Abstract
Dust in homes can contain phthalates that may adversely affect child development, but whether residential interventions and dust removal can prevent children’s exposure to phthalates is unknown. We quantified the influence of a residential lead hazard intervention and dust control on children’s urinary phthalate metabolite concentrations. Between 2003 and 2006, The Health Outcomes and Measures of the Environment (HOME) Study randomized 355 pregnant women to receive an intervention to reduce either residential lead or injury hazards before delivery. We quantified eight urinary phthalate metabolites in 288 children at ages 1, 2, or 3 years (680 observations). During yearly home visits, we assessed dust accumulation in housing units. Children in the lead intervention group had 11–12% lower concentrations of the sum of di(2-ethylhexyl) phthalate metabolites, monocarboxyoctyl phthalate, and monocarboxynonyl phthalate compared to the injury intervention group. Monoethyl phthalate concentrations did not differ by group. In observational analyses, children living in housing units that appeared clean had 12–17% lower concentrations of these phthalate metabolites and monobenzyl phthalate, compared to children living in housing units with more dust accumulation. Features of this lead hazard intervention and measures to control dust may reduce children’s exposure to phthalates found in building materials and household furnishings.
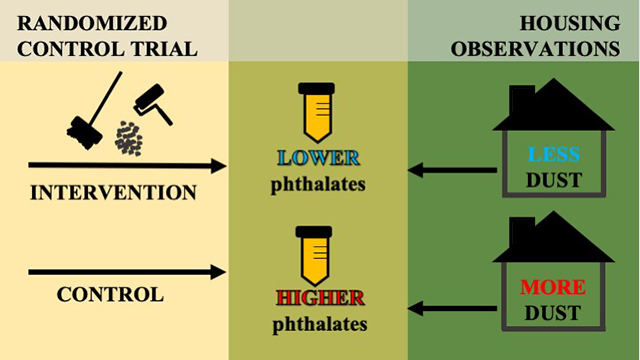
Graphical Abstract
INTRODUCTION
Phthalates, commonly used as plasticizers in household products and building materials, can be released into indoor air and settle in household dust.1 While diet and personal care product use are major routes of exposure to many phthalates, ingestion and inhalation of phthalate-containing dust may also be an important exposure pathway, especially during early childhood.
Some prior studies have reported correlations between dust concentrations of some phthalates and children’s urinary phthalate metabolite concentrations,2 as well as associations between phthalate exposure in household dust and an increased risk of asthma, developmental delay, and rhino-conjunctivitis.3–5 Gestational and childhood exposure to phthalates may also contribute to an increased risk of allergies, early-onset eczema, obesity, and attention-deficit/hyperactivity disorder in children.6–10
Residential modifications, such as paint stabilization and dust control measures, are routinely implemented to reduce childhood lead exposure. Given that these measures aim to lower lead exposure by reducing levels of contaminated dust, we hypothesized that these interventions could also lower children’s exposure to phthalates released from paints and building materials into dust. Thus, we conducted a secondary analysis of a randomized trial designed to evaluate the effect of a lead hazard intervention on urinary phthalate metabolite concentrations at ages 1, 2, and 3 years. We further explored the relationship between cleaning in housing units and phthalate exposure, by evaluating the association between repeated observations of housing dust accumulation and urinary phthalate metabolite concentrations.
METHODS
Participant Recruitment and Intervention Assignment
Between 2003 and 2006, research staff recruited pregnant women living in the Cincinnati, Ohio metropolitan area from nine local prenatal clinics. Eligibility criteria included: English fluency; 18 years of age or older; 16 ± 3 weeks gestation; plan to continue prenatal care and deliver at collaborating clinics and hospitals; residence in a home built in or prior to 1978 which was not a mobile or trailer home; plan to live in the greater Cincinnati metropolitan area for at least one year; HIV-negative; not taking medications for seizures or thyroid disorders; not diagnosed with diabetes, bipolar disorder, schizophrenia, or cancer. We targeted women living in homes built before 1978, the year residential lead paints were banned in the United States, and we oversampled women self-identifying as non-Hispanic black because of their children’s increased risk of lead exposure.
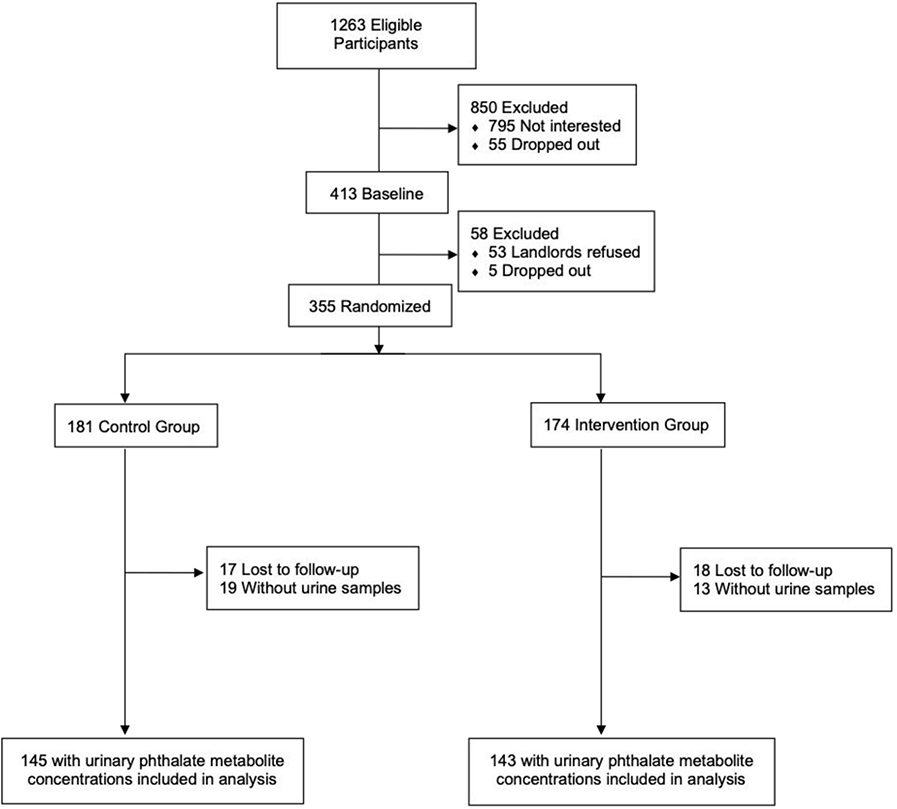
Of 1,263 eligible women, 468 (37%) agreed to participate; 60 (13%) dropped out before delivery of their infant and 53 (11%) had landlords refuse to participate (Figure 1). The remaining 355 women were assigned in blocks of 10 to receive a residential intervention to reduce lead hazards or injury hazards. We sealed assignment codes in radio-opaque envelopes until research assistants confirmed participant’s eligibility. Of the 355 families who participated in the trial, 288 children had urinary phthalate metabolite concentrations measured at ages 1, 2, or 3 years. Participants were included in this analysis if urinary phthalate concentrations were quantified at one or more of these three timepoints.
