Abstract
Introduction:
Over 14% of Canadians use cannabis, with nearly 60% of these individuals reporting daily or weekly use. Inhalation of cannabis vapour has recently gained popularity, but the effects of this exposure on neural activity remain unknown. In this study, we assessed the impact of acute exposure to vapourized Δ9-tetrahydrocannabinol (THC) on neural circuit dynamics in rats.
Objectives:
We aimed to characterize the changes in neural activity in the dorsal striatum (dStr), orbitofrontal cortex (OFC), and prefrontal cortex (PFC), after acute exposure to THC vapour.
Methods:
Rats were implanted with electrode arrays targeting the dStr, OFC, and PFC. Rats were administered THC (or vehicle) using a Volcano® vapourizer and local field potential recordings were performed in a plexiglass chamber in a cross-over design with a week-long washout period.
Results:
Decreased spectral power was observed within the dStr, OFC, and PFC in the gamma range (>32-100 Hz) following vapourized THC administration. Most changes in gamma were still present 7 days after THC administration. Decreased gamma coherence was also observed between the OFC-PFC and dStr-PFC region-pairs.
Conclusion:
A single exposure to vapourized THC suppresses cortical and dorsal striatal gamma power and coherence, effects that appear to last at least a week. Given the role of gamma hypofunction in schizophrenia, these findings may provide mechanistic insights into the known psychotomimetic effects of THC.
Keywords: limbic, vapor, cannabis, circuitry, psychosis
INTRODUCTION
In 2019, the World Health Organization (WHO) reported an estimated 147 million users of cannabis globally 1. In Canada, national census data revealed that 15% of individuals aged 15 years and older had consumed cannabis in the third quarter of 2018 2, and the Canadian Centre for Substance Abuse 3 reported that nearly 30% of youth in grades 7 to 12 (approximately 12 to 18 years of age) had reported consuming cannabis in the past 12 months. Considering the prevalence of cannabis and the shifting landscape of legalization for recreational purposes 4, research that aims to elucidate the causal effects of cannabis on brain and behaviour is needed. One emerging trend is the use of alternative routes of administration such as vapourized cannabis; in 2018, the Monitoring the Future survey recently reported that the frequency of “vaping” cannabis had increased by approximately 50-60% in high school since it was first measured in 20175.
Escalations in the frequency of use and cannabis potency, combined with novel delivery methods, call for a more in-depth understanding of the differential effects of varying routes and durations of exposure to cannabis on brain function and circuitry. The reported and observed cognitive effects of cannabis vary greatly depending on the route of administration, the frequency of use, and the concentration of Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD), two of the main phytocannabinoids found in cannabis 6, 7. Generally, consuming cannabis results in a “stoned” or “calm and relaxed” feeling; the distinctive ‘high’ experienced by cannabis users 4, 8. In contrast, greater amounts of THC in cannabis often produce psychotomimetic feelings of paranoia, anxiety, and may lead to an increased risk for psychiatric illness 8, 9, 10.
Previous studies have used electrophysiological assessments to explore changes in neural activity following THC or cannabinoid receptor agonist administration (intravenous in humans and intraperitoneal in rodents), reporting acute suppression of gamma and theta signal in hippocampal, parahippocampal, and cortical regions 11, 12, 13, suggesting this as a possible mechanism for the psychotomimetic effects of cannabis due to consistent findings in patients with schizophrenia 14. However, human studies often recruit subjects with prior, albeit minimal, cannabis use, making it difficult to assess the acute impacts of THC in cannabis-naïive individuals 9, 15, 16. Furthermore, the responsiveness of individuals to THC, including the appearance of psychotomimetic effects, varies greatly depending on age 17, sex 18, socioeconomic status 19, 20, 21, genetics 22, and education, amongst other environmental measures. These confounding factors make it difficult to assess the causal effects of cannabis constituents on brain circuitry.
