Abstract
Purpose
Radioresistance is a vital obstacle for the prognosis of human nasopharyngeal carcinoma (NPC), but the underlying mechanism is still unknown. Here, we explored the role of the NRF2/KEAP1 pathway in radioresistance of NPC cell lines.
Materials and Methods
We selected NPC cell lines CNE-1 and CNE-2, treated them with ionization, and subsequently determined the levels of NRF2, KEAP1, antioxidant enzymes, and ROS. We then evaluated the effect of NRF2 or KEAP1 inhibition on cell proliferation, colony formation, and radiosensitivity in CNE2 cells.
Results
We discovered that the NRF2/KEAP1 signaling pathway can be activated by radiotherapy in NPC cells, while NRF2 knockdown enhances the sensitivity of CNE-2 cells to radiation treatment. In contrast, the silencing of KEAP1 inhibits the sensitivity of CNE-2 cells to radiation treatment.
Conclusion
Our results suggest that NRF2/KEAP1 signaling may serve as an essential regulator of the radioresistance of NPC and may be applied as a novel therapeutic approach for the sensitization of NPC to radiation.
Keywords: nasopharyngeal carcinoma, NRF2, KEAP1, radiosensitivity
Introduction
Nasopharyngeal carcinoma (NPC), which originates from the mucosal epithelium of nasopharynx, is a widespread disease in southern China and Southeast Asia.1 Although radiotherapy is a powerful and primary method for the treatment of NPC because of its excellent local control in most patients, radioresistance-induced local recurrence and distant metastasis is still the major cause of deaths in advanced stages.2 Therefore, it is urgently needed to elucidate the mechanisms for radioresistance and develop new radiosensitizers.3
Mechanistically, radiotherapy causes tissue damage in two different ways. One is the direct damage caused by ionization itself, while an indirect effect results from ionization mediated by the products of water radiolysis.4 It is noteworthy that approximately two-thirds of radiation-mediated tissue damage is caused by the indirect effect of accumulated reactive oxygen species (ROS).5 The enhanced ability of cancer cells to inactivate ROS is a key mechanism for radioresistance. As such, inhibition of the ability of NPC cells to inactivate ROS during radiotherapy is essential.
Interestingly, the NRF2 (erythroid-2 related factor 2)/KEAP1 (Kelch-like ECH-associated protein 1) regulatory system functions in cytoprotection from ROS via the upregulation of various antioxidant response elements (ARE) genes, such as GCLC, NQO1, and GSTm1.6 It has been reported that the NRF2/KEAP1 signaling pathway is impaired and associated with radioresistance in numerous types of cancer, including gastric cancer,7 esophageal cancer,8 and lung cancer.7,9 Our earlier study also demonstrated that the aberrant expression of NRF2 and KEAP1 correlates with poor prognosis of NPC patients.10 However, little is known about the biological function of the NRF2/KEAP1 signaling pathway in the radioresistance of NPC.
In this study, we sought to dissect the role of the NRF2/KEAP1 pathway in the radiotherapy of NPC. We first examined the expression levels of NRF2 and KEAP1 in NPC cell lines CNE1 and CNE2 that are representative of radiosensitivity and radioresistance.11 We then used shRNA-mediated NRF2 or KEAP1 silencing to evaluate the effects of inhibiting this pathway on radiotherapy of the CNE2 cell lines. The results from our study revealed the molecular mechanism underlying NPC radioresistance and may improve the therapeutic efficacy against radioresistance.
Materials and Methods
Cell Culture
Two human NPC cell lines (CNE1 and CNE2) were purchased from Shanghai Fuheng Biological Technology Co., Ltd. (Shanghai, China). These cells were cultured in RPMI-1640 medium with 10% fetal bovine serum (Gibco, 10099141), 2 mM glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin and incubated in a humid atmosphere containing 5% CO2 at 37 °C.
Retrovirus Production and Infection
For shRNA-mediated knockdown of KEAP1 and NRF2, pLKO.1-puro-shRNA containing lentiviruses were used. The KEAP1 and NRF2 shRNA sequences are shown in Table 1. Lentiviruses were generated as previously described,12 added to the cells with 10μg/mL polybrene (Shanghai Yeasen Biotechnology, 40804ES76), and incubated for 20 hours, after which fresh growth medium was provided. Cells were selected with 5 μg/mL puromycin (Shanghai Yeasen Biotechnology, 60210ES25) 2 days after the infection.
Table 1.
