Abstract
Background
NVX-CoV2373 is a recombinant severe acute respiratory syndrome coronavirus 2 (rSARS-CoV-2) nanoparticle vaccine composed of trimeric full-length SARS-CoV-2 spike glycoproteins and Matrix-M1 adjuvant.
Methods
We initiated a randomized, placebo-controlled, phase 1–2 trial to evaluate the safety and immunogenicity of the rSARS-CoV-2 vaccine (in 5-μg and 25-μg doses, with or without Matrix-M1 adjuvant, and with observers unaware of trial-group assignments) in 131 healthy adults. In phase 1, vaccination comprised two intramuscular injections, 21 days apart. The primary outcomes were reactogenicity; laboratory values (serum chemistry and hematology), according to Food and Drug Administration toxicity scoring, to assess safety; and IgG anti–spike protein response (in enzyme-linked immunosorbent assay [ELISA] units). Secondary outcomes included unsolicited adverse events, wild-type virus neutralization (microneutralization assay), and T-cell responses (cytokine staining). IgG and microneutralization assay results were compared with 32 (IgG) and 29 (neutralization) convalescent serum samples from patients with Covid-19, most of whom were symptomatic. We performed a primary analysis at day 35.
Results
After randomization, 83 participants were assigned to receive the vaccine with adjuvant and 25 without adjuvant, and 23 participants were assigned to receive placebo. No serious adverse events were noted. Reactogenicity was absent or mild in the majority of participants, more common with adjuvant, and of short duration (mean, ≤2 days). One participant had mild fever that lasted 1 day. Unsolicited adverse events were mild in most participants; there were no severe adverse events. The addition of adjuvant resulted in enhanced immune responses, was antigen dose–sparing, and induced a T helper 1 (Th1) response. The two-dose 5-μg adjuvanted regimen induced geometric mean anti-spike IgG (63,160 ELISA units) and neutralization (3906) responses that exceeded geometric mean responses in convalescent serum from mostly symptomatic Covid-19 patients (8344 and 983, respectively).
Conclusions
At 35 days, NVX-CoV2373 appeared to be safe, and it elicited immune responses that exceeded levels in Covid-19 convalescent serum. The Matrix-M1 adjuvant induced CD4+ T-cell responses that were biased toward a Th1 phenotype. (Funded by the Coalition for Epidemic Preparedness Innovations; ClinicalTrials.gov number, NCT04368988).
Coronavirus disease 2019 (Covid-19) has spread globally at a rapid pace since the novel coronavirus was first reported in late December 2019 in Wuhan, China, and was declared a pandemic by the World Health Organization on March 11, 2020.1,2 As of August 1, 2020, more than 17 million cases and over 675,000 deaths due to Covid-19 have been reported worldwide,3 caused by infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).4,5 NVX-CoV2373 contains Matrix-M1 adjuvant6 and a recombinant SARS-CoV-2 (rSARS-CoV-2) nanoparticle vaccine, constructed from the full-length (i.e., including the transmembrane domain), wild-type SARS-CoV-2 spike glycoprotein, which mediates attachment of the virus to the human angiotensin-converting enzyme 2 (hACE2) receptor of host cells for cellular entry and serves as a key target for development of antibodies and vaccines.7,8 In rodent and nonhuman primate challenge models, NVX-CoV2373 induced high titers of antibodies measured against anti-spike protein that blocked hACE2 receptor binding and achieved neutralization of wild-type virus that exceeded the magnitude of responses measured in human convalescent serum and that provided protection against SARS-CoV-2 challenge.9,10 In addition, polyfunctional CD4+ and CD8+ T-cell responses were induced with a T helper 1 (Th1) dominant phenotype.9
We report here the findings of the phase 1 part of a randomized, placebo-controlled, phase 1–2 trial that commenced in May 2020 to evaluate the safety and immunogenicity of 5-μg and 25-μg doses of rSARS-CoV-2 with or without Matrix-M1 adjuvant (50-μg dose) in healthy adults younger than 60 years of age.
Methods
Trial Design and Oversight
Our phase 1 trial was conducted at two sites in Australia (Nucleus Network, Herston, Queensland, and Melbourne, Victoria). Eligible participants were healthy men and nonpregnant women, 18 to 59 years of age, with a body-mass index (the weight in kilograms divided by the square of the height in meters) of 17 to 35. Healthy status, assessed during the screening period, was based on medical history and clinical laboratory findings, vital signs, and physical examination. Participants with a history of SARS or Covid-19 or who tested positive at screening (by real-time polymerase-chain-reaction [RT-PCR] assay or enzyme-linked immunosorbent assay [ELISA]) along with participants exposed to persons with confirmed SARS-CoV-2 or working in an occupation at high risk for exposure to SARS-CoV-2 were excluded. (Details of the trial design, conduct, oversight, and analyses are provided in the protocol and statistical analysis plan, available with the full text of this article at NEJM.org.) All participants provided written informed consent before enrollment in the trial.