To test the effects of acute vapourized THC exposure on neural circuitry in cannabis-na’ive animals, we employed an established rodent model of vapourized THC exposure 23, 24, 25 and acquired electrophysiological recordings of local field potentials (LFPs) from corticostriatal brain circuitry after THC exposure. In this study, we targeted the dorsal striatum (dStr), prefrontal cortex (PFC), and orbitofrontal cortex (OFC) because these regions are often implicated in the cognitive and psychotomimetic effects of THC. Specifically, clinical studies have previously highlighted the impact of cannabis consumption on decision-making 26, 27, attention and memory 4, 15; cognitive tasks that involve the OFC and PFC, in addition to the psychotomimetic effects correlated with cortical electrophysiology measures mentioned above 13. The dStr was targeted because it is a brain region that is commonly implicated in the rewarding effects of cannabis use and dependency 28, 29, and is known to communicate with the PFC and OFC 30, 31. We hypothesized that vapourized THC would acutely reduce neural synchrony and power between the dStr and cortical regions (PFC and/or OFC).
METHODS
Animals:
Adult male Sprague Dawley rats weighing approximately 400 grams at the start of the experiment were used. Rats were housed individually in polyethylene cages in a colony room maintained on a 12-hour light:dark cycle with ad libitum access to food and water. Rats were handled for two minutes daily for 5 days before the start of experiments to habituate them to the experimental manipulations. All treatments were performed during the dark phase of the 12-hour reverse light:dark cycle. All procedures complied with the guidelines described in the Guide to the Care and Use of Experimental Animals (Canadian Council on Animal Care, 1993) and the Animal Care Committee at the University of Guelph.
Surgeries:
Eight rats were anesthetized with isoflurane, administered the analgesic carprofen (5 mg/kg, subcutaneous injection) and secured in a stereotaxic frame. Body temperature was maintained at 37 °C with a warming pad. Electrodes (A-M Systems, Washington, United States) were implanted bilaterally into the dStr (AP: +1.9, ML: ±2.6, DV: −4.4), OFC (AP: +3.2, ML: ±2.6, DV: −5.5), and PFC (AP: +3.2, ML: ±0.6, DV: −3.8), and grounded by attaching (using silver paint) a reference wire to a screw fixed into the skull below lambda. Additional anchor screws were attached to the skull and electrodes secured with dental cement to the anchor screws. The animals received an additional injection of carprofen 24- and 48-hours following surgery and recovered individually in their home cage for a minimum of 7 days before the experiments were performed. Electrode placement was validated post-mortem with 40-micron brain slices stained using cresyl violet.
Vapourized THC Administration:
Effects of vapourized THC on neural activity was evaluated using a crossover design as has previously been performed in human subjects 9. Rats were randomized to two groups (n=4/group), receiving either vapourized THC or vehicle (1:1:18 TWEEN-80:ethanol:saline) in the first session. After a 7-day washout period, rats that initially received THC were administered vehicle, and rats that initially received vehicle received THC. A Volcano® vapourizer (Storz and Bickel, GmbH and Co., Tuttlingen, Germany) was used as described previously 23, 24, 25: THC (10 mg/pad; 250μL of 40mg/mL solution for 2 rats) was vapourized at approximately 226 °C and channeled into detachable plastic bags (with a total volume of approximately 25L) through an attached valve. The bags were manually constricted to expel the vapour into an enclosed Plexiglas chamber (with approximate dimensions of 15 × 10 × 15 cm3) using a small port in one face of the chamber. It is reported that this method delivers ~50% of the THC on the wire pad into the bag with a pulmonary uptake similar to smoking cannabis (402 ng/ml/kg in whole blood, 20 minutes after 10 mg/pad exposure) 25. Rats were administered THC or vehicle vapour individually and LFP recordings were collected for 30 minutes beginning 10 minutes post-administration.
Electrophysiology:
All LFPs were acquired using a wireless electrophysiology recording system (W2100, Multichannel Systems, Reutlingen, Germany) and were performed in awake, freely moving animals. Data were recorded for 30 minutes and sampled at a rate of 1000 samples/second. The spectral power of LFP oscillations and coherence analyzed using routines from the Chronux software package for MATLAB (MathWorks, Massachusetts, United States). LFP data were segmented, detrended and low-pass filtered to remove frequencies greater than 100 Hz. Continuous multitaper spectral power (tapers = [5 9]) for each region was calculated for each segment in the following frequency bands: delta (1–4 Hz), theta (>4–12 Hz), beta (>12–32 Hz), slow gamma (>32–60 Hz), and fast gamma (>60–100 Hz). The LFP spectral power from each group was normalized to the respective total spectral power for each rat within each treatment.
