Abstract
Introduction
Oxaliplatin (L-OHP) is a well-known third-generation platinum anticancer drug with severe systemic- and neuro-toxicity. The main objective of the current research was to develop a targeted long-circulating thermosensitive smart-release liposome (LCTL) system for better therapeutic efficacy and less toxicity.
Methods
The reverse-phase evaporation method (REV) was used to prepare L-OHP loaded LCTL (L-OHP/LCTL). The physical characteristics were evaluated including encapsulation efficiency (EE), size, zeta potential and stability. The release behavior, cytotoxicity and in vivo evaluation were also carried out.
Results
EE of LCTL was around 25% with a uniform size distribution, and LCTL achieved almost complete release at 42°C while it was only 10% at 37°C. Moreover, the LCTL showed significantly higher cytotoxicity at 42°C than that at 37°C. The in vivo results indicated LCTL could target tumors and enhance retention for more than 24 h, thereby enhancing anti-tumor efficacy on 4T1-bearing mice.
Discussion
These results indicated that LCTL not only possessed a prolonged circulation time but it also enhanced accumulation and achieved selective release at the tumor sites. Conclusively, LCTL could serve as a promising carrier for oxaliplatin delivery to treat solid tumors.
Keywords: delivery, oxaliplatin, thermosensitive, pharmacokinetic properties, tumor targeting
Introduction
Oxaliplatin (L-OHP) is a third-generation platinum-based drug which is usually used as first-line chemotherapy for metastatic colorectal cancer. The mechanism of L-OHP to inhibit tumor growth was blocking DNA replication and transcription of cells.1 Compared with cisplatin and carboplatin, L-OHP has advantages of higher anticancer activity and lower toxicity. However, L-OHP still appears seriously neurological toxicity, system toxicity and gastrointestinal toxicity. In its clinical application, nearly three-fourths of patients presented neutropenia with different symptoms including nausea,2 vomiting and other adverse reactions.3 In addition, as references reported, L-OHP showed a low concentration in the plasma because of its high irreversible binding to plasma, tissue proteins and erythrocytes4 which causes serious limitations to reach desirable drug concentrations in the tumor tissue and subsequent therapeutic efficacy. Recently, to solve these problems, novel strategies aimed at modifying L-OHP biodistribution and pharmacokinetics have been researched,5 such as the nanocarrier of oxaliplatin or other platinum prodrugs6,7 which had been investigated widely in the field of biomedical material. Almost all these reports indicated that the nano formulation could decrease the toxicity of platinum significantly.
Among the nanoscale carriers, liposomes have been widely studied with promising applications for platinum based drugs.8 For example, MBP426 (L-OHP) and Lipoplatin (cisplatin) have reached Phase I and Phase III clinical trials, respectively. Liposomes could also protect the drugs from degradation and improve drug accumulation in the tumor area by the EPR effect.9,10 Besides, liposomes have a hydrophilic core which can encapsulate hydrophilic drugs since the solubility in water of L-OHP is 7.9 mg·mL−1. PEGylated-liposomes can reduce the uptake of liposomes by the reticuloendothelial system and prolong the half-life of drugs in blood.11 Referred to some reports, there published many researches on PEG-coated liposomes to delivery L-OHP and co-delivery of oxaliplatin and other chemical drugs for combination therapy which showed superior effect in enhancing the tumor accumulation and modifying the intratumor distribution.12–15
Despite advances with prolonged circulation and increased accumulation in the tumor area, the absence of an active trigger to release drugs from liposome provides an explanation for their rejection of antitumor activity,16 such as the clinical failure for platinum liposome (SPI-077),17 even though it has been found that the liposomes accumulate substantially in the tumor tissue. An elusive and paradoxical problem still existing is how to obtain high local drug bioavailability while maintaining the stability of the liposomes in circulation. In 1978, triggered drug release from thermosensitive liposomes was first proposed by Yatvin et al.18 Since then, different thermosensitive liposomes have been researched.19 The original thermosensitive liposome was composed of DPPC (Tm=41.4°C), which could exhibit smart release by phase transitions. In addition, some reports revealed that DPPC-based thermosensitive liposomes with the inclusion of another lipid components such as MSPC or mPEG2000-DSPE could also result in rapid release of drugs upon heating to the phase transition (42°C~45°C). With different lipid compositions, their thermosensitivity can be successfully tuned between 41°C and 43°C which is clinically preferable. The phase transition at the Tm is a result of a conformational change in the alkyl chains of the lipids.20 So that the drug release from LCTL relied mostly on the increased permeability after heating to the temperature above the average Tm of the lipid mixture.
The objective of our research was to design PEGylated thermosensitive liposomes which will result in selective and controlled drug release due to a narrow temperature range with mild local hyperthermia. The DPPC as main thermosensitive material and PEG2000-DSPE as the long-circulating material were selected to construct long-circulating thermosensitive liposomes (LCTL). Besides, the mild local hyperthermia at 41–43 °C is known to increase tumor blood flow, microvascular permeability and trigger quick release of the drug from liposomes in tumors which decrease normal tissue side effects.21 Taking into account that most of the publications about liposomes of oxaliplatin,12–15 we found that although this PEG-coated liposome system was already developed for L-OHP delivery, our work still have some significant meanings in improving its therapeutic efficiency. Despite advances with prolonged circulation and increased accumulation in the tumor area, an active trigger to release drugs is still a key characteristic, so our work firstly carried out the research on the PEG-coated thermosensitive liposome system for smart delivery of L-OHP. In this study, we propose an efficient drug delivery system using PEGylated thermosensitive liposomes for the targeted delivery of L-OHP to tumor followed by heat-triggered drug release (Scheme 1). Based on this design, we report on the different preparation methods, characterization, release behaviors and stability by L-OHP-loaded targeted thermosensitive liposomes. LCTL were applied in combination with mild hyperthermia (HT) to different tumor cells and normal cells in order to test their cellular toxicity. In addition, we studied the pharmacokinetics of LCTL and their targeting ability by in vivo imaging. Finally, we investigated the effect of HT on the anti-tumor efficiency of LCTL based on weight, tumor inhibition and survival time of mice.
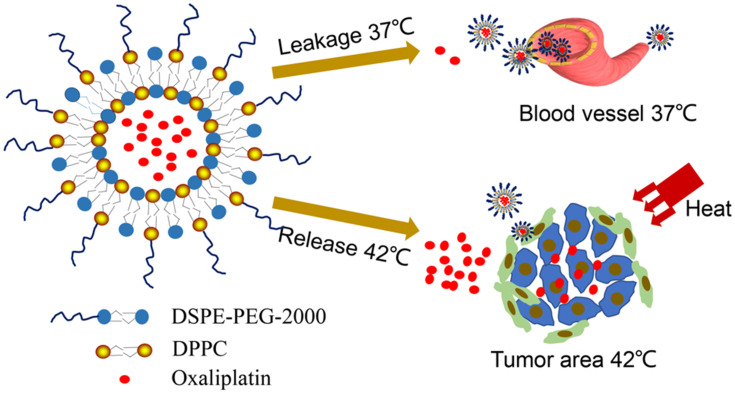
Scheme 1.
Schematic diagram of the heat-triggered release from long-circulating L-OHP-loaded thermosensitive liposomes.
Materials and Methods
Materials
L-OHP was obtained from Shandong Boyuan Chemical Co. (Shandong, China). 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene glycol)-2000] (DSPE-PEG2000) were purchased from Avanti polar lipids Inc. (Alabaster,Alabama, USA). Soybean phosphatidylcholine (SPC) was purchased from Tywei company in Shanghai. MTT ((3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide) assays were purchased from the Shanghai Institute of Cell Research (Shanghai, China). DiR (1,1ʹ-dioctadecyl-3,3,3ʹ,3ʹ-tetramethyl indotricarbocyanine Iodide) was obtained from Sigma Chemical Co, Ltd. (Saint Louis, MO). All the reagents were of analytical grade and used without further purification.
