Abstract
Background
The emergence of the COVID-19 pandemic has significantly impacted global healthcare systems and this may affect stroke care and outcomes. This study examines the changes in stroke epidemiology and care during the COVID-19 pandemic in Zanjan Province, Iran.
Methods
This study is part of the CASCADE international initiative. From February 18, 2019, to July 18, 2020, we followed ischemic and hemorrhagic stroke hospitalization rates and outcomes in Valiasr Hospital, Zanjan, Iran. We used a Bayesian hierarchical model and an interrupted time series analysis (ITS) to identify changes in stroke hospitalization rate, baseline stroke severity [measured by the National Institutes of Health Stroke Scale (NIHSS)], disability [measured by the modified Rankin Scale (mRS)], presentation time (last seen normal to hospital presentation), thrombolytic therapy rate, median door-to-needle time, length of hospital stay, and in-hospital mortality. We compared in-hospital mortality between study periods using Cox-regression model.
Results
During the study period, 1,026 stroke patients were hospitalized. Stroke hospitalization rates per 100,000 population decreased from 68.09 before the pandemic to 44.50 during the pandemic, with a significant decline in both Bayesian [Beta: -1.034; Standard Error (SE): 0.22, 95% CrI: -1.48, -0.59] and ITS analysis (estimate: -1.03, SE = 0.24, p < 0.0001). Furthermore, we observed lower admission rates for patients with mild (NIHSS < 5) ischemic stroke (p < 0.0001). Although, the presentation time and door-to-needle time did not change during the pandemic, a lower proportion of patients received thrombolysis (-10.1%; p = 0.004). We did not see significant changes in admission rate to the stroke unit and in-hospital mortality rate; however, disability at discharge increased (p < 0.0001).
Conclusion
In Zanjan, Iran, the COVID-19 pandemic has significantly impacted stroke outcomes and altered the delivery of stroke care. Observed lower admission rates for milder stroke may possibly be due to fear of exposure related to COVID-19. The decrease in patients treated with thrombolysis and the increased disability at discharge may indicate changes in the delivery of stroke care and increased pressure on existing stroke acute and subacute services. The results of this research will contribute to a similar analysis of the larger CASCADE dataset in order to confirm findings at a global scale and improve measures to ensure the best quality of care for stroke patients during the COVID-19 pandemic.
Key Words: Stroke, COVID-19, Epidemiology, Outcome, Mortality, Disability, Stroke care
Introduction
In December 2019, a new and contagious strain of coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was identified among patients with pneumonia in Wuhan, China. The disease caused by this virus is now named coronavirus disease 2019 (COVID-19).1 The disease burden has extended beyond what would be expected from a contagious disease. COVID-19 incidence and mortality are significantly correlated with non-communicable diseases (NCDs), including cerebro- and cardiovascular diseases2 and dementia.3 Globally, NCDs account for more than 70% of all deaths.4 Therefore, any changes in incidence, prevalence, and mortality of these conditions may dramatically affect the burden of disease worldwide. During the pandemic, many countries have altered the delivery of healthcare in response to the need for additional capacity and resources through national hospital system restructuring.5 This reorganization has resulted in detrimental effects, including decreased patient capacity at stroke units and interruptions in the delivery of specialized stroke care services.6 Such changes in the previously established delivery of care, particularly among older adults, may lead to increased mortality related to NCDs. For example, between March 13 and April 17, 2020, England and Wales reported an additional 11,334 deaths (8,753 related to COVID-19) compared to their five-year average.7
In an attempt to understand the changes in stroke epidemiology and care related to COVID-19, we initiated a multicenter initiative known as the Call to Action: SARS-CoV-2 and CerebrovAscular DisordEr (CASCADE) study.8 This is an international study consisting of 110 stroke centers in 24 countries. The present report provides information from one of the CASCADE centers and describes the statistical analysis plan used in the CASCADE cohort. This paper specifically examines changes in stroke hospitalization and care during the COVID-19 pandemic in a CASCADE center in Zanjan Province, Iran. Furthermore, this manuscript will serve as a model for analysis in order to harmonize the results across all other CASCADE centers.