Data Analysis:
Quantification of LFP power and coherence data at each frequency is reported as the mean ± SEM. Comparisons were performed to evaluate between-subject or within-subject changes between THC and VEH treatment groups using a Student’s t-test or paired t-tests, respectively. Computations were performed using the SPSS/PC+ statistical software package.
RESULTS
Exposure to vapourized THC reduced the amplitude of the gamma frequency band in dStr, OFC and PFC, compared to vehicle (Figure 1A). Reductions in gamma frequency (>32-100 Hz) spectral power following acute THC exposure was evident in all three brain regions (Figure 1 B–D). Quantification of the power spectra demonstrated an approximate 35% decrease in low gamma (>32-60 Hz) power in the dStr and OFC (Figure 1E and F) with a 22% decrease in the PFC (Figure 1G). Similarly, a 40% to 50% reduction in high gamma (>60-100 Hz) power following THC administration was observed in each of the three brain regions (Figure 1B–G). We did not observe any cross-over order effect on spectral power at each frequency between the two THC-treated groups, in that the group that received THC in week one was not different from the group that received THC in week two (Figure 1E–G). However, unlike the two THC groups, a cross-over order effect was evident in rats that received THC in week one and VEH in week two, indicative of an extended effect of THC on neural gamma oscillations (Figure 1H–J). One week after THC administration, only the dStr high gamma deficits appeared to normalize (Figure 1H), whereas no significant increases in gamma power in any of the other regions was evident (Figure 1I and J). In order to control for any confounding effects of sedation, we assessed the effects of THC exposure on delta power and delta/gamma correlations, and saw no significant effects of THC on both measures (data not shown).
Figure 1.
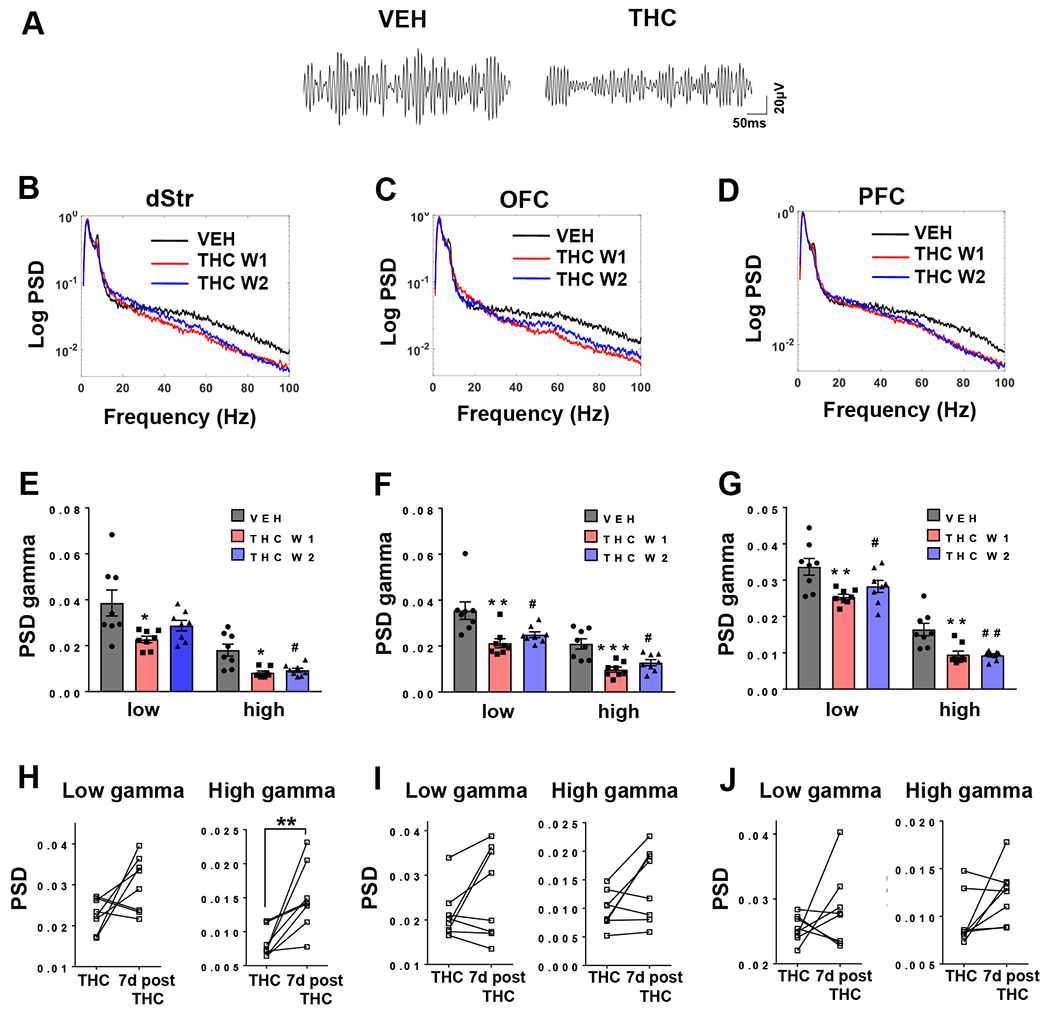
