Abstract
Background
The performance of magnetic resonance imaging (MRI), the effect of patient factors, and resulting surgical management in underserved and ethnically diverse breast cancer (BC) patient populations have been understudied.
Methods
We retrospectively analyzed the data of 1116 consecutive patients who were newly diagnosed with in situ or invasive BC with preoperative staging MRI. Non-index lesions (NILs) were defined as abnormal MRI findings with BI-RADS score of 4 or 5 in breast or axillary nodes not previously detected by conventional imaging. Occult cancers (OCs) were NILs found to be malignant by biopsy or surgery. Logistic regression was used to examine associations between probabilities of NILs or OCs and patient characteristics.
Results
Staging MRI detected NILs and OCs in 24% and 7.5% of patients, respectively. Of 1116 patients, 271 (24%) had 327 NILs, and 84 (7.5%) had 87 OCs. Follow-up information was available for 306 NILs. Ipsilateral breast NILs (n = 124) were seen in 115 patients (10.3%), with OCs (n = 51) seen in 48 patients (4.4%). Contralateral breast NILs (n = 134) were seen in 118 (10.6%) patients, with OCs (n = 20) seen in 20 patients (1.8%). Laterality (p < 0.001) and disease stage (p = 0.018) were associated with probability of OC. Patients without BRCA mutations had a significantly higher probability of having NILs (p = 0.003) but not OCs.
Conclusions
Our study provides useful estimates of the rates of NILs and OCs anticipated in a younger, uninsured, ethnically diverse population. Prospective trials and larger pooled retrospective analyses are needed to define the long-term impacts of MRI staging after a BC diagnosis.
Keywords: Magnetic resonance imaging, Non-index lesions, Occult cancer, Screening, Risk factors, BRCA, Body mass index, Breast density, Neoadjuvant therapy, Underserved populations
Introduction
Magnetic resonance imaging (MRI) of the breast is a potentially valuable screening and diagnostic tool for obtaining information beyond that provided by conventional breast imaging with mammography and ultrasonography. MRI is also increasingly used for treatment selection [1–3] and BC staging, yet there is wide variability in its use. Despite breast MRI being used in multiple settings, there is still heterogeneity in practice as most published reports are from single institution and focus on specific populations, without definitive randomized trials assessing long-term outcomes having been reported [4]. Case series have shown that in patients diagnosed with BC, staging MRI can detect occult BCs with a false-positive detection rate of 10.9% and a relatively low risk of detecting benign disease on biopsy (9.4%) [5]. The reported incidence of additional MRI-detected disease in the ipsilateral breast is 3–34% [6]and a 3–10% in the contralateral breast [7, 8].
In contrast, in the randomized controlled Comparative Effectiveness of MRI in Breast Cancer (COMICE) trial, the reoperation rate of 816 patients assigned to preoperative MRI (19%) and that of 807 patients assigned to no MRI (19%) did not differ significantly, a result that does not support the addition of MRI to conventional assessment with physical examination, mammography, and ultrasonography [9]. However, this study did not address the role of MRI in detecting additional cancers over and above conventional imaging, as it was designed to assess reoperation rate and lacked clinical follow-up data.
Another randomized trial study from Utrecht, Netherlands showed that MRI does not reduce the number of surgical procedures but had unexpected finding of a higher re-excision rate when MRI was performed prior to diagnostic biopsy [10]. This study again was not intended to evaluate the role of MRI in detecting occult tumor foci at sites other than index lesion as the MRI group consisted of only 74 patients with 83 cancers.
To analyze the impact of MRI at diagnosis, we first defined non-index lesions (NILs) as suspicious findings in the ipsilateral and contralateral breast and axillary nodes that were not initially detected by physical examination and standard imaging with diagnostic bilateral mammography and ultrasound that included axillary nodal basins. Occult cancers (OCs) were defined as NILs found to be invasive or in situ cancer by core needle biopsy and/or surgical specimens of breast or axillary nodes.
Our aim was to assess the impact of NILs and/or OCs on BC management and to determine the clinical and pathological characteristics associated with NILs and OCs. Specifically, our study focused on an ethnically diverse, heterogeneous and underserved population known to present with key factors such as higher stage of disease, younger age, and higher body mass index (BMI) that could affect the performance of MRI staging.
Patients and methods
This retrospective study was performed with the approval of the University of Southern California’s Institutional Review Board [HS-10–00611] of patients from Los Angeles County Medical Center (LAC + USC) and Norris Comprehensive Cancer Center (NCCC) that cares for a diverse patient population at our two hospitals with shared faculty and breast cancer management algorithms. The LAC + USC is a public hospital considered to be the safety net for healthcare access for Los Angeles County residents. It is the largest single provider of healthcare for the area’s medically underserved community. Most newly diagnosed BC patients at LAC + USC are uninsured, and 80% are Hispanic, whereas at NCCC, a private academic practice, most are insured and Caucasian, thus providing an opportunity to study the impact of NILs and OCs in this heterogeneous population.