Figure 1.
Flow diagram of participant recruitment and randomization in The HOME Study (Cincinnati, OH; 2003–06).
The primary analysis of the effects of the residential lead and injury hazard interventions have been previously published.11,12 For this analysis, we treated participants receiving the injury hazard intervention as the control group and participants receiving the lead hazard intervention as the intervention group. The institutional review boards (IRB) of Cincinnati Children’s Hospital Medical Center (CCHMC) and the participating prenatal clinics and delivery hospitals approved the HOME Study. The Centers for Disease Control and Prevention (CDC) and Brown University deferred to the CCHMC IRB as the IRB of record. Women provided written informed consent for themselves and their children after research assistants explained study protocols during face-to-face visits. The trial protocol was registered on August 11, 2005 (ClinicalTrials.gov identifier: NCT00129324).
Residential Intervention
In the intervention group, we implemented a series of measures to remove or reduce lead hazards in paint, dust, water, and soil in and around the homes of women prior to 32 weeks gestation and delivery. The intervention included paint stabilization which involved repairing any deteriorating or water-damaged wall material, removing loose or peeling paint, reapplying new paint, and thoroughly cleaning the area using wet methods. In addition, study contractors took measures to reduce dust accumulation in the housing unit by installing window trough liners, as well as creating smooth and cleanable floors and windows so that dust could be removed from surfaces by normal cleaning. For example, creating smooth and cleanable floors involved repairing or replacing damaged floor tiles and refinishing wooden floors. The lead hazard intervention also involved covering exposed lead-contaminated soil with groundcover; installing tap filters for drinking water lead sources; repairing areas with deteriorating lead-based paint; and replacing windows with deteriorating lead-based paint. We did extensive clean-up and dust removal after the intervention was completed. We adapted the intervention based on the condition of each home and attempted to implement the intervention in new housing units when families moved before the child was 23 months of age. Before children in the control group turned 6 months old, we installed injury prevention devices or conducted residential modifications to reduce residential injury hazards.12 The types of injury prevention devices that we installed included stair gates, cabinet locks, and smoke detectors. We did not expect any of the devices installed to have an impact on the dust accumulation in the housing unit. If children in the control group moved before 23 months of age, we attempted to implement the injury hazard intervention in their new housing unit.
Characterization of Dust Accumulation and Cleaning in the Housing Unit
During home visits at baseline, 1, 2, and 3 years of age, research assistants assessed cleaning and the accumulation of dust and debris in the entire home, including the kitchen, main activity room, bathrooms, child’s bedroom, and stairwells using standardized criteria. Based on the presence of dust, soiled carpeting, cobwebs, and indications of cleaning in all rooms, the research assistant classified the housing unit into one of three groups: appears clean, some evidence of cleaning, and no evidence of cleaning (see Table S1). Due to the small number of housing units with no evidence of cleaning, we combined the latter two groups into one, which we refer to as “does not appear clean” in the main analyses. We assessed how combining the two categories in the main analysis impacted our results by conducting sensitivity analyses with all three of the original categories.
Quantification of Urinary Phthalate Metabolite Concentrations
Of the 174 women assigned to the intervention group and 181 women assigned to the control group, 143 (82%) and 145 (80%), had children with urinary phthalate metabolite concentrations measured at age 1, 2, or 3 years, respectively (see Table S2). In these urine samples, we examined metabolites of phthalates that have been quantified in household dust, including di(2-ethylhexyl) phthalate (DEHP), benzylbutyl phthalate (BBzP), di-isononyl phthalate (DiNP), and di-isodecyl phthalate (DiDP). We also included a negative control, diethyl phthalate (DEP), which has not been detected frequently in household dust.13–19
We collected spot urine samples from children at ages 1, 2, and 3 years. Prior to the sample collection, the caregiver wiped the child’s genitals with a phthalate-free wipe. We collected urine samples from children who did not use the toilet by placing an insert in a clean diaper. After collection, the insert was placed into a polypropylene cup, and urine was later expressed from the insert using a syringe. For children who were learning how to use the toilet, we used training toilets lined with inserts to collect urine samples. Caregivers helped children who used the toilet to collect urine directly into the cup. All samples were stored at −20 °C until they were shipped overnight on dry ice to the CDC for analysis.
Using previously described methods, we quantified concentrations of mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), and mono-2-ethyl-5-carboxypentyl phthalate (MECPP), which are metabolites of DEHP, as well as monobenzyl phthalate (MBzP), monocarboxyoctyl phthalate (MCOP), monocarboxynonyl phthalate (MCNP), and monoethyl phthalate (MEP) which are metabolites of BBzP, DiNP, DiDP, and DEP, respectively.20 These metabolites, specific to select parent compounds, have been detected in the urine of the majority of the U.S. general population since the mid-2000s.21 We were not able to assess urinary concentration of mono-2-ethylhexyl phthalate (MEHP), mono-n-butyl phthalate (MnBP), or monoisobutyl phthalate (MiBP) due to phthalate contamination from diaper inserts. Laboratory staff were blinded to participant’s intervention status. We created a summary DEHP measure (ΣDEHP) by dividing MEHHP, MEOHP, and MECPP concentrations by their respective molar mass, summing the molar metabolite concentrations, and then multiplying by the molar mass of MECPP to express the sum in units of ng/mL. We quantified urinary creatinine concentrations using enzymatic methods to account for individual urine dilution.
Participant and Housing Unit Characteristics
We documented participant’s age and season at the time of urine collection, as well as their sex, race, and ethnicity at birth. We conducted structured interviews at each home visit to gather information about maternal education, household income, and marital status, as well as housing characteristics and behaviors that could potentially confound the relationship between dust accumulation in the housing unit and urinary phthalate metabolite concentrations. We calculated the average urinary ΣDEHP, MBzP, and MEP concentrations in maternal samples collected at 16- and 26-weeks gestation to estimate maternal exposure to the parent phthalates at baseline. At the time of the prenatal analysis, the assay for MCOP and MCNP was not yet developed. We also documented the year the participant’s home was built and the type of flooring visible in the main activity room (high carpet, mid carpet, low carpet, wood/tile/ceramic, or vinyl). We dichotomized flooring type into carpeting and no carpeting groups due to the low frequency of some types of flooring (i.e. vinyl flooring, high carpet, and wood/tile/ceramic).
Statistical Analysis
Intervention and Phthalate Metabolite Concentrations
For the intention-to-treat analysis of the secondary trial, we first calculated proportions for categorical variables as well as means for continuous variables and compared participant characteristics at baseline between the intervention and control groups. We also compared characteristics of houses that appeared clean to those that did not appear clean at baseline.
We log10-transformed urinary phthalate metabolite concentrations and compared the distribution of repeated urinary phthalate metabolite concentrations measured at ages 1, 2, and 3 years. Because season could be associated with both dust accumulation and dust phthalate concentrations, we compared the proportions of urine samples collected by season in the intervention and control groups. Using linear mixed models with a compound symmetry covariance matrix, identified as the optimal covariance structure based on the Akaike Information Criteria, we estimated differences in the geometric mean urinary phthalate metabolite concentrations by intervention group. We adjusted these models for the children’s age at urine collection and log10-transformed creatinine concentrations to control for urine dilution. In a per protocol analysis, we included only participants who did not move from their baseline residence before age 3 years (56% of total participants (n=161); 57% of participants in control group (n=83); 55% of participants in the intervention group (n=78)) and repeated all analyses.