Animals and Ethical Statement
Rabbits weighing 2~2.5kg and BALB/c nude mice (seven weeks old, 20–25 g) were purchased from Qinglongshan Farms (Nanjing, China). The ethical and legal approval from the Animal Welfare and Research Ethics Committee of China Pharmaceutical University (No.2019–08-003) was obtained prior to the commencement of animal experiments. All the animal experiments were conducted in full compliance with the ethical guidelines of China Pharmaceutical University.
Cell Culture and Cell Lines
The RKO colonic cancer cell, L02 normal human liver cell and 4T1 breast cancer cell were used, which were all were brought from the American Type Culture Collection (ATCC). The cells were cultured in RPMI 1640 medium with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin ll cells were cultured in RPMI1640 medium with 10% fetal bovine serum (FBS).
The Preparation of Thermosensitive Liposomes
Incubation of Blank Liposome Method (IBL)
Lipids (DPPC, DSPE-PEG2000, w/w, 10:1) solubilized in a mixture of chloroform were placed on a rotary evaporator to remove the organic solvent under reduced pressure (0.1 MPa) and to obtain a film on the wall of the flask. The film was hydrated at 55°C ºC with a 9% sucrose solution[16]. The mixture was sonicated at 100W for 3 min which made the suspension a clear blank liposome. The liposomes were extruded through a polycarbonate membrane to control the particle diameter. L-OHP was added into the liposome and incubated for 3h at 55°C. The amount of non-encapsulated L-OHP was removed from the formulation by dialysis (3500kDa) for 12h. The final liposome with DPPC, DSPE-PEG2000 and L-OHP were prepared (LCTL) with the lipid to drug ratio of 7.5:1. The liposome only with DPPC and L-OHP(TL) and the liposome with SPC, DSPE-PEG2000, cholesterol and L-OHP(LCL) were prepared using the same methods and same ratio of lipid to drug (7.5:1, w/w).
Reverse-Phase Evaporation Method (REV)
REV, as the most common technique, is used to encapsulate water-soluble drugs inside liposomes. Firstly, lipids (DPPC, DSPE-PEG2000, w/w, 10:1) were dissolved in 6mL organic solvents (chloroform: ether=2:1, v/v). Two mililiter L-OHP solution (4 mg/mL) in glucose 5% [17] was added into above organic solvents and the mixture was sonicated at 200W for 5min. After that, the organic solvents in mixture was removed by rotary evaporation.REV is the most common technique used to encapsulate platinum derivatives. Lipids were dissolved in chloroform. The aqueous phase containing L-OHP (4 mg/mL) dissolved in glucose 5% was added into the organic phase at a ratio of 3:1 (v/v) between the organic and aqueous phase. The lipid to drug ratio was setted as 7.5:1 (w/w). The mixture was sonicated at 200 W for 5 min and then the chloroform was removed on a rotary evaporator. The clear liposome formed during the evaporation. The liposomes were extruded through a 0.22μm membrane and dialyzed similar with using the incubation of IBL blank liposome method.
Size Distribution, Zeta Potential and Encapsulation Efficiency (EE, %)
The particle size, PDI and zeta potential of LCTL in HEPES buffer solution were measured using a Malvern Zetasizer Nano-ZS90 (Malvern instruments, UK). All of the dynamic light scattering (DLS) measurements were performed at 25°C and at a scattering angle of 90° detection angle. The morphology was observed by transmission electron microscopy (TEM, H-600, Hitachi, Japan).
The L-OHP encapsulation was measured by HPLC using a validated method. The liposomes after the dialysis were diluted with 4-times volume of 10% triton solution, and shaken at 60°C for 10 minutes to ensure that the liposome structure was fully disrupted and L-OHP had been released. After cooling to room temperature, the solution was diluted with 9% sucrose solution for further detection. Another solution of initial oxaliplatin liposome without dialysis was performed in the same way. The content of oxaliplatin was determined by HPLC for the liposomes before and after dialysis. The HPLC conditions were included in the following details. The chromatographic column is Zorbax SB-18 (4.6nmX250nm, 5 μm), mobile phase was water-methanol (95:5), detection wavelength is 250nm, column temperature is 30°C, flow rate is 1.0mL/min and injection volume is 20μL. Then, the EE expressed in percentage (%) was calculated by the following formula, while: m1 represents the total weight of L-OHP in the solution and m2 represents the free L-OHP which was not loaded inside liposomes. 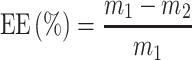
Differential Scanning Calorimetry (DSC)
The blank LCTL and L-OHP/LCTL were prepared according to the REV method. DSC is a thermal-analytical technique used to measure the melting process of a substance and to determine the glass transition temperature, melting point, and crystallinity used to observe fusion and crystallization events. The experiments were conducted on Thermograms of the samples were obtained in a DSC-204 system (Netzsch, Germany). All experiments with 6mg samples were operated from 20°C to 200°C at a heating rate of 10°C/min under constant purging of nitrogen at 20 mL/min.
Lyophilization Assay
The LCTL was prepared according to method 2.4.2. Then different lyoprotectants including mannitol and trehalose were added into LCTL solution, respectively. After the lyophilization process, the formulation was again characterized by determining size, PDI and EE Formulations were lyophilized with different cryoprotectants, such as mannitol (2–10%, w/v), or with trehalose (2–10%, w/v) and with a different hydration medium. After the lyophilization process, the formulation was again characterized by determining size, PDI and EE.
Stability Study
Physical or colloidal stability based on size distribution and EE under storage conditions as well as in a biological medium must be considered. For this, the liposomes were placed at 4°C for one month and the EE was measured to calculate the leakage.
Release Behavior of LCTL in vitro
The release rate of L-OHP in vitro was determined using the dialysis method. Briefly, 0.5 mL L-OHP/LCTL was added to a dialysis bag (3500 Da), and dialyzed for 24h against 15mL of a 9% sucrose solution on a horizontal shaker (110 rpm) at 37°C and 42°C. At predetermined intervals, 2 mL aliquots were withdrawn and replaced with an equal volume of fresh medium. The concentrations of L-OHP in the collected samples were analyzed by HPLC. The cumulative release (%) was calculated by the following formula. Cn is the concentration of L-OHP in the medium at the n sampling point and Ci is the concentration of L-OHP in the medium at the i sampling point. V is the total medium (15mL) and Vi is the withdrawn volume every time (2mL).
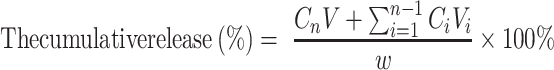 |
MTT Assays
The cytotoxicity of L-OHP/LCTL was evaluated using the MTT colorimetric test by calculating cell viability percentage. RKO, 4T1 and L02 cells were seeded in a 96-well plate at a density of 8000 cells per well in RPMI1640 medium containing 10% FBS separately. For toxicity at 37 °C and 42 °C, different preparations were firstly diluted into serial concentrations and added to the 96-well plate to incubate with the cells. After the addition, the cell plates were placed at 37°C or 42°C for 30 minutes, respectively, and then were incubated in a 37°C incubator for 24 hours and 48 hours, respectively. The LCTL was diluted into serial concentrations after incubation under a 37°C and 42°C water bath for 30min separately. After the incubation of serial concentrations (0.1–100 μM) in each well at 37°C for 48 h, 20 μL of MTT (5 mg/mL) was added to each well and then incubated for 4h at 37°C to form formazan crystals. The medium was removed and dimethyl sulfoxide (DMSO) was added to each well. The L-OHP solution was used as the reference. The absorbance of dissolved formazan was measured at 570 nm using a Microplate Reader. Cell inhibition percent was calculated using the following equation:
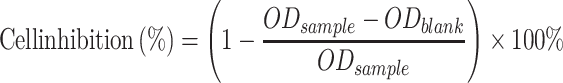 |
where ODsample represents the absorbance of formazan in live cells incubated with nanoparticles and ODblank represents the absorbance of the blank sample.