We included all consecutive patients newly diagnosed with a biopsy-proven in situ or non-metastatic invasive BC stage 0–III, between January 1, 2006, and December 31, 2013 and who underwent preoperative MRI within 3 months of BC diagnosis for evaluation of extent of disease at LAC + USC or NCCC prior to receiving any surgical or systemic therapy. Cases were identified through a review of electronic radiology and medical records. All patients must have undergone our standard breast assessment of diagnostic bilateral mammography and ultrasound that include axillary nodal basins. Patients who received preoperative (neoadjuvant) chemotherapy were included, as our standard practice was to evaluate any abnormal imaging prior to medical treatment. Patients with any past history of treated primary BC or those found to have metastatic (Stage IV) disease were excluded from the study. Patients who underwent MRI for reasons other than disease extent such as evaluation of implants or high-risk screening were excluded. All patient information was abstracted and coded to protect patients’ privacy and managed using Research Electronic Data Capture [11].
Non-index lesions (NILs) were defined as those detected only by MRI and were not index lesions in breast or axillary nodes known to be malignant nor presenting with abnormal findings on clinical examination nor by our standard imaging as defined by Breast Imaging-Reporting and Data System (BI-RADS) [12] scores of 4 or 5. In addition, NILs had to be in a quadrant or sector different from that of the index lesion and at least 5 cm away from the index lesion. Occult cancers (OCs) were defined as NILs found to be invasive or in situ cancer by analysis of core or excisional biopsy specimens and/or surgical specimens of breast or nodal tissue from patients for whom follow-up information was available. Similarly, when mastectomy followed biopsy or excision, the diagnosis made with tissue from mastectomy was used as the definitive evaluation result for the lesion. Patients’ genetic data were collected if individuals met and consented according to National Comprehensive Cancer Network (NCCN) testing guidelines and the breast density was scored as ‘a’ (almost entirely fatty), ‘b’ (scattered areas of fibroglandular density), ‘c’ (heterogeneously dense), and ‘d’ (extremely dense) [13].
MRI
Bilateral diagnostic MRI was performed prior to surgery for staging the BC with a 1.5-T scanner (Siemens Healthcare, Germany) at NCCC and a 1.5-T scanner (General Electric Healthcare, USA) at LAC + USC using a dedicated breast coil with patients in the prone position. For dynamic sequences, 15 ml of gadopentetate dimeglumine (Magnevist, Bayer) was injected into an antecubital vein at a rate of 3 ml/second using a power injector followed by a 20-ml saline flush. The following bilateral sequences were obtained: precontrast axial T2 STIR, axial T1, coronal STIR (body coil), and dynamic axial 3D gradient T1 FS precontrast followed by axial 3D gradient T1 FS postcontrast, with 5 series at 1-min intervals beginning 60 s after contrast injection. Images were interpreted by fellowship trained, experienced board-certified radiologists, specializing in breast from the division of women’s imaging at the University of Southern California with expertise in contrast-enhanced breast MRI. NILs were interpreted from these MRI reports and if ambiguous or for special cases were re-reviewed by radiologists. All MRI studies were evaluated using commercially available CAD software (CADstream, Merge Healthcare).
Statistical analysis
We examined predictors including demographic, radiographic, and pathologic data points including age, race, body mass index (BMI), mammographic density, biopsy histology, receptor biomarkers, and genetic susceptibility testing (BRCA 1 and 2) with respect to OCs and NILs in all evaluable patients. To demonstrate that our cohort was representative of all patients seen at both institutions, we obtained registry data of all patients with stage I–III BC diagnosed in the same timeframe as this study seen between January 1, 2006, and December 31, 2013 for cohort comparison.
To assess the association between patient or lesion characteristics and the probability of a patient having an NIL, the probability of an NIL being an OC, and the probability of a patient having an OC, we used logistic regression models as described previously [14]. For each of these three outcomes, we first included the variables of interest mentioned above in a multivariable logistic regression model and then used a backward stepwise model selection method to select a final model by successively dropping nonsignificant variables from the model and re-fitting the reduced models until all remaining variables were statistically significant at p ≤ 0.20 [15]. Because a patient could have more than one NIL, we accounted for this intra-patient correlation in the logistic regression analyses of the probability of an NIL being OC.
The lesions that did not have a final surgical diagnostic evaluation included (1) those in patients who were lost to follow-up (n = 18) and (2) those which were negative on targeted ultrasonography studies and for which treating physicians considered a surgical procedure unnecessary. In the main analyses, these lesions were considered not to be OC. To assess the degree to which the inclusion of these lesions would have influenced the results, we also performed analyses in which these lesions were considered to be OC. These secondary analyses gave results equivalent to those of the main analyses (not reported).
All statistical computations were performed using STATA software (release 13; StataCorp, College Station, TX). All reported p-values are two-sided.
Results
Among the 2280 patients diagnosed from 2006 to 2013 (Supplementary Table 1) who had been newly diagnosed with in situ or non-metastatic invasive BC between January 1, 2006, and December 31, 2013, we found no significant differences in age, ethnicity, or stage distribution from our 1116-patient cohort (48.95%) that met our inclusion criteria.
