we show that serological biomarkers for degradation of type IV collagen at baseline could identify patients with active CD who will respond to anti-TNF therapy. MMP-2–, MMP-9–, and MMP-12–degraded type IV collagen (C4M) levels measured at baseline could predict response to anti-TNF in both IFX- and ADA-treated patients. Furthermore, MMP-9–degraded C3M levels measured during the induction phase (weeks 6–8) could predict response to anti-TNF. These results were be reproduced in 2 independent cohorts of 2 different ant-TNF treatments in which patient characteristics and response based on reproducible HBI scores were well defined (28). Because of small sample sizes and differences between the cohorts, predictive values are based on different cutoff values within each cohort and therefore need further validation.
None of the markers that are currently used in clinical practice to monitor inflammation are direct derivates of tissue inflammation. CRP is the most commonly used inflammatory marker; however, CRP is produced and secreted by hepatocytes during inflammation on stimulation by IL-1 and IL-6 and by TNF originating from the site of inflammation (14). In contrast, serological markers for posttranslational modification of the ECM (both formation and degradation) are produced and released by the disease-affected tissue directly (29). As shown in this study, biomarkers that reflect posttranslational modifications of the ECM can be used to predict response to anti-TNF. Since CRP has been extensively validated as a biomarker of active inflammation for patients with CD, the combination of CRP and serological markers for formation and degradation of type IV collagen might be optimal for clinical use and in prediction of response to anti-TNF in patients with CD.
Serological markers that indicate collagen degradation are produced by MMP-mediated cleavage of collagens at the site of inflammation. The antibodies used in these assays recognize the MMP-cleaved neoepitope specifically. For C4M, the neoepitope is cleaved off by MMP-2, MMP-9, and MMP-12. For C3M, the neoepitope is cleaved off by MMP-9. MMP-9 protein activity is upregulated in inflamed IBD mucosa compared with noninflamed IBD and control mucosa (16,30). Mucosal MMP expression in response to IFX was previously investigated by Di Sabatino et al. They showed that mucosal MMP-3, MMP-12 mRNA, and protein expression decreased after treatment in patients with CD who responded to IFX. Furthermore, they showed that no change in MMP expression was found in nonresponders and that downregulation of MMP-3 and MMP-12 correlates with improvement of the histology score (31). Another study showed that serum levels of MMP-9 decrease on IFX induction treatment in patients with active luminal CD or fistulizing CD and observed a trend for lower MMP-9 levels at weeks 6 and 10 in responders to IFX (32). These data are in line with the decreasing concentrations of MMP-cleaved degradation products of collagen III and IV measured in responders in this study. De Bruyn et al. (33,34) showed that MMP-9 is a surrogate marker to assess mucosal healing in CD. These results are in line with the decreased C3M levels (a surrogate marker for MMP-9 activity) in responders in the IFX and ADA cohort in our study.
Our results show that responders could be differentiated from nonresponders, based on levels of degradation markers of type IV collagen (C4M), already at baseline, before anti-TNF therapy was administered. Baseline PRO-C4 concentrations were different between responders and nonresponders in both cohorts, but the prediction model was only statistically significant in the IFX cohort. Nonfibrillar type IV collagen is, as everywhere in the body, the main component of the basement membrane forming the barrier between the epithelium on the intestinal luminal side and the lamina propria of the intestine (35). Serum concentrations of collagen IV are decreased in patients with IBD as previously reported by Koutroubakis et al. (36) They suggest altered type IV collagen metabolism in patients with IBD, but do not clarify whether a decrease in circulating type IV collagen corresponds to a decrease in serum formation or degradation products of type IV collagen. Our results show increased serum levels of formation and degradation products of type IV collagen in nonresponders compared with responders, indicating increased turnover of type IV collagen in nonresponders. Increased turnover of type IV collagen might reflect a decrease in the amount of type IV collagen that is deposited in the basement membrane of the affected intestine and may indicate a more severe disease phenotype in which the mucosa is severely affected. Intestinal barrier function is impaired in patients with CD compared with healthy controls (37). An important role for TNF in intestinal homeostasis, barrier function, and pathogenesis has been suggested (38). The role of anti-TNF was confirmed in studies that showed that intestinal permeability normalizes following successful anti-TNF (IFX) therapy in patients with active CD (39). Our data indicate that an increase in type IV collagen turnover in nonresponders (and therefore a decrease in net deposited type IV collagen) is associated with nonresponse to anti-TNF, perhaps due to a decrease in deposited type IV collagen leading to impaired intestinal barrier function (36). Nonresponse to anti-TNF might be explained by the inability of the intestine to restore the intestinal basement membrane, which predominantly consists of type IV collagen, and thereby the inability to restore its barrier.
