Abstract
Burkholderia pseudomallei is the causative agent for melioidosis. Because of its intracellular nature, the bacterium is capable of replicating within a plethora of eukaryotic cell lines. B. pseudomallei can remain dormant within host cells without symptoms for years, causing recrudescent infections. Here, we investigated the pathogenesis mechanism behind the suppression of T cell responses by B. pseudomallei . Peripheral blood mononuclear cells (1×106 cells/well) isolated by Ficoll Paque (Sigma-Aldrich) density gradient centrifugation were incubated with optimized concentrations of bacterial crude culture filtrate antigens (CFAs) (10 ug ml−1) and heat-killed bacteria [1 : 10 multiplicity of infection (m.o.i.)]. Following incubation, cells were investigated for surface expression of coinhibitory molecules by flow cytometry. We found that B. pseudomallei induced the upregulation of programmed death 1 (PD-1), a molecule responsible for T cell exhaustion, on T cells in vitro following exposure to crude CFAs of B. pseudomallei . This upregulation of PD-1 probably contributes to poor immune surveillance and disease pathogenesis.
Keywords: Burkholderia pseudomallei, PD-1, culture filtrate, immune responses
INTRODUCTION
Burkholderia pseudomallei causes melioidosis, a deadly infectious disease of humans and animals leading to significant mortality rates that is often reported from parts of Southeast Asia and northern Australia [1–3]. Over the past few decades, it has become a major focus of global concern [4, 5]. The intrinsic ability of B. pseudomallei to resist various antibiotics makes it less vulnerable to antibiotic therapy, meaning there is an urgent need to develop an effective vaccine against melioidosis [6]. B. pseudomallei , a category B bioterrorism agent [7], is acquired via inhalation of aerosolized bacteria, ingestion of contaminated water or by cutaneous inoculation [8]. Clinical manifestations include localized infections, with the lungs being the most commonly affected organ, followed by the liver and spleen [9]. Other protean manifestations, such as pneumonia and septic shock, are associated with high mortality rates. B. pseudomallei is a facultative intracellular pathogen that can invade, multiply and thrive within phagocytic and non-phagocytic cells [10–12]. This ability allows them to remain quiescent in the host, resulting in the recurrence of symptoms for several years after infection [13–15].
A recent study on a murine macrophage-like cell line showed that B. pseudomallei can combat host proteases in the phagosome by releasing a serine protease inhibitor called ecotin [16]. This, together with the type 3 secretion system (T3SS), allows the bacteria to escape phagosomal killing by macrophages [17, 18]. Upon escape, the bacteria spread to surrounding cells and form multinucleated giant cells [19]. Another mechanism adopted by B. pseudomallei to escape phagocytosis is the induction of caspase-1-dependant cell death in macrophages [20]. However, stimulation of macrophages with IFN-γ notably enhances antibacterial ability, limiting the intracellular survival of B. pseudomallei [21–23]. This is because macrophage-mediated killing requires optimal levels of IFN-γ via T cell and NK cell activation and inadequate levels would allow B. pseudomallei to escape innate immune responses [24, 25]. Since B. pseudomallei can evade macrophages, the more versatile cell-mediated immunity (CMI) involving the expansion of T cells could be essential. The fact that clinical melioidosis samples show reduced T cell counts indicates that B. pseudomallei could be impairing T cell activation to evade immune surveillance [26, 27]. Nonetheless, the exact mechanism behind T cell suppression during melioidosis is poorly understood.
T cells are activated by dendritic cells (DCs) and the engagement of co-signalling molecules on both the cells helps to positively (co-stimulation) and negatively (co-inhibition) regulate T cell activation. PD-1 receptor is found on the surface of CD4+ and CD8+ T cells and is upregulated within 24–72 h of TCR stimulation [28]. Further engagement of PD-1 with its ligand(s) PD-L1/L2 inhibits T cell proliferation and cytokine secretion [29]. In an acute infection, such an inhibitory signal would limit the number of effector T cells during T cell expansion [30], whereas during chronic infection, due to persistent exposure to antigens, this interaction renders the T cells unresponsive, leading to T cell exhaustion [31]. Increasing evidence suggests that PD-1 has a role in the inhibition of effector T cell responses in persistent Mycobacterium tuberculosis [32] and persistent viral infections [33–35], which we demonstrated in a murine model of persistent B. pseudomallei infection [36]. Upregulation of PD-L1 on polymorphonuclear neutrophils (PMNs) of melioidosis patients has been shown to impair T cell functions [37]. Nonetheless, there is no evidence to date regarding PD-1 upregulation on T cells following exposure to crude culture filtrate antigens of B. pseudomallei . Here, we investigated the pathogenesis mechanisms behind the suppression of T cell responses by B. pseudomallei .