The Sequences of NRF2 and KEAP1 shRNA for pLKO.1-Puro-shRNA Containing Lenti-Viruses
| Name | Sequence | |
|---|---|---|
| NRF2-sh1 | Forward | GATCCGGCTCCTACTGTGATGTGAAATTTCAAGAGAATTTCACATCACAGTAGGAGCTTTTTTG |
| Reverse | AATTCAAAAAAGCTCCTACTGTGATGTGAAATTCTCTTGAAATTTCACATCACAGTAGGAGCCG | |
| NRF2-sh2 | Forward | GATCCGCCGGCATTTCACTAAACACAATTCAAGAGATTGTGTTTAGTGAAATGCCGGTTTTTTG |
| Reverse | AATTCAAAAAACCGGCATTTCACTAAACACAATCTCTTGAATTGTGTTTAGTGAAATGCCGGCG | |
| KEAP1-sh1 | Forward | GATCCGGCGAATGATCACAGCAATGAATTCAAGAGATTCATTGCTGTGATCATTCGCTTTTTTG |
| Reverse | AATTCAAAAAAGCGAATGATCACAGCAATGAATCTCTTGAATTCATTGCTGTGATCATTCGCCG | |
| KEAP1-sh2 | Forward | GATCCGGCACTGCAAATAACCCATCTTTTCAAGAGAAAGATGGGTTATTTGCAGTGCTTTTTTG |
| Reverse | AATTCAAAAAAGCACTGCAAATAACCCATCTTTCTCTTGAAAAGATGGGTTATTTGCAGTGCCG | |
Ionization Radiation
The cells were irradiated with X-rays at room temperature. The X-ray linear accelerator (MBR-1505R; Hitachi Medical Co., Tokyo, Japan) was operated at 210 kV and 10 mA, with 0.5 mm Al external filtration. The dose rate was 1.8 Gy/min. In addition, 1.5 cm tissue compensation gum was placed on the culture medium.
Colony Formation Assay
Cells were irradiated at 0 Gy, 2 Gy, 4 Gy, 6 Gy, and 8 Gy. Afterwards, the cells were further cultured for 14 days. The colonies were fixed with methanol, stained with crystal violet, naturally dried, and photographed using a GelDoc XR+ Imaging Analysis System (Bio-Rad, USA). The clone numbers were counted, and the mean clone number was calculated. The cell clusters containing over 50 cells were regarded as colonies.
Cell Growth Assay
Cell growth was analyzed using the Cell Counting Kit-8 assay (Dojindo, Japan) according to the manufacturer’s instructions. Briefly, cells were seeded at a density of 3000 cells/well in a 96-well plate and cultured overnight. The cells that had been cultured for 0 h, 24 h, 48 h, 72 h, 96 h, and 120 h were incubated with CCK-8 solutions at 37°C for 2 hours, and the absorbance was measured at 450 nm with a microplate reader.
RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
Total RNA was isolated from cultured cells using Trizol reagent (Takara, Dalian, China) and was then reverse-transcribed to cDNA with PrimeScript Reverse Transcriptase (Takara, Dalian, China) by following the manufacturer’s protocol. RT-qPCR was performed using the Roche Light Cycler 480 Real-Time PCR detector (Roche Diagnostics International Ltd, Forrenstrasse2,6343 Rotkreuz, Switzerland). The gene-specific primers used were as follows:
NQO1, 5ʹ- GAAGAGCACTGATCGTACTGGC-3ʹ (sense),
5ʹ- GGATACTGAAAGTTCGCAGGG-3ʹ (antisense);
GCLC, 5ʹ- GGCACAAGGACGTTCTCAAGT-3ʹ (sense),
5ʹ- CAGACAGGACCAACCGGAC-3ʹ (antisense);
GSTm1, 5ʹ- TCTGCCCTACTTGATTGATGGG-3ʹ (sense),
5ʹ- TCCACACGAATCTTCTCCTCT-3ʹ (antisense);
ACTIN, 5ʹ-GGAGCGAGATCCCTCCAAAAT-3ʹ (sense),
5ʹ-GGCTGTTGTCA TACTTCTCATGG-3ʹ (antisense).
Both the target and reference (ACTIN) genes were amplified in separate wells in triplicate. Gene expression was calculated using the comparative threshold cycle (2−ΔΔCT) method.
Nuclear and Cytoplasmic Extraction Assay
Cells cultured in plates (6 cm) were collected after radiation for 24 h and washed twice with cold PBS. 200 μL Cytoplasmic Extraction Reagent I was added and incubated with cells for 10 min on ice, and 11 μL Cytoplasmic Extraction Reagent II was added and incubated for 1 min on ice. The cells were centrifuged at 16,000g for 5 min, and supernatants (Cytoplasmic extract) were collected. 100 μL Nuclear Extraction Reagent was added and incubated with the extracts for 40 min on ice with vortex every five minutes. Nuclei were separated by centrifugation at 16,000g for 5 min, and supernatants (Nuclear extract) were transferred to a clean tube. Cocktail protease inhibitors were added to all buffers.
Western Blot Analysis
The cells were rinsed with pre-cooling phosphate-buffered saline (PBS) three times and lysed with RIPA lysis buffer (Beyotime Biotechnology Co., Ltd., Shanghai, China). The protein samples were analyzed with SDS-PAGE followed by immunoblotting. Proteins were visualized with ECL according to the manufacturer’s instructions (Millipore, WBKLS0050). The following antibodies were used: Anti-NRF2 antibody (Abcam, ab62352, dilution ratio of 1:1000), anti-KEAP1 antibody (Affinity, BF0010, dilution ratio of 1:1000), and anti-ACTB antibody (Transgen Biotech, HC-201, dilution ratio of 1:5000).
Apoptosis Assay
Apoptotic cells were identified by using the Annexin V-FITC and propidium iodide (PI) apoptosis detection kit (BD, San Diego, USA) according to the manufacturer’s instructions. After being washed twice with PBS, the cells were resuspended in 400 µL 1 × binding buffer and stained with 5 µL Annexin V-FITC and 10 µL PI for 15 min at 4°C in the dark. Apoptosis was analyzed with a BD FACS CantoTM II flow cytometer (BD Bioscience, California, USA).