Dust Accumulation and Urinary Phthalate Metabolite Concentrations
We used linear mixed models with an unstructured covariance matrix and random intercept to estimate the association between time-varying repeated measures of dust accumulation and urinary phthalate concentrations at ages 1, 2, and 3 years. We identified covariates to include in the linear mixed models based on prior knowledge about characteristics which could be related to dust accumulation in housing units and phthalate exposure. We adjusted all models for child age, sex, race/ethnicity, log10-transformed urinary creatinine concentration (time-varying), intervention group, house flooring type, maternal education, household income (time-varying), and season of urine collection (time-varying). We also constructed adjusted linear mixed models considering the three categories of dust accumulation (time-varying) and conducted a trend test by treating the categories as a continuous variable.
Dust Accumulation and Intervention Effectiveness
In the randomized trial and observational analyses, we assessed if dust accumulation pre- and post-intervention impacted the effectiveness of the lead hazard intervention. To evaluate if the effectiveness of the intervention varied based on dust accumulation in the housing unit prior to the trial, we included a product interaction term between intervention group and dust accumulation at baseline (appears clean or does not appear clean). We also stratified the sample by dust accumulation at baseline and evaluated if the effect of the intervention on urinary phthalate metabolite concentrations varied in housing units appearing clean compared to those that did not appear clean.
We evaluated if the effectiveness of the intervention varied based on dust accumulation characterized at 1, 2, and 3 years post-trial. First, we added an intervention and post-trial dust accumulation product interaction term to fully adjusted models to assess relative reductions in urinary phthalate metabolites due to additive interaction. Next, we parameterized a combined variable with four categories: 1) Control group and does not appear clean; 2) Control group and appears clean; 3) Intervention group and does not appear clean; 4) Intervention group and appears clean. We treated the “Control group and does not appear clean” category as the reference group and assessed the relationship of this variable with urinary phthalate metabolite concentrations in adjusted models.
Sensitivity Analysis
In sensitivity analyses, we compared results from statistical models adjusting for log10-transformed creatinine concentrations as a covariate to models using creatinine-standardized urinary phthalate metabolite concentrations as the outcome variable. Urinary phthalate metabolite concentrations were creatinine-standardized by dividing each phthalate metabolite concentration by urinary creatinine concentrations and multiplying by 100. In the dust accumulation analyses, we assessed the robustness of our model by comparing our results from a fully adjusted model to the results from minimally-adjusted models only including child age, sex, and intervention status as covariates. We also assessed if other behaviors or characteristics not captured by covariates included in the adjusted models may confound our main results. In sensitivity analyses we constructed models adjusting for additional behavioral characteristics, including breastfeeding duration, pacifier use, toy mouthing behaviors, and consumption of pre-packaged fruits and vegetables.
RESULTS
Participant and Housing Unit Characteristics
Compared to pregnant women in the control group, women in the intervention group had higher median urinary ΣDEHP metabolite concentrations at baseline. Urinary concentrations of other phthalate metabolites did not statistically differ between the two groups (Table S5). In the intervention group, approximately 50% of children were males and 66% self-identified as non-Hispanic white, while 43% of children in the control group were males and 75% were non-Hispanic white. The majority of children in both groups were living in a household with at least two adults. Approximately 43% and 45% of participants in the control and intervention groups had an annual income greater than $70,000, respectively (Table 1). There was no notable difference between the intervention and control groups in the proportion of home ownership, year the home was built, or the type of housing (single-family versus multi-family). Overall the majority of housing units appeared clean at baseline (64% and 63% of the intervention and control groups, respectively). For housing units that appeared clean, the mean year of construction was 1941 (SD=47). On average, housing units that did not appear clean were older (mean year constructed=1927; SD=28). Housing units that appeared clean at baseline were more likely to be single-family homes or townhouses (86% vs. 70%) and owner-occupied (85% vs. 56%).
Table 1.
HOME Study participant characteristics at baseline for control and lead hazard intervention groups.
| Total | Control Group | Intervention Group | ||||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | |
| Total | 288 | (100) | 145 | (100) | 143 | (100) |
| Child Sex | ||||||
| Male | 135 | (47) | 63 | (43) | 72 | (50) |
| Female | 153 | (53) | 82 | (57) | 71 | (50) |
| Child Race/Ethnicity | ||||||
| Non-Hispanic white | 202 | (70) | 108 | (75) | 94 | (66) |
| Non-Hispanic black | 66 | (23) | 29 | (20) | 37 | (26) |
| Hispanic | 7 | (2) | 3 | (2) | 4 | (3) |
| Other | 13 | (5) | 5 | (3) | 8 | (5) |
| Marital Status | ||||||
| Married, or living with someone | 241 | (84) | 125 | (87) | 116 | (81) |
| Not married, living alone | 47 | (16) | 20 | (14) | 27 | (19) |
| Household Income | ||||||
| Less than $20,000 | 46 | (16) | 22 | (15) | 24 | (17) |
| $20,000 to less than $40,000 | 39 | (14) | 20 | (14) | 19 | (13) |
| $40,000 to $70,000 | 75 | (26) | 40 | (28) | 35 | (24) |
| Greater than $70,000 | 128 | (44) | 63 | (43) | 65 | (45) |
| Maternal Education | ||||||
| Bachelor’s degree or higher | 171 | (59) | 85 | (59) | 86 | (60) |
| Some college or technical school | 72 | (25) | 40 | (28) | 32 | (22) |
| High school or less | 45 | (16) | 20 | (14) | 25 | (17) |
| Cleaning/ Dust accumulation | ||||||
| Appears clean | 183 | (64) | 92 | (63) | 91 | (64) |
| Some or no cleaning | 105 | (36) | 53 | (37) | 52 | (36) |
| Home Ownership | ||||||
| Own | 214 | (75) | 111 | (77) | 103 | (72) |
| Public | 13 | (5) | 5 | (3) | 8 | (6) |
| Rent | 55 | (19) | 25 | (17) | 30 | (21) |
| Other | 5 | (2) | 3 | (2) | 2 | (1) |
| Year Home was Constructed | ||||||
| 1924 or earlier | 93 | (32) | 47 | (32) | 46 | (32) |
| 1925 to 1955 | 107 | (37) | 56 | (39) | 51 | (36) |
| 1955 to 1978 | 87 | (30) | 42 | (29) | 45 | (32) |
| House Type | ||||||
| Single family or townhouse | 231 | (80) | 119 | (82) | 112 | (78) |
| Multi-family, apartment, or other | 57 | (20) | 26 | (18) | 31 | (22) |
Phthalate Metabolite Concentrations
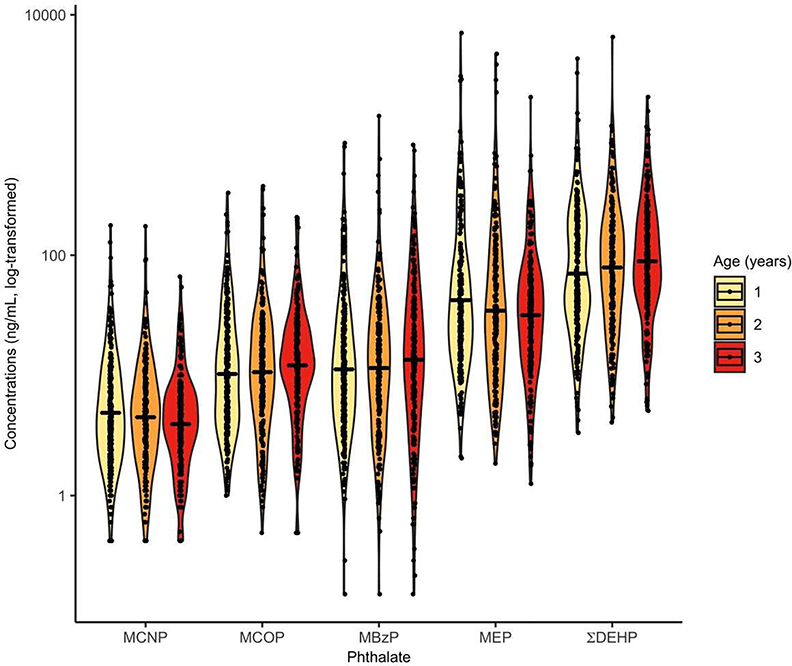
The proportion of urine samples collected from participants at ages 1, 2, and 3 years, as well as the season of collection, was similar for the intervention and control groups (see Table S2 and Table S3). The geometric means of urinary phthalate metabolite concentrations were relatively stable across samples collected at ages 1, 2, and 3 years (Figure 2 and see Table S4). Overall, geometric mean urinary phthalate concentrations were highest for ΣDEHP, followed by MEP, MBzP, MCOP, and MCNP.