As C4M formation and degradation markers are not drug specific but might reflect the state of the intestinal barrier (and whether an anti-inflammatory drug is able to restore this), these markers may be suitable to monitor response to other biologicals (within the anti-TNF class or to another biological (e.g., anti-IL12/23, JAK/STAT inhibitors, anti-α4β7, sphingosine-1-phosphate inhibitors, and anti–MAdCAM-1) in patients with CD or Ulcerative colitis (UC) as well. Further studies might show that patients with high C4M at baseline could better be treated with, e.g., anti-IL12/23 compared with anti-TNF or that patients having a C4M serum level above a certain threshold are predicted to not respond to biologicals and are better offered an ileocecal resection.
Our results furthermore show that response to anti-TNF can be predicted based on serum levels of MMP-9–degraded C3M at weeks 6–8 after the start of therapy. Increased C3M concentrations in nonresponders to anti-TNF indicate less suppression of MMP-9 activity on induction therapy and thereby less suppression of tissue inflammation compared with responders. This is in line with C4M serum concentrations measured in responders vs nonresponders. These results (C3M and C4M) further confirm that tissue inflammation (i.e., MMP activity) is reduced on anti-TNF therapy and that the degree of reduction of tissue inflammation is indicative of response to anti-TNF. Based on serum concentrations of biomarkers for interstitial C3M determined during the anti-TNF induction phase, one cannot conclude whether anti-TNF is pro- or anti-fibrotic on the long term. Measuring markers reflecting formation and degradation of interstitial collagen (type I and III collagens) in a cohort of patients with CD in remission might be suitable to answering this question. Ideally, concentrations of formation and degradation markers of interstitial collagens would be correlated (and thereby validated) to quantification of fibrosis by imaging (by ultrasound, computed tomography enterography, or magnetic resonance enterography). Unfortunately, intestinal fibrosis cannot be adequately quantified by imaging so far (40).
Furthermore, the predictive value of the delta between baseline biomarker levels and biomarker levels measured during the induction phase was inferior to the predictive value of the absolute biomarker concentrations. Therefore, ORs calculated from absolute biomarker concentrations are shown.
A major limitation of this study is its sample size. Although the results observed in the IFX cohort could be reproduced in a comparable ADA cohort, these cohorts would ideally have been larger. Patients with different disease location (ileal, colonic, or ileocolonic) and disease behavior (nonstricturing/nonpenetrating [majority], stricturing [9.5%–14.3%]) were combined in this pilot study, causing variation. We have previously observed differences in serological biomarkers of ECM formation and degradation between nonstricturing/nonpenetrating, stricturing, and penetrating disease in patients with solely ileal disease (16). The surface area of the inflammation-affected region presumably correlates with biomarker levels (especially levels of the by MMP-activity formed neoepitopes). Ideally, one would validate the predicting value of these biomarkers in response to anti-TNF for each of the phenotypes within the Montreal classification (behavior and location) in a larger cohort. Because the sample sizes of both cohorts were small, high variation in marker levels between the 2 cohorts was observed. Differences in baseline biomarker concentrations between the cohorts lead to differences in cutoff concentrations (used to determine sensitivity and specificity) between the 2 cohorts, as these were calculated per cohort per marker. This variation might be explained by the difference in steroid use at baseline between the 2 cohorts. Furthermore, this study focused on the 2 for CD most widely used anti-TNF therapies. These results should be separately validated for other anti-TNF therapies (certolizumab, etanercept, and golimumab) used for IBD and other (rheumatic) diseases. The generalizability of this study remains therefore to be validated. Also, correlation to endoscopic mucosal healing, fecal calprotectin, imaging, histology of the basement membrane in biopsies from endoscopy, and response profiles for each of the disease phenotypes (inflammatory, stricturing, and penetrating CD) are needed before these biomarkers can be used in clinical practice. This pilot study intended to be a proof of concept showing that biomarkers of ECM turnover can predict response to anti-TNF and was not intended to define final reference concentrations.