The Cell Uptake Study
To evaluate the uptake of L-OHP in cells, 4T1 cells were selected as the cell line to determine the uptake behavior. The cells were seeded in a 6-well plate at a density of 3*105 cells per well in RPMI1640 medium containing 10% FBS separately. After 24h of incubation, drugs were added with a final concentration of L-OHP as 20μM and content of lipids as 9.81mg/mL. Then, separately after 2h, 4h, 6h, 8h, 10h, 12h, the cells were washed for three times and were collected. Four hundred microliter of cell lysate buffer were added for 20min on an ice bath followed by using BCA protein detection. The remaining cells were digested with concentrated nitric acid for 10min, and diluted with 1mL of 3% nitric acid to determine the platinum content by the graphite furnace atomic absorption spectrometry method.
Tumor-Targeting Properties of LCTL by in vivo Imaging
To evaluate the prolonged-circulation ability of LCTL, 1,1ʹ-dioctadecyl-3,3,3ʹ,3ʹ-tetramethyl indotricarbocyanineiodide (DiR), a near-infrared (NIR) fluorescent dye was loaded instead of L-OHP into LCTL, TL was obtained at 0.20mg/mL of the final concentration. The antitumour study was performed using 4T1-bearing mice.
Approximately 1×105 4T1 cells were injected subcutaneously into the armpit region of BALB/c nude mice (seven weeks old, 20–25 g). Two weeks after the cell injection and when the tumors grew to 1–2 cm3 in diameter, the animals were divided into three groups: DiR-LCTL group, DiR-TL group and the control free DiR. The liposomes were injected into tumor-bearing mice at a dose of 1.5 mg DiR/kg by the tail vein. NIRF imaging experiments were performed at 1h, 6h, 8h and 24h after injection. After 24h, the mice with different treatments were sacrificed and the main organs containing including heart, liver, spleen, lung, kidney and the tumor were obtained. Then, the fluorescence intensity was detected quantitatively determined by a Kodak in vivo imaging system FX PRO (Kodak, USA) equipped with an excitation band pass filter at 720 nm and an emission at 790 nm. All experiments were conducted in strict accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Pharmacokinetics Study of LCTL
Male mice (5 weeks old, SD rats, 20–22 g) were given a single intravenous (i.v.) injection of free L-OHP (5 mg/kg) or LCTL at an equivalent dose of 5 mg/kg L-OHP via the tail vein. The blood (0.15 mL) was collected into a heparinized microtube from the orbital vein at 15min, 30min, 1h, 2h, 4h, 8h, 12h, and 24 h after the injection. The blood samples were centrifuged at 4000rpm for 10 min at room temperature to get the plasma.
In vivo Study
To evaluate the anti-tumor ability of LCTL with HT, LCTL without HT, TL with HT and L-OHP solutions, the L-OHP/liposomes were first prepared by the REV method. The antitumour study was performed using 4T1 models.
Approximately 1×105 4T1 cells were injected subcutaneously into the armpit region of BALB/c nude mice (seven weeks old, 20–25 g). Two weeks after the cell injection and when the volume of tumor was around 150 mm3, the animals were randomly divided into four groups. The mice received a total of four doses of L-OHP-loaded liposomes (5 mg L-OHP/kg per dose) at four-day intervals by intravenous injection. The control group was administrated with physical saline. After the injection, infrared radiation was performed to the tumor area of the LCTL group for 30min every day for four times. The tumor volume and weight were measured every other day. The tumor volume and anti-tumor efficiency were calculated by the following equation:
Tumor volume (mm3) = 0.5 × length × width2
Body weight was measured simultaneously and was taken as a parameter to determine systemic toxicity.
The Hemolysis Assay
Fresh rabbit blood was collected into a conical flask firstly and stirred gently for 10 min with a glass bar to remove the formed fibrinogen. The remaining red blood sample cells (3mL) were diluted with 30mL 10 times the volume of physiological saline followed by the centrifugation at 1500 rpm for 10 min. Then, the supernatant was decanted and the blood cells were rinsed with physiological saline three times. The blood cells obtained were resuspended in physiological saline until the final concentration was 2% (v/v). The TL solution, LCTL solution or L-OHP solution, distilled water, physiological saline and the rabbit blood cell suspension were added according to Table 1. The resulting mixtures were incubated at 37°C ºC for 3 h and then centrifuged at 1500 rpm for 10 min. The supernatant was measured at 540 nm to determine the degree of hemolysis. Tube 6 and tube 7 represent zero hemolysis and 100% hemolysis. The percentage of hemolysis was determined as shown in Table 1.
Table 1.
Experimental Arrangement of Hemolysis Assay
| Tube | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| 2% blood cell suspension | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Physiological saline | 2.0 | 2.1 | 2.2 | 2.3 | 2.4 | 2.5 | – |
| Distilled water | – | – | – | – | – | – | 2.5 |
| L-OHP solution | 0.5 | 0.4 | 0.3 | 0.2 | 0.1 | ||
| SPC liposome | 0.5 | 0.4 | 0.3 | 0.2 | 0.1 | ||
| mPEG liposome | 0.5 | 0.4 | 0.3 | 0.2 | 0.1 | ||
The TL and LTCL were prepared according to the REV method. The rabbits were administrated by the intravenous injection from the ear at 1-day interval with free L-OHP solution, TL, and LCTL at a dose of 5 mg/kg for L-OHP and equivalent volume of physiological saline as the control. The animals were sacrificed 3 days post-injection. The ears were harvested and stored in a 10% formaldehyde solution overnight for fixation before slide mounting with hematoxylin and staining with eosin.
Statistical Analysis
Statistical analysis was performed by SPSS using a standard Student’s t-test (comparing only two individual groups) with a minimum confidence level of 0.05 for the significant statistical difference. All values are reported as mean ± standard deviation (SD).
Results
Characterization of LCTL
The physicochemical characterization of liposomes prepared by the different methods in this study were listed in Table 2, which included particle size, polydispersity index (PDI) and zeta potential.
Table 2.