Measurement of Intracellular ROS
Cellular ROS levels were measured using the Reactive Oxygen Species Assay Kit (Shanghai Yeasen Biotechnology, 50101ES01). Briefly, the medium was removed and washed three times with serum-free medium. 100 uL of diluted DCFH-DA (1:1000) working solution was added to each well of cells, which were subsequently incubated at 37°C for 30 min in the dark. The cells were washed twice with serum-free medium to thoroughly remove DCFH-DA that failed to enter the cells. Fluorescence intensity was measured using an excitation wavelength of 488 nm and an emission wavelength of 525 nm with a multi-mode detection platform (Tecan Austria GmbH, Untersbergstr.1A, A-5082 Grödig, Austria).
Immunofluorescence
Cells were cultured on glass coverslips in the six-well plates, fixed with 4% paraformaldehyde for 15 min, and permeabilized with 0.1% Triton X-100 in PBS. They were then blocked with 5% bovine serum albumin (BSA) followed by the incubation with anti-NRF2 primary antibody at 4°C overnight and secondary antibody at room temperature for 2 h. The secondary antibody used was Alexa Fluor 594 goat anti-rabbit IgG (H + L; 1:500, Life Technologies). The DAPI solution was added to stain and count the cells on the coverslips, and images were captured with a FluoView microscope (Nikon, Japan).
Statistical Analysis
SPSS 17.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 5.0 (GraphPad Software Inc., SanDiego, CA, USA) statistical softwares were employed for the analysis. Data were presented as mean ± standard deviation (SD), and statistical differences between the two experimental groups were determined by using Student’s two-tailed non-paired t-test. Values of P<0.05 were considered statistically significant.
Results
CNE2 Cells are More Resistant to Radiotherapy Than CNE1 Cells
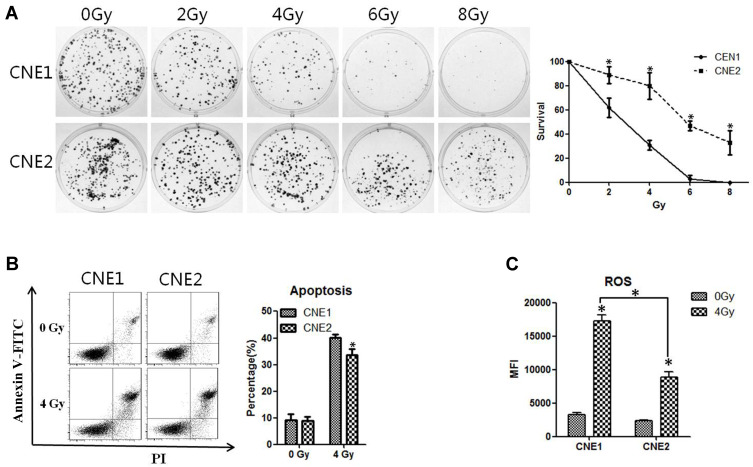
To investigate the radioresistant ability of CNE1 and CNE2 cell lines in vitro, we first employed the clonogenic assay to measure the colony formation abilities of these two cell lines, leading us to discover that colony formation was markedly reduced when the dose of X-ray radiation was gradually elevated. However, compared with the CNE2 cells, the CNE1 cells showed significantly reduced colony formation (P < 0.05, Figure 1A). Moreover, the CNE1 cells displayed increased apoptosis in comparison with the CNE2 cells after 48 h of 4 Gy radiation (P < 0.05, Figure 1B). Thus, X-ray radiation causes the inhibition of cell proliferation in a dose-dependent manner, while CNE2 cells are more resistant to radiotherapy, consistent with earlier studies.11,13
Figure 1.
CNE2 cells are more resistant to radiotherapy than CNE1. (A), CNE1 and CNE2 cells were plated on six-well plates at 400 cells per well. After 24 hours of culture, they received 0 Gy, 2 Gy, 4 Gy, 6 Gy, and 8 Gy radiation. After the replacement with a fresh medium, the cultivation continued for 14 days, and the cells were subjected to crystal violet staining and photographed. (B), CNE1 and CNE2 cells were irradiated with 4 Gy radiation, and the apoptosis rate was detected with flow cytometry 48 hours later. (C), CNE1 and CNE2 cells were plated on 96-well plates, respectively, at 10,000 per well. After 24 hours, they were exposed to 0 Gy and 4 Gy radiation. After another 24 hours, ROS levels were detected. All experiments were repeated three times with similar results. The data are the mean ± SD of values from three experiments. *P< 0.05.