As a safety measure, 6 participants were initially randomly assigned in a 1:1 ratio to the 5-μg and 25-μg rSARS-CoV-2 plus Matrix-M1 groups (groups C and D), vaccinated in an open-label manner, and observed for reactogenicity for 48 hours. Thereafter, the remaining 125 participants were randomly assigned, in a 1:1:1:1:1 ratio and in a blinded manner to one of five vaccine groups according to pregenerated randomization schedules, without stratification (Figure 1).
Figure 1. Vaccine Regimens and Key Trial Assessments.
Shown are the planned randomization schema and associated vaccine regimens administered in the trial (Panel A), along with timing of the key safety and immunogenicity assessments (Panel B).
The trial was designed by Novavax, with funding support from the Coalition for Epidemic Preparedness Innovations. The trial protocol was approved by the Alfred Hospital Human Research Ethics Committee (Melbourne) and was performed in accordance with the International Council for Harmonisation Good Clinical Practice guidelines. Safety oversight for specific vaccination pause rules and for advancement to phase 2 was performed by an independent safety monitoring committee. The manuscript was written by the authors, with the first author as the overall lead author. No one who is not an author contributed to writing the manuscript. All authors had full access to the data on a vaccine-group level only. The authors assume responsibility for the accuracy and completeness of the data and for the fidelity of the trial to the protocol.
Trial Vaccine, Adjuvant, and Placebo
rSARS-CoV-2, developed by Novavax and manufactured at Emergent Biosolutions, is a recombinant nanoparticle vaccine constructed from the full-length (i.e., including the transmembrane domain), wild-type SARS-CoV-2 spike glycoprotein (GenBank accession number, MN908947; nucleotides 21563–25384) optimized in the established baculovirus Spodoptera frugiperda (Sf9) insect cell-expression system.9 rSARS-CoV-2 is generated with 682-QQAQ-685 mutations at the S1/S2 cleavage sites to confer protease resistance and two proline substitutions at residues K986P and V987P at the top of the heptad repeat 1/central helix in the S2 subunit to stabilize the construct in a prefusion conformation. rSARS-CoV-2 is resistant to proteolytic cleavage, binds with high affinity to the hACE2 receptor, and demonstrates thermostability.9,11 Matrix-M1, a saponin-based adjuvant,12 was manufactured by Novavax. Both vaccine and adjuvant were stored at 2°C to 8°C. Placebo was sterile 0.9% normal saline.
rSARS-CoV-2 and Matrix-M1 were mixed just before use. Each participant received two intramuscular injections of trial vaccine or placebo (injection volume, 0.6 ml) in the deltoid muscle, one injection on day 0 and one on day 21. Participants and trial site personnel managing the conduct of the trial remained unaware of vaccine assignment, with the exception of sentinel dosing (described below). Vaccination pause rules were in place to monitor participants’ safety (see the Supplementary Appendix, available at NEJM.org).
Safety Assessments
Safety and immunogenicity were evaluated at specified time points throughout the trial (Figure 1). The primary safety outcomes were the number and percentage of participants with solicited local and systemic reactogenicity according to the Food and Drug Administration (FDA) toxicity grading scale (Table S2 in the Supplementary Appendix)13 and their duration and peak intensity for 7 days after vaccination (days 0 to 7 and days 21 to 28) and laboratory values (serum chemistry and hematology) according to FDA toxicity scoring (Table S5)13,14 at 7 days after vaccination (days 7 and 28). Reported secondary safety outcomes were laboratory values at day 21, unsolicited adverse events during the first 35 days according to Medical Dictionary for Regulatory Activities (MedDRA) classification, severity score (mild, moderate, or severe), and relatedness to the vaccine; vital sign measurements assessed according to FDA toxicity scoring (Table S6)13 after vaccination; and adverse events of special interest, which included SARS-CoV-2 infection, Covid-19 disease manifestations (Table S3),14,15 and potential immune-mediated medical conditions (Table S4). In addition, participants underwent nasopharyngeal swab testing for SARS-CoV-2 on day 35 or any time they reported symptoms suggestive of possible infection.
Participants were observed for at least 30 minutes after each vaccination for assessment of reactogenicity and were instructed to continue monitoring these events at home daily, for 7 days after each vaccination, using a diary. Predefined local (injection site) reactogenicity included pain, tenderness, erythema, and swelling; systemic reactogenicity included fever, nausea or vomiting, headache, fatigue, malaise, myalgia, and arthralgia.
Immunogenicity Assessments
The primary immunogenicity outcome was the anti-spike IgG ELISA unit responses to rSARS-CoV-2 protein antigens, measured on days 0, 7, 21, 28, and 35. Reported secondary immunogenicity assessments were the wild-type virus microneutralization assay (MN) with an inhibitory concentration of >99% (MN IC>99%) on days 0, 21, and 35 and intracellular cytokine staining of antigen-specific CD4+ T cells at days 0 and 28 in a randomly selected subgroup of 16 participants, 4 participants each from groups A, B, C, and D. Details of these assays are provided in the Supplementary Appendix.