Figure 2.
Distribution of urinary phthalate metabolite concentrations by annual visit at ages 1, 2, 3 years (The HOME Study; Cincinnati, OH).
Solid line indicates geometric mean of each distribution. Jittered dots are individual observations. Smoothed lines are density functions of urinary phthalate metabolite concentrations.
Total participants = 288 (680 observations).
Abbreviations: summary DEHP (ΣDEHP) = sum of the molar mass of mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), and mono-2-ethyl-5-carboxypentyl phthalate (MECPP); monobenzyl phthalate (MBzP); monocarboxyoctyl phthalate (MCOP); monocarboxynonyl phthalate (MCNP); monoethyl phthalate (MEP)
Intervention and Phthalate Metabolite Concentrations
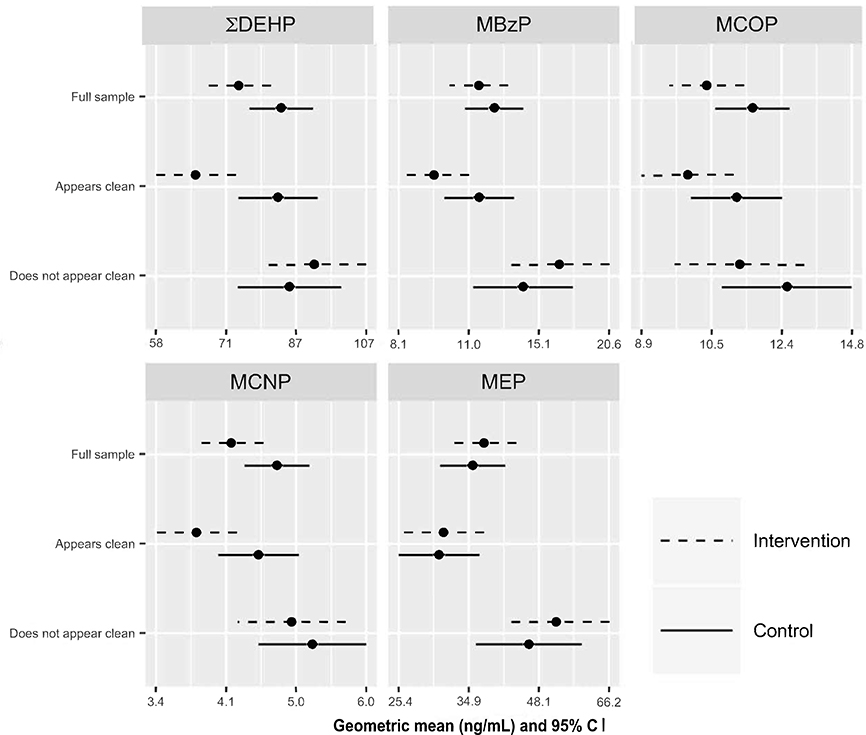
Urinary concentrations of ΣDEHP, MCOP, and MCNP were 12% (95% Cl= −23, 1), 11% (95% Cl= −21, 2), and 12% (95%= −22, 0) lower, respectively, among children in the intervention group compared with those in the control group (Figure 3 and see Table S6). In contrast, urinary MEP concentrations were similar for participants in the two groups, with children in the intervention group having 5% higher concentrations (95% Cl= −15, 30).
Figure 3.
Adjusted geometric mean (95% confidence interval) childhood urinary phthalate metabolite concentration (ng/mL) by intervention group in the full sample and by baseline dust accumulation (The HOME Study; Cincinnati, OH).a
a Model adjusted for log10-transformed urinary creatinine concentrations and age (years).
Total participants = 288 (145 control and 143 intervention group).
Abbreviations: summary DEHP (ΣDEHP) = sum of the molar mass of mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), and mono-2-ethyl-5-carboxypentyl phthalate (MECPP); monobenzyl phthalate (MBzP); monocarboxyoctyl phthalate (MCOP); monocarboxynonyl phthalate (MCNP); monoethyl phthalate (MEP).
In the per protocol analysis, estimates of the intervention effect on urinary phthalate metabolite concentrations were generally less precise but similar in magnitude to the estimates from the intention-to-treat analysis (see Table S7 and Table S8). Participants who lived in the same home throughout the study were mostly non-Hispanic white (85%), living in a household with at least two adults (94%), had a mother with a college degree (72%) who owned the home (91%) and had an annual income greater than $70,000 (61%). In addition, they were more likely to live in a housing unit that appeared clean at baseline (73%).
Dust Accumulation and Phthalate Metabolite Concentrations
The housing units of participants in the intervention group were not more likely to appear clean at 1, 2, and 3 year visits compared to participants in the control group (60% compared to 58%, respectively). Compared to mothers who lived in housing units with slight to heavy dust accumulation, mothers who lived in housing that appeared clean were more likely to have a college degree (75% vs 40%) and an annual household income over $70,000 (63% vs 35%; Table S9).