The Characteristics of LCTL Prepared by REV and IBL Methods
| Method | REV | IBL |
|---|---|---|
| Particle size | 190 2.7nm 2.7nm |
120 1.3nm 1.3nm |
| PDI | 0.09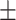 0.01 0.01 |
0.16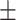 0.01 0.01 |
| Zeta potential | −21.2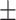 0.34mV 0.34mV |
−20.2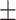 0.46mV 0.46mV |
| EE | 26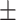 2.9% 2.9% |
6.8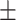 2.4% 2.4% |
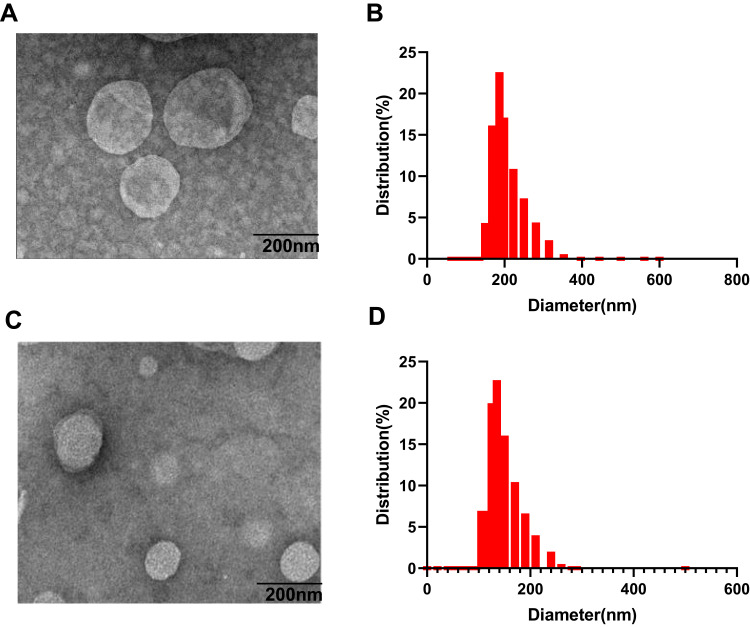
REV is the most common technique used to encapsulate platinum derivatives[18]. All liposomal formulations prepared by REV showed a particle size around 190 nm with the 26.5% encapsulation efficiency (EE) while liposomes prepared by IBL were around 120nm with a very low EE. The zeta potential was negative at about −21mV which was similar between both methods. As shown in the TEM and DLS images (Figure 1), it is clearly found that the although the particle diameter by IBL is smaller than that by REV, the polydispersity index showed a significant decline for the particle by REV compared with that by IBL (Figure 1). This data indicated that the LCTL prepared by REV possessed a more uniform distribution. Moreover, the EE for the IBL was only 6%~10% because the L-OHP was loaded into the hydrophilic cavity only by weak passive diffusion caused by the concentration difference which is not unfavorable for the in vivo evaluations.
Figure 1.
The TEM images and a plot curve of the DLS-determined hydrodynamic diameter of LCTL. (A) The TEM images of LCTL prepared by REV. (B) The DLS-curve of LCTL prepared by REV. (C) The TEM images of LCTL prepared by IBL. (D) The DLS-curve of LCTL prepared by IBL.
Several initial conditions were tested to increase the drug loading such as L-OHP/lipid ratio, ultrasonic time and the volume ratio of the organic and water phase. The optimal conditions for the REV are listed in Table 3. Based on the above results, the REV method was selected for the next studies.
Table 3.
The Optimal Conditions for REV
| Method | Conditions |
|---|---|
| Organic phase/water phase (V/V) | 3:1 |
| Ultrasonic power | 200 W for 5 min |
| CL-OHP | 4 mg/mL |
| DSPE-PEG2000 (molar ratio of total lipids) | 5% |
DSC
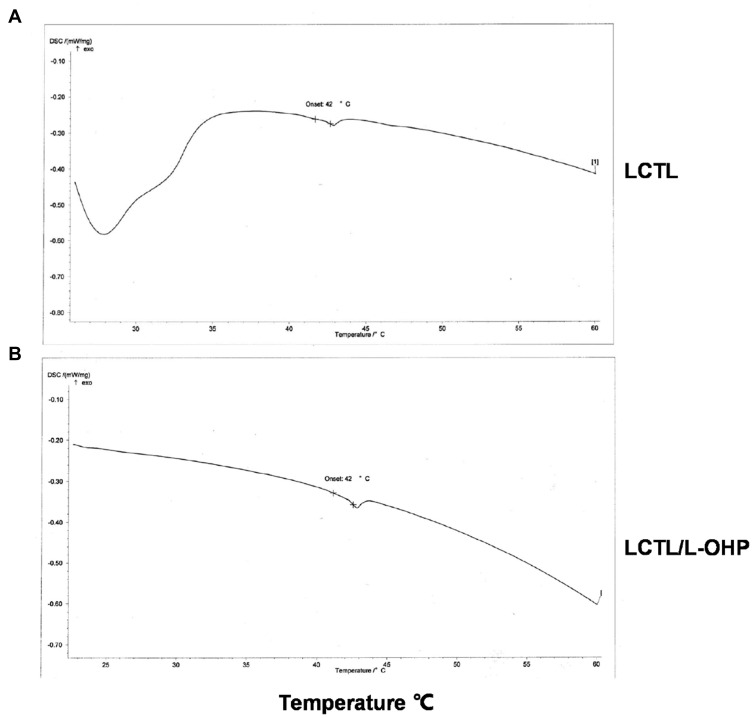
Figure 2 shows a thermal curve with a sharp transition peak at about 42°C for both LCTL and L-OHP/LCTL. As seen from Figure 2, the phase transition temperature of blank LCTL was 42°C, which is consistent with relevant conclusions of many literatures.19 In our study, it was found that the loading of oxaliplatin had negligible effect on the phase transition temperature for LCTL membrane, which was still maintained at 41.9°C.
Figure 2.
DSC thermograms of blank LCTL (A) and LCTL/L-OHP (B).
Lyophilization Assay and Stability Studies
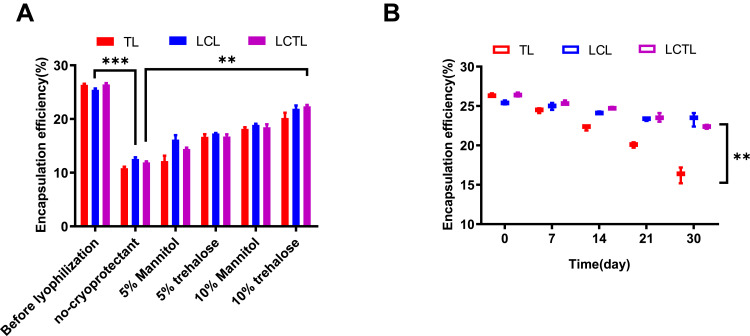
Lyophilization is an effective way to improve the long-term stability of liposomal formulations. However, the processes of pre-freezing and drying involved in lyophilization are not unfavorable to the stability of the structure for liposome. Based on the reports, the lyoprotectants can effectively reduce the influence by these lyophilization process.22 (Figure 3). Two different cryoprotectants, trehalose and mannitol, were used to prevent liposome instability during freeze-drying. The results showed that the lyophilization process would still cause almost 40% leakage when the cryoprotectants were increased to 5%. In addition, the continuous increment of cryoprotectants to 10% leads to the stability of L-OHP inside the LCTL after reconstitution. As shown in Figure 3A, when the trehalose was added to 10%, more than 70% of L-OHP was kept inside the LCTL.
Figure 3.
(A) The change of EE for TL, LCL, LCTL after lyophilization with different cryoprotectants. (B) The change of EE for TL, LCL, LCTL after storage at 4°C for one month. Data are represented as the mean±SD from three independent experiments. **p < 0.01, ***p < 0.001.
The stability of LCTL stored at 4°C was also studied. Table 4 shows that at 4°C, the LCTL were stable at least for one month with regards to size, zeta potential and EE, but the TL appeared to have almost a 60% leakage after 30 days while LCL and LCTL appeared to have only less than 20% of leakage (Figure 3B). In Figure 3B, it was found that the PEG2000 increased the stability of liposomes during storage at 4°C. According to the references,23 the electrical neutrality, hydrophilicity and large steric hindrance of the polyethylene glycol (PEG) chain can effectively prevent the recognition and combination with the opsin protein and plasma proteins which thereby reduced the phagocytosis of RES in the body.23
Table 4.