Immunogenicity (IgG and MN) results were compared with a control panel of 32 (IgG) and 29 (MN) convalescent serum specimens collected from patients with PCR-confirmed Covid-19, obtained from Baylor College of Medicine, which were classified according to clinical severity at the same institution. Covid-19 clinical severity was classified as asymptomatic, exposed (sample collected from contact exposure assessment), symptomatic outpatient (sample collected from outpatients discharged from the emergency department; see Table S1 for a list of symptoms), and hospitalized (sample collected from hospitalized patients, including those receiving supportive measures in the intensive care unit). Disease classification was conducted independent of and before the study assays and analysis.
Statistical Analysis
The sample size for the trial was based on clinical and practical considerations, not on a formal statistical power calculation. Most end points were summarized with geometric means and 95% confidence intervals that were based on the t distribution of the log-transformed values. No adjustments for multiplicity were made in these analyses, including the calculation of confidence intervals.
The primary safety and immunogenicity analyses were conducted after all participants had been followed through day 35. Safety data on the sentinel participants (who received open-label vaccination) were analyzed separately from other participants because of potential investigator bias; immunogenicity data were combined for all participants. Sponsor personnel who were involved in the analysis of data were not provided with individual data; they received only vaccine-group assignment data.
Results
Trial Population
The trial was initiated on May 26, 2020; 134 participants underwent randomization between May 27 and June 6, 2020, including 3 participants who were to serve as backups for sentinel dosing and who immediately withdrew from the trial without being vaccinated (Fig. S1). Of the 131 participants who received injections, 23 received placebo (group A), 25 received 25-μg doses of rSARS-CoV-2 (group B), 29 received 5-μg doses of rSARS-CoV-2 plus Matrix-M1, including three sentinels (group C), 28 received 25-μg doses of rSARS-CoV-2 plus Matrix-M1, including three sentinels (group D), and 26 received a single 25-μg dose of rSARS-CoV-2 plus Matrix-M1 followed by a single dose of placebo (group E). All 131 participants received their first vaccination on day 0, and all but 3 received their second vaccination at least 21 days later; exceptions include 2 in the placebo group (group A) who withdrew consent (unrelated to any adverse event) and 1 in the 25-μg rSARS-CoV-2 + Matrix-M1 group (group D) who had an unsolicited adverse event (mild cellulitis; see below). Demographic characteristics of the participants are presented in Table 1. Of note, missing data were infrequent.
Table 1. Demographic Characteristics of the Participants in the NVX-CoV2373 Trial at Enrollment.*.
| Variable | Group A | Group B | Group C | Group C (Sentinel) |
Group D | Group D (Sentinel) |
Group E | Total |
|---|---|---|---|---|---|---|---|---|
| rSARS-CoV-2 dose 1, dose 2 — μg | 0, 0 | 25, 25 | 5, 5 | 5, 5 | 25, 25 | 25, 25 | 25, 0 | — |
| Matrix-M1 dose 1, dose 2 — μg | 0, 0 | 0, 0 | 50, 50 | 50, 50 | 50, 50 | 50, 50 | 50, 0 | — |
| No. of participants | 23 | 25 | 26 | 3 | 25 | 3 | 26 | 131 |
| Sex — no. (%) | ||||||||
| Male | 11 (47.8) | 12 (48.0) | 13 (50.0) | 2 (66.7) | 17 (68.0) | 2 (66.7) | 9 (34.6) | 66 (50.4) |
| Female | 12 (52.2) | 13 (52.0) | 13 (50.0) | 1 (33.3) | 8 (32.0) | 1 (33.3) | 17 (65.4) | 65 (49.6) |
| Age — yr | 30.3±10.92 | 27.2±9.38 | 29.5±7.99 | 23.7±7.37 | 35.6±12.50 | 25.0±4.58 | 33.0±8.91 | 30.8±10.20 |
| Race or ethnic group — no. (%)† | ||||||||
| American Indian or Alaska Native | 1 (4.3) | 1 (4.0) | 2 (7.7) | 0 | 1 (4.0) | 0 | 2 (7.7) | 7 (5.3) |
| Asian | 2 (8.7) | 0 | 6 (23.1) | 1 (33.3) | 3 (12.0) | 1 (33.3) | 4 (15.4) | 17 (13.0) |
| Black or African American | 0 | 0 | 0 | 0 | 1 (4.0) | 0 | 1 (3.8) | 2 (1.5) |
| Multiracial | 1 (4.3) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.8) |
| Native Hawaiian or other Pacific Islander | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3.8) | 1 (0.8) |
| Not reported | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| White | 19 (82.6) | 24 (96.0) | 18 (69.2) | 2 (66.7) | 20 (80.0) | 2 (66.7) | 18 (69.2) | 103 (78.6) |
| Hispanic or Latino | 2 (8.7) | 3 (12.0) | 6 (23.1) | 0 | 3 (12.0) | 0 | 5 (19.2) | 19 (14.5) |
| Body-mass index‡ | 24.94±3.418 | 25.59±4.217 | 24.10±3.872 | 21.43±2.401 | 26.18±3.454 | 25.67±2.294 | 25.52±3.342 | 25.19±3.672 |
Plus-minus values are means ±SD.
Race or ethnic group was reported by the participant.
The body-mass index is the weight in kilograms divided by the square of the height in meters. The calculation was based on the weight and height measured at the time of screening.