After adjusting for covariates, children who lived in housing units that appeared clean had 12% (95% Cl= −24, 1), 17% (95%= −30, −2), 12% (95%CI= −23, 1) and 16% (95% Cl= −25, −5) lower urinary ΣDEHP, MBzP, MCOP, and MCNP concentrations compared with children who lived in housing units that did not appear clean. Urinary MEP concentrations of participants who lived in housing that appeared clean were similar to those of participants who lived in housing that did not appear clean (0% difference; 95% Cl= −16, 19); see Table S10).
In adjusted models considering the three categories of dust accumulation, we found evidence that lower levels of dust accumulation were monotonically associated with lower urinary concentrations of ΣDEHP (p-value for trend=0.014), MBzP (p-value for trend=0.005), MCOP (p-value for trend=0.023), and MCNP (p-value for trend=0.005) (see Table S11). For instance, children living in households that appeared clean (observations=382) or had slight dust accumulation (observations=241) had 39% lower (95% Cl= −57, −12) and 31% lower (95% Cl= −51, −3) urinary ΣDEHP concentrations, respectively, compared to children who lived in housing units with heavy dust accumulation (observations= 27).
Dust Accumulation and Intervention Effectiveness
Dust accumulation in housing units prior to the trial modified the effect of the intervention on ΣDEHP and MBzP concentrations (intervention by baseline dust accumulation interaction p-values = 0.03 and 0.06, respectively). Among participants who lived in housing units that appeared clean at baseline, children in the intervention group had 21% lower ΣDEHP concentrations (95% Cl= −33, −7) and 18% lower MBzP concentrations (95% Cl= −34, 2) compared with children in the control group (Figure 3; see Table S6). Among participants who lived in housing units that did not appear clean at baseline, children in the intervention group had 8% higher ΣDEHP concentrations (95% Cl= −13, 34) and 17% higher MBzP concentrations (95% Cl= −15, 61).
In adjusted models, we did not find a greater than additive effect of the intervention and dust accumulation post-trial for urinary MBzP (intervention by dust accumulation product interaction term p= 0.99), MCOP (intervention by dust accumulation product interaction term p= 0.85), MCNP (intervention by dust accumulation product interaction term p= 0.76), or MEP (intervention by dust accumulation product interaction term p=0.64) concentrations. In adjusted models, we found evidence of a greater than additive effect of the intervention and living in a housing unit that appeared clean post-trial on urinary ΣDEHP concentrations (intervention by dust accumulation product interaction term p=0.07). Participants in the intervention group who lived in housing that appeared clean post-trial, had 21% lower urinary concentrations of ΣDEHP (95% Cl= −35, −4) compared to participants in the control group who lived in housing that did not appear clean post-intervention. On average, urinary MBzP, MCOP, and MCNP concentrations were lowest among participants in the intervention group who also lived in housing units that appeared clean post-trial (Table 2).
Table 2.
Adjusted geometrie mean childhood urinary phthalate metabolite concentrations (ng/mL) and percent differences by combined intervention and post-trial household cleanliness category (The HOME Study; Cincinnati, OH). a
| ∑DEHP | MBzP | MCOP | MCNP | MEP | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention / Cleanliness Category | GM | %Difference (95% CI) | p-valueb | GM | %Difference (95% CI) | p-valueb | GM | %Difference (95% CI) | p-valueb | GM | %Difference (95% CI) | p-valueb | GM | %Difference (95% CI) | p-valueb |
| Control / Does not appear clean | 93 | Ref | 0.020 | 19 | Ref | 0.14 | 13 | Ref | 0.21 | 5.3 | Ref | 0.021 | 57 | Ref | 0.97 |
| Control / Appears clean | 91 | −1 (−18, 19) | 16 | −17 (−34, 4) | 11 | −13 (−27, 4) | 4.4 | −17 (−29, −3) | 60 | 4 (−18, 31) | |||||
| Intervention / Does not appear clean | 94 | 2 (−17, 25) | 17 | −7 (−28, 19) | 12 | −7 (−24, 12) | 4.8 | −9 (−24, 9) | 59 | 2 (−21, 33) | |||||
| Intervention / Appears clean | 73 | −21 (−35, −4) | 14 | −23 (−39, −2) | 10 | −17 (−31, −1) | 4.2 | −22 (−34, −7) | 56 | −2 (−23, 26) | |||||
Model adjusted for child age (continuous), sex (male vs. female), race/ethnicity (Non-Hispanic white vs. Non-Hispanic Black vs. Hispanic or other race/ethnicity), log10-transformed urinary creatinine concentration (continuous), flooring type (carpet vs. non-carpet), maternal education (Bachelor’s degree or higher vs. Tech/some college vs. High school or less), household income ( <$20,000 vs. $20,000 to <$40,000 vs. $40,000 to $70,000 vs. >$70,000), and season (spring vs. summer vs. fall vs, winter).
p-value for effect of combined intervention/cleanliness categorical variable.
Observations: Control/ Does not appear clean = 137; Control/Appears clean = 189; Intervention/ Does not appear clean = 131; Intervention/Appears clean = 193. Abbreviations: GM = geometrie mean; summary DEHP (∑DEHP) = sum of the molar mass of mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), and mono-2-ethyl-5-carboxypentyl phthalate (MECPP); monobenzyl phthalate (MBzP); monocarboxyoctyl phthalate (MCOP); monocarboxynonyl phthalate (MCNP); monoethyl phthalate (MEP).
Sensitivity Analyses
Our results were not meaningfully different when we used creatinine-standardized urinary concentrations of phthalate metabolites for analyses (see Table S11 and Table S12). The results of the dust accumulation analyses were not meaningfully different in minimally-adjusted models or models adjusted for additional covariates (breastfeeding duration, pacifier use, toy mouthing behaviors, and consumption of pre-packaged fruits and vegetables; see Table S13).
DISCUSSION
In this randomized trial, children who lived in housing units that received a residential lead hazard intervention, which included extensive dust control, had lower early childhood urinary concentrations of ΣDEHP, MCOP, and MCNP. At baseline, ΣDEHP concentrations were higher among pregnant women in the intervention group compared to the control group. Urinary concentrations of other phthalate metabolites were not different between pregnant women in the intervention and control groups. The higher maternal DEHP metabolite concentrations in the intervention group suggest that the effect of the intervention on children’s DEHP metabolite concentrations may not be due to baseline differences in DEHP exposure sources.
Consistent with the results of the randomized trial, we found that living in a housing unit that appeared clean was associated with lower urinary concentrations of ΣDEHP, MBzP, MCOP, and MCNP. For ΣDEHP, we found that the intervention was most effective in housing units that also appeared clean pre- and post-trial. As hypothesized, we did not find that the randomized intervention or dust accumulation was associated with lower urinary MEP concentrations. Our results from sensitivity analyses adjusting for additional behavioral characteristics that could be associated with cleaning or dust accumulation and phthalate exposure did not meaningfully differ from our main results. This consistency suggests that the association between dust accumulation and phthalate metabolite concentrations persists when accounting for behavioral factors.