Furthermore, in this retrospective cohort study, endoscopic data were not available because colonoscopy was not routinely performed after remission induction with IFX/ADA. Only from a few patients, fecal calprotectin levels were available after remission induction with anti-TNF. In prospective studies validating these markers as markers for mucosal healing, correlation to the simple endoscopic score for CD and fecal calprotectin is needed. It is known that the simple endoscopic score–CD correlates closest with fecal calprotectin, followed by CRP, blood leukocytes, and the Crohn's Disease Activity Index (CDAI) (41). These data were, however, not available in this pilot study.
Next to this, this study included patients who received ADA or IFX for at least 14 weeks. Hereby, we did not include primary nonresponders who ended treatment before week 14. Ideally, this subgroup would be included, and a biomarker would be valid to discriminate between all subgroups, namely primary nonresponders, nonresponders at weeks 8–14, and responders.
Furthermore, this study lacks the correlation and correction of biomarker levels and IFX/ADA drug concentrations in plasma. This might explain part of the observed variation. Determining trough levels and antibodies against IFX/ADA was not considered necessary in the patients included in this study within the first 14 weeks of anti-TNF treatment as patients had sufficient clinical response. The probability that patients developed antibodies to IFX within the first 14 weeks is small (42). However, we cannot exclude that responders had higher drug levels compared with nonresponders.
In summary, we are the first to show that clinical response to anti-TNF for patients with active CD can be predicted at baseline based on a serological marker for MMP-degraded type IV collagen (C4M) at baseline and based on MMP-9–degraded C3M measured during the induction phase (weeks 6–8). The results of this study are promising but need validation.
CONFLICTS OF INTEREST
Guarantor of the article: Gerard Dijkstra, MD, PhD.
Specific author contributions: Wouter T. van Haaften, MD, PhD, Joachim H. Mortensen, PhD, MSc, Tina Manon-Jensen, PhD, and Gerard Dijkstra, MD, PhD, contributed equally to this work. W.T.v.H. and J.H.M.: concept and design of the study, acquisition of data, analysis and interpretation of data, and drafting the article and revising it critically for important intellectual content. G.D., A.K.D., H.G., and C.L.H.: collecting patient samples. A.K.D., H.G., C.L.H., A.-C.B.J, M.A.K., P.O., T.M.-J., and G.D.: concept and design of the study, interpretation of data and revising critically for important intellectual content. All authors approved to the final graft before the manuscript was submitted.
Financial support: None to report.
Potential competing interests: W.T.v.H. has received funding to print his thesis from Ferring B.V., Teva B.V., Tramedico B.V., and Mylan B.V. G.D. reports, outside the submitted work, grants from Takeda and AbbVie, fees for advisory boards from Cosmo Pharma and Mundipharma, and speaker fees from Pfizer, Janssen Pharmaceutical, and Takeda. The work was supported by a ZonMW grant, number 114021010, obtained by P.O. for animal free research techniques and by the Danish Research Foundation. J.H.M., M.A.K., and A.-C.B.J. are full-time employees at Nordic Bioscience. M.A.K., A.-C.B.J., and T.M.-J. hold stocks in Nordic Bioscience. Nordic Bioscience is a privately owned medium-sized enterprise, partly focused on the development of biomarkers for connective tissue disorders and rheumatic diseases. None of the authors received any kind of financial benefits or other bonuses for the work described in this manuscript.
Study Highlights.
WHAT IS KNOWN
✓ Anti-TNF is effective in inducing remission in CD in 60% of patients.
✓ No serological biomarkers are available, which can predict early response to anti-TNF.
WHAT IS NEW HERE
✓ Response to anti-TNF therapy within the first 14 weeks of treatment can be predicted based on baseline levels of basement membrane marker C4M.
✓ Further validation is needed.
TRANSLATIONAL IMPACT
✓ These markers could be used as biomarkers for response to anti-TNF and could aid in early therapy decision making.