METHODS
Ethical approval
All experiments involving humans were performed in accordance with the relevant guidelines and regulations and under examination by the Medical Ethics Committee (MEC) of University Malaya Medical Centre (UMMC), Kuala Lumpur, Malaysia (ref. no. 1017.23), and were conducted per the guidelines of the International Conference on Harmonization Guidelines and the Declaration of Helsinki. All individuals involved in the study were over 18 years of age and provided informed consent to participate in the study.
Blood samples
Blood samples (10 ml) from healthy subjects at UMMC, Malaysia were collected in sodium heparin BD Vacutainers (BD Biosciences, Franklin Lakes, NJ, USA). Peripheral blood mononuclear cells were isolated within 8 h of phlebotomy by Ficoll Paque (Sigma-Aldrich) density gradient centrifugation. Cells were counted using the trypan blue exclusion method. PBMCs were seeded in six-well tissue culture plates at 1×106 cells/well.
Bacterial strains
Three bacterial strains were used: a clinical isolate of B. pseudomallei (THE), obtained from the spleen of a patient admitted to UMMC; a virulent environmental isolate, Burkholderia thailandensis (ATCC); and the K96243 strain (mouse spleen). The clinical isolate was identified as B. pseudomallei through its ability to grow on Ashdown agar, and also by molecular confirmation using groEL-specific primer for genus detection and mprA-specific primer for species characterization. We also performed substrate utilization tests using the API20NE test according to the manufacturer’s instructions.
Twenty-four hour growth curves, colony-forming units (c.f.u.) ml−1 and multiplicity of infection (m.o.i.) were determined for all strains (data not shown). Heat inactivation of bacteria was performed as described previously [38–40]. Briefly, all strains were grown in Luria–Bertani (LB) broth and incubated overnight (37 °C at 200 r.p.m.) in the shaking incubator. The OD at 590 nm for each tube was checked the following day and all cultured tubes were adjusted to the same OD using phosphate-buffered saline (PBS). The samples were serially diluted and plated to determine the number of viable cells. The cells were harvested and washed twice using PBS. The bacterial suspension was heat-inactivated (HI) at 80 °C in 5 mM PBS (pH 7.3) in a water bath. The bacterial cells were harvested by centrifugation and resuspended in PBS and stored at 4 °C until use.
Extraction of culture filtrate antigens
Crude CFA was extracted as described previously [41]. Briefly, the strains were grown in LB broth. The culture was centrifuged at 20 000 g for 40 min. The supernatant was harvested and filtered through a 0.22 µM filter (Sartorius, Goettingen, Germany). The filtered supernatant was concentrated 50-fold using a Pierce Concentrator 9K (Thermo Scientific, USA). The protein content was estimated by Bradford assay against a bovine serum albumin (Biowest, USA) standard [42]. The preparations were stored at −20 °C until use. PBMCs (1×106 cells) were seeded into six-well plates and incubated with optimized concentrations of CFA (10 ug ml−1) and heat-killed bacteria (1 : 10 m.o.i.) for 36 h. Antigen-unexposed mock cells were used as a negative control, and cells stimulated with phytohaemagglutin (PHA) (1.5 % v/v) were used as a positive control.
Flow cytometry
Following incubation with antigens, the cells were investigated for surface expression of co-inhibitory receptors. All antibodies were pretitrated to determine appropriate working concentrations. Cells were stained with Fixable Viability Stain (FVS510, BD Biosciences; clone R35-95) and incubated for 20 min. Monoclonal antibodies directed against CD3 (BD Biosciences clone UCHT1), CD4 (BD Biosciences clone SK7), CD8 (BD Biosciences clone SK1) and PD-1 (BD Biosciences clone MIH4), CTLA-4 (BD Biosciences clone BNI3) and TIM-3 (R and D Systems clone #344823) were added and incubated for 30 min. The samples were washed twice prior to acquisition on a FACSCanto II Immunocytometry system (BD Biosciences) and the data were analysed using FlowJo software version 10 (Ashland, OR, USA).