Radiotherapy Enhances Anti-Oxidase Gene Expression in NPC Cells
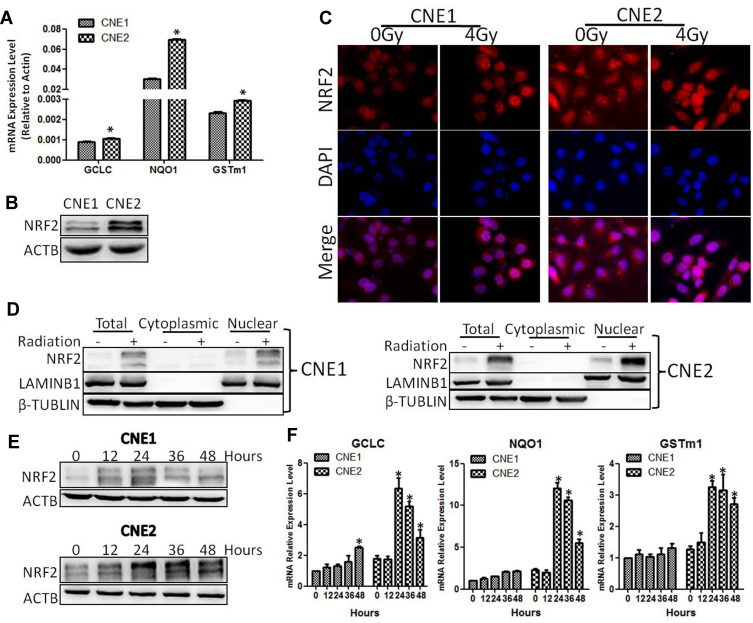
Because ROS plays an important role in radiotherapy-induced cell death,14 we decided to explore the effect of X-ray on the level of ROS. CNE1 and CNE2 cells were irradiated at 0 Gy or 4 Gy, and the ROS level was determined using a Reactive Oxygen Species Assay Kit. We found that the levels of ROS in CNE1 and CNE2 cells were significantly elevated after 24 h of radiation. However, compared with the CNE1 group, the CNE2 group exhibited reduced ROS levels (P < 0.05, Figure 1C). Because ROS is metabolized by antioxidant enzymes in the cells,15 we further assessed the mRNA levels of GCLC, NQO1, and GSTm1 by using qRT-PCR, allowing us to find that the levels were significantly elevated in both NPC cells, while in CNE2 cells there was a stronger response (P < 0.05, Figure 2A). Thus, X-ray radiation enhances the levels of ROS, GCLC, NQO1, and GSTm1, while the elevation of antioxidant enzymes reverses the level of ROS in NPC cells. Together, these results suggest that the ROS level correlates inversely with radioresistance, while the level of antioxidant enzymes correlates positively with radioresistance.
Figure 2.
CNE2 cells have higher ROS inhibitory ability. (A), the levels of anti-ROS genes were detected with qPCR. (B), The protein levels of NRF2 were detected with Western blotting. (C), CNE1 and CNE2 cells were exposed to 0 Gy and 4 Gy radiation for 24 hours, and NRF2 protein was detected with immunofluorescence. (D), CNE1 and CNE2 were exposed to 0 Gy and 4 Gy rays, and 24 hours later, cytoplasmic and nuclear proteins were extracted, while NRF2 were detected by Western blotting. (E), CNE1 and CNE2 cells were exposed to 0 Gy and 4 Gy radiation, and NRF2 protein was detected with Western blotting. (F), CNE1 and CNE2 cells were exposed to 0 Gy and 4 Gy radiation, and the mRNA level of anti-ROS genes were detected with qPCR. All experiments were repeated three times with similar results. The data are the mean ± SD of values from three experiments. *P< 0.05.
Radiotherapy Activates NRF2 in NPC Cells
It has been reported that the NRF2/KEAP1 signaling pathway is impaired and associated with radioresistance in numerous tumors7–9 and correlates with poor prognosis of NPC patients.10 These findings prompted us to examine the level of NRF2 in NPC cells. As shown in Figure 2B, the NRF2 level was higher in CNE2 cells than that in CNE1 cells (P < 0.05). Next, we examined the impact of radiotherapy on NRF2 subcellular localization. Upon radiotherapy treatment, we detected more NRF2 in the nuclei by using immunofluorescence analysis and the Nuclear and Cytoplasmic Protein Extraction assay (Figure 2C and D). To further verify the impact of radiotherapy on NRF2 and antioxidase genes, we exposed CNE1 and CNE2 cells to 0 Gy and 4 Gy of X-ray radiation and examined the time-course expression by using Western blot or qRT-PCR analysis. We found that radiotherapy significantly increased the NRF2 protein level and the levels of antioxidase mRNAs in both NPC cells in a time-dependent manner. However, the levels of NRF2 and antioxidase genes were much higher in CNE2 cells than that in CNE1 cells with radiotherapy treatment (Figure 2E and F). Thus, the activation of NRF2 and the downstream antioxidase genes contributes to the increased resistance of CNE2 cells to radiation.