Safety Outcomes
No serious adverse events or adverse events of special interest were reported, and vaccination pause rules were not implemented. As noted above, one participant did not receive a second vaccination owing to an unsolicited adverse event, mild cellulitis, that was associated with infection after an intravenous cannula placement to address an unrelated mild adverse event that occurred during the second week of follow-up. Second vaccination was withheld because the participant was still recovering and receiving antibiotics. This participant remains in the trial.
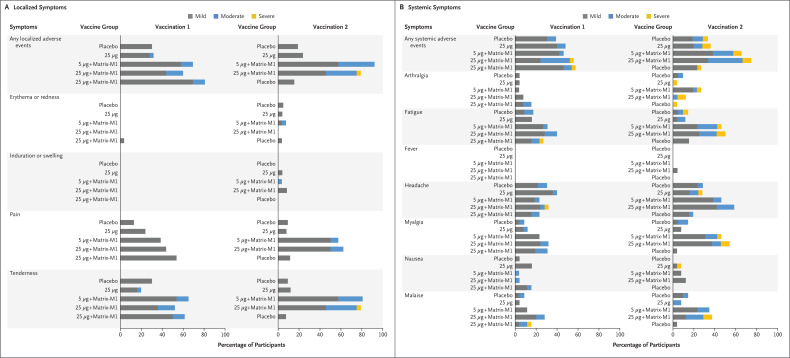
Overall reactogenicity was largely absent or mild, and second vaccinations were neither withheld nor delayed due to reactogenicity. After the first vaccination, local and systemic reactogenicity was absent or mild in the majority of participants (local: 100%, 96%, 89%, 84%, and 88% of participants in groups A, B, C, D, and E, respectively; systemic: 91%, 92%, 96%, 68%, and 89%) who were unaware of treatment assignment (Figure 2 and Table S7). Two participants (2%), one each in groups D and E, had severe adverse events (headache, fatigue, and malaise). Two participants, one each in groups A and E, had reactogenicity events (fatigue, malaise, and tenderness) that extended 2 days after day 7. After the second vaccination, local and systemic reactogenicity were absent or mild in the majority of participants in the five groups (local: 100%, 100%, 65%, 67%, and 100% of participants, respectively; systemic: 86%, 84%, 73%, 58%, and 96%) who were unaware of treatment assignment. One participant, in group D, had a severe local event (tenderness), and eight participants, one or two participants in each group, had severe systemic events; the most common severe systemic events were joint pain and fatigue. Only one participant, in group D, had fever (temperature, 38.1°C) after the second vaccination, on day 1 only. No adverse event extended beyond 7 days after the second vaccination. Of note, the mean duration of reactogenicity events was 2 days or less for both the first vaccination and second vaccination periods.
Figure 2. Solicited Local and Systemic Adverse Events.
The percentage of participants in each vaccine group (groups A, B, C, D, and E) with adverse events according to the maximum FDA toxicity grade (mild, moderate, or severe) during the 7 days after each vaccination is plotted for solicited local (Panel A) and systemic (Panel B) adverse events. There were no grade 4 (life-threatening) events. Participants who reported 0 events make up the remainder of the 100% calculation (not displayed). Excluded were the three sentinel participants in groups C (5 μg + Matrix-M1, 5 μg + Matrix-M1) and D (25 μg + Matrix-M1, 25 μg + Matrix-M1), who received the trial vaccine in an open-label manner (see Table S7 for complete safety data on all participants).
Laboratory abnormalities of grade 2 or higher occurred in 13 participants (10%): 9 after the first vaccination and 4 after the second vaccination (Table S8). Abnormal laboratory values were not associated with any clinical manifestations and showed no worsening with repeat vaccination. Six participants (5%; five women and one man) had grade 2 or higher transient reductions in hemoglobin from baseline, with no evidence of hemolysis or microcytic anemia and with resolution within 7 to 21 days. Of the six, two had an absolute hemoglobin value (grade 2) that resolved or stabilized during the testing period. Four participants (3%), including one who had received placebo, had elevated liver enzymes that were noted after the first vaccination and resolved within 7 to 14 days (i.e., before the second vaccination). Vital signs remained stable immediately after vaccination and at all visits.
Unsolicited adverse events (Table S9) were predominantly mild in severity (in 71%, 91%, 83%, 90%, and 82% of participants in groups A, B, C, D, and E, respectively) and were similarly distributed across the groups receiving adjuvanted and unadjuvanted vaccine. There were no reports of severe adverse events.
Immunogenicity Outcomes
ELISA anti-spike IgG geometric mean ELISA units (GMEUs) ranged from 105 to 116 at day 0. By day 21, responses had occurred for all adjuvanted regimens (1984, 2626, and 3317 GMEUs for groups C, D, and E, respectively), and geometric mean fold rises (GMFRs) exceeded those induced without adjuvant by a factor of at least 10 (Figure 3 and Table S10). Within 7 days after the second vaccination with adjuvant (day 28; groups C and D), GMEUs had further increased by a factor of 8 (to 15,319 and 20,429, respectively) over responses seen with the first vaccination, and within 14 days (day 35), responses had more than doubled yet again (to 63,160 and 47,521, respectively), achieving GMFRs that were approximately 100 times greater than those observed with rSARS-CoV-2 alone. A single vaccination with adjuvant achieved GMEU levels similar to those in asymptomatic (exposed) patients with Covid-19 (1661), and a second vaccination with adjuvant achieved GMEU levels that exceeded those in convalescent serum from symptomatic outpatients with Covid-19 (7420) by a factor of at least 6 and rose to levels similar to those in convalescent serum from patients hospitalized with Covid-19 (53,391). The responses in the two-dose 5-μg and 25-μg adjuvanted vaccine regimens were similar, a finding that highlights the role of adjuvant dose sparing.