Statistical analysis
Statistical analysis were performed using the non-parametric Kruskal–Wallis test using GraphPad Prism software version 7. Differences were considered statistically significant at a P value of <0.05.
RESULTS
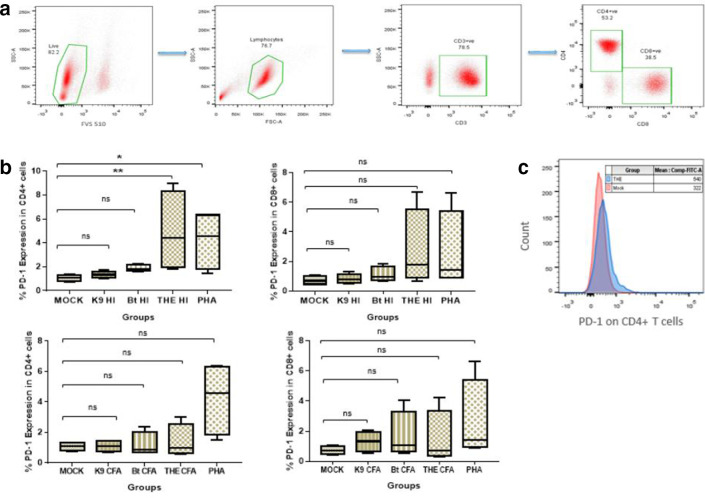
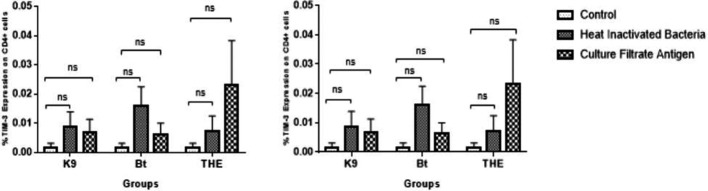
PD-1 was recently shown to be upregulated in persistent B. pseudomallei infections in mice [36], which nonetheless has not been proven in humans. Here, healthy PBMCs were exposed to B. pseudomallei and B. thailandensis antigens in vitro and, following 18 h of incubation, cells were assessed for PD-1 and TIM-3 expression. We found that PD-1 was significantly upregulated upon exposure to HI B. pseudomallei strain THE compared to control, whereas B. pseudomallei strain K9 and B. thailandensis did not alter PD-1 expression. Interestingly, only CD4+ T cell subsets showed upregulated PD-1 upon exposure to HI THE strain compared to both control and B. thailandensis (Fig. 1). However, the CFAs did not alter PD-1 expression on both the T cell subsets (Fig. 1c). Next, we investigated the expression of TIM-3 on both the T cell subsets. There was no statistical significance in TIM-3 expression when compared to control (Fig. 2). We also observed the CD4+ and CD8+ T cell numbers and observed that there was no significant change upon exposure to B. pseudomallei and B. thailandensis antigens (data not shown).
Fig. 1.
PD-1 expression upon exposure to HI bacteria. Peripheral blood mononuclear cells from healthy controls were stimulated in vitro with HI whole bacteria at an m.o.i. f 1 : 10 from the strains THE, B. thailandensis and K96243. (a) Gating strategy used for identifying T cell subsets. All gates were set using appropriate isotype controls. (b) PD-1 expression in CD4+ and CD8+ T cells following 18 h incubation with HI bacteria and culture filtrate antigen. Statistical analysis was performed using the Kruskal–Wallis test. P*<0.0125, P**<0.0025, P***<0.00025 with four Bonferroni comparisons. (c) An overlay histogram plot comparing mean fluorescence intensities of PD-1 in HI THE exposed and antigen-unexposed mock PBMCs. The data presented are representative of four individual experiments (n=4).
Fig. 2.