Silencing of NRF2 Enhances the Sensitivity of NPC Cells to Radiation
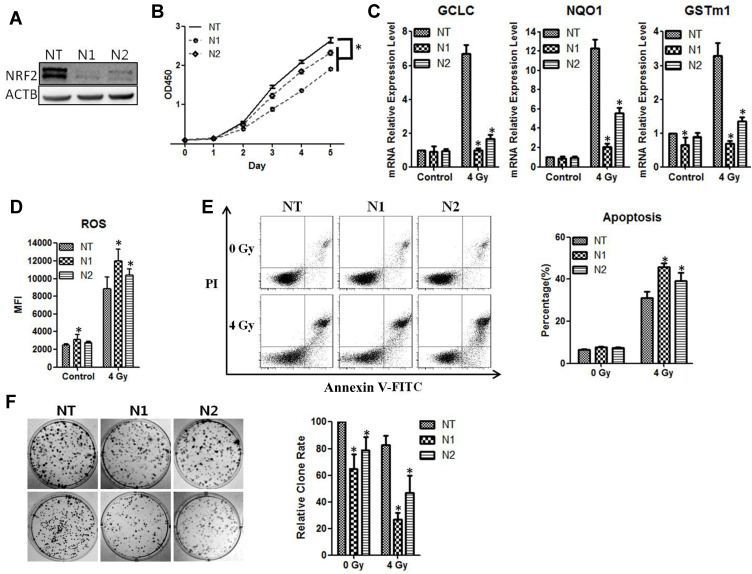
To verify the function of NRF2 in radioresistance, we knocked down NRF2 with shRNAs in the CNE2 cells. Compared with the negative control group (NC-shRNA), the expression of NRF2 protein (Figure 3A) was significantly reduced after infection with retroviruses containing NRF2 shRNAs (shRNA1 or shRNA2). The results from the CCK-8 assays showed that the cell growth rate of CNE-2 cells in the NRF2-shRNA1 and NRF2-shRNA2 group was reduced (P < 0.05, Figure 3B). We then examined the effect of NRF2 depletion on ROS and anti-ROS gene expression after the exposure of cells to 4 Gy of radiation. As shown in Figure 3C and D, the anti-ROS genes GCLC, NQO1, and GSTm1 were markedly down-regulated with the treatment of NRF2-shRNAs, and the ROS level was significantly augmented. We then used flow cytometry to detect the apoptosis rate of cells after 4Gy radiation, which was significantly higher in the groups with NRF2 depletion than those with radiation only (Figure 3E). The apoptosis rates for shRNA1, shRNA2 and the control were 45.68%±2.14%, 39.15%±4.22% and 30.97%±3.01%, respectively (P < 0.05). Moreover, NRF2 depletion markedly reduced colony formation of CNE-2 cells when compared with the control (P < 0.05, Figure 3F). Thus, the silencing of NRF2 attenuates tumorigenic activities in CNE-2 cells, and the combined treatment of NRF2 depletion and radiation leads to a larger reduction of surviving CNE-2 cells than radiation treatment alone. We also knocked down NRF2 in CNE-1 cells and observed similar phenotypes with CNE2 (Supplementary Figure 1).
Figure 3.
CNE2 has enhanced sensitivity to radiotherapy with NRF2 knockdown. (A), The CNE2 cells were infected with retroviruses containing NRF2 shRNAs. The efficiency of knockdown was examined with Western blotting. (B), The cell proliferation of NRF2-depleted CNE2 cells was measured with the CCK-8 assay. (C), The CNE2 cells with NRF2 depletion were exposed to 4Gy radiation. 24 hours later total RNA was extracted, and the mRNA level of anti-ROS genes was detected with qPCR. (D), The NRF2–depleted CNE2 cells were exposed to 4 Gy radiation. 24 hours later the level of ROS was detected. (E), The NRF2-depleted CNE2 cells were exposed to 4 Gy radiation. 48 hours later cell apoptosis was detected with flow cytometry. (F), The NRF2-depleted CNE2 cells were plated on six-well plates at 500 cells per well. After 24 hours of culture, they received 4 Gy radiation. After the replacement with fresh medium, the cells were cultured continuously for 14 days and were subjected to crystal violet staining. All experiments were repeated three times with similar results. The data are the mean ± SD of values from three experiments. *P< 0.05.
Knockdown of KEAP1 Inhibits the Sensitivity of CNE-2 Cells to Radiation
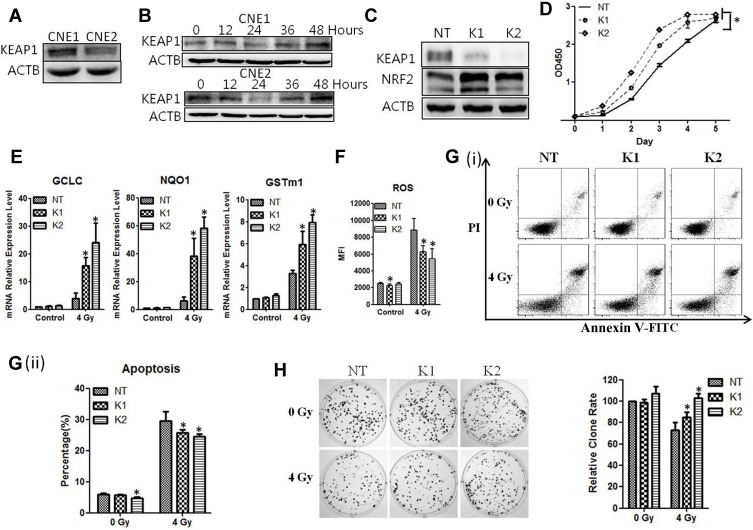
It has been shown previously that the association of KEAP1 with NRF2 promotes NRF2 degradation and prevents it from translocating to the nucleus and activating its target genes.6 We therefore sought to assess the level of KEAP1 expression in NPC cells. We found that the level of KEAP1 was lower in CNE2 cells than CNE-1 cells (P < 0.05, Figure 4A). We also examined the impact of radiotherapy on KEAP1 expression and discovered that the KEAP1 protein level was reduced with 4 Gy of radiation in both NPC cell lines. The change in KEAP1 expression was time-dependent and correlated inversely with NRF2 expression (Figure 4B).