Figure 3. SARS-CoV-2 Anti-Spike IgG and Neutralizing Antibody Responses.
Shown are geometric mean anti-spike IgG enzyme-linked immunosorbent assay (ELISA) unit responses to recombinant severe acute respiratory syndrome coronavirus 2 (rSARS-CoV-2) protein antigens (Panel A) and wild-type SARS-CoV-2 microneutralization assay at an inhibitory concentration greater than 99% (MN IC>99%) titer responses (Panel B) at baseline (day 0), 3 weeks after the first vaccination (day 21), and 2 weeks after the second vaccination (day 35) for the placebo group (group A), the 25-μg unadjuvanted group (group B), the 5-μg and 25-μg adjuvanted groups (groups C and D, respectively), and the 25-μg adjuvanted and placebo group (group E). Diamonds and whisker endpoints represent geometric mean titer values and 95% confidence intervals, respectively. The Covid-19 human convalescent serum panel includes specimens from PCR-confirmed Covid-19 participants, obtained from Baylor College of Medicine (29 specimens for ELISA and 32 specimens for MN IC>99%), with geometric mean titer values according to Covid-19 severity. The severity of Covid-19 is indicated by the colors of the dots for hospitalized patients (including those in intensive care), symptomatic outpatients (with samples collected in the emergency department), and asymptomatic patients who had been exposed to Covid-19 (with samples collected during contact and exposure assessment). Mean values (in black) for human convalescent serum are depicted next to (and of same color as) the category of Covid-19 patients, with the overall mean shown above the scatter plot (in black). For each trial vaccine group, the mean at day 35 is depicted above the scatterplot.
Neutralizing antibodies were undetectable before vaccination and had patterns of response similar to those of anti-spike antibodies after vaccination with adjuvant (Figure 3 and Table S11). After the first vaccination (day 21), GMFRs were approximately 5 times greater with adjuvant (5.2, 6.3, and 5.9 for groups C, D, and E, respectively) than without adjuvant (1.1). By day 35, second vaccinations with adjuvant induced an increase more than 100 times greater (195 and 165 for groups C and D, respectively) than single vaccinations without adjuvant. When compared with convalescent serum, second vaccinations with adjuvant resulted in GMT levels approximately 4 times greater (3906 and 3305 for groups C and D, respectively) than those in symptomatic outpatients with Covid-19 (837) and approached the magnitude of levels observed in hospitalized patients with COVID-19 (7457).
At day 35, ELISA anti-spike IgG GMEUs and neutralizing antibodies induced by the two-dose 5-μg and 25-μg adjuvanted vaccine regimens were 4 to 6 times greater than the geometric mean convalescent serum measures (8344 and 983, respectively).
A strong correlation was observed between neutralizing antibody titers and anti-spike IgG GMEUs with adjuvanted vaccine at day 35 (correlation, 0.95) (Figure 4), a finding that was not observed with unadjuvanted vaccine (correlation, 0.76) but was similar to that of convalescent serum (correlation, 0.96). Two-dose regimens of 5-μg and 25-μg rSARS-CoV-2 plus Matrix-M1 produced similar magnitudes of response, and every participant had seroconversion according to either assay measurement. Reverse cumulative-distribution curves for day 35 are presented in Figure S2.
Figure 4. Correlation of Anti-Spike IgG and Neutralizing Antibody Responses.
Shown are scatter plots of 100% wild-type neutralizing antibody responses and anti-spike IgG ELISA unit responses at 3 weeks after the first vaccination (day 21) and 2 weeks after the second vaccination (day 35) for the two-dose 25-μg unadjuvanted vaccine (group B; Panel A), the combined two-dose 5-μg and 25-μg adjuvanted vaccine (groups C and D, respectively; Panel B), and convalescent serum from patients with Covid-19 (Panel C). In Panel C, the severity of Covid-19 is indicated by the colors of the dots for hospitalized patients (including those in intensive care), symptomatic outpatients (with samples collected in the emergency department), and asymptomatic patients who had been exposed to Covid-19 (with samples collected during contact and exposure assessment).