The Stability of LCTL at 4°C After One Month
| Before | After One Month | |
|---|---|---|
| Particle size | 190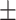 2.7 nm 2.7 nm |
192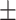 1.7 nm 1.7 nm |
| PDI | 0.09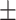 0.01 0.01 |
0.10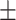 0.13 0.13 |
| Zeta potential | −21.2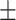 0.34 mV 0.34 mV |
−23.2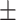 0.17 mV 0.17 mV |
| EE | 26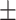 2.9% 2.9% |
24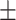 3.9% 3.9% |
| Appearance | Clear solution with blue opalescence | Clear solution with blue opalescence |
The Release Study
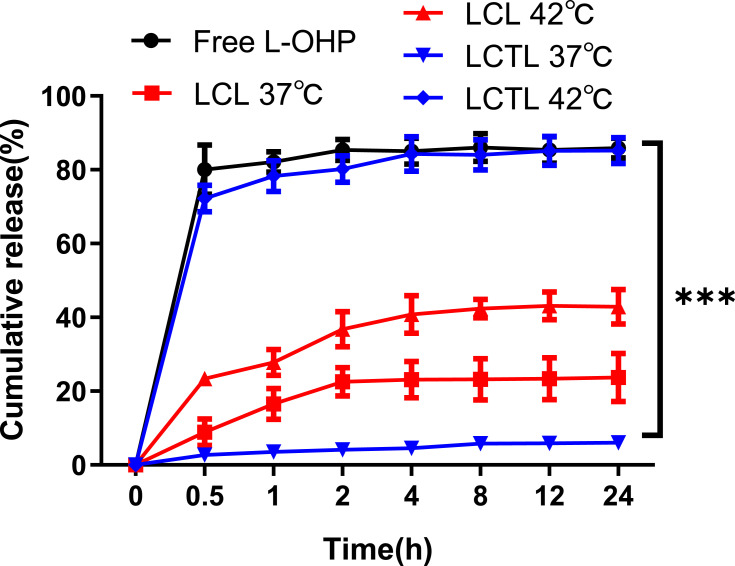
According to the standard curve (Figure S1), the cumulative release curves of L-OHP from LCL and LCTL in PBS at 37°C and 42°C are shown in Figure 4. First, the L-OHP showed an almost complete release from the L-OHP solution within 5min and an initial burst release was observed at 5 min triggered by temperature as expected for LCTL. Then, the release from the LCTL achieved, ie,70% of drug release within 0.5h at 42 °C and ie 80% of drug release within 4h at 42 °C. The release behavior indicated that the LCTL achieved an almost total release at 42 °C while that was only 10% at 37 °C after 24h which exhibited superior smart-release. A liposome formulation with specifically fast release at mild hyperthermic temperatures is particularly advantageous in delivering L-OHP to the heterogeneously heated tumor. The enhanced release within tumor could prevent the lost of L-OHP in the plasma because the blood transit time through a tumor is usually very rapid. As seen in Figure 3, the LCL could release L-OHP at about 30% at different temperatures. So, the classic liposomes composed of SPC induced higher leakage when used to load L-OHP in the physical blood at 37 °C, but the DPPC increased the rigidity of the liposomal membrane which reduced leakage at 37 °C.
Figure 4.
The accumulative release percentage of L-OHP from free L-OHP solution, LCL, LCTL in PBS at 37°C and 42°C. Results are represented as the mean±SD from three independent experiments. ***p < 0.001.
MTT Assays
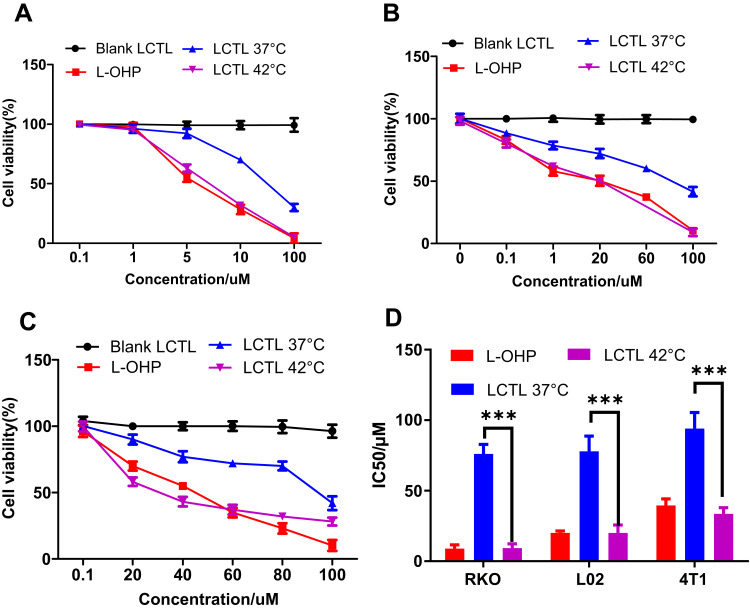
The LCTL prepared with the 26.5% EE was used for the MTT studies for the 4T1, RKO and L02 cell lines. As illustrated by Figure 5A–C, the empty LCTL liposome showed slight cytotoxicity on all cell lines tested after incubation for 48h which indicated that the liposome itself is relatively safe to the tissue. The IC50 values for the LCTL at 37°C and 42°C are presented in Figure 5D. The cytotoxicity of LCTL at 42°C displayed no difference compared to the free L-OHP on the cells while the LCTL at 37°C showed decreased cytotoxicity, leading to a ninefold increased IC50 for the RKO cell lines, 3.5-fold increase for the L02 cell and a threefold increase for the 4T1 cells.
Figure 5.
Cell viability of different cells after the treatment of LCTL to RKO (A), L02 (B), and 4T1 cell lines (C) at 37°C and 42°C after incubation for 48h. (D) The IC50 of the LCTL to RKO, L02 and 4T1 cells. Results are expressed as the mean±SD from five independent experiments. ***p < 0.001.
In vitro Cellular Uptake Test
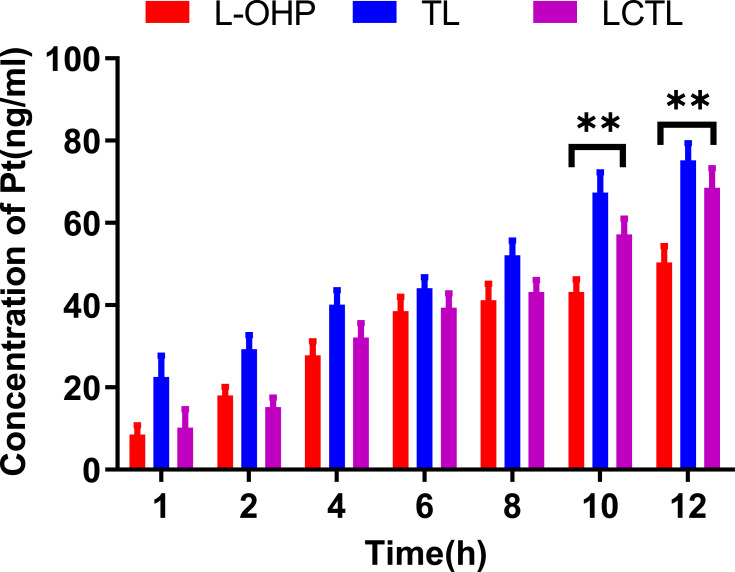
To verify the cellular uptake of the L-OHP solution, TL and LCTL at different incubation times, the content of Pt inside the cells was determined after incubation. After treatment of 4T1 cells with the L-OHP solution, TL and LCTL, the uptake of L-OHP all were proportionally increased which exhibited time-dependent behavior (Figure 6). Notably, the uptake of L-OHP for TL was significantly higher than that for the L-OHP solution. The results may be due to the endocytosis caused by liposome nano-formulation which increases the uptake of the L-OHP in the cell.15 Moreover, specifically, lipids with longer (16:0, DPPC) acyl chains also showed greater uptake according to some reports.24 Besides, LCTL showed an almost similar uptake during the first 8h with the L-OHP solution which may be due to the steric hindrance of PEG. But after 8h, the LCTL showed a significant increase compared with the L-OHP solution which is explained by the advantages of the liposomes to the free drug.