TIM-3 expression in CD4+ and CD8+ T cells following 18 h of exposure to HI bacteria and culture filtrate antigens. Peripheral blood mononuclear cells from healthy controls were stimulated in vitro with crude culture filtrates (10 ug ml−1) and HI whole bacteria at an m.o.i. of 1 : 10 from the strains THE, B. thailandensis and K96243. Statistical analysis was performed using the Kruskal–Wallis test. P*<0.0125, P**<0.0025, P***<0.00025 with four Bonferroni comparisons. The data presented are representative of four individual experiments (n=4).
DISCUSSION
Recent research shows that the PD-1 pathway is emerging as an important mechanism exploited by many viruses and intracellular bacteria to dampen T cell responses [33–35]. We previously showed that PD-1 is highly upregulated during persistent B. pseudomallei infection in a murine model [36]. Although animal studies allow a closer approximation to human responses, we sought to validate if such PD-1 upregulation is translated in humans. We used PBMCs derived from healthy donors to provide a better understanding of immune responses during human melioidosis. The inclusion of the closely related non-virulent species B. thailandensis in our study helped to illustrate the prominence of PD-1 upregulation by virulent B. pseudomallei . Our findings showed that PD-1 is significantly upregulated by HI B. pseudomallei in vitro. The fact that PD-1 upregulation was seen in CD4+ T cells alone is contradictory to our recent reports on a mouse model, where both CD4+ and CD8+ T cells showed significant PD-1 upregulation [43]. However, previous studies have shown that CD4+ T cell proliferation alone was inhibited upon exposure to human PMNs pulsed with B. pseudomallei antigens [37]. We infer that the CD4+ phenotype of the T cells is important for host resistance against melioidosis in humans [37]. According to our results, only HI whole bacteria led to PD-1 upregulation and not culture filtrate antigen. B. pseudomallei is known to enter a viable but non-culturable (VBNC) state in response to environmental stress [44–46]. The incubation of B. pseudomallei at high temperatures for heat inactivation would have led them to enter a VBNC state and a possible resuscitation upon culturing with PBMCs would have allowed them to regain their ability to cause infection. However, the K96243 isolate did not cause any changes to PD-1 expression. It is unclear why these two strains of B. pseudomallei elicit varying degrees of T cell responses. The co-expression of PD-1 and TIM-3 has previously been reported in chronic viral infections [47, 48] and in M. tuberculosis infection [49, 50]. We investigated the possibility of PD-1 and TIM-3 co-expression in PBMCs exposed to B. pseudomallei and found that no T cells expressed TIM-3. Our study points to the likely role of PD-1 in regulating immune responses, especially those involving T cells in melioidosis, and supports the adoption of strategies to target PD-1 for developing newer therapeutic molecules for use in clinical treatment of melioidosis.
Funding information
The authors acknowledge the funding provided by the Ministry of Higher Education (MOHE), Malaysia under the High Impact Research (HIR)-MOHE project (E000013-20001), the Ministry of Science, Innovation and Technology (MOSTI), Malaysia under the Science Fund (55-02-03-1002) and the University of Malaya research grant (RG029-09HTM) titled ‘Immunology of persistent bacterial infections using Burkholderia pseudomallei as a model’ for J. V. and E. M. S.
Acknowledgements
The authors acknowledge the support provided by the laboratory attendants, and also Ms. Valli Ramanathan for the administrative support rendered throughout the project.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical statement
All experiments involving humans were performed in accordance with the relevant guidelines and regulations and under examination by the Medical Ethics Committee (MEC) of University Malaya Medical Centre (UMMC), Kuala Lumpur, Malaysia (ref. no. 1017.23), and were conducted per the guidelines of the International Conference on Harmonization Guidelines and the Declaration of Helsinki. All individuals involved in the study were over 18 years of age and provided informed consent to participate in the study.
Footnotes
Abbreviations: CFA, cultured filterate antigen; CFU, colony forming units; CMI, cell mediated immunity; DC, dentritic cell; FVS, fixable viability stain; HI, heat inactivated; IFN, interferon; LB, Luria Bertani; MEC, medical ethics committee; NK, natural killer; OD, optical density; PBS, phosphate buffered saline; PD-1, programmed death-1; PHA, phytohaemagglutinin; PMN, polymorphonuclear neutrophil; TCR, T-cell receptor; T3SS, type 3 secretion system; UMMC, University Malaya Medical Centre; VBNC, viable but non-cultivable.