Acute vapourized THC suppresses gamma power in cortical and striatal regions. A) Representative gamma frequency tracing from dStr showing reduced amplitude following THC exposure. B-D) Power spectral density (PSD) curves for LFPs from dSTR, OFC and PFC showing reduced gamma power following THC administration (blue and red lines, W1 = Week 1 THC, W2 = Week 2 THC) compared to VEH-treated animals (black line). E-G) Quantification (mean ± SEM) of low and high gamma power for each treatment in all three regions. (n=8; *p<0.05, ** p<0.01, *** p<0.001, between-subject comparison to vehicle-treated group in week one [students t-test], #p<0.05, ##p<0.01 within subject comparison to baseline (vehicle) treatment [paired t-test]). H-J) Within subject comparison showing the long-lasting effects (7 days) of THC on suppression of gamma power. Normalization of high gamma power was observed in the dSTR only (** p<0.01) compared to THC administration (paired t-test).
Due to the cross-over order effect observed with the power measures, oscillatory coherence between brain regions was evaluated within-subjects for those animals that received VEH in week one and THC in week two (n=4). There were no effects of THC on dStr-OFC coherence (Figure 2A and B) with changes occurring selectively in dStr-PFC (Figure 2C and D) and OFC-PFC coherence (Figure 2E and F). Like the observations in spectral power, THC-induced differences in coherence occurred predominantly within the gamma frequency range. Specifically, analysis of dStr-PFC coherence showed reduced coherence selectively in high gamma (Figure 2C and D). With OFC-PFC coherence, THC induced a reduction in the low and high gamma ranges, as well as in the theta frequency range (Figure 2E and F).
Figure 2.
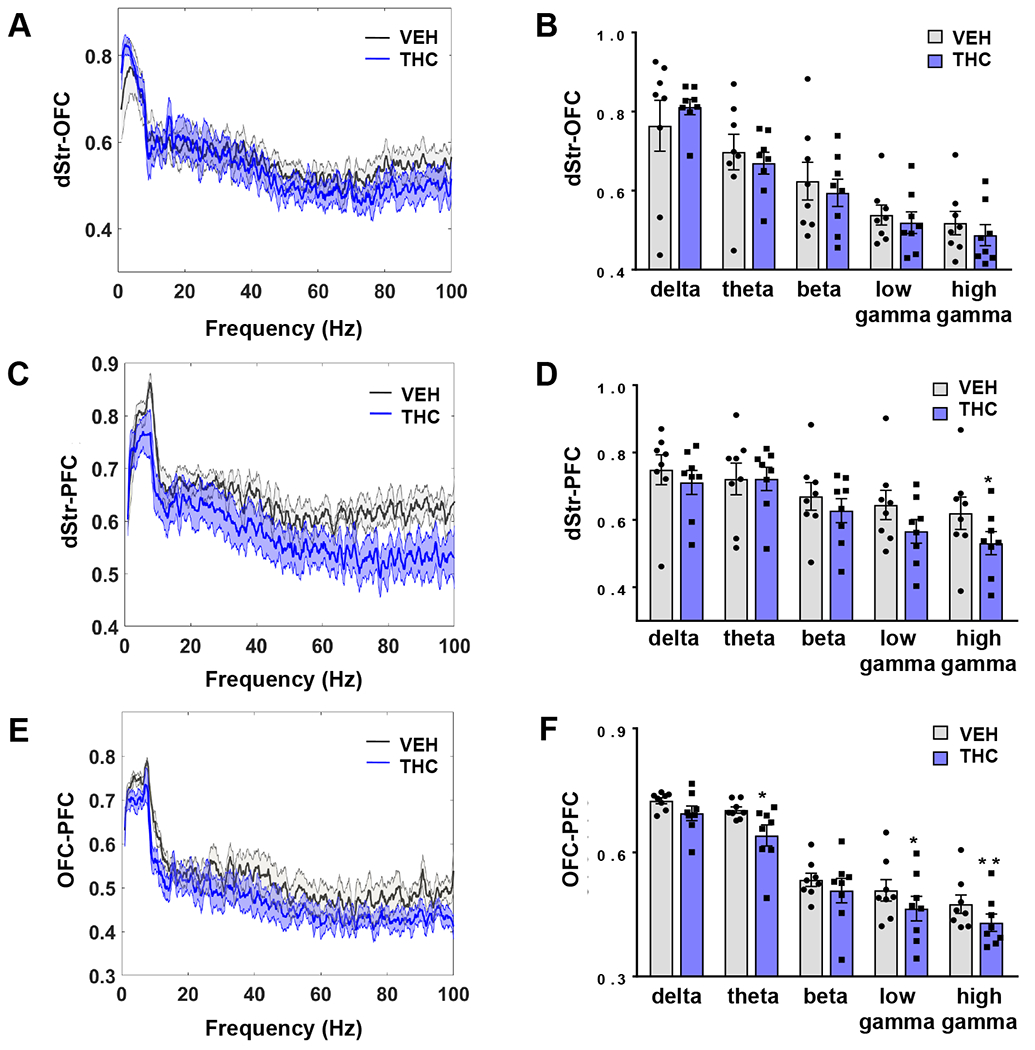
Acute vapourized THC exposure reduces corticostriatal coherence. A-B) Coherence curves and quantification (mean ± SEM) showing no effects of THC (blue or black) on dSTR-OFC coherence (mean ± SEM) compared to VEH (grey or white). C-D) THC administration reduced dStr-PFC coherence selectively in the high gamma range compared to baseline (vehicle) treatment. E-F) Reduced OFC-PFC theta and gamma coherence was observed following THC treatment (n=4 with bilateral electrodes; *p<0.05, ** p<0.01).
DISCUSSION