Figure 6.
The cellular uptake of platinum after the incubation with free L-OHP solution, TL and LCTL on 4T1 cells at different times of 1h, 2h, 4h, 6h, 8h, 10h, 12h. The data are represented as the mean±SD from three independent experiments. **p < 0.01.
In vivo Imaging
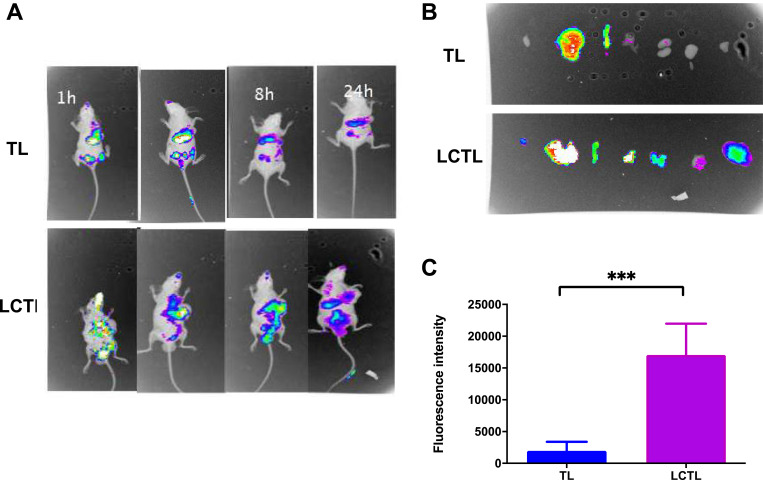
To evaluate the targeting ability of LCTL, the DiR-loaded LCTL were i.v. injected into 4T1-bearing mice. As shown in Table 5, the DiR-loaded TL and LCTL possessed similar physical characteristics with L-OHP loaded TL and LCTL. Figure 7 shows the real-time images of TL and LCTL in mice, in which the whole bodies of live mice were monitored at 1h, 6h, 8h and 24 h after administration by a non-invasive near-infrared optical imaging technique. The fluorescence intensity in tumor of LCTL was higher than that of TL at 6h. A stronger fluorescent signal from 1h to 8h was detected in the tumor region in mice treated with the LCTL while the fluorescent signal reduced from 6h for the TL (Figure 7A). A suitable reason is the enhanced prolonged circulation by a PEG coating which could reduce the elimination of the complex and retention (EPR) effect by a suitable LCTL size. The fluorescence intensity of tissue harvested from mice at 24h proved that LCTL could achieve at least a 24h-retention in tumors (Figure 7B and C). The in vivo images showed that the LCTL could target tumors and enhance retention for more than 24 h, which should result in an intense concentration effect in the tumor area.
Table 5.
The Characteristics of Different DiR-Loaded Liposomes
| Liposomes | TL | LCTL |
|---|---|---|
| Particle size | 182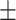 3.1nm 3.1nm |
186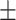 3.5nm 3.5nm |
| PDI | 0.17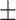 0.03 0.03 |
0.12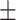 0.01 0.01 |
| Zeta potential | −24.2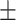 0.41mV 0.41mV |
−22.6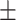 0.49mV 0.49mV |
| EE | 98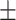 1.3% 1.3% |
98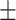 1.9% 1.9% |
Figure 7.
(A) In vivo imaging of 4T1-bearing mice after administration of DiR-loaded TL and LCTL at 1h, 6h, 8h and 24h. (B) In vivo imaging of tissue from mice at 24h. (C) The quantitative analysis of the fluorescence intensity in tumors at 24h. ***p < 0.001. Results are expressed as the mean±SD from five independent experiments.
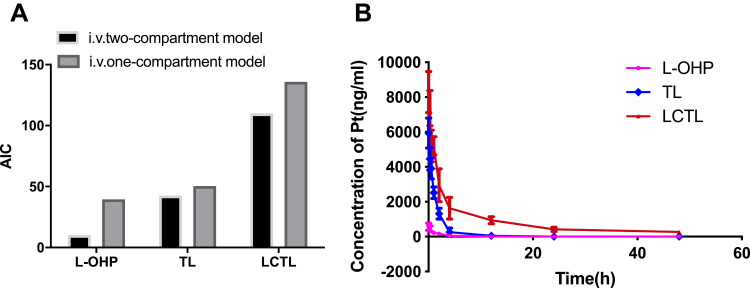
The Pharmacokinetic Study
AIC (Akaike’s Information Criterion) is a better parameter developed in recent years for fitting linear pharmacokinetic models. The smaller the AIC value is, the better the model is fit. Pharmacokinetic results indicated the in vivo behavior of LCTL, TL and free L-OHP compiled with the two-compartment model (Figure 8A). The consistence of compartment model between the liposome formulations and the free L-OHP indicates that the application of the liposome carrier does not alter the distribution and metabolism of L-OHP itself in vivo. However, LCTL showed superior AUC in vivo compared to that of TL or L-OHP solution which increased the long-time circulation and bioavailability significantly (p<0.05) (Figure 8B). At the same time, the CL of LCTL was significantly low as 99.28 L•h•kg-1 while that was 6943.49 L•h•kg-1 for L-OHP solution and 521.32 L•h•kg-1 for TL. All the data indicated that the LCTL could effectively prolong the circulation time of oxaliplatin in the body (Table 6). This enhanced long-circulation in the plasma could increase the drug content accumulated in the tumor area which would support the anti-tumor effect in the further study.
Figure 8.
(A) The AIC of the L-OHP solution, LCTL, and TL for the one-compartment model and two-compartment model. (B) Mean plasma concentration–time profiles of the platinum after the treatment of L-OHP solution, LCTL, and TL (n=5).
Table 6.
Pharmacokinetic Parameters of the L-OHP Solution, TL, and LCTL After Injection (n=5)
| L-OHP | TL | LCTL | |
|---|---|---|---|
| AUC (ng·h·mL-1) | 633.69 | 7726.67 | 43,447.38** |
| T1/2α (h) | 0.2253 | 0.7392 | 1.03485 |
| T1/2β (h) | 3.0827 | 2.186 | 18.0441** |
| CL (L·h·kg-1) | 6943.49 | 521.32 | 99.28** |
Note: **p < 0.01, compared with the L-OHP group.
Abbreviations: AUC, area under the curve; T1/2α, distribution half-life; T1/2β, elimination of half-life; CL, clearance rate.
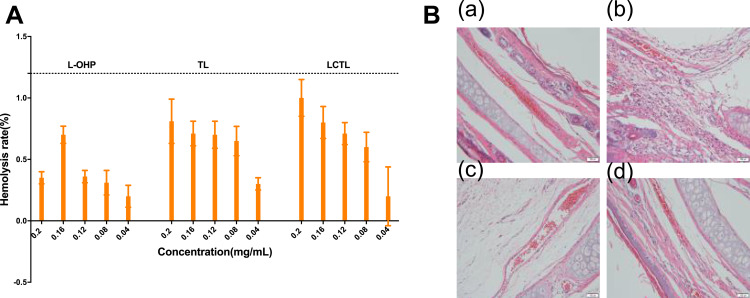
Hemolytic Study and Irritation Study
The results showed that the hemolysis of all of the L-OHP solution and liposome formulations were less than 5% (Figure 9A) which provides evidence that the LCTL are safe for carrying oxaliplatin. In the irritation studies, except for the L-OHP solution, all the liposomes caused no influence to the vessels. The vessels were intact and smooth for all liposome formulations while there was a small amount of inflammatory cell infiltration for the L-OHP solution (Figure 9B). It has been reported that the local reactions (such as phlebitis) are a common local reaction when the L-OHP was used for chemotherapy. Its main symptoms include redness along the vein, swelling, heat, pain and vein enlargement, as well as hardened and inelastic vessels which not only bring pain to the patient but also lead to subsequently difficult intravenous treatment. In a way, the LCTL is a safe protectant for the anti-tumor drug delivery to patients.