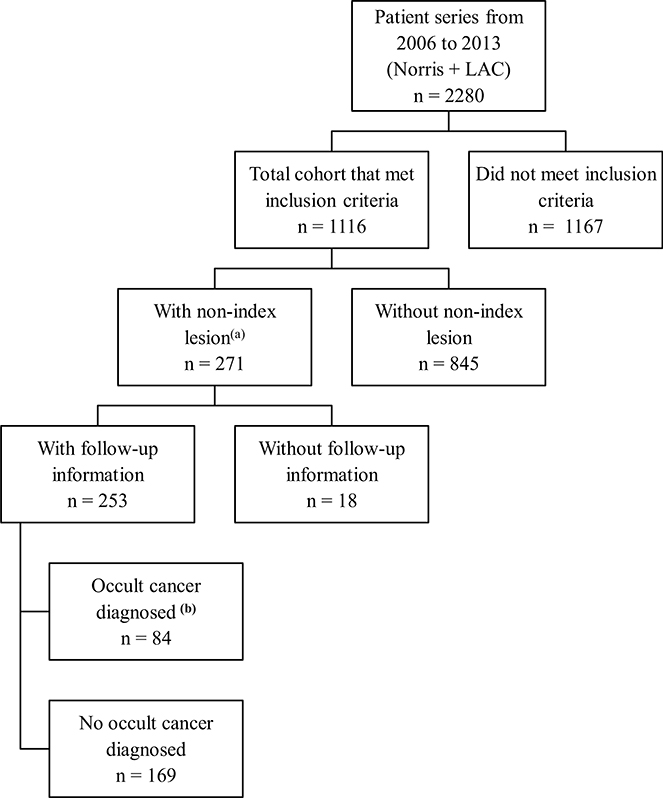
Of the 1116 patients in our cohort, 271 (24%) had a total of 327 NILs, and 84 (7.5%) had 87 OCs. Follow-up information for 306 NILs was available.
Demographic and clinical characteristics—patient level
Patients’ clinical characteristics are summarized in Table 1. Our patient cohort had a younger age at diagnosis and greater ethnic diversity than previously reported series. However, body mass index (BMI), BRCA status, and mammographic density were similar. Neoadjuvant chemotherapy was administered in 20% of patients. Among the 271 patients with NILs, 221 (20%), 45 (4%), 4 (0.4%), and 1 (0.09%) had 1, 2, 3, and 4 NILs, respectively. In the overall cohort, 523 patients (47%) were Hispanic, 422 (38%) were younger than 50 years, 381 (34%) had a BMI ≥ 30, 352 (32%) underwent BRCA testing, and 38 (3%) had deleterious mutations in BRCA1 (n = 23; 16 Hispanic, 3 Asian, and 4 Caucasian patients) or BRCA2 (n = 15; 10 Hispanic, 3 Asian, and 2 Caucasian or other patients). Interestingly, only one of the OCs detected was among the 38 known BRCA mutation carriers. Among patients who were tested for BRCA mutations, BRCA mutation status (BRCA 1 or BRCA 2 vs. normal) was not associated with the probability of having MRI-detected NILs (Fisher exact test, p = 0.41) or OCs (p = 0.71). Mammographically fatty/scattered fibroglandular breast tissue was reported in 474 patients (42%), and dense/heterogeneous dense breast tissue was reported in 624 (56%) patients. There were 52 prophylactic mastectomies, which yielded 1 incidental case of DCIS not seen on MRI. Eighteen patients with NILs did not undergo tissue diagnostic evaluation because they were lost to follow-up or had negative follow-up targeted ultrasound or MRI.
Table 1.
Patient demographic and clinical characteristics
| Variable | All patients (N = 1116) | Patients with non-index lesions (n = 271) | Patients with follow-up informationa (n = 253) | Patients with occult cancers (n = 84) |
|---|---|---|---|---|
| Institution | ||||
| LAC + USC | 693 (62.1) | 171 (63.1) | 162 (64.0) | 55 (65.5) |
| Norris | 423 (37.9) | 100 (36.9) | 91 (36.0) | 29 (34.5) |
| Race/ethnicity | ||||
| Hispanic | 523 (46.9) | 124 (45.8) | 116 (46.0) | 40 (47.6) |
| Asian | 154(13.8) | 48(17.7) | 44(17.3) | 9(10.7) |
| Caucasian | 323 (28.9) | 73 (26.9) | 69 (27.2) | 28 (33.3) |
| African American | 79 (7.1) | 21 (7.8) | 19(7.5) | 6 (7.2) |
| Others/unknown | 37 (3.3) | 5(1.8) | 5 (2.0) | 1 (1.2) |
| Age at diagnosis (years) | ||||
| <40 | 142(12.7) | 35(12.9) | 33(13.0) | 8 (9.5) |
| 40-49 | 280 (25.1) | 73 (26.9) | 70 (28.0) | 22 (26.2) |
| 50-64 | 518(46.4) | 132 (48.7) | 124 (49.0) | 42 (50.0) |
| ≥65 | 176(15.8) | 31 (11.5) | 26(10.0) | 12(14.3) |
| BMI | ||||
| 0-18.5 | 19(1.7) | 3(1.1) | 3(1.2) | 0 |