The results of our study revealed that vapourized THC exposure in rats leads to acute decreases in gamma power within the dStr, OFC, and PFC, and a decrease in gamma coherence between OFC-PFC and dStr-PFC. Interestingly, rats that received THC in week one and vehicle in week two showed a cross-over effect, indicating that the THC-induced decreases in gamma power last for at least one week after exposure. One important caveat in attributing the source of these oscillations to the anatomical targets of electrodes is that there are known limitations in localizing the source of the LFP signal 32, 33, which is mitigated by the consistent effects seen across the anatomical targets of the electrodes.
The decrease in gamma signal that was observed in this study is supported by similar observations after cannabinoid exposure in rodents and humans: a decrease in both power and coherence of gamma and theta signals in the rat hippocampus was observed acutely after THC and after a cannabinoid receptor 1 agonist, CP55940, was administered intraperitoneally or intracranially 11, 12. Aberrant gamma power and coherence has also been measured after intravenous THC-administration in humans with a history of cannabis use and was associated with a greater score on the Positive and Negative Syndrome Scale (PANSS), a clinical survey commonly used in the diagnosis of schizophrenia 13. Previously, psychotomimetic behaviours have been associated with a dysfunctional gamma signal: 1) directly, as recorded in patients with schizophrenia compared to healthy controls 14, 34; and 2) indirectly, through shared behavioural manifestations such as sensorimotor gating deficits in heavy cannabis users 35 and in patients with schizophrenia 12. Thus, gamma hypofunction arising from acute THC exposure may explain some of the psychotomimetic effects of THC and provide a potential mechanistic commonality between acute negative consequences of cannabis use (especially high THC dose variants) and schizophrenia phenotypes. Subsequent studies will be designed to explore this relationship and to determine whether gamma hypofunction contributes causally to the psychotomimetic effects of cannabis.