The results of this study provide further evidence that preventing and removing dust build-up may reduce childhood phthalate exposure. We are not aware of any prior studies evaluating if lead hazard controls, such as paint stabilization, and dust control measures, such as cleaning, can reduce phthalate exposure in young children. Previous research suggests that some phthalates can be released from household materials and accumulate in dust, particularly DEHP, BBzP, DiNP, and DiDP, which are less volatile and have a higher molecular weight.13–19,22 On the other hand, previous studies generally detected relatively low household dust concentrations of phthalates with higher volatility and lower molecular weights, such as DEP, because these phthalates tend to be found in the gas phase when released from household products.13,18,19,22–24 While there are significant sources of DEP exposure in indoor environments, such as from personal care products,25 we do not expect that either this residential lead intervention or cleaning would substantially remove indoor DEP exposure sources.
Young children may have increased risk of phthalate exposure through dust ingestion and inhalation because they frequently engage in hand-to-mouth behaviors, breathe air closer to the ground, and play on carpets and floors.26–28 Children are also exposed to phthalates through other sources, including ingestion of packaged foods and personal care product use.27,29,30 Available evidence suggests that exposure to higher molecular weight phthalates indoors marginally contributes to an individual’s total intake. However, dust is hypothesized to be the primary source of exposure in the indoor environment.31,32 Previous studies that estimated the correlation between phthalate ingestion from concentrations measured in urine and dust report inconsistent results.2,5,33 However, these inconsistences may be partially attributable to the wide range in age of participants studied, 3 to 14 years. Younger children, especially those under the age of three, may have a higher risk of exposure to phthalates in dust from mouthing behaviors.
While we are not aware of any studies that have evaluated the association between dust removal or cleaning and children’s phthalate exposure, previous research suggests that some typical cleaning methods may reduce the concentration of some phthalates, as well as other chemicals such as polycyclic aromatic hydrocarbons and flame retardants, in household dust.34–36 However, other studies reported no association between cleaning behaviors and lower phthalate dust concentrations.22
We speculate that children in the residential lead hazard intervention group had lower urinary phthalate metabolite concentrations because paint stabilization and dust control measures reduced levels of phthalate-contaminated house dust. As part of the paint stabilization process, we removed degraded or water-damaged building materials, such as paint-covered or plastic laminate flooring and wallboard, which could release phthalates into the indoor environment.37 Furthermore, we applied new paint to areas which may have been previously covered with worn or chipped phthalate-containing paints.38 After paint stabilization, we also conducted extensive cleaning. Due to the design of the study we were unable to identify the individual or combined aspect(s) of the intervention necessary to reduce phthalate exposure. However, the positive association between dust accumulation and urinary phthalate metabolite concentrations we observed provides indirect evidence that dust is an important source for the uptake of phthalates.
Some researchers have raised concerns about certain cleaning methods increasing exposure to phthalates in dust.13 Exposure to phthalates could occur if phthalate-containing cleaning products are used, as well as when cleaning methods resuspend phthalate-containing dust particles in the air, but do not fully remove them. While we found that the intervention followed by extensive cleaning led to a reduction in some urinary phthalate metabolites in young children, painting and renovation could increase exposure if the materials used contained phthalates and these processes were not followed by extensive cleaning or dust control. In our study, we found that in housing units which did not appear clean at baseline, children in the intervention group had higher urinary concentration of ΣDEHP and MBzP compared to the control group, suggesting increased exposure could be possible in some situations.
Among children living in housing units that did not appear clean at baseline, we hypothesize that housing characteristics could have also impacted the effectiveness of the intervention in reducing urinary phthalate metabolite concentrations. Housing units that did not appear clean at baseline were older, renter-occupied, and multi-family units. These characteristics could be related to higher exposure to phthalates in building materials, as well as greater dust accumulation or differences in ventilation.15,37 These types of units may require additional dust control measures, repeated cleanings, or more extreme housing modifications to reduce children’s exposure to phthalates in dust. Future studies could identify typical cleaning methods or renovations that are most effective in reducing exposure and characterize the types of housing units where these methods may need to be adapted to reduce exposure risk.
This study has some limitations. Because this intervention was not specifically designed to reduce phthalate exposure, we did not intentionally identify and remove phthalate-containing building materials or other sources of phthalates in the home. We also did not measure phthalates in dust, therefore we do not know if the intervention reduced phthalate levels in dust. Other socioeconomic factors and behavioral characteristics associated with cleaning or dust accumulation and phthalate exposure that we did not consider could contribute to residual confounding of the association in the observational analysis. Furthermore, we did not consider children’s time-activity patterns or adjust for time spent indoors in our observational analysis. We used phthalate metabolite concentrations quantified in a spot urine sample collected at ages 1, 2, and 3 years to assess phthalate exposure. Because phthalates are non-persistent in the body, metabolites measured in a spot urine sample may result in exposure misclassification, which we expect to be non-differential. We also were not able to evaluate how the presence of polyvinyl chloride (PVC) flooring, subflooring materials, or water pipes in housing impacted our results. Finally, our results may not be generalizable to other populations because the study participants lived in homes built before 1978 and were predominately non-Hispanic white, well-educated, and had household incomes near the regional median.
Our study has several strengths. We were able to test our hypotheses using both a randomized trial of a residential intervention and observational study of dust accumulation and cleaning. Research staff who implemented the residential interventions and assessed dust and debris accumulation in the housing unit were blinded to mother and children’s urinary phthalate metabolite concentrations. We also used repeated measures of phthalate metabolite concentrations and dust accumulation at several timepoints. Additionally, the HOME Study collected longitudinal behavioral and housing characteristic data that we considered in our observational analyses. We were able to include urinary MEP concentrations in our analyses as a negative control outcome, and the results of this analysis were consistent across both the randomized trial and observational analyses. Finally, urinary phthalate metabolite concentrations among these children were similar to other populations in the United States at this time.39,40
In conclusion, our results suggest that removing phthalate contaminated dust through methods such as paint stabilization and extensive cleaning could reduce young children’s phthalate exposure. Additional research is necessary to identify modification of specific building materials and dust control measures that are most effective in reducing phthalate exposure from dust. Future interventions could consider incorporating more extensive and repeated cleanings, as well as identifying and removing household products and worn phthalate-containing building materials to ensure that the intervention is effective in all types of households.
Supplementary Material
Acknowledgments
FUNDING SOURCES: This work was supported by grants R01 ES024381, P01 ES011261, R01 ES014575, R01 ES020349, and P42 ES013660 from the National Institutes of Environmental Health Sciences, grant RD-83544201 from the US Environmental Protection Agency, and a grant from the US Department of Housing and Urban Development (HUD). The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the sponsoring organizations.
Footnotes
COMPETING FINANCIAL INTERESTS: Dr. Lanphear reported serving as an expert witness in childhood lead poisoning cases, for which he has not received any personal compensation. Dr. Braun was financially compensated for serving as an expert witness for plaintiffs in litigation related to tobacco smoke exposures. The other authors declare they have no actual or potential competing financial interests.
DISCLAIMER: The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services. The authors declare no competing financial interest.