Figure 9.
(A) The hemolytic rate of red blood cells after incubation with different concentration of L-OHP, TL, LCTL formulations. (B) The irritation to the vessels after the administration of saline (a), L-OHP solution (b), TL (c) and LCTL (d).
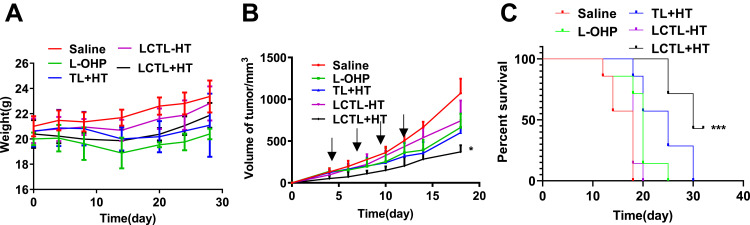
In vivo Efficiency
The mice in the control group treated with saline appeared in slow action and quick depilation of the head and abdomen. In addition, the median survival time was the shortest. At the same time, the mice treated with the L-OHP showed a significant decline in weight which indicated that the anti-tumor drug would cause side effects (Figure 10A). For the anti-tumor effect, the LCTL with HT presented the best inhibition toward the tumor growth. However, the therapeutic effects of LCTL without HT was weaker than the free L-OHP solution which was consistent with the release result in vitro (Figure 10B). Moreover, the TL with HT and LCTL with HT groups both induced a better delay in tumor growth than the free L-OHP, reflecting the stability and ability of the liposomes to reach the tumor area. All the liposome formulations showed superior advantages with an increase in weight after 14 days which indicated the enhanced security and decreased toxicity compared with commercial L-OHP in solution.25,26 The anti-tumor activity for this formulation was evident in vivo, which is supported by the research of others14 (Figure 10C). Among the formulations, LCTL showed the strongest effect on the survival rate of tumor-bearing mice: 42.8% of the mice (3 out of 7) became long-term survivors (>30 days) (p<0.01).
Figure 10.
The evaluation of anti-tumor effect on 4T1-bearing mice. (A) The weight changes of mice after the administration with physical saline, free L-OHP, TL+HT, LCTL with HT and LCTL without HT (n=7). (B) Tumor growth of mice bearing 4T1 tumor, which were intravenously administrated with physical saline, free L-OHP, TL+HT, LCTL with HT and LCTL without HT (n=7). (C) The survival curves after administration with physical saline, free L-OHP, TL+HT, LCTL with HT and LCTL without HT. Each symbol represents the mean of 7 animals and the bars correspond to the standard deviation. The local tumors of mice were heated at 42°C for 30 min each time for +HT groups. *p < 0.05, ***p < 0.001. Results are expressed as the mean±SD from five independent experiments.
Discussion
In this study, the L-OHP thermosensitive liposome was prepared by two different methods. Based on the physical characteristics, the REV was more complex due to the number of steps used for the preparation compared with the IBL, and exhibited a larger particle size, but the drug loading and PDI of REV provided greater advantages than the IBL. In our study, we found the EE of L-OHP was limited by IBL which lead to a higher ratio of lipids to L-OHP. As we know, ratio of lipid to drug was a key point for the liposome, because if the ratio is too high, the lipid would nourish tumor cells and then decrease the cytotoxicity on cells for L-OHP liposome. Moreover, with higher EE, the dose could be decreased for in vivo application which could improve the safety of formulation. Furthermore, the recent report27 demonstrated that particle size between 100nm to 200nm could achieve expected tumor targeting and much more complex factors would influence the EPR effect which including tumor perfusion, lymphatic function, interstitial penetration, vascular permeability except the particle size, so we chose a REV method with a larger particle size for preparation in our study. In the future, the processes and prescriptions that can reduce the particle size by REV method still need to be explored.
The LCTL could achieve a gel-to-liquid transition with a mild HT at the tumor, but it would maintain an integrated structure at 37°C which inhibits drug leakage. The storage at 4°C proved the stability again in vitro. In addition, the release study at 42°C and 37 °C for LCTL indicated the heat-responsive release for L-OHP.28 The low release at 37°C might be due to the fact that L-OHP was loaded in the hydrophilic core which further caused difficulty for release. Further, the decomposition of the phospholipid molecular layer was the only factor that restricted the release rate of the drugs. On the other hand, this further prevent the leakage of L-OHP in the plasma which decreased the side-effect to normal tissue. This showed a pharmaceutically more stable formulation with faster drug release kinetics at the tumor which was consistent with the DSC and stability tests. Tested on different cells, the RKO was the most sensitive cells to the L-OHP. The significant stronger cytotoxicity was found at 42°C instead of at 37°C. The reduction of cytotoxicity for the L-OHP-LCTL at 37°C was due to the negligible release from the liposomes which inhibited the efficiency of the drugs. These results are also consistent with the results reported by several authors regarding the IC50 for the free drug vs the liposomal formulation.13,14 This better suggested that LCTL could decrease the systemic toxicity in the body.
LCTL was also proved to achieve long-circulating effect in this work by in vivo study. Moreover, for anti-tumor efficiency, the LCTL showed best anti-tumor effect. But the therapeutic effects of LCTL without HT was weaker than the free L-OHP solution which was explained by the low release inside tumor. We therefore considered that LCTL could deliver L-OHP to the tumor tissue through the EPR effect, but were not effectively internalized into the cytoplasm since the blood passage time through a tumor was so rapid that the liposome circulated out of the tumor rapidly with only a minor release of L-OHP without HT. Moreover, the burst release in a short time in tumor was essential for anti-tumor effect by LCTL with HT. This result suggests that LCTL with HT could act as a depot of L-OHP in the tumor area, inhibiting its circulation out of the tumor since the LCTL showed a burst release with HT which achieved retention in the tumor. In addition, as many literatures mentioned,25,26 the elevated body weight of mice in LCTL may explain the decreased systemic toxicity to some extent including neurologically toxicity, blood system toxicity, gastrointestinal toxicity and so on. Meanwhile, the accurate evaluation for neurologically toxicity, blood system toxicity, gastrointestinal toxicity would be researched in the future.
Conclusion
In conclusion, this study indicated that LCTL not only prolonged circulation time, but also enhanced retention and achieved selective release at the tumor. We found that L-OHP/LCTL with HT could achieve remarkable tumor growth suppression and increase survival time for the tumor-bearing mice without hemolysis and irritation to the vessels. In summary, the long-circulating thermosensitive liposome with heat-triggered release may have strong potential to overcome some of the major shortcomings of conventional chemotherapeutic strategies. Besides, our work could demonstrate the effectiveness and safety of thermosensitive L-OHP liposomes, which could accelerate the industrial production development and extend its clinical applications.