Figure 4.
KEAP1 depletion reduces the radiosensitivity of the cells. (A), The levels of KEAP1 in CNE1 and CNE2 cells were detected with Western blotting. (B), The CNE1 and CNE2 cells were exposed to 0 Gy and 4 Gy radiation, and proteins were extracted at the indicated time. KEAP1 protein was subsequently detected with Western blotting. (C), The CNE2 cells were infected with retroviruses containing KEAP1 shRNAs. The efficiency of knockdown was detected with Western blotting. (D), Cell proliferation of KEAP1-depleted CNE2 cells was measured with the CCK-8 assay. (E), The KEAP1–depleted CNE2 cells were exposed to 4Gy radiation. 24 hours later total RNA was extracted, and the mRNA levels of anti-ROS genes were detected with qPCR. (F), The KEAP1–depleted CNE2 cells were exposed to 4Gy radiation. 24 hours later the ROS level was measured. (G), The KEAP1 RNAi CNE2 cells were exposed to 4 Gy radiation. 48 hours later cell apoptosis was detected with flow cytometry. (H), The KEAP1-depleted CNE2 cells were plated on six-well plates at 300 cells per well. After 24 hours of culture, they received 4 Gy radiation. After the replacement with fresh medium, the cells were cultured continuously for 14 days and were subjected to crystal violet staining. All experiments were repeated three times with similar results. The data are the mean ± SD of values from three experiments. *P< 0.05.
We next examined the function of KEAP1 by constructing retroviruses containing KEAP1 shRNAs and examining the effect of KEAP1 knockdown. We found that two different shRNA sequences (KEAP1-shRNA1 and KEAP1-shRNA2) significantly reduced KEAP1 levels while causing NRF2 expression to increase significantly (Figure 4C). Experiments with the CCK-8 assays showed that the rate of cell growth was elevated in CNE-2 cells treated with KEAP1-shRNA1 and KEAP1-shRNA2 (P < 0.05, Figure 4D).
Furthermore, when exposed to 4 Gy of radiation, cells with KEAP1 depletion exhibited elevated levels of GCLC, NQO1, and GSTm1 mRNA and reduced ROS (P < 0.05, Figure 4E and F). Experiments with flow cytometry analysis showed that the apoptosis rate of CNE-2 cells with KEAP1 depletion was lower than that of the cells without depletion (Figure 4G). The apoptosis rates for cells with shRNA1, shRNA2 and control shRNA were 26.80%±1.13%, 24.54%±0.87%, and 30.97%±3.01%, respectively (P < 0.05). Moreover, the ability of CNE-2 cells to form colonies was significantly enhanced with KEAP1 depletion (P < 0.05, Figure 4H). Thus, the silencing of KEAP1 enhances the tumorigenic response in CNE-2 cells and promotes radioresistance of CNE2 cells to radiation. Similar phenotypes were observed for CNE1 cells (Supplementary Figure 1).
Discussion
Despite recent advances in radio-therapeutic strategies, intrinsic or acquired radioresistance remains a major obstacle to successful treatment in about 20% of NPC patients.2 Thus, the discovery of novel radiosensitizers is urgently needed to improve the radiotherapeutic efficiency. Numerous studies have identified various agents such as Osthole,16 Salinomycin,17 and Berberine18 that are capable of sensitizing NPC cells to radiation. Unfortunately, most of these studies have failed to provide potential radiosensitizers for clinical applications. Therefore, it is essential to explore novel molecular targets for the development of more effective therapeutic strategies for patients with NPC.
It is well known that ROS-mediated DNA damages are the principal mechanism for radiotherapy to kill cancer cells.5 Thus, it is a viable strategy to sustain high levels of ROS via the inactivation of antioxidant function to maintain the efficiency of radiotherapy. The NRF2/KEAP1 signaling pathway has both a positive and a negative effect on cancer.19 Although this pathway can inhibit malignant transformation, it offers resistance to therapy once the tumor is formed.20 Accumulating evidence suggests that the NRF2/KEAP1 signaling pathway is strongly implicated in the development of radioresistance.21 It has also been reported that NRF2 hyperactivation can be induced by radiation,22 and constitutive activation of NRF2 promotes cancer development as well as resistance to chemotherapy and radiotherapy.23 Therefore, targeted inhibition of NRF2 together with traditional radiotherapy may be a promising approach to improving the survival rates of the NPC patients, because CNE1 and CNE2 cells are representative NPC cell types for radiosensitivity and radioresistance.11 In the present study, we first examined NRF2 and KEAP1 expression in these two cell lines and found that NRF2 expression was higher in CNE2 cells than in CNE1 cells, while KEAP1 expression was lower in CNE2 cells than in CNE1 cells. These differences were further exacerbated by radiation. We also demonstrated that the scavengers of ROS, such as GCLC, NQO1, and GSTm1, were substantially up-regulated in CNE2 cells followed by the radiotherapy treatment, suggesting that the activation of NRF2 and its downstream antioxidases are major contributors to render CNE2 cells more radioresistant than CNE1 cells.