Gamma-band oscillatory activity is thought to result from competing excitatory (i.e. glutamatergic) and inhibitory (i.e. GABAergic) activity 36, with most research highlighting a strong association between GABA concentrations and gamma activity 37. However, previous research in humans and rodent models has suggested that gamma activity is also associated with glutamate neurotransmitter concentrations in specific brain regions, including the lateral occipital cortex 38 and the anterior cingulate cortex 39, 40. Glutamate concentrations can affect distal brain structures as well: glutamate activity in the hippocampus was shown to be predictive of theta activity in the PFC 41. Recent studies have revealed that glutamate levels in the striatum decrease after exposure to THC, and relate to the psychotomimetic effects produced by THC 10. Combined with the results of our study, it is plausible that corticostriatal gamma hypofunction resulting from acute THC exposure could possibly be a product of changes in glutamatergic signalling; future studies combining LFP recordings with microdialysis will help to characterize the relationship between glutamatergic and GABAergic signalling with the gamma hypofunction and psychotomimetic effects produced by vapourized THC exposure.
The cross-over effect observed in our animals that received THC one week before vehicle indicates that the THC-induced gamma suppression may last at least up to one week after a single exposure to vapourized THC in otherwise na’ive animals; lasting differences in gamma activity has previously been observed in chronic cannabis users 42. These findings provide important considerations for designing cross-over studies with THC in animals and in humans, and ensuring sufficient wash-out durations. It is unclear whether this long-lasting suppression is due to pharmacokinetic or pharmacodynamics factors related to vapourized THC. Inhalation of vapourized THC, like smoking, results in rapid increased in plasma THC concentration, with some indications that it produces higher plasma THC levels compared to smoking 43, 44. In future studies, we will assess later time-points and both plasma and brain levels, to establish the role of pharmacokinetic factors in this long-lasting effect.
Taken together, these studies indicate that acute exposure to vapourized THC in na’ive animals can produce lasting changes in brain circuit dynamics, and the overlap between these signatures and those observed in patients with schizophrenia may provide a potential mechanism for the psychotomimetic effects of THC. Future studies, combining electrophysiology with behaviour, will aim to determine how long this gamma suppression lasts, and, using circuit manipulation techniques, whether it serves as a causal factor in the psychotomimetic or cognitive effects of THC; one that could be targeted to develop treatments for acute THC-induced psychosis.
ACKNOWLEDGEMENTS
We would like to thank Dr. Paul Mallet (from Wilfrid Laurier University) for providing the THC vapourization apparatus for use in this study.
REFERENCES
- 1.Management of Substance Abuse. 2019; Available from: https://www.who.int/substance_abuse/facts/cannabis/en/.
- 2.Cannabis use in the past three months by province. 2018.
- 3.Cannabis Use, Harms and Perceived Risks among Canadian Students. 2019.
- 4.Blaes SL, Orsini CA, Holik HM, et al. , Enhancing effects of acute exposure to cannabis smoke on working memory performance. Neurobiol Learn Mem, 2018. 157: p. 151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamilton AD, Jang JB, Patrick ME, Schulenberg JE, and Keyes KM, Age, period and cohort effects in frequent cannabis use among US students: 1991-2018. Addiction, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson ES and Schenk S, Variability in subjective responses to marijuana: Initial experiences of college students. Addictive Behaviors, 1994. 19(5): p. 531–538. [DOI] [PubMed] [Google Scholar]
- 7.Feeney DM, The marijuana window: A theory of cannabis use. Behavioral Biology, 1976. 18(4): p. 455–471. [DOI] [PubMed] [Google Scholar]