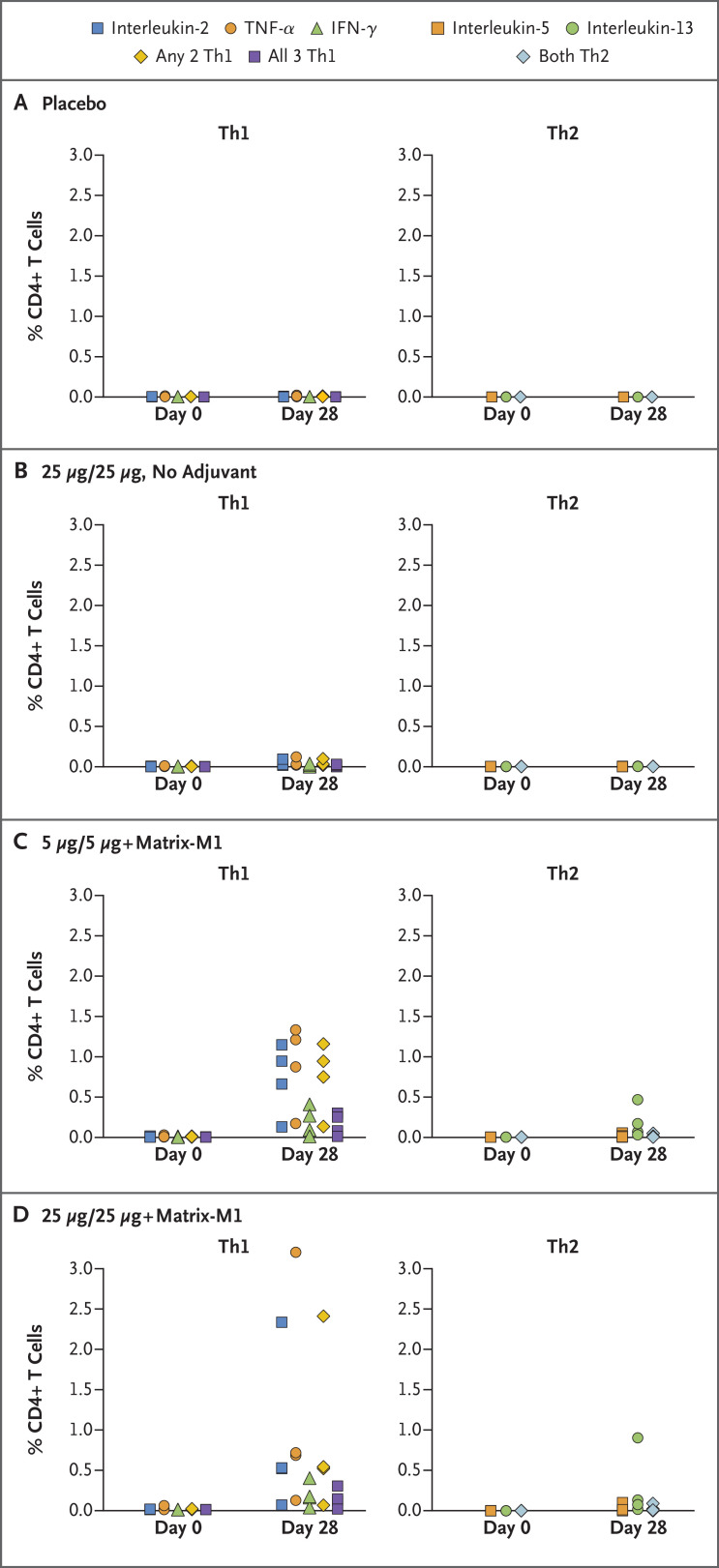
T-cell responses in 16 participants who were randomly selected from groups A through D, 4 participants per group, showed that adjuvanted regimens induced antigen-specific polyfunctional CD4+ T-cell responses that were reflected in IFN-γ, IL-2, and TNF-α production on spike protein stimulation. A strong bias toward this Th1 phenotype was noted; Th2 responses (as measured by IL-5 and IL-13 cytokines) were minimal (Figure 5).
Figure 5. rSARS-CoV-2 CD4+ T-cell Responses with or without Matrix-M1 Adjuvant.
Frequencies of antigen-specific CD4+ T cells producing T helper 1 (Th1) cytokines interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), and interleukin-2 and for T helper 2 (Th2) cytokines interleukin-5 and interleukin-13 indicated cytokines from four participants each in the placebo (group A), 25-μg unadjuvanted (group B), 5-μg adjuvanted (group C), and 25-μg adjuvanted (group D) groups at baseline (day 0) and 1 week after the second vaccination (day 28) after stimulation with the recombinant spike protein. “Any 2Th1” indicates CD4+ T cells that can produce two types of Th1 cytokines at the same time. “All 3 Th1” indicates CD4+ T cells that produce IFN-γ, TNF-α, and interleukin-2 simultaneously. “Both Th2” indicates CD4+ T cells that can produce Th2 cytokines interleukin-5 and interleukin-13 at the same time.
Discussion
The primary safety and immunogenicity analyses indicate that in healthy adult participants 18 to 59 years of age, two-dose regimens of 5 μg and 25 μg of rSARS-CoV-2 plus the Matrix-M1 adjuvant had acceptable safety findings and induced high immune responses, with levels of neutralizing antibodies that closely correlated with anti-spike IgG. Furthermore, neutralizing antibody responses after the second vaccination with rSARS-CoV-2 plus Matrix-M1 exceeded values seen in symptomatic Covid-19 outpatients and were of the magnitude seen in convalescent serum from hospitalized patients with Covid-19. The benefit of the Matrix-M1 adjuvant was clear in the magnitude of the antibody and the T-cell response, the induction of functional antibodies, and antigen dose sparing. The value of the second dose on day 21 for the two-dose rSARS-CoV-2 plus Matrix-M1 regimen is clearly demonstrated and warrants the use of this vaccination schedule.