Supplementary Material
ACKNOWLEDGEMENT
The UMCG thanks the Parelsnoer Institute for providing the Biobank Infrastructure to contribute to this study.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A372
REFERENCES
- 1.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn's disease: The ACCENT I randomised trial. Lancet 2002;359(9317):1541–9. [DOI] [PubMed] [Google Scholar]
- 2.Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med 1999;340(18):1398–405. [DOI] [PubMed] [Google Scholar]
- 3.Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med 2004;350(9):876–85. [DOI] [PubMed] [Google Scholar]
- 4.Bouhnik Y, Carbonnel F, Laharie D, et al. Efficacy of adalimumab in patients with Crohn's disease and symptomatic small bowel stricture: A multicentre, prospective, observational cohort (CREOLE) study. Gut 2017;67(1):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: The CHARM trial. Gastroenterology 2007;132(1):52–65. [DOI] [PubMed] [Google Scholar]
- 6.Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: The CLASSIC-I trial. Gastroenterology 2006;130(2):323–33. [DOI] [PubMed] [Google Scholar]
- 7.Ding NS, Hart A, De Cruz P. Systematic review: Predicting and optimising response to anti-TNF therapy in Crohn's disease—Algorithm for practical management. Aliment Pharmacol Ther 2016;43(1):30–51. [DOI] [PubMed] [Google Scholar]
- 8.Gomollón F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn's disease 2016: Part 1: Diagnosis and medical management. J Crohns Colitis 2017;11(1):3–25. [DOI] [PubMed] [Google Scholar]
- 9.Ponsioen CY, de Groof EJ, Eshuis EJ, et al. Laparoscopic ileocaecal resection versus infliximab for terminal ileitis in Crohn's disease: A randomised controlled, open-label, multicentre trial. Lancet Gastroenterol Hepatol 2017;2(11):785–92. [DOI] [PubMed] [Google Scholar]
- 10.Vermeire S, Van Assche G, Rutgeerts P. C-reactive protein as a marker for inflammatory bowel disease. Inflamm Bowel Dis 2004;10(5):661–5. [DOI] [PubMed] [Google Scholar]
- 11.Reinisch W, Wang Y, Oddens BJ, et al. C-reactive protein, an indicator for maintained response or remission to infliximab in patients with Crohn's disease: A post-hoc analysis from ACCENT I. Aliment Pharmacol Ther 2012;35(5):568–76. [DOI] [PubMed] [Google Scholar]
- 12.Magro F, Rodrigues-Pinto E, Santos-Antunes J, et al. High C-reactive protein in Crohn's disease patients predicts nonresponse to infliximab treatment. J Crohns Colitis 2014;8(2):129–36. [DOI] [PubMed] [Google Scholar]
- 13.Jürgens M, Mahachie John JM, Cleynen I, et al. Levels of C-reactive protein are associated with response to infliximab therapy in patients with Crohn's disease. Clin Gastroenterol Hepatol 2011;9(5):421–7.e1. [DOI] [PubMed] [Google Scholar]
- 14.Nijsten MW, Olinga P, The TH, et al. Procalcitonin behaves as a fast responding acute phase protein in vivo and in vitro. Crit Care Med 2000;28(2):458–61. [DOI] [PubMed] [Google Scholar]
- 15.Shimshoni E, Yablecovitch D, Baram L, et al. ECM remodelling in IBD: Innocent bystander or partner in crime? The emerging role of extracellular molecular events in sustaining intestinal inflammation. Gut 2014;64:367–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Haaften WT, Mortensen JH, Karsdal MA, et al. Misbalance in type III collagen formation/degradation as a novel serological biomarker for penetrating (Montreal B3) Crohn's disease. Aliment Pharmacol Ther 2017;46(1):26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mortensen JH, Godskesen LE, Jensen MD, et al. Fragments of citrullinated and MMP-degraded vimentin and MMP-degraded type III collagen are novel serological biomarkers to differentiate Crohn's disease from ulcerative colitis. J Crohns Colitis 2015;9(10):863–72. [DOI] [PubMed] [Google Scholar]
- 18.Sand JM, Larsen L, Hogaboam C, et al. MMP mediated degradation of type IV collagen alpha 1 and alpha 3 chains reflects basement membrane remodeling in experimental and clinical fibrosis: Validation of two novel biomarker assays. PLoS One 2013;8(12):e84934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karsdal MA, Nielsen SH, Leeming DJ, et al. The good and the bad collagens of fibrosis–their role in signaling and organ function. Adv Drug Deliv Rev 2017;121:43–56. [DOI] [PubMed] [Google Scholar]
- 20.Barascuk N, Veidal SS, Larsen L, et al. A novel assay for extracellular matrix remodeling associated with liver fibrosis: An enzyme-linked immunosorbent assay (ELISA) for a MMP-9 proteolytically revealed neo-epitope of type III collagen. Clin Biochem 2010;43(10–11):899–904. [DOI] [PubMed] [Google Scholar]