Acknowledgments
The authors would like to thank China Pharmaceutical University and Northeastern University for providing funding and facilities. This work was supported by the Ministry of Science and Technology of China (NO.2017ZX09101001-005-003), the National Natural Science Foundation of China (NO. 81972892, NO.81673364 and NO.81760760), the Priority Academic Program Development of Jiangsu Higher Education Institutions and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX17_0674), and the Applied Technology Research and Development Project of the Inner Mongolia Autonomous Region (2019GG035).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Stein A, Arnold D. Oxaliplatin: a review of approved uses. Expert Opin Pharmacother. 2012;13(1):125–137. doi: 10.1517/14656566.2012.643870 [DOI] [PubMed] [Google Scholar]
- 2.Grothey A. Oxaliplatin-safety profile: neurotoxicity. Semin Oncol. 2003;30(4 Suppl 15):5–13. doi: 10.1016/S0093-7754(03)00399-3 [DOI] [PubMed] [Google Scholar]
- 3.Pendyala L, Creaven PJ. In vitro cytotoxicity, protein binding, red blood cell partitioning, and biotransformation of oxaliplatin. Cancer Res. 1993;53(24):5970–5976. [PubMed] [Google Scholar]
- 4.Levi F, Metzger G, Massari C, Milano G. Oxaliplatin - pharmacokinetics and chronopharmacological aspects. Clin Pharmacokinet. 2000;38(1):1–21. doi: 10.2165/00003088-200038010-00001 [DOI] [PubMed] [Google Scholar]
- 5.Zalba S, Garrido MJ. Liposomes, a promising strategy for clinical application of platinum derivatives. Expert Opin Drug Del. 2013;10(6):829–844. doi: 10.1517/17425247.2013.778240 [DOI] [PubMed] [Google Scholar]
- 6.Chen Q, Yang Y, Lin X, et al. Platinum(iv) prodrugs with long lipid chains for drug delivery and overcoming cisplatin resistance. Chem Commun. 2018;54(42):5369–5372. doi: 10.1039/C8CC02791A [DOI] [PubMed] [Google Scholar]
- 7.Liang C, Wang H, Zhang M, et al. Self-controlled release of Oxaliplatin prodrug from d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) functionalized mesoporous silica nanoparticles for cancer therapy. J Colloid Interface Sci. 2018;525:1–10. doi: 10.1016/j.jcis.2018.04.058 [DOI] [PubMed] [Google Scholar]
- 8.Zhang R, Song X, Liang C, et al. Catalase-loaded cisplatin-prodrug-constructed liposomes to overcome tumor hypoxia for enhanced chemo-radiotherapy of cancer. Biomaterials. 2017;138:13–21. doi: 10.1016/j.biomaterials.2017.05.025 [DOI] [PubMed] [Google Scholar]
- 9.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/S0065-2571(00)00013-3 [DOI] [PubMed] [Google Scholar]
- 10.Gill PS, Espina BM, Muggia F, et al. Phase I/II clinical and pharmacokinetic evaluation of liposomal daunorubicin. J Clin Oncol. 1995;13(4):996–1003. doi: 10.1200/JCO.1995.13.4.996 [DOI] [PubMed] [Google Scholar]
- 11.Suzuki R, Takizawa T, Kuwata Y, et al. Effective anti-tumor activity of oxaliplatin encapsulated in transferrin-PEG-liposome. Int J Pharm. 2008;346(1–2):143–150. doi: 10.1016/j.ijpharm.2007.06.010 [DOI] [PubMed] [Google Scholar]
- 12.Zalba S, Navarro I, Troconiz IF, Tros de Ilarduya C, Garrido MJ. Application of different methods to formulate PEG-liposomes of oxaliplatin: evaluation in vitro and in vivo. Eur J Pharm Biopharm. 2012;81(2):273–280. doi: 10.1016/j.ejpb.2012.02.007 [DOI] [PubMed] [Google Scholar]
- 13.Franzen U, Nguyen TT, Vermehren C, Gammelgaard B, Ostergaard J. Characterization of a liposome-based formulation of oxaliplatin using capillary electrophoresis: encapsulation and leakage. J Pharm Biomed Anal. 2011;55(1):16–22. doi: 10.1016/j.jpba.2010.12.037 [DOI] [PubMed] [Google Scholar]
- 14.Zhang B, Wang T, Yang S, et al. Development and evaluation of oxaliplatin and irinotecan co-loaded liposomes for enhanced colorectal cancer therapy. J Control Release. 2016;238:10–21. doi: 10.1016/j.jconrel.2016.07.022 [DOI] [PubMed] [Google Scholar]
- 15.Nakamura H, Doi Y, Abu Lila A, Nagao A, Ishida T, Kiwada H. Sequential treatment of oxaliplatin-containing PEGylated liposome together with S-1 improves intratumor distribution of subsequent doses of oxaliplatin-containing PEGylated liposome. Eur J Pharm Biopharm. 2014;87(1):142–151. [DOI] [PubMed] [Google Scholar]
- 16.Crommelin DJA, Florence AT. Towards more effective advanced drug delivery systems. Int J Pharm. 2013;454(1):496–511. doi: 10.1016/j.ijpharm.2013.02.020 [DOI] [PubMed] [Google Scholar]
- 17.Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65(1):36–48. doi: 10.1016/j.addr.2012.09.037 [DOI] [PubMed] [Google Scholar]
- 18.Yatvin MB, Weinstein JN, Dennis WH, Blumenthal R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science. 1978;202(4374):1290–1293. doi: 10.1126/science.364652 [DOI] [PubMed] [Google Scholar]
- 19.Dou Y, Hynynen K, Allen C. To heat or not to heat: challenges with clinical translation of thermosensitive liposomes. J Control Release. 2017;249:63–73. doi: 10.1016/j.jconrel.2017.01.025 [DOI] [PubMed] [Google Scholar]
- 20.Tsong TY. Kinetics of the crystalline-liquid crystalline phase transition of dimyristoyl L-alpha-lecithin bilayers. Proc Natl Acad Sci U S A. 1974;71(7):2684–2688. doi: 10.1073/pnas.71.7.2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolosnjaj-Tabi J, Marangon I, Nicolas-Boluda A, Silva AKA, Gazeau F. Nanoparticle-based hyperthermia, a local treatment modulating the tumor extracellular matrix. Pharmacol Res. 2017;126:123–137. doi: 10.1016/j.phrs.2017.07.010 [DOI] [PubMed] [Google Scholar]
- 22.Sylvester B, Porfire A, Van Bockstal PJ, et al. Formulation optimization of freeze-dried long-circulating liposomes and in-line monitoring of the freeze-drying process using an NIR spectroscopy tool. J Pharm Sci. 2018;107(1):139–148. doi: 10.1016/j.xphs.2017.05.024 [DOI] [PubMed] [Google Scholar]
- 23.Caddeo C, Pucci L, Gabriele M, et al. Stability, biocompatibility and antioxidant activity of PEG-modified liposomes containing resveratrol. Int J Pharm. 2018;538(1–2):40–47. doi: 10.1016/j.ijpharm.2017.12.047 [DOI] [PubMed] [Google Scholar]
- 24.Pennington ER, Funai K, Brown DA, Shaikh SR. The role of cardiolipin concentration and acyl chain composition on mitochondrial inner membrane molecular organization and function. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864(7):1039–1052. doi: 10.1016/j.bbalip.2019.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang C, Tu K, Gao H, et al. The novel platinum(IV) prodrug with self-assembly property and structure-transformable character against triple-negative breast cancer. Biomaterials. 2020;232:119751. doi: 10.1016/j.biomaterials.2019.119751 [DOI] [PubMed] [Google Scholar]
- 26.Li D, Lu B, Huang Z, et al. A novel melphalan polymeric prodrug: preparation and property study. Carbohydr Polym. 2014;111:928–935. doi: 10.1016/j.carbpol.2014.04.062 [DOI] [PubMed] [Google Scholar]
- 27.Kalyane D, Raval N, Maheshwari R, Tambe V, Kalia K, Tekade RK. Employment of enhanced permeability and retention effect (EPR): nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater Sci Eng C Mater Biol Appl. 2019;98:1252–1276. doi: 10.1016/j.msec.2019.01.066 [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Hu Y, Huang L. Influence of polyethylene glycol density and surface lipid on pharmacokinetics and biodistribution of lipid-calcium-phosphate nanoparticles. Biomaterials. 2014;35(9):3027–3034. doi: 10.1016/j.biomaterials.2013.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]