- 8.Sharma P, Murthy P, and Bharath MM, Chemistry, metabolism, and toxicology of cannabis: clinical implications. Iran J Psychiatry, 2012. 7(4): p. 149–56. [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan CJA, Freeman TP, Hindocha C, Schafer G, Gardner C, and Curran HV, Individual and combined effects of acute delta-9-tetrahydrocannabinol and cannabidiol on psychotomimetic symptoms and memory function. Transl Psychiatry, 2018. 8(1): p. 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colizzi M, Weltens N, McGuire P, et al. , Delta-9-tetrahydrocannabinol increases striatal glutamate levels in healthy individuals: implications for psychosis. Mol Psychiatry, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robbe D, Montgomery SM, Thome A, Rueda-Orozco PE, McNaughton BL, and Buzsaki G, Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nature Neuroscience, 2006. 9(12): p. 1526–1533. [DOI] [PubMed] [Google Scholar]
- 12.Hajos M, Hoffmann WE, and Kocsis B, Activation of cannabinoid-1 receptors disrupts sensory gating and neuronal oscillation: relevance to schizophrenia. Biol Psychiatry, 2008. 63(11): p. 1075–83. [DOI] [PubMed] [Google Scholar]
- 13.Cortes-Briones J, Skosnik PD, Mathalon D, et al. , Delta9-THC Disrupts Gamma (gamma)-Band Neural Oscillations in Humans. Neuropsychopharmacology, 2015. 40(9): p. 2124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, and McCarley RW, Abnormal neural synchrony in schizophrenia. The Journal of neuroscience : the official journal of the Society for Neuroscience, 2003. 23(19): p. 7407–7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Souza DC, Perry E, MacDougall L, et al. , The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology, 2004. 29(8): p. 1558–72. [DOI] [PubMed] [Google Scholar]
- 16.Roitman P, Mechoulam R, Cooper-Kazaz R, and Shalev A, Preliminary, open-label, pilot study of add-on oral Delta9-tetrahydrocannabinol in chronic post-traumatic stress disorder. Clin Drug Investig, 2014. 34(8): p. 587–91. [DOI] [PubMed] [Google Scholar]
- 17.De Hert M, Wampers M, Jendricko T, et al. , Effects of cannabis use on age at onset in schizophrenia and bipolar disorder. Schizophr Res, 2011. 126(1-3): p. 270–6. [DOI] [PubMed] [Google Scholar]
- 18.Bassir Nia A, Mann C, Kaur H, and Ranganathan M, Cannabis Use: Neurobiological, Behavioral, and Sex/Gender Considerations. Current Behavioral Neuroscience Reports, 2018. 5(4): p. 271–280. [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JO, Hill KG, Hartigan LA, et al. , Unemployment and substance use problems among young adults: Does childhood low socioeconomic status exacerbate the effect? Soc Sci Med, 2015. 143: p. 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergen SE, Gardner CO, Aggen SH, and Kendler KS, Socioeconomic status and social support following illicit drug use: causal pathways or common liability? Twin Res Hum Genet, 2008. 11(3): p. 266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Legleye S, Beck F, Khlat M, Peretti-Watel P, and Chau N, The influence of socioeconomic status on cannabis use among French adolescents. J Adolesc Health, 2012. 50(4): p. 395–402. [DOI] [PubMed] [Google Scholar]
- 22.Khokhar JY, Dwiel LL, Henricks AM, Doucette WT, and Green AI, The link between schizophrenia and substance use disorder: A unifying hypothesis. Schizophr Res, 2018. 194: p. 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manwell LA, Charchoglyan A, Brewer D, Matthews BA, Heipel H, and Mallet PE, A vapourized Delta(9)-tetrahydrocannabinol (Delta(9)-THC) delivery system part I: development and validation of a pulmonary cannabinoid route of exposure for experimental pharmacology studies in rodents. J Pharmacol Toxicol Methods, 2014. 70(1): p. 120–7. [DOI] [PubMed] [Google Scholar]
- 24.Manwell LA, Ford B, Matthews BA, Heipel H, and Mallet PE, A vapourized Delta(9)-tetrahydrocannabinol (Delta(9)-THC) delivery system part II: comparison of behavioural effects of pulmonary versus parenteral cannabinoid exposure in rodents. J Pharmacol Toxicol Methods, 2014. 70(1): p. 112–9. [DOI] [PubMed] [Google Scholar]
- 25.Hazekamp A, Ruhaak R, Zuurman L, van Gerven J, and Verpoorte R, Evaluation of a vaporizing device (Volcano) for the pulmonary administration of tetrahydrocannabinol. J Pharm Sci, 2006. 95(6): p. 1308–17. [DOI] [PubMed] [Google Scholar]