Methods
The study was approved by the Ethics Committee of the Zanjan University of Medical Sciences; IR.ZUMS.REC.1399.066.
Populations
This is the first report of the CASCADE initiative from one of the participating centers, Valiasr Hospital, Zanjan, Iran. Valiasr hospital is a major stroke referral center with an annual catchment area of approximately 520,000 patients (Census data 2016). We included all ischemic and hemorrhagic strokes, and excluded patients with transient ischemic attacks due to difficulties in their diagnosis and variable outcomes.9 Stroke is defined according to the Stroke Council of the American Heart Association/American Stroke Association census definition and categorized based on neuroimaging findings, such as ischemic vs. hemorrhagic strokes.10 The first officially confirmed case and death of COVID-19 in Iran were reported on 18 February 2020.11 The first case and death in Zanjan province were registered on February 29, 2020. On March 13, 2020, Iran implemented a national lockdown, which ended on May 3, 2020. We selected a study period from February 18, 2019 (the 1-year calendar month before the first confirmed COVID-19 case in Iran), and followed stroke trends until July 18, 2020, as per CASCADE initiative protocols.8
Statistical analysis
Variables of interest included hospitalization rate per week per 100,000 population (total ischemic and hemorrhagic strokes), male-to-female ratio, severity of stroke at admission [measured by the National Institutes of Health Stroke Scale (NIHSS)], functional status at admission and at discharge [defined by modified Rankin Scale (mRS) > 2], presentation time (last known well to hospital admission), the proportion of patients who received intravenous tissue plasminogen activator (IV tPA) to total ischemic stroke incidence, door-to-needle time, door-to-CT time, hospital length-of-stay, and in-hospital mortality per admissions ratio (death before discharge).
Data are presented as mean ± standard deviation (SD), absolute numbers with percentages (%), and median with interquartile range (IQR) according to the pattern of distribution. A Chi-Square test and independent-sample median test were used to compare the variables of interest within the study periods. Due to the non-normally distributed pattern of our data, we used the Spearman correlation coefficient to examine the association among variables of interest.
To compare the variables of interest between the COVID-19 period to the corresponding months in the previous year, we used two approaches: 1) the Bayesian hierarchical model (time series Bayesian multilevel) and 2) the interrupted time series analysis (ITS), to reduce the uncertainty that may occur when an outcome exhibits a time trend that might confound the intervention effect.
In the Bayesian hierarchical model, we classified the study period into three groups: a) The COVID-19 period, defined from the first confirmed case to the last day of data analysis (i.e., February 18, 2020, to June 30, 2020), b) the months corresponding to COVID-19 in 2019 (i.e., February 18, 2019 to June 30, 2019), and c) the remainder of the study period (i.e., July 1, 2019, to January 31, 2020). The Bayesian hierarchical model allowed the control of the effects of independent variables (e.g., mRS and NIHSS at baseline and the in-hospital mortality), as well as random effects properties, and compared variables of interest in the three selected study periods. According to the pattern of data, we used Bernoulli, Poisson, Gaussian, exGaussian, and asymmetric Laplace family distribution. All results were shown with the 95% credible interval (CrI), estimated based on the Hamiltonian Monte Carlo method. In brief, CrI in the Bayesian models is an analog to the confidence interval (CI) in traditional frequentist analysis, including 95% of the probability distribution of the mean.12 We also used an interrupted time series analysis (ITS) with segmented regression analysis to examine the possible effects of the COVID-19 pandemic on major stroke trends. The weekly rate of stroke data was included in the interrupted time series model. We used the autocorrelation function, partial autocorrelation function, Ljung–Box, Durbin–Watson, and Dickey-Fuller unit root tests to examine the assumptions of time series data. We used the auto-regressive integrated moving average (ARIMA) model to account for autocorrelation. In addition, using the Cox-regression model, we estimated the hazard ratio (HR) with 95% CI of in-hospital mortality. We used the multiple logistic regression model to compare the presentation time to hospital and door-to-needle time of cases with stroke stratified according to the severity of stroke before and during COVID-19.