| 18.6-25 | 290 (26) | 66 (24.4) | 61 (24.1) | 17 (20.2) |
| 26-30 | 352 (31.5) | 98 (36.2) | 94 (37.0) | 33 (39.3) |
| ≥31 | 381 (34.2) | 83 (30.5) | 78(31.0) | 25 (29.8) |
| Unknown | 74 (6.6) | 21 (7.8) | 17 (6.7) | 9(10.7) |
| BRCA | ||||
| BRCA 1 | 23 (2.1) | 2 (0.7) | 2 (0.8) | 0 |
| BRCA2 | 15(1.3) | 4(1.5) | 4(1.6) | 1(1.2) |
| Normal | 314(28.1) | 73 (26.9) | 68 (26.8) | 20 (23.8) |
| Unknown/untested | 764 (68.5) | 192 (70.9) | 179 (70.8) | 63 (75.0) |
| Biomarkers | ||||
| HER2-, ER or PR+ | 608 (54.5) | 159 (58. 7) | 150(59.3) | 57 (67.9) |
| Triple negative | 143(12.8) | 25 (9.2) | 22 (8.7) | 6(7.1) |
| HER2+ | 204(18.3) | 48(17.7) | 46(18.2) | 14(16.7) |
| HER2 missing, ER or PR+ | 9 (0.8) | 4(1.5) | 3(1.2) | 1(1.2) |
| HER2 missing, ER or PR missing or - | 152(13.6) | 35(12.9) | 32(12.6) | 6(7.1) |
| Mammogram density | ||||
| Fatty | 45 (4) | 6 (2.2) | 6 (2.4) | 3 (3.6) |
| Scattered fibroglandular | 429 (38.4) | 98 (36.2) | 90 (35.6) | 31 (36.9) |
| Heterogeneously dense | 582 (52.2) | 151 (55.7) | 142 (56.1) | 47 (56.0) |
| Extremely dense | 42 (3.8) | 13(4.8) | 12(4.7) | 2 (2.3) |
| Unknown | 18(1.6) | 3(1.1) | 3(1.2) | 1 (1.2) |
| Index lesion histology | ||||
| Invasive lobular | 149(13.4) | 35(12.9) | 34(13.4) | 14(16.6) |
| Invasive ductal/ductal carcinoma | 761 (68.1) | 191 (70.5) | 177 (70.0) | 64 (76.3) |
| DCIS/LCIS | 206(18.5) | 45(16.6) | 42(16.6) | 6(7.1) |
| Neoadjuvant therapy | ||||
| No | 894(80.1) | 218(80.4) | 206 (81.4) | 70 (83.4) |
| Yes | 222 (19. 9) | 53(19. 6) | 47(18.6) | 14(16.6) |
| No. of non-index lesions | ||||
| 0 | 845 (75.7) | 0 | - | - |
| 1 | 221 (19.8) | 221 (81.5) | 205 (81.0) | 67 (79.8) |
| 2 | 45 (4) | 45(16.6) | 43(17.0) | 17 (20.2) |
| 3 | 4 (0.4) | 4(1.5) | 4(1.6) | 0 |
| 4 | 1 (0.1) | 1 (0.4) | 1 (0.4) | 0 |
All data are no. of patients (%) calculated for each variable relative to the overall cohort numbers in all patients, patients with non-index lesions, patients with follow-up information, and patients with occult cancers, respectively
Of the 271 patients who had non-index lesions, 18 were lost to follow-up
Demographic and clinical characteristics—lesion level
The distribution of NILs and OCs by patients’ clinical characteristics is shown in Table 2. The MRI BI-RADS scores corresponding to NILs were 4a in 180 cases (59%), 4b in 65 (21%), 4c in 26 (8%), and 5 in 35 (11%). For OCs, the corresponding numbers were 36 (12%), 19 (6%), 13 (4%), and 19 (6%), respectively. Of the final assessments of NILs, 151 were performed by core or excisional biopsy, 42 by breast-conserving surgery, 75 by mastectomy, and 38 by node dissection. Of the final assessments of OCs, 10 were performed by biopsy, 14 by breast-conserving surgery, 48 by mastectomy, and 15 by node dissection.
Table 2.