- 26.Churchwell JC, Lopez-Larson M, and Yurgelun-Todd DA, Altered frontal cortical volume and decision making in adolescent cannabis users. Front Psychol, 2010. 1: p. 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant JE, Chamberlain SR, Schreiber L, and Odlaug BL, Neuropsychological deficits associated with cannabis use in young adults. Drug Alcohol Depend, 2012. 121(1-2): p. 159–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou F, Zimmermann K, Xin F, et al. , Shifted balance of dorsal versus ventral striatal communication with frontal reward and regulatory regions in cannabis-dependent males. Hum Brain Mapp, 2018. 39(12): p. 5062–5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodman J and Packard MG, The influence of cannabinoids on learning and memory processes of the dorsal striatum. Neurobiol Learn Mem, 2015. 125: p. 1–14. [DOI] [PubMed] [Google Scholar]
- 30.McCutcheon RA, Abi-Dargham A, and Howes OD, Schizophrenia, Dopamine and the Striatum: From Biology to Symptoms. Trends Neurosci, 2019. 42(3): p. 205–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bloomfield MA, Ashok AH, Volkow ND, and Howes OD, The effects of Delta(9)-tetrahydrocannabinol on the dopamine system. Nature, 2016. 539(7629): p. 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carmichael JE, Gmaz JM, and van der Meer MAA, Gamma Oscillations in the Rat Ventral Striatum Originate in the Piriform Cortex. J Neurosci, 2017. 37(33): p. 7962–7974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bastos AM and Schoffelen JM, A Tutorial Review of Functional Connectivity Analysis Methods and Their Interpretational Pitfalls. Front Syst Neurosci, 2015. 9: p. 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen M, Solowij N, and Carr V, Cannabis, Cannabinoids and Schizophrenia: Integration of the Evidence. Australian & New Zealand Journal of Psychiatry, 2008. 42(5): p. 357–368. [DOI] [PubMed] [Google Scholar]
- 35.Edwards CR, Skosnik PD, Steinmetz AB, O’Donnell BF, and Hetrick WP, “Sensory gating impairments in heavy cannabis users are associated with altered neural oscillations”: Correction. Behavioral Neuroscience, 2009. 123(5): p. 1065–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atallah BV and Scanziani M, Instantaneous modulation of gamma oscillation frequency by balancing excitation with inhibition. Neuron, 2009. 62(4): p. 566–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duncan NW, Wiebking C, and Northoff G, Associations of regional GABA and glutamate with intrinsic and extrinsic neural activity in humans-a review of multimodal imaging studies. Neurosci Biobehav Rev, 2014. 47: p. 36–52. [DOI] [PubMed] [Google Scholar]
- 38.Lally N, Mullins PG, Roberts MV, Price D, Gruber T, and Haenschel C, Glutamatergic correlates of gamma-band oscillatory activity during cognition: a concurrent ER-MRS and EEG study. Neuroimage, 2014. 85 Pt 2: p. 823–33. [DOI] [PubMed] [Google Scholar]
- 39.Walter M, Henning A, Grimm S, et al. , The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch Gen Psychiatry, 2009. 66(5): p. 478–86. [DOI] [PubMed] [Google Scholar]
- 40.Schmaal L, Goudriaan AE, van der Meer J, van den Brink W, and Veltman DJ, The association between cingulate cortex glutamate concentration and delay discounting is mediated by resting state functional connectivity. Brain Behav, 2012. 2(5): p. 553–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallinat J, Kunz D, Senkowski D, et al. , Hippocampal glutamate concentration predicts cerebral theta oscillations during cognitive processing. Psychopharmacology (Berl), 2006. 187(1): p. 103–11. [DOI] [PubMed] [Google Scholar]
- 42.Skosnik PD, Krishnan GP, D’Souza DC, Hetrick WP, and O’Donnell BF, Disrupted gamma-band neural oscillations during coherent motion perception in heavy cannabis users. Neuropsychopharmacology, 2014. 39(13): p. 3087–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grotenhermen F, Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet, 2003. 42(4): p. 327–60. [DOI] [PubMed] [Google Scholar]
- 44.Spindle TR, Cone EJ, Schlienz NJ, et al. , Acute Effects of Smoked and Vaporized Cannabis in Healthy Adults Who Infrequently Use Cannabis: A Crossover Trial. JAMA Netw Open, 2018. 1 (7): p. e184841. [DOI] [PMC free article] [PubMed] [Google Scholar]