We assessed the model adequacy using Rhat, Gelman-Rubin diagnostic plots, and Loo. We used the Stan, 'ggplot2′, 'brms', ConsReg, and survival packages to perform statistical analysis in the R v.4 environment.13 , 14
Results
Demographic data and clinical variables
During the study period, 1,026 stroke patients (mean age: 68.82 ± 14.31 years, male-to-female ratio: 1.2) were hospitalized. The median weekly admission rate was 14 ± 4.6 patients. Of these cases, 858 had an ischemic stroke (mean age: 69.03 ± 14.3 years; male-to-female ratio: 1.16) and 168 had a hemorrhagic stroke (mean age: 67.75 ± 14.3 years; male-to-female ratio: 1.43). Table 1 provides a comparison between stroke hospitalization trends and care before and during COVID-19. While a shift towards a younger age was seen at the onset of COVID-19 in comparison to the two months immediately prior to the start of the pandemic (Supplemental Fig. 1), there was no significant difference in the age of admitted cases. In addition, we did not observe any difference in male-to-female ratio and in-hospital mortality rates. In the Spearman correlation analysis, while the hospitalization rate of ischemic stroke reduced during the pandemic (r = -0.50, p = < 0.0001), we did not see a significant change in the hospitalization rate of hemorrhagic stroke (r = 0.016, p = 0.891). We observed a significant reduction in the proportion of those with ischemic stroke receiving IV tPA (r = -0.027, p < 0.0001). There was no change in the door-to-needle time during the pandemic (r = -0.12, p = 0.29). Disability at discharge (measured by mRS) increased during the pandemic in both ischemic (r = 0.46, p < 0.0001) and hemorrhagic strokes (r = 0.46, p < 0.0001). We did not observe any changes in the presentation time, length of stay and in-hospital mortality rate in both ischemic and hemorrhagic strokes.
Table 1.
Baseline demographic and major epidemiological information
| Variables | Study Period: February 18, 2019 to July 18, 2020 |
p | |||
|---|---|---|---|---|---|
| Pandemic Period: (February 18, 2020 to July 18, 2020)(n = 232) | Corresponding month to COVID-19 in 2019: (February 18, 2019, July 18, 2019)(n = 355) | Remainder of study period: (July 19, 2019 to February 17, 2020)(n = 439) | |||
| Mean age (Years ± SD) | 69.21 ± 13.88 | 68.74 ± 14.49 | 68.67 ± 14.41 | 0.891 | |
| Proportion of patients aged ≤ 55 | 31/232 (13.36%) | 62/301 (20.6%) | 75/439 (17.08%) | 0.367 | |
| Proportion of patients aged < 70*1 | 122/232 (52.59%) | 179/355 (50.42%) | 231/439 (52.62%) | 0.801 | |
| Male-to-Female ratio | 128/104 (55.17%) | 196/159 (55.21%) | 236/203 (53.75%) | 0.901 | |
| Hospitalization rate of stroke (per 100,000 population per week) | Total | 2.206 | 3.356 | 2.685 | <0.0001 |
| Ischemic | 1.674 | 2.694 | 2.209 | <0.0001 | |
| Hemorrhagic | 0.436 | 0.4396 | 0.420 | 0.973*2 | |
| In-hospital mortality ratio | Total | 24/229 (10.48%) | 38/352 (10.8%) | 58/377 (13.3%) | 0.426 |
| Ischemic | 16/180 (8.89%) | 24/302 (7.95%) | 41/325 (11.2%) | 0.363 | |
| Hemorrhagic | 7/48 (14.58%) | 14/50 (28%) | 17/52 (24.6%) | 0.253 | |
| Disability ratio*3 | At admission | 16/230 (6.96%) | 34/355 (9.58%) | 19/420 (4.3%) | 0.013 |
| At discharge | 103/190 (54.21%) | 40/155 (25.81%) | 43/155 (21.7%) | <0.0001 | |
| Proportion of NIHSS < 5 *4 | 33/144 (18.6%) | 33/177 (18.64%) | 90/301 (29.9%) | 0.017 | |
| Stroke Unit admission ratio | 134/232 (57.76%) | 221/355 (62.25%) | 295/438 (67.35%) | 0.042 | |
| IV tPA to ischemic stroke ratio | 104/183 (56.83%) | 204/305 (66.89%) | 262/369 (71%) | 0.004 | |
| Length of stay (days; median, IQR) | 3.587 (2.11, 7.3) | 4.415 (2.31, 8.04) | 4.152 (2.68, 8.02) | 0.313 | |
| In-hospital survival (days; median, IQR) | 8.44 (4.2, 16.56) | 5.18 (2.52, 9.8) | 6.43 (3.11, 21.2) | 0.394 | |
| Presentation time (hours; median, IQR) | 4.5 (2.34, 10.91) | 4.2 (1.84, 10.57) | 3.16 (1.59, 9.10) | 0.003 | |
| Door-to-needle time (minutes; median, IQR) | 20 (15, 28) | 21 (15.5, 30) | 19 (13.75, 26) | 0.347 | |
Numbers are presented as No. / Total (%) or median (IQR) according to their distribution patterns.