References
- 1.White NJ. Melioidosis. Lancet. 2003;361:1715–1722. doi: 10.1016/S0140-6736(03)13374-0. [DOI] [PubMed] [Google Scholar]
- 2.Currie BJ, Jacups SP, Cheng AC, Fisher DA, Anstey NM, et al. Melioidosis epidemiology and risk factors from a prospective whole-population study in northern Australia. Trop Med Int Health. 2004;9:1167–1174. doi: 10.1111/j.1365-3156.2004.01328.x. [DOI] [PubMed] [Google Scholar]
- 3.Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18:383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dance DA. Melioidosis: the tip of the iceberg? Clin Microbiol Rev. 1991;4:52–60. doi: 10.1128/CMR.4.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Limmathurotsakul D, Golding N, Dance DAB, Messina JP, Pigott DM, et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol. 2016;1:pii: 15008. doi: 10.1038/nmicrobiol.2015.8. [DOI] [PubMed] [Google Scholar]
- 6.Kenny DJ, Russell P, Rogers D, Eley SM, Titball RW. In vitro susceptibilities of Burkholderia mallei in comparison to those of other pathogenic Burkholderia spp. Antimicrob Agents Chemother. 1999;43:2773–2775. doi: 10.1128/AAC.43.11.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bioterrorism Agents/Diseases, by category. http://emergency.cdc.gov/agent/agentlist.asp
- 8.Lim C, Peacock SJ, Limmathurotsakul D. Association between activities related to routes of infection and clinical manifestations of melioidosis. Clin Microbiol Infect. 2016;22:79.e1–7979.:e1-79.e3. doi: 10.1016/j.cmi.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Churuangsuk C, Chusri S, Hortiwakul T, Charernmak B, Silpapojakul K. Characteristics, clinical outcomes and factors influencing mortality of patients with melioidosis in southern Thailand: a 10-year retrospective study. Asian Pac J Trop Med. 2016;9:256–260. doi: 10.1016/j.apjtm.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 10.Pruksachartvuthi S, Aswapokee N, Thankerngpol K. Survival of Pseudomonas pseudomallei in human phagocytes. J Med Microbiol. 1990;31:109–114. doi: 10.1099/00222615-31-2-109. [DOI] [PubMed] [Google Scholar]
- 11.Jones AL, Beveridge TJ, Woods DE. Intracellular survival of Burkholderiapseudomallei . Infect Immun. 1996;64:782–790. doi: 10.1128/IAI.64.3.782-790.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harley VS, Dance DA, Drasar BS, Tovey G. Effects of Burkholderia pseudomallei and other Burkholderia species on eukaryotic cells in tissue culture. Microbios. 1998;96:71–93. [PubMed] [Google Scholar]
- 13.Ngauy V, Lemeshev Y, Sadkowski L, Crawford G. Cutaneous melioidosis in a man who was taken as a prisoner of war by the Japanese during World War II. J Clin Microbiol. 2005;43:970–972. doi: 10.1128/JCM.43.2.970-972.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Currie BJ, Fisher DA, Anstey NM, Jacups SP. Melioidosis: acute and chronic disease, relapse and re-activation. Trans R Soc Trop Med Hyg. 2000;94:301–304. doi: 10.1016/S0035-9203(00)90333-X. [DOI] [PubMed] [Google Scholar]
- 15.Chaowagul W, Suputtamongkol Y, Dance DA, Rajchanuvong A, Pattara-arechachai J, et al. Relapse in melioidosis: incidence and risk factors. J Infect Dis. 1993;168:1181–1185. [PubMed] [Google Scholar]
- 16.Ireland PM, Marshall L, Norville I, Sarkar-Tyson M. The serine protease inhibitor Ecotin is required for full virulence of Burkholderia pseudomallei . Microb Pathog. 2014;67-68:55–58. doi: 10.1016/j.micpath.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Stevens MP, Wood MW, Taylor LA, Monaghan P, Hawes P, et al. An Inv/Mxi-Spa-like type III protein secretion system in Burkholderia pseudomallei modulates intracellular behaviour of the pathogen. Mol Microbiol. 2002;46:649–659. doi: 10.1046/j.1365-2958.2002.03190.x. [DOI] [PubMed] [Google Scholar]