SUPPORTING INFORMATION: description of dust accumulation and cleaning classifications, distribution of phthalate metabolites and urine samples collected by year and season, baseline participant characteristics by dust accumulation classification, adjusted geometric mean and percent differences in childhood urinary phthalate metabolite concentrations from per protocol and sensitivity analyses.
REFERENCES
- (1).Sukiene V; Gerecke AC; Park Y-M; Zennegg M; Bakker MI; Delmaar CJE; Hungerbühler K; von Goetz N Tracking SVOCs’ Transfer from Products to Indoor Air and Settled Dust with Deuterium-Labeled Substances. Environ. Sci. Technol 2016, 50(8), 4296–4303. 10.1021/acs.est.5b05906. [DOI] [PubMed] [Google Scholar]
- (2).Langer S; Bekö G; Weschler CJ; Brive LM; Toftum J; Callesen M; Clausen G Phthalate Metabolites in Urine Samples from Danish Children and Correlations with Phthalates in Dust Samples from Their Homes and Daycare Centers. Int. J. Hyg. Environ. Health 2014, 217 (1), 78–87. https://doi.Org/10.1016/j.ijheh.2013.03.014. [DOI] [PubMed] [Google Scholar]
- (3).Bornehag C-G; Sundell J; Weschler CJ; Sigsgaard T; Lundgren B; Hasselgren M; Hägerhed-Engman L The Association between Asthma and Allergic Symptoms in Children and Phthalates in House Dust: A Nested Case–Control Study. Environ. Health Perspect 2004, 112 (14), 1393–1397. https://doi.Org/10.1289/ehp.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Philippat C; Bennett DH; Krakowiak P; Rose M; Hwang H-M; Hertz-Picciotto I Phthalate Concentrations in House Dust in Relation to Autism Spectrum Disorder and Developmental Delay in the CHildhood Autism Risks from Genetics and the Environment (CHARGE) Study. Environ. Health 2015, 14 (1). https://doi.Org/10.1186/s12940-015-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Ait Bamai Y; Araki A; Kawai T; Tsuboi T; Saito I; Yoshioka E; Cong S; Kishi R Exposure to Phthalates in House Dust and Associated Allergies in Children Aged 6–12years. Environ. Int 2016, 96, 16–23. https://doi.Org/10.1016/j.envint.2016.08.025. [DOI] [PubMed] [Google Scholar]
- (6).Braun JM Early-Life Exposure to EDCs: Role in Childhood Obesity and Neurodevelopment. Nat. Rev. Endocrinol 2017, 13 (3), 161–173. https://doi.Org/10.1038/nrendo.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Engel SM; Villanger GD; Nethery RC; Thomsen C; Sakhi AK; Drover SSM; Hoppin JA; Zeiner P; Knudsen GP; Reichborn-Kjennerud T; et al. Prenatal Phthalates, Maternal Thyroid Function, and Risk of Attention-Deficit Hyperactivity Disorder in the Norwegian Mother and Child Cohort. Environ. Health Perspect 2018, 126(5), 057004 10.1289/EHP2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Katsikantami I; Sifakis S; Tzatzarakis MN; Vakonaki E; Kalantzi O.-l.; Tsatsakis AM; Rizos AK A Global Assessment of Phthalates Burden and Related Links to Health Effects. Environ. Int 2016, 97, 212–236. https://doi.Org/10.1016/j.envint.2016.09.013. [DOI] [PubMed] [Google Scholar]
- (9).Whyatt RM; Perzanowski MS; Just AC; Rundle AG; Donohue KM; Calafat AM; Hoepner LA; Perera FP; Miller RL Asthma in Inner-City Children at 5–11 Years of Age and Prenatal Exposure to Phthalates: The Columbia Center for Children’s Environmental Health Cohort. Environ. Health Perspect 2014, 722 (10), 1141–1146. 10.1289/ehp.1307670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Just AC; Whyatt RM; Perzanowski MS; Calafat AM; Perera FP; Goldstein IF; Chen Q; Rundle AG; Miller RL Prenatal Exposure to Butylbenzyl Phthalate and Early Eczema in an Urban Cohort. Environ. Health Perspect 2012, 120 (10), 1475–1480. 10.1289/ehp.1104544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Braun JM; Hornung R; Chen A; Dietrich KN; Jacobs DE; Jones R; Khoury JC; Liddy-Hicks S; Morgan S; Vanderbeek SB; et al. Effect of Residential Lead-Hazard Interventions on Childhood Blood Lead Concentrations and Neurobehavioral Outcomes: A Randomized Clinical Trial. JAMA Pediatr. 2018, 172 (10), 934 10.1001/jamapediatrics.2018.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Phelan KJ; Khoury J; Xu Y; Liddy S; Hornung R; Lanphear BP A Randomized Controlled Trial of Home Injury Hazard Reduction: The HOME Injury Study. Arch. Pediatr. Adolesc. Med 2011, 165 (4). 10.1001/archpediatrics.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Bi C; Maestre JP; Li H; Zhang G; Givehchi R; Mahdavi A; Kinney KA; Siegel J; Horner SD; Xu Y Phthalates and Organophosphates in Settled Dust and HVAC Filter Dust of U.S. Low-Income Homes: Association with Season, Building Characteristics, and Childhood Asthma. Environ. Int 2018, 121, 916–930. https://doi.Org/10.1016/j.envint.2018.09.013. [DOI] [PubMed] [Google Scholar]
- (14).Gaspar FW; Castorina R; Maddalena RL; Nishioka MG; McKone TE; Bradman A Phthalate Exposure and Risk Assessment in California Child Care Facilities. Environ. Sci. Technol 2014, 48(13), 7593–7601. 10.1021/es501189t. [DOI] [PubMed] [Google Scholar]
- (15).Kim W; Choi I; Jung Y; Lee J; Min S; Yoon C Phthalate Levels in Nursery Schools and Related Factors. Environ. Sci. Technol 2013, 47 (21), 12459–12468. 10.1021/es4025996. [DOI] [PubMed] [Google Scholar]
- (16).Kubwabo C; Rasmussen PE; Fan X; Kosarac I; Wu F; Zidek A; Kuchta SL Analysis of Selected Phthalates in Canadian Indoor Dust Collected Using ACS Paragon Plus Environment Household Vacuum and Standardized Sampling Techniques. Indoor Air 2013, 23 (6), 506–514. 10.1111/ina.12048. [DOI] [PubMed] [Google Scholar]
- (17).Larsson K; Lindh CH; Jönsson BA; Giovanoulis G; Bibi M; Bottai M; Bergström A; Berglund M Phthalates, Non-Phthalate Plasticizers and Bisphenols in Swedish Preschool Dust in Relation to Children’s Exposure. Environ. Int 2017, 102, 114–124. https://doi.Org/10.1016/j.envint.2017.02.006. [DOI] [PubMed] [Google Scholar]
- (18).Rudel RA; Camann DE; Spengler JD; Korn LR; Brody JG Phthalates, Alkylphenols, Pesticides, Polybrominated Diphenyl Ethers, and Other Endocrine-Disrupting Compounds in Indoor Air and Dust. Environ. Sci. Technol 2003, 37 (20), 4543–4553. 10.1021/es0264596. [DOI] [PubMed] [Google Scholar]
- (19).Subedi B; Sullivan KD; Dhungana B Phthalate and Non-Phthalate Plasticizers in Indoor Dust from Childcare Facilities, Salons, and Homes across the USA. Environ. Pollut 2017, 230, 701–708. https://doi.Org/10.1016/j.envpol.2017.07.028. [DOI] [PubMed] [Google Scholar]
- (20).Silva M; Samandar E; Preaujr J; Reidy J; Needham L; Calafat A Quantification of 22 Phthalate Metabolites in Human Urines☆. J. Chromatogr. B 2007, 860(1), 106–112. 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- (21).Centers for Disease Control and Prevention. National Report on Human Exposure to Environmental Chemicals; January 2019. Updated Tables. https://www.cdc.gov/exposurereport/ (accessed Nov 26, 2019).