Distribution of non-index lesions and the occult cancers by clinical characteristics
| Variable | Non-index lesions among 271 patients (n = 327) | Non-index lesions with follow-up informationa (n = 306) | Occult cancers (n = 87) |
|---|---|---|---|
| Institution | |||
| LAC + USC | 204 (62.4) | 195 (63.7) | 57 (65.5) |
| Norris | 123 (37.6) | 111 (36.3) | 30 (34.5) |
| Race/ethnicity | |||
| Hispanic | 150 (45.9) | 142 (46.4) | 42 (48.2) |
| Asian | 58 (17.7) | 53 (17.3) | 10 (11.5) |
| Caucasian | 89 (27.3) | 83 (27.1) | 28 (32.3) |
| African American | 24 (7.3) | 22 (7.2) | 6 (6.9) |
| Others/unknown | 6 (1.8) | 6 (2.0) | 1 (1.1) |
| Age at diagnosis (years) | |||
| <40 | 38 (11.6) | 36 (11.8) | 8 (9.2) |
| 40-49 | 92 (28.2) | 88 (28.8) | 23 (26.4) |
| 50-64 | 156 (47.7) | 148 (48.4) | 43 (49.4) |
| ≥65 | 41 (12.5) | 34 (11.0) | 13 (15.0) |
| BMI | |||
| 0-18.5 | 3 (0.9) | 3 (1.0) | 0 |
| 18.6-25 | 82 (25.1) | 74 (24.2) | 17 (19.5) |
| 26-30 | 122 (37.3) | 118 (38.6) | 33 (37.9) |
| ≥31 | 95 (29.1) | 90 (29.3) | 27 (31.1) |
| Unknown | 25 (7.6) | 21 (6.9) | 10 (11.5) |
| BRCA | |||
| BRCA 1 | 2 (0.6) | 2 (0.7) | 0 |
| BRCA 2 | 4 (1.2) | 4 (1.3) | 1 (1.1) |
| Normal | 88 (26.9) | 82 (26.8) | 20 (23.0) |
| Unknown | 233 (71.3) | 218(71.2) | 66 (75.9) |
| Biomarkers | |||
| HER2-. ER. orPR+ | 198 (60.6) | 186 (60.8) | 58 (66.7) |
| Triple negative | 28 (8.6) | 25 (8.2) | 7 (8.0) |
| HER2+ | 52 (15.8) | 50 (16.3) | 15 (17.3) |
| HER2 missing, ER. or PR+ | 4 (1.2) | 3 (1.0) | 1 (1.1) |
| HER2 missing. ER or PR missing or - | 45 (13.8) | 42 (13.7) | 6 (6.9) |
| Mammographic density | |||
| Fatty | 7 (2.1) | 7 (2.9) | 3 (3.5) |
| Scattered fibroglandular | 117 (35.8) | 108 (35.3) | 31 (35.6) |
| Heterogeneously dense | 185 (56.6) | 174 (56.2) | 50 (57.5) |
| Extremely dense | 15 (4.6) | 14 (4.6) | 2 (2.3) |
| Unknown | 3 (0.9) | 3 (1.0) | 1 (1.1) |
| Index lesion histology | |||
| Invasive lobular | 41 (12.6) | 40 (13.1) | 14(16.1) |
| Invasive ductal/ductal carcinoma | 227 (69.4) | 211 (68.9) | 67 (77.0) |
| DCIS/LCIS | 59 (18) | 55 (18.0) | 6 (6.9) |
| Type of surgery | |||
| Lumpectomy | 42 (12.9) | 42 (13.7) | 14(16.1) |
| Mastectomy | 75 (22.9) | 75 (24.5) | 48 (55.2) |
| Node surgery | 38 (11.6) | 38 (12.4) | 15 (17.2) |
| None | 169 (51.7) | 148 (48.4) | 10 (11.5) |
| Awaiting surgery | 3 (0.9) | 3 (1.0) | 0 |
| Type of node surgery | |||
| SLN | 31 (9.5) | 31 (10.1) | 11 (12.6) |
| ALND | 9 (2.8) | 9 (3.0) | 8 (9.2) |
| SLN and ALND | 8 (2.4) | 8 (2.6) | 4 (4.6) |
| None | 279 (85.3) | 258 (84.3) | 64 (73.6) |
| Grade | |||
| 0 | 3 (0.9) | 3 (1.0) | 0 |
| 1 | 73 (22.3) | 65 (21.2) | 19 (21.8) |
| 2 | 129 (39.4) | 124 (40.5) | 36 (41.4) |
| 3 | 114 (34.9) | 107 (35.0) | 30 (34.5) |
| Unknown | 8 (2.5) | 7 (2.3) | 2 (2.3) |
All data are no. of lesions (%) calculated for each variable relative to the overall cohort numbers in non-index lesion among 271 patients, non-index with follow-up information, and occult cancers, respectively
Of the 327 non-index lesions, 21 from 18 unique patients did not have a surgical diagnostic evaluation, either due to negative follow-up targeted ultrasonography, or MRI, or to loss to follow-up
Distribution of NILs and OCs by laterality
The distribution of NILs and OCs by laterality is given in Table 3. Of the 165 ipsilateral NILs for which follow-up information was available, 124 arose from the breast, and 41 arose from the axillary nodes, and in 150 patients (13.4%), 87 (7.9%) were false positive, and 63 (5.7%) were true positive (58 [5.3%] invasive and 5 [0.46%] in situ outside the index cancer). Of the 141 contralateral NILs for which follow-up information was available, 134 arose from the breast, and 7 arose from the axillary nodes, and in 124 (11.1%) patients (11.1%), 103 (9.4%) were false positive, and 21 (1.9%) were true positive (16 [1.46%] invasive and 5 [0.46%] in situ). The distribution of patients with NILs and OCs is shown in Fig. 1.
Table 3.