- 18.Kang W-T, Vellasamy KM, Chua E-G, Vadivelu J. Functional characterizations of effector protein BipC, a type III secretion system protein, in Burkholderia pseudomallei pathogenesis. J Infect Dis. 2015;211:827–834. doi: 10.1093/infdis/jiu492. [DOI] [PubMed] [Google Scholar]
- 19.Suparak S, Kespichayawattana W, Haque A, Easton A, Damnin S, et al. Multinucleated giant cell formation and apoptosis in infected host cells is mediated by Burkholderia pseudomallei type III secretion protein BipB. J Bacteriol. 2005;187:6556–6560. doi: 10.1128/JB.187.18.6556-6560.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun GW, Lu J, Pervaiz S, Cao WP, Gan Y-H. Caspase-1 dependent macrophage death induced by Burkholderia pseudomallei . Cell Microbiol. 2005;7:1447–1458. doi: 10.1111/j.1462-5822.2005.00569.x. [DOI] [PubMed] [Google Scholar]
- 21.Charoensap J, Utaisincharoen P, Engering A, Sirisinha S. Differential intracellular fate of Burkholderia pseudomallei 844 and Burkholderia thailandensis UE5 in human monocyte-derived dendritic cells and macrophages. BMC Immunol. 2009;10:20. doi: 10.1186/1471-2172-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santanirand P, Harley VS, Dance DA, Drasar BS, Bancroft GJ. Obligatory role of gamma interferon for host survival in a murine model of infection with Burkholderia pseudomallei . Infect Immun. 1999;67:3593–3600. doi: 10.1128/IAI.67.7.3593-3600.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lauw FN, Simpson AJ, Prins JM, Smith MD, Kurimoto M, et al. Elevated plasma concentrations of interferon (IFN)-gamma and the IFN-gamma-inducing cytokines interleukin (IL)-18, IL-12, and IL-15 in severe melioidosis. J Infect Dis. 1999;180:1878–1885. doi: 10.1086/315155. [DOI] [PubMed] [Google Scholar]
- 24.Ulett GC, Ketheesan N, Hirst RG. Macrophage-lymphocyte interactions mediate anti-Burkholderia pseudomallei activity. FEMS Immunol Med Microbiol. 1998;21:283–286. doi: 10.1111/j.1574-695X.1998.tb01175.x. [DOI] [PubMed] [Google Scholar]
- 25.Lertmemongkolchai G, Cai G, Hunter CA, Bancroft GJ. Bystander activation of CD8+ T cells contributes to the rapid production of IFN-gamma in response to bacterial pathogens. J Immunol. 2001;166:1097–1105. doi: 10.4049/jimmunol.166.2.1097. [DOI] [PubMed] [Google Scholar]
- 26.Tanphaichitra D, Srimuang S. Cellular immunity (T-cell subset using monoclonal antibody) in tuberculosis, melioidosis, pasteurellosis, penicilliosis; and role of levamisole and Isoprinosine. Dev Biol Stand. 1984;57:117–123. [PubMed] [Google Scholar]
- 27.Ramsay SC, Ketheesan N, Norton R, Watson A-M, LaBrooy J. Peripheral blood lymphocyte subsets in acute human melioidosis. Eur J Clin Microbiol Infect Dis. 2002;21:566–568. doi: 10.1007/s10096-002-0768-3. [DOI] [PubMed] [Google Scholar]
- 28.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 29.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown KE, Freeman GJ, Wherry EJ, Sharpe AH. Role of PD-1 in regulating acute infections. Curr Opin Immunol. 2010;22:397–401. doi: 10.1016/j.coi.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 32.Singh A, Mohan A, Dey AB, Mitra DK. Inhibiting the programmed death 1 pathway rescues Mycobacterium tuberculosis-specific interferon γ-producing T cells from apoptosis in patients with pulmonary tuberculosis. J Infect Dis. 2013;208:603–615. doi: 10.1093/infdis/jit206. [DOI] [PubMed] [Google Scholar]
- 33.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, et al. Pd-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, et al. Pd-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 35.Shankar EM, Che KF, Messmer D, Lifson JD, Larsson M. Expression of a broad array of negative costimulatory molecules and Blimp-1 in T cells following priming by HIV-1 pulsed dendritic cells. Mol Med. 2011;17:229–240. doi: 10.2119/molmed.2010.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.See J-X, Chandramathi S, Abdulla MA, Vadivelu J, Shankar EM. Persistent infection due to a small-colony variant of Burkholderia pseudomallei leads to PD-1 upregulation on circulating immune cells and mononuclear infiltration in viscera of experimental BALB/c mice. PLoS Negl Trop Dis. 2017;11:e0005702. doi: 10.1371/journal.pntd.0005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buddhisa S, Rinchai D, Ato M, Bancroft GJ, Lertmemongkolchai G. Programmed death ligand 1 on Burkholderiapseudomallei-infected human polymorphonuclear neutrophils impairs T cell functions. J Immunol. 2015;194:4413–4421. doi: 10.4049/jimmunol.1402417. [DOI] [PubMed] [Google Scholar]