- 21.Bay-Jensen AC, Platt A, Byrjalsen I, et al. Effect of tocilizumab combined with methotrexate on circulating biomarkers of synovium, cartilage, and bone in the LITHE study. Semin Arthritis Rheum 2014;43(4):470–8. [DOI] [PubMed] [Google Scholar]
- 22.Schaeffer DF, Walsh JC, Kirsch R, et al. Distinctive histopathologic phenotype in resection specimens from patients with Crohn's disease receiving anti-TNF-α therapy. Hum Pathol 2014;45(9):1928–35. [DOI] [PubMed] [Google Scholar]
- 23.De Bruyn JR, Becker MA, Steenkamer J, et al. Intestinal fibrosis is associated with lack of response to infliximab therapy in Crohn's disease. PLoS One 2018;13(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielsen MJ, Nedergaard AF, Sun S, et al. The neo-epitope specific PRO-C3 ELISA measures true formation of type III collagen associated with liver and muscle parameters. Am J Transl Res 2013;5(3):303–15. [PMC free article] [PubMed] [Google Scholar]
- 25.Leeming DJ, Karsdal MA, Rasmussen LM, et al. Association of systemic collagen type IV formation with survival among patients undergoing hemodialysis. PLoS One 2013;8(8):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dige A, Støy S, Thomsen KL, et al. Soluble CD163, a specific macrophage activation marker, is decreased by anti-TNF-alfa antibody treatment in active inflammatory bowel disease. Scand J Immunol 2014;80(6):417–23. [DOI] [PubMed] [Google Scholar]
- 27.Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet 1980;1(8167):514. [DOI] [PubMed] [Google Scholar]
- 28.Vermeire S, Schreiber S, Sandborn WJ, et al. Correlation between the Crohn's disease activity and Harvey-Bradshaw indices in assessing Crohn's disease severity. Clin Gastroenterol Hepatol 2010;8(4):357–63. [DOI] [PubMed] [Google Scholar]
- 29.Karsdal MA, Nielsen MJ, Sand JM, et al. Extracellular matrix remodeling: The common denominator in connective tissue diseases. Possibilities for evaluation and current understanding of the matrix as more than a passive architecture, but a key player in tissue failure. Assay Drug Dev Technol 2013;11(2):70–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meijer MJ, Mieremet-Ooms MA, Sier CF, et al. Matrix metalloproteinases and their tissue inhibitors as prognostic indicators for diagnostic and surgical recurrence in Crohn's disease. Inflamm Bowel Dis 2009;15(1):84–92. [DOI] [PubMed] [Google Scholar]
- 31.Di Sabatino A, Jackson CL, Pickard KM, et al. Transforming growth factor beta signalling and matrix metalloproteinases in the mucosa overlying Crohn's disease strictures. Gut 2009;58(6):777–89. [DOI] [PubMed] [Google Scholar]
- 32.Gao Q, Meijer MJ, Schlüter UG, et al. Infliximab treatment influences the serological expression of matrix metalloproteinase (MMP)-2 and -9 in Crohn's disease. Inflamm Bowel Dis 2007;13:693–702. [DOI] [PubMed] [Google Scholar]
- 33.De Bruyn M, Breynaert C, Arijs I, et al. Inhibition of gelatinase B/MMP-9 does not attenuate colitis in murine models of inflammatory bowel disease. Nat Commun 2017;8:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Bruyn M, Arijs I, De Hertogh G, et al. Serum neutrophil gelatinase B-associated lipocalin and matrix metalloproteinase-9 complex as a surrogate marker for mucosal healing in patients with Crohn's disease. J Crohns Colitis 2015;9(12):1079–87. [DOI] [PubMed] [Google Scholar]
- 35.Li AC, Thompson RP. Basement membrane components. J Clin Pathol 2003;56(12):885–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koutroubakis IE, Petinaki E, Dimoulios P, et al. Serum laminin and collagen IV in inflammatory bowel disease. J Clin Pathol 2003;56:817–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martínez-gonzález O, Cantero-hinojosa J, Paule-sastre P, et al. Intestinal permeability in patients with ankylosing spondylitis and their healthy relatives. Rheumatology 1994;33(7):644–7. [DOI] [PubMed] [Google Scholar]
- 38.Gibson PR. Increased gut permeability in Crohn's disease: Is TNF the link? Gut 2004;53(12):1724–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suenaert P, Bulteel V, Lemmens L, et al. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn's disease. Am J Gastroenterol 2002;97(8):2000–4. [DOI] [PubMed] [Google Scholar]
- 40.Stidham RW, Higgins PDR. Imaging of intestinal fibrosis: Current challenges and future methods. United Eur Gastroenterol J 2016;4(4):515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn's disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol 2010;105(1):162–9. [DOI] [PubMed] [Google Scholar]
- 42.Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015;148(7):1320–9.e3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.