Based on these findings, we focused our attention on NRF2-mediated transcriptional silencing of antioxidases as a means to improve radiotherapy. To achieve this goal, we depleted NRF2 and found that the silencing of NRF2 has an anticancer effect on CNE-2 cells and that combined treatment of NRF2 depletion and radiation significantly reduces the survival of CNE2 cells compared with those that receive radiation treatment alone. That is because NRF2 knockdown significantly diminishes X-ray-induced antioxidant responses by simultaneously suppressing GCLC, NQO1, and GSTm1. Consequently, the cellular ROS level is elevated. Thus, we conclude that lentiviruses containing NRF2-shRNA may be used as a potential radiosensitizer for NPC treatment.
Because KEAP1 plays a central role in the regulation of NRF2 activity,24 epigenetic silencing of the KEAP1 gene has been found to cause NRF2 upregulation and further activate the antioxidases.25 In our current study, we also examined the impact of radiotherapy on KEPA1 expression and found that radiotherapy can reduce KEAP1 levels in both NPC cell lines. The expression of KEAP1 correlates inversely with NRF2 expression. Under radiotherapeutic conditions, KEAP1 knockdown further enhances NRF2 expression, leading to the reduction of the ROS level. Thus, the silencing of KEAP1 increases the resistance of CNE2 cells to radiation.
In summary, we demonstrated that activation of the NRF2/KEAP1 signaling pathway renders CNE2 cells more radioresistant than CNE1 cells. We further showed that NRF2 knockdown enhances the sensitivity of CNE-2 cells to radiation treatment. However, the silencing of KEAP1 reduces the sensitivity of CNE-2 cells to radiation treatment (Figure 5). These results have improved the understanding of the molecular mechanism for NPC radioresistance and may provide an innovative therapeutic approach for NPC radiotherapy.
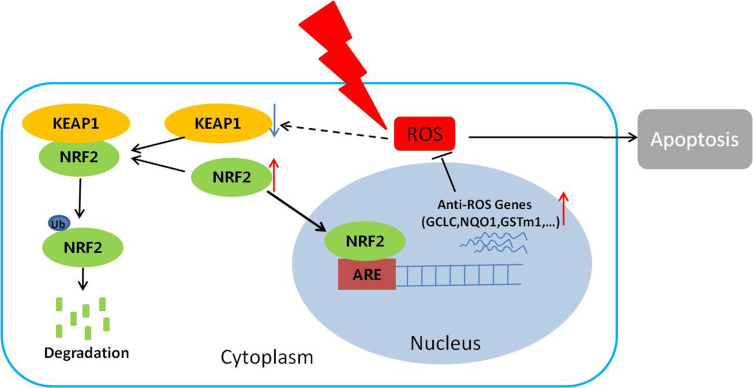
Figure 5.
The proposed mechanism underlying NRF2 regulation of cellular radiosensitivity. NRF2 binds to KEAP1 in the cytoplasm and is ubiquitinated for further degradation. After irradiation, the KEAP1 level is reduced, resulting in reduced degradation and increased accumulation of NRF2 in the nuclei. As a result, the expression of the anti-ROS genes downstream of NRF2 is up-regulated, leading to the reduction of ROS, cell apoptosis, and ultimately the radiosensitivity of the cells.
Funding Statement
This study was supported by the Fundamental Research Program Funding of Ninth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine under Grant JYZ005 (Jieyu Zhou), National Natural Science Foundation of China under Grant 81970094 (Ding Li) and The Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning (TP2015022, Dong Li).
Disclosure
The authors declare that they have no competing interests for this work.