Distribution of non-index lesions and occult cancers by laterality
| Laterality | Non-index lesions (n = 327) | Non-index lesions with follow-up information (n = 306) | Occult cancers (n = 87) | Invasive occult cancers (n = 77) | In situ occult cancers (n = 10) |
|---|---|---|---|---|---|
| Ipsilateral | 133 | 124 | 51 (41%) | 46 | 5 |
| Ipsilateral node | 42 | 41 | 15 (37%) | 15 | 0 |
| Contralateral | 143 | 134 | 20(15%) | 15 | 5 |
| Contralateral node | 9 | 7 | 1 (14%) | 1 | 0 |
Data are no. of lesions (%) calculated for occult cancers relative to the total no. follow-up information (n = 306) for each laterality
Fig. 1.
Contains the distribution of patients from the total series to patients with non-index lesions (NIL) and occult cancers, where (a) Laterality of patients with NIL were 150 ipsilateral (150/1116 = 13.4%), 124 contralateral (124/1116 = 11.1%). Laterality of occult cancers (b) were 63 ipsilateral (63/1098) 5.7% with (58/1098) 5.3% invasive, (5/1098) 0.46 % in situ and 21 contralateral (21/1098)1.9 % with (16/1098) 1.5 % were invasive, (5/1098) 0.46% in situ. (NOTE the denominator for OC’s represents those not lost to follow up)
Multivariate analysis
The OC probabilities based on patients’ clinical and pathological indices are shown in Table 4. All variables were considered in a multivariable logistic regression model. Clinical stage (trend test p-value = 0.002) and biomarker subtype (overall p-value = 0.062) were the only variables that were statistically significant in predicting OC status.
Table 4.
Results of the univariate and multivariable analyses for associations between clinical characteristics and probability of occult cancer
| Variable | Univariate |
Multivariable |
||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Institution | 0.50 | |||
| LAC + USC | 1.00 | Excluded from the model | ||
| Norris | 0.85 (0.54, 1.36) | |||
| Race/ethnicity | 0.58a | |||
| Hispanic | 1.00 | Excluded from the model | ||
| Asian | 0.75 (0.36, 1.58) | |||
| Caucasian | 1.15 (0.69, 1.90) | |||
| African American | 0.99 (0.41, 2.42) | |||
| Others/unknown | 0.34 (0.045.2.51) | |||
| Age at diagnosis (years) | 0.68b | |||
| <40 | 1.00 | Excluded from the model | ||
| 40-49 | 1.43 (0.62, 3.29) | |||
| 50-64 | 1.48 (0.68, 3.22) | |||
| ≥65 | 1.23 (0.49, 3.09) | |||
| BMI | 0.16b | |||
| 0-18.5 | Small sample size | Excluded from the model | ||
| 18.6-25 | 1.00 | |||
| 26-30 | 1.66 (0.91, 3.05) | |||
| ≥31 | 1.13(0.60, 2.13) | |||
| Unknown | ||||
| Biomarkers | 0.040a | .062a | ||
| HER2-, ER or PR+ | 1.00 | 1.00 | ||
| Triple negative | 0.42(0.18, 1.01) | 0.38 (0.16, 0.90) | ||
| HER2+ | 0.71 (0.39, 1.31) | 0.65 (0.35, 1.20) | ||
| HER2 missing | 0.44 (0.20 0.98) | 1.19(0.45, 3.16) | ||
| Mammogram density | 0.670 | |||
| Fatty/scattered fibroglandular | 1.00 | Excluded from the model | ||
| Heterogeneously dense/extremely dense | 1.10(0.70, 1.74) | |||
| Unknown | ||||
| Index lesion histology | 0.008a | |||
| Invasive ductal/ductal carcinoma | 1.00 | Excluded from the model | ||
| Invasive lobular | 1.13 (0.62, 2.07) | |||
| DCIS/LCIS | 0.33 (0.14, 0.77) | |||
| Index lesion grade | 0.27b | |||
| 1 | 1.00 | Excluded from the model | ||
| 2 | 0.88 (0.49, 1.58) | |||
| 3 | 0.72 (0.39, 1.32) | |||
| Unknown | ||||
| Clinical stage | 0.004b | .002b | ||
| 0 | 0.24(0.056, 1.06) | 0.19(0.036, 1.01) | ||
| I | 1.00 | 1.00 | ||
| II | 1.88(1.07, 3.30) | 2.06(1.17, 3.64) | ||
| III | 1.46 (0.74, 2.89) | 1.69 (0.84, 3.38) | ||
| Unknown | ||||
OR odds ratio, CI confidence interval
Overall p-value
Trend test p-value
Supplementary Table 2 shows all variables included in the multivariable logistic regression model. Race/ethnicity (overall p-value = 0.16), age (trend test p-value = 0.098), and biomarkers (overall p-value = 0.099) were included in the final prediction model for an NIL, with triple-negative BC patients having a lower probability of an NIL.
The multivariable logistic model revealed that stage (p = 0.018) and laterality (p ≤ 0.001) were associated with the probability of an NIL being an OC shown in Supplementary Table 3.