- 38.Sarkar-Tyson M, Smither SJ, Harding SV, Atkins TP, Titball RW. Protective efficacy of heat-inactivated B. thailandensis, B. mallei or B. pseudomallei against experimental melioidosis and glanders. Vaccine. 2009;27:4447–4451. doi: 10.1016/j.vaccine.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 39.Cuccui J, Milne TS, Harmer N, George AJ, Harding SV, et al. Characterization of the BurkholderiapseudomalleiK96243 capsular polysaccharide I coding region. Infect Immun. 2012;80:1209–1221. doi: 10.1128/IAI.05805-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elvin SJ, Healey GD, Westwood A, Knight SC, Eyles JE, et al. Protection against heterologous Burkholderiapseudomallei strains by dendritic cell immunization. Infect Immun. 2006;74:1706–1711. doi: 10.1128/IAI.74.3.1706-1711.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collins FM, Lamb JR, Young DB. Biological activity of protein antigens isolated from Mycobacterium tuberculosis culture filtrate. Infect Immun. 1988;56:1260–1266. doi: 10.1128/IAI.56.5.1260-1266.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 43.See J-X, Samudi C, Saeidi A, Menon N, Choh L-C, et al. Experimental persistent infection of BALB/c mice with small-colony variants of Burkholderia pseudomallei leads to concurrent upregulation of PD-1 on T cells and skewed Th1 and Th17 responses. PLoS Negl Trop Dis. 2016;10:e0004503. doi: 10.1371/journal.pntd.0004503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim HS, Schell MA, Yu Y, Ulrich RL, Sarria SH, et al. Bacterial genome adaptation to niches: divergence of the potential virulence genes in three Burkholderia species of different survival strategies. BMC Genomics. 2005;6:174. doi: 10.1186/1471-2164-6-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oliver JD. Recent findings on the viable but nonculturable state in pathogenic bacteria. FEMS Microbiol Rev. 2010;34:415–425. doi: 10.1111/j.1574-6976.2009.00200.x. [DOI] [PubMed] [Google Scholar]
- 46.Inglis TJJ, Sagripanti J-L. Environmental factors that affect the survival and persistence of Burkholderia pseudomallei . Appl Environ Microbiol. 2006;72:6865–6875. doi: 10.1128/AEM.01036-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, et al. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J Clin Invest. 2010;120:4546–4557. doi: 10.1172/JCI43127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Z-N, Zhu M-L, Chen Y-H, Fu Y-J, Zhang T-W, et al. Elevation of Tim-3 and PD-1 expression on T cells appears early in HIV infection, and differential Tim-3 and PD-1 expression patterns can be induced by common γ -chain cytokines. Biomed Res Int. 2015;2015:916936. doi: 10.1155/2015/916936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Day CL, Abrahams DA, Bunjun R, Stone L, de Kock M, et al. PD-1 expression on Mycobacterium tuberculosis – specific CD4 T cells is associated with bacterial load in human tuberculosis. Front Jmmunol. 1995;2018:9. doi: 10.3389/fimmu.2018.01995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jayaraman P, Jacques MK, Zhu C, Steblenko KM, Stowell BL, et al. TIM3 mediates T cell exhaustion during Mycobacterium tuberculosis infection. PLoS Pathog. 2016;12:e1005490. doi: 10.1371/journal.ppat.1005490. [DOI] [PMC free article] [PubMed] [Google Scholar]