Although the effector mechanisms that might lead to protection with a Covid-19 vaccine are yet not known, it is presumed that neutralizing antibodies will be associated with protection; this has led to the use of microneutralization testing in all recent human Covid-19 vaccine trials.16 In Covid-19 nonhuman primate challenge models, wild-type neutralizing antibodies are correlated with protection.17 It is notable that studies of respiratory syncytial virus, which is similar to Covid-19 as it infects cells through a type 1 fusion protein mechanism, have clearly shown reduction in disease through the presence of both naturally induced neutralizing antibodies18 and prophylactically injected monoclonal antibodies; the key features of these antibodies are their ability to neutralize virus, and they have been shown to be highly protective in multiple studies using multiple constructs.19-21 In lieu of Covid-19-specific data that clearly correlate immunogenicity to efficacy, vaccine developers have compared vaccine responses with human convalescent serum from Covid-19 patients. Here, we show that NVX-CoV2373 induced immune responses that compared well with anti-spike IgG and microneutralization in a Covid-19 population with clinically significant illness. We also found that when the human convalescent serum was stratified according to illness severity, anti-spike IgG and microneutralization titers closely resembled findings in other Covid-19 studies in which illness severity and the magnitude of microneutralization responses are proportional,22 a finding that suggests that the selection of comparative populations has to be interpreted in light of the clinical severity of disease. Although the comparative immunogenicity of NVX-CoV2373 and convalescent serum from clinically ill Covid-19 patients must be interpreted with caution, the level of vaccine-induced immunogenicity appears promising and in line with protective responses seen in a similar respiratory disease.18 The theoretical concern for vaccine-induced enhanced disease is in part addressed in this trial by the use of an adjuvant, which stimulates both high neutralizing antibody responses and T cells with a predominant Th1 phenotype, both of which are suggested to be important in vaccine candidate selection.23,24
Novavax has accumulated safety data on more than 14,000 participants in various nanoparticle vaccine trials, including children, pregnant women, and older adults25-28 and more than 4300 participants exposed to Matrix-M1 adjuvant, from 5 months to 85 years of age. The current study demonstrates a safety and immunogenicity profile that is acceptable and comparable to past studies with the platform technologies. Most relevant to Covid-19, seasonal influenza hemagglutinin nanoparticle plus Matrix-M1 has demonstrated induction functional immunity and T-cell responses in older adults, including those with a modest level of coexisting conditions, a population most at risk for severe Covid-19 disease.28-30
Limitations of this trial include the small size of the trial, the limited ethnic diversity (particularly the low number of Black and Latinx participants), the younger age of participants, the short period of follow-up, and the participants’ good health status. The populations at greatest risk for serious Covid-19 include people with coexisting conditions and older adults, groups that will be included in our phase 2 program. Data collected during follow-up beyond 35 days in the current cohort will be analyzed after the participants have been monitored for 105 days.
Taken together, our findings indicate that the adjuvanted, recombinant, full-length spike protein nanoparticle vaccine NVX-CoV2373 is a promising candidate that warrants testing in efficacy studies. Phase 2 has commenced on the basis of the safety results of the day 35 primary analysis, and phase 3 is in preparatory stages.
Acknowledgments
We thank the members of the NVX-CoV2373 Study Team (see the Supplementary Appendix) for their many and invaluable contributions. We are especially grateful to the participants (and the support of their families) for their participation in this trial.
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
This article was published on September 2, 2020, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by the Coalition for Epidemic Preparedness Innovation (CEPI), which provided funding for the clinical trial and for the manufacture of rSARS-CoV-2 and the Matrix-M1 adjuvant. Novavax provided rSARS-CoV-2 and Matrix-M1 adjuvant for use in this trial but not provide any financial support. Employees of Novavax, including those listed as authors, contributed substantially to the development and implementation of the trial and the analysis of the data.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Wang Y, Zhang Y, Yu Q, Zhu K. Convalescent plasma coupled with medications for the treatment of a severe COVID-19 patient: drugs analysis and pharmaceutical care based on the newly established guidelines for COVID-19 remedy. Front Pharmacol 2020;11:966-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Pneumonia of unknown cause — China. 2020. (https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china).
- 3.World Health Organization. Coronavirus disease (COVID-19): situation report 194: data as received by WHO from national authorities by 10:00 CEST, 1 August 2020. 2020. (https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200801-covid-19-sitrep-194.pdf?sfvrsn=401287f3_2).
- 4.World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. 2020. (https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it).