References
- 1.Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80. doi: 10.1016/S0140-6736(19)30956-0 [DOI] [PubMed] [Google Scholar]
- 2.Zhao Y, Shen L, Huang X, et al. High expression of Ki-67 acts a poor prognosis indicator in locally advanced nasopharyngeal carcinoma. Biochem Biophys Res Commun. 2017;494(1–2):390–396. doi: 10.1016/j.bbrc.2017.09.118 [DOI] [PubMed] [Google Scholar]
- 3.Lu J, Tang M, Li H, et al. EBV-LMP1 suppresses the DNA damage response through DNA-PK/AMPK signaling to promote radioresistance in nasopharyngeal carcinoma. Cancer Lett. 2016;380(1):191–200. doi: 10.1016/j.canlet.2016.05.032 [DOI] [PubMed] [Google Scholar]
- 4.Szumiel I. Ionizing radiation-induced oxidative stress, epigenetic changes and genomic instability: the pivotal role of mitochondria. Int J Radiat Biol. 2015;91(1):1–12. doi: 10.3109/09553002.2014.934929 [DOI] [PubMed] [Google Scholar]
- 5.Holley AK, Miao L, St Clair DK, St Clair WH. Redox-modulated phenomena and radiation therapy: the central role of superoxide dismutases. Antioxid Redox Signal. 2014;20(10):1567–1589. doi: 10.1089/ars.2012.5000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paunkov A, Chartoumpekis DV, Ziros PG, Sykiotis GP. A Bibliometric Review of the Keap1/Nrf2 Pathway and its Related Antioxidant Compounds. Antioxidants. 2019;8(9). 10.3390/antiox8090353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bao J, Li J, Li D, Li Z. Correlation between expression of NF-E2-related factor 2 and progression of gastric cancer. Int J Clin Exp Med. 2015;8(8):13235–13242. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Wang Z, Zhang J, Li M, Kong L, Yu J. The expression of p-p62 and nuclear Nrf2 in esophageal squamous cell carcinoma and association with radioresistance. Thorac Cancer. 2020;11(1):130–139. doi: 10.1111/1759-7714.13252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva MM, Rocha CRR, Kinker GS, Pelegrini AL, Menck CFM. The balance between NRF2/GSH antioxidant mediated pathway and DNA repair modulates cisplatin resistance in lung cancer cells. Sci Rep. 2019;9(1):10.1038/s41598-019-54065-6. PubMed PMID: 31776385; PubMed Central PMCID: PMCPMC6881285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma XK, Liu JS. [Expression patterns and prognostic values of Nrf2 and Keap1 in nasopharyngeal carcinoma]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2018;32(9):678–682. Chinese. doi: 10.13201/j.issn.1001-1781.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Tu Z, Shen Z, et al. Quantitative measurement of optical attenuation coefficients of cell lines CNE1, CNE2, and NP69 using optical coherence tomography. Lasers Med Sci. 2013;28(2):621–625. doi: 10.1007/s10103-012-1124-1 [DOI] [PubMed] [Google Scholar]
- 12.Zhou P, Li Y, Li B, et al. NMIIA promotes tumor growth and metastasis by activating the Wnt/beta-catenin signaling pathway and EMT in pancreatic cancer. Oncogene. 2019;38(27):5500–5515. [DOI] [PubMed] [Google Scholar]
- 13.Pan Y, Zhang Q, Atsaves V, Yang H, Claret FX. Suppression of Jab1/CSN5 induces radio- and chemo-sensitivity in nasopharyngeal carcinoma through changes to the DNA damage and repair pathways. Oncogene. 2013;32(22):2756–2766. doi: 10.1038/onc.2012.294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zou Z, Chang H, Li H, Wang S. Induction of reactive oxygen species: an emerging approach for cancer therapy. Apoptosis. 2017;22(11):1321–1335. doi: 10.1007/s10495-017-1424-9. [DOI] [PubMed] [Google Scholar]
- 15.He L, He T, Farrar S, Ji L, Liu T, Ma X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol Biochem. 2017;44(2):532–553. doi: 10.1159/000485089. [DOI] [PubMed] [Google Scholar]
- 16.Peng L, Huang YT, Chen J, et al. Osthole sensitizes with radiotherapy to suppress tumorigenesis of human nasopharyngeal carcinoma in vitro and in vivo. Cancer Manag Res. 2018;10:5471–5477. doi: 10.2147/CMAR.S182798;. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Zhang G, Wang W, Yao C, Ren J, Zhang S, Han M. Salinomycin overcomes radioresistance in nasopharyngeal carcinoma cells by inhibiting Nrf2 level and promoting ROS generation. Biomed Pharmacother. 2017;91:147–154. doi: 10.1016/j.biopha.2017.04.095 [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Kang M, Wen Q, et al. Berberine sensitizes nasopharyngeal carcinoma cells to radiation through inhibition of Sp1 and EMT. Oncol Rep. 2017;37(4):2425–2432. doi: 10.3892/or.2017.5499 [DOI] [PubMed] [Google Scholar]
- 19.Qin JJ, Cheng XD, Zhang J, Zhang WD. Dual roles and therapeutic potential of Keap1-Nrf2 pathway in pancreatic cancer: a systematic review. Cell Commun Signal. 2019;17(1):121. doi: 10.1186/s12964-019-0435-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basak P, Sadhukhan P, Sarkar P, Sil PC. Perspectives of the Nrf-2 signaling pathway in cancer progression and therapy. Toxicol Rep. 2017;4:306–318. doi: 10.1016/j.toxrep.2017.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu S, Lu H, Bai Y. Nrf2 in cancers: A double-edged sword. Cancer Med. 2019;8(5):2252–2267. doi: 10.1002/cam4.2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panieri E, Saso L. Potential Applications of NRF2 Inhibitors in Cancer Therapy. Oxid Med Cell Longev. 2019;2019:8592348. doi: 10.1155/2019/8592348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taguchi K, Yamamoto M. The KEAP1-NRF2 System in Cancer. Front Oncol. 2017;7:85. doi: 10.3389/fonc.2017.00085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimta AA, Cenariu D, Irimie A, et al. The Role of Nrf2 Activity in Cancer Development and Progression. Cancers. 2019;11(11):1755. doi: 10.3390/cancers11111755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang R, An J, Ji F, Jiao H, Sun H, Zhou D. Hypermethylation of the Keap1 gene in human lung cancer cell lines and lung cancer tissues. Biochem Biophys Res Commun. 2008;373(1):151–154. doi: 10.1016/j.bbrc.2008.06.004. [DOI] [PubMed] [Google Scholar]