- (22).Ait Bamai Y; Araki A; Kawai T; Tsuboi T; Saito I; Yoshioka E; Kanazawa A; Tajima S; Shi C; Tamakoshi A; et al. Associations of Phthalate Concentrations in Floor Dust and Multi-Surface Dust with the Interior Materials in Japanese Dwellings. Sci. Total Environ 2014, 468–469, 147–157. https://doi.Org/10.1016/j.scitotenv.2013.07.107. [DOI] [PubMed] [Google Scholar]
- (23).Langer S; Weschler CJ; Fischer A; Bekö G; Toftum J; Clausen G Phthalate and PAH Concentrations in Dust Collected from Danish Homes and Daycare Centers. Atmos. Environ 2010, 44 (19), 2294–2301. https://doi.Org/10.1016/j.atmosenv.2010.04.001. [Google Scholar]
- (24).Weschler CJ; Nazaroff WW SVOC Partitioning between the Gas Phase and Settled Dust Indoors. Atmos. Environ 2010, 44 (30), 3609–3620. https://doi.Org/10.1016/j.atmosenv.2010.06.029. [Google Scholar]
- (25).Just AC; Adibi JJ; Rundle AG; Calafat AM; Camann DE; Hauser R; Silva MJ; Whyatt RM Urinary and Air Phthalate Concentrations and Self-Reported Use of Personal Care Products among Minority Pregnant Women in New York City. J. Expo. Sci. Environ. Epidemiol 2010, 20 (7), 625–633. 10.1038/jes.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Bearer CF How Are Children Different from Adults? Environ. Health Perspect 1995, 103, 6, 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Sathyanarayana S; Karr CJ; Lozano P; Brown E; Calafat AM; Liu F; Swan SH Baby Care Products: Possible Sources of Infant Phthalate Exposure. PEDIATRICS 2008, 121 (2), e260–e268. 10.1542/peds.2006-3766. [DOI] [PubMed] [Google Scholar]
- (28).Wormuth M; Scheringer M; Vollenweider M; Hungerbuhler K What Are the Sources of Exposure to Eight Frequently Used Phthalic Acid Esters in Europeans? Risk Anal. 2006, 26 (3), 803–824. https://doi.Org/10.1111/j.1539-6924.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- (29).Lewis RC; Meeker JD; Peterson KE; Lee JM; Pace GG; Cantoral A; Tellez-Rojo MM Predictors of Urinary Bisphenol A and Phthalate Metabolite Concentrations in Mexican Children. Chemosphere 2013, 93 (10), 2390–2398. https://doi.Org/10.1016/j.chemosphere.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Watkins DJ; Eliot M; Sathyanarayana S; Calafat AM; Yolton K; Lanphear BP; Braun JM Variability and Predictors of Urinary Concentrations of Phthalate Metabolites during Early Childhood. Environ. Sci. Technol 2014, 48 (15), 8881–8890. 10.1021/es501744v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Bekö G; Weschler CJ; Langer S; Callesen M; Toftum J; Clausen G Children’s Phthalate Intakes and Resultant Cumulative Exposures Estimated from Urine Compared with Estimates from Dust Ingestion, Inhalation and Dermal Absorption in Their Homes and Daycare Centers. PLoS ONE 2013, 8 (4), e62442 10.1371/journal.pone.0062442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Guo Y; Kannan K Comparative Assessment of Human Exposure to Phthalate Esters from House Dust in China and the United States. Environ. Sci. Technol 2011, 45 (8), 3788–3794. 10.1021/es2002106. [DOI] [PubMed] [Google Scholar]
- (33).Becker K; Seiwert M; Angerer J; Heger W; Koch HM; Nagorka R; Roßkamp E; Schlüter C; Seifert B; Ullrich D DEHP Metabolites in Urine of Children and DEHP in House Dust. Int. J. Hyg. Environ. Health 2004, 207 (5), 409–417. https://doi.Org/10.1078/1438-4639-00309. [DOI] [PubMed] [Google Scholar]
- (34).Kolarik B; Bornehag C-G; Naydenov K; Sundell J; Stavova P; Nielsen OF The Concentrations of Phthalates in Settled Dust in Bulgarian Homes in Relation to Building Characteristic and Cleaning Habits in the Family. Atmos. Environ 2008, 42 (37), 8553–8559. https://doi.Org/10.1016/j.atmosenv.2008.08.028. [Google Scholar]
- (35).Sugeng EJ; de Cock M; Leonards PEG; van de Bor M Electronics, Interior Decoration and Cleaning Patterns Affect Flame Retardant Levels in the Dust from Dutch Residences. Sci. Total Environ 2018, 645, 1144–1152. https://doi.Org/10.1016/j.scitotenv.2018.07.127. [DOI] [PubMed] [Google Scholar]
- (36).Yu CH; Yiin L-M; (Tina) Fan Z-H; Rhoads GG Evaluation of HEPA Vacuum Cleaning and Dry Steam Cleaning in Reducing Levels of Polycyclic Aromatic Hydrocarbons and House Dust Mite Allergens in Carpets. J Env. Monit 2009, 11 (1), 205–211. 10.1039/B807821A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Bornehag C-G; Lundgren B; Weschler CJ; Sigsgaard T; Hagerhed-Engman L; Sundell J Phthalates in Indoor Dust and Their Association with Building Characteristics. Environ. Health Perspect 2005, 113 (10), 1399–1404. https://doi.Org/10.1289/ehp.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).IARC (International Agency for Research on Cancer). A review of human carcinogens. Part F: chemical agents and related occupations. https://monographs.iarc.fr/ (accessed Jun 10, 2019).
- (39).Silva MJ; Barr DB; Reidy JA; Malek NA; Hodge CC; Caudill SP; Brock JW; Needham LL; Calafat AM Urinary Levels of Seven Phthalate Metabolites in the U.S. Population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ. Health Perspect 2004, 112 (3), 331–338. 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Trasande L; Attina TM; Sathyanarayana S; Spanier AJ; Blustein J Race/Ethnicity-Specific Associations of Urinary Phthalates with Childhood Body Mass in a Nationally Representative Sample. Environ. Health Perspect 2013, 121 (4), 501–506. 10.1289/ehp.1205526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.