- 5.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed 2020;91:157-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bengtsson KL, Song H, Stertman L, et al. Matrix-M adjuvant enhances antibody, cellular and protective immune responses of a Zaire Ebola/Makona virus glycoprotein (GP) nanoparticle vaccine in mice. Vaccine 2016;34:1927-1935. [DOI] [PubMed] [Google Scholar]
- 7.Du L, He Y, Zhou Y, Liu S, Zheng B-J, Jiang S. The spike protein of SARS-CoV — a target for vaccine and therapeutic development. Nat Rev Microbiol 2009;7:226-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tai W, He L, Zhang X, et al. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol 2020;17:613-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian J-H, Patel N, Haupt R, et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 elicits immunogenicity in baboons and protection in mice. June 30, 2020. (https://www.biorxiv.org/content/10.1101/2020.06.29.178509v1). preprint. [DOI] [PMC free article] [PubMed]
- 10.Mandolesi M, Sheward DJ, Hanke L, et al. SARS-CoV-2 protein subunit vaccination elicits potent neutralizing antibody responses. July 31, 2020. (https://www.biorxiv.org/content/10.1101/2020.07.31.228486v1). preprint. [DOI] [PMC free article] [PubMed]
- 11.Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020;367:1260-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reimer JM, Karlsson KH, Lövgren-Bengtsson K, Magnusson SE, Fuentes A, Stertman L. Matrix-M adjuvant induces local recruitment, activation and maturation of central immune cells in absence of antigen. PLoS One 2012;7(7):e41451-e41451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials: guidance for industry. Rockville, MD: Food and Drug Administration, September 2007. [Google Scholar]
- 14.Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events, corrected version 2.1. Bethesda, MD: National Institute of Allergy and Infectious Diseases, July 2017. [Google Scholar]
- 15.Law B, Sturkenboom M. D2.3 priority list of adverse events of special interest: COVID-19. SPEAC. 2020. (https://brightoncollaboration.us/wp-content/uploads/2020/06/SPEAC_D2.3_V2.0_COVID-19_20200525_public.pdf).
- 16.Mulligan MJ, Lyke KE, Kitchin N, et al. Phase 1/2 study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020. August 12 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 17.Mercado NB, Zahn R, Wegmann F, et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 2020. July 30 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buchwald AG, Graham BS, Traore A, et al. RSV neutralizing antibodies at birth predict protection from RSV illness in infants in the first three months of life. Clin Infect Dis 2020. May 28 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pedraz C, Carbonell-Estrany X, Figueras-Aloy J, Quero J. Effect of palivizumab prophylaxis in decreasing respiratory syncytial virus hospitalizations in premature infants. Pediatr Infect Dis J 2003;22:823-827. [DOI] [PubMed] [Google Scholar]
- 20.Carbonell-Estrany X, Simões EA, Dagan R, et al. Motavizumab for prophylaxis of respiratory syncytial virus in high-risk children: a noninferiority trial. Pediatrics 2010;125(1):e35-e51. [DOI] [PubMed] [Google Scholar]
- 21.Griffin MP, Yuan Y, Takas T, et al. Single-dose nirsevimab for prevention of RSV in preterm infants. N Engl J Med 2020;383:415-425. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, To KK-W, Chan K-H, et al. High neutralizing antibody titer in intensive care unit patients with COVID-19. Emerg Microbes Infect 2020;9:1664-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arvin AM, Fink K, Schmid MA, et al. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature 2020;584:353-363. [DOI] [PubMed] [Google Scholar]
- 24.Center for Biologics Evaluation and Research. Development and licensure of vaccines to prevent COVID-19: guidance for industry. Rockville, MD: Food and Drug Administration, June 2020. [Google Scholar]
- 25.Fries L, Shinde V, Stoddard JJ, et al. Immunogenicity and safety of a respiratory syncytial virus fusion protein (RSV F) nanoparticle vaccine in older adults. Immun Ageing 2017;14:8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muňoz FM, Swamy GK, Hickman SP, et al. Safety and immunogenicity of a respiratory syncytial virus fusion (F) protein nanoparticle vaccine in healthy third-trimester pregnant women and their infants. J Infect Dis 2019;220:1802-1815. [DOI] [PubMed] [Google Scholar]
- 27.Glenn GM, Fries LF, Thomas DN, et al. A randomized, blinded, controlled, dose-ranging study of a respiratory syncytial virus recombinant fusion (F) nanoparticle vaccine in healthy women of childbearing age. J Infect Dis 2016;213:411-422. [DOI] [PubMed] [Google Scholar]
- 28.Shinde V, Cai R, Plested J, et al. Induction of cross-reactive hemagglutination inhibiting antibody and polyfunctional CD4+ T-cell responses by a recombinant Matrix-M-adjuvanted hemagglutinin nanoparticle influenza vaccine. May 18, 2020. (https://www.medrxiv.org/content/10.1101/2020.05.11.20098574v1). preprint. [DOI] [PMC free article] [PubMed]
- 29.Shinde V, Fries L, Wu Y, et al. Improved titers against influenza drift variants with a nanoparticle vaccine. N Engl J Med 2018;378:2346-2348. [DOI] [PubMed] [Google Scholar]
- 30.Shinde V, Cho I, Plested JS, et al. Comparison of the safety and immunogenicity of a novel Matrix-M-adjuvanted nanoparticle influenza vaccine with a quadrivalent seasonal influenza vaccine in older adults: a randomized controlled trial. May 18, 2020. (https://www.medrxiv.org/content/10.1101/2020.08.07.20170514v1). preprint. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.