Discussion
Overall, MRI detected NILs and OCs in 24% and 7.5% of patients, respectively. These data complement the growing body of literature showing that preoperative breast MRI can detect OCs [16]. The incidence of OCs is on the lower side of the reported literature, possible due to the younger age of our patients and the routine use of bilateral breast and regional nodal ultrasound.
Our analysis included two distinct populations—a mostly insured population at an academic practice and a largely underserved minority population at an urban public/county health system that is distinct from most published data comprised of a primarily Caucasian population [17]—seen over the same time span using similar diagnostic procedures, equipment, and care providers. In our two institutions, a private comprehensive cancer center and a public safety net hospital, the same faculty treats patients in a multidisciplinary manner and allows more consistent interpretation and management. Most newly diagnosed BC patients at LAC + USC are uninsured, and 80% are Hispanic and 15% are Asian; whereas at NCCC, most newly diagnosed BC patients are Caucasian showing the impact of NILs and OC in this heterogeneous population. In addition, routine MRI staging was adopted in 2005 as standard practice at both institutions that served as a basis for this consecutive series.
Despite the wealth of clinical data we analyzed, we found no specific risk factors for NILs or OCs other than laterality: Ipsilateral NILs were 4 times as likely as contralateral NILs to be OCs. The detection rates of ipsilateral NILs (13%), ipsilateral OCs (6%), contralateral NILs (11%), and contralateral OCs (2%) in our population were lower than those in previous studies, whose detection rates of non-index ipsilateral occult malignant foci and contralateral occult malignancies were 6–27% and 3–9%, respectively [18]. The low rates in the present study may have been due to the systematic mammography and ultrasonography methods our radiologists used to successfully identify the lesions prior to MRI. Other studies have shown that MRI detects additional disease in the affected breast in 16% of BC patients [19]. Lehman et al. reported that MRI detected an occult contralateral malignancy in 30 (3.1%) of 969 patients with a false-positive rate of 10.9% [5]. In contrast, we found a lower false-positive rate (7.5%), which may have been due to our patients’ considerably younger age at diagnosis (median age, 42 years) compared with that of the general population (61 years) and the associated lower incidence of contralateral OC [20].
In the present study, lesions classified by MRI as suspicious were confirmed as invasive cancer or DCIS in about 27% of lesions. Previous studies have shown that the sensitivity of MRI is superior to that of mammography for invasive cancer but not for DCIS [21] hence supporting the importance of MRI across all histologies.
In the present study, we did not observe the expected higher rate of OCs in patients with genetic susceptibility risk factors or increased breast density. This may be explained in part by the smaller sample size of BRCA mutation carriers; however, a national Dutch study supported the use of MRI screening in addition to current standard-of-care imaging in women with a familial or genetic predisposition for BC [22]. More recently, a pooled analysis of women aged 50 years or older with BRCA1 and BRCA2 mutations found that the combination of MRI and mammography had higher sensitivity than either modality alone [23]. However, the prevalence of OCs and associated risk factors in diverse and underserved populations and the accompanying surgical management remain unclear. For patients lost to follow-up (n = 18) and those with a negative targeted second-look ultrasound where a surgical procedure was not necessary, these lesions were considered not be OC. A sensitivity analysis assuming all those lost to follow-up actually had OCs which rendered similar results to our main analyses and did not change our main findings.
Our study is novel in its description of a previously uncharacterized, underserved, ethnically diverse, primarily Hispanic population with preoperative MRI and BRCA testing data available which have major implications in identifying optional management pathways and projecting the likelihood of OC in this population.
The principle limitation of our study was its retrospective nature and its inability to capture intended treatment and the extent to which MRI findings altered such treatment. All potentially relevant variables were included in analysis although this could have been confounded by lesion size, tumor focality, NIL location, and tumor response to neoadjuvant chemotherapy. There were also some cases for which follow-up tissue information was not available, but these numbers were low, reflecting real-life practice. This analysis is unique because of the diverse population, the inclusion of consecutive patients who met the eligibility criteria assessed with MRI in a practice setting in which routine MRI staging was used for both populations, and the wealth of clinical data, including BRCA status, breast density, and BMI.
Our study does not look at re-excision or recurrence rates, but existing literature of patient-level meta-analysis had no difference in 8-year local recurrence-free survival rates between MRI (97%) and no-MRI groups (95%) or in distant recurrence rates (89% and 93%, respectively) [24]. The ongoing Alliance A011104 prospective randomized trial comparing MRI with standard imaging in BC patients undergoing breast-conserving surgery will help determine the value of MRI in predicting locoregional recurrence and may point to specific clinical factors that may predict OCs and subsequent clinical outcomes [25].
In conclusion, our study provides useful estimates of the rates of NILs and OCs anticipated in a younger, uninsured, primarily Hispanic population to determine their impact on management which also reflect current standard in axillary imaging with ultrasound in conjunction with MRI. Prospective trials and larger pooled retrospective analyses are needed to define the long-term benefits of MRI staging after a BC diagnosis and identify the specific populations that would benefit from such staging.
Supplementary Material
Acknowledgements
Joe Munch in MD Anderson’s Department of Scientific Publications edited the manuscript.
Funding This study was supported by the Women’s Cancer Program, USC Norris Comprehensive Cancer Center, and by the National Cancer Institute through MD Anderson’s Cancer Center Support Grant (P30CA016672). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare no potential conflicts of interest.
Ethical approval This article does not contain any studies with human participants or animals.
Informed consent This study was approved by the institutional review board at University of Southern California, and waivers for obtaining informed consent were granted.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10549-018-05084-w) contains supplementary material, which is available to authorized users.
References
- 1.Warner E, Hill K, Causer P et al. (2011) Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol 29:1664–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnaout A, Catley C, Booth CM et al. (2015) Use of preoperative magnetic resonance imaging for breast cancer: a Canadian population-based study. JAMA Oncol 1:1238–1250 [DOI] [PubMed] [Google Scholar]
- 3.Bedrosian I, Mick R, Orel SG et al. (2003) Changes in the surgical management of patients with breast carcinoma based on preoperative magnetic resonance imaging. Cancer 98:468–473 [DOI] [PubMed] [Google Scholar]
- 4.Wernli KJ, DeMartini WB, Ichikawa L et al. (2014) Patterns of breast magnetic resonance imaging use in community practice. JAMA Intern Med 174:125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehman CD, Gatsonis C, Kuhl CK et al. (2007) MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med 356:1295–1303 [DOI] [PubMed] [Google Scholar]
- 6.Iacconi C, Galman L, Zheng J et al. (2015) Multicentric cancer detected at breast MR imaging and not at mammography: important or not? Radiology 279(2):378–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bitencourt AG, Pereira NP, França LK et al. (2015) Role of MRI in the staging of breast cancer patients: does histological type and molecular subtype matter? Br J Radiol 88:20150458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehman CD, Gatsonis C, Kuhl CK et al. (2007) MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med 2007:1295–1303 [DOI] [PubMed] [Google Scholar]
- 9.Turnbul L, Brown S, Harvey I et al. (2010) Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial [DOI] [PubMed]
- 10.Peters N, Van Esser S, van den Bosch M et al. (2011) Preoperative MRI and surgical management in patients with nonpalpable breast cancer: the MONET–randomised controlled trial. Eur J Cancer 47:879–886 [DOI] [PubMed] [Google Scholar]
- 11.Harris PA, Taylor R, Thielke R et al. (2009) Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sickles E, D’Orsi C, Bassett L et al. (2013) ACR BI-RADS® Atlas Breast Imaging Reporting and Data System. American College of Radiology, Reston [Google Scholar]
- 13.D’Orsi DBBWR (2003) VA: American College of Radiology; 2003. Breast Imaging Reporting and Data System. Mammography, BI-RADS [Google Scholar]
- 14.Cox DR (1989) Analysis of binary data, Chapman & Hall, London [Google Scholar]
- 15.Hocking RR (1976) The Analysis and Selection of Variables in Linear Regression
- 16.Gutierrez RL, DeMartini WB, Silbergeld JJ et al. (2011) High cancer yield and positive predictive value: outcomes at a center routinely using preoperative breast MRI for staging. Am J Roentgenol 196:W93–W99 [DOI] [PubMed] [Google Scholar]
- 17.Chlebowski RT, Chen Z, Anderson GL et al. (2005) Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst 97:439–448 [DOI] [PubMed] [Google Scholar]
- 18.Orel S (2008) Who should have breast magnetic resonance imaging evaluation? J Clin Oncol 26:703–711 [DOI] [PubMed] [Google Scholar]
- 19.Houssami N, Ciatto S, Macaskill P et al. (2008) Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol 26:3248–3258 [DOI] [PubMed] [Google Scholar]
- 20.Houssami N, Turner R, Morrow M (2013) Preoperative magnetic resonance imaging in breast cancer: meta-analysis of surgical outcomes. Ann Surg 257:249–255 [DOI] [PubMed] [Google Scholar]
- 21.Rijnsburger AJ, Obdeijn IM, Kaas R et al. (2010) BRCA1-associated breast cancers present differently from BRCA2-associated and familial cases: long-term follow-up of the Dutch MRISC Screening Study. J Clin Oncol 28:5265–5273 [DOI] [PubMed] [Google Scholar]
- 22.Kriege M, Brekelmans CT, Boetes C et al. (2001) MRI screening for breast cancer in women with familial or genetic predisposition: design of the Dutch National Study (MRISC). Fam Cancer 1:163–168 [DOI] [PubMed] [Google Scholar]
- 23.Phi XA, Houssami N, Obdeijn IM et al. (2015) Magnetic resonance imaging improves breast screening sensitivity in BRCA mutation carriers age ≥ 50 years: evidence from an individual patient data meta-analysis. J Clin Oncol 33:349–356 [DOI] [PubMed] [Google Scholar]
- 24.Houssami N, Turner R, Macaskill P et al. (2014) An individual person data meta-analysis of preoperative magnetic resonance imaging and breast cancer recurrence. J Clin Oncol 32:392–401 [DOI] [PubMed] [Google Scholar]
- 25. https://clinicaltrials.gov/ct2/show/NCT01805076.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.