*1-Median age prior to the COVID-19 pandemic.
*2- Non-parametric median test
*3- Defined as modified ranking scale ≥2
*4- National Institutes of Health Stroke Scale at admission
Stroke hospitalization rate
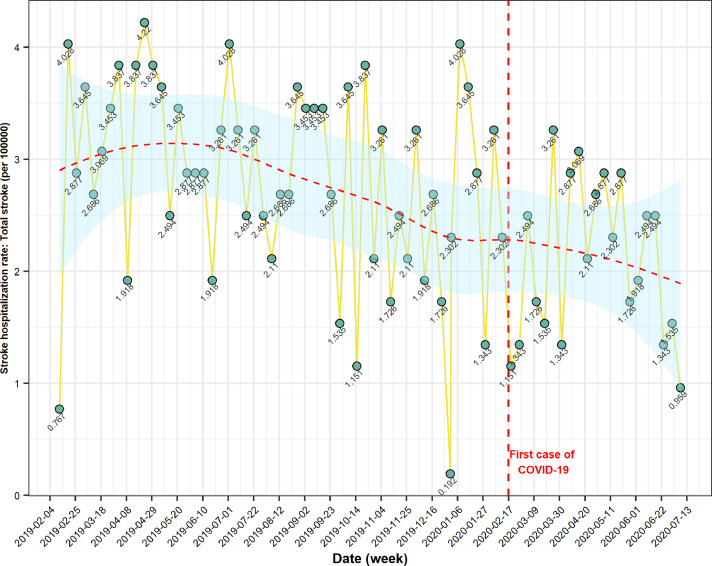
Stroke hospitalization during COVID-19 reduced from 68.09 per 100,000 population, in February 2019, to 44.50 in July 2020 (Fig. 1 ). Changes in total stroke hospitalization rate were also found in the Bayesian analysis [Beta: -1.035; Standard Error (SE): 0.269, 95% CrI: (-1.56, -0.49)] and ITS analysis (estimate: -1.03, SE: 0.24, t: -4.19, p < 0.0001). The stroke hospitalization rate for ischemic stroke per 100,000 population reduced from 58.5 in February to July 2019 to 35.1 in February to July 2020 (p < 0.0001). There was no significant change in the hospitalization rate of hemorrhagic stroke (Tables 2 & 3 ).
Fig. 1.
Stroke hospitalization rate per 100,000 population before and during the COVID-19 pandemic. Results from the CASCADE initiative. Recruiting centre Valiasr Hospital, Zanjan, Iran.
Table 2.
Changes in the stroke epidemiological figures after COVID-19 pandemic: The results of Bayesian hierarchical model.
| Variables | Study period | Beta | Standard Error | 95% credible interval for Beta |
|---|---|---|---|---|
| Average age (yrs) | Pandemic Period | 0.626 | 1.6 | [-2.44, 3.95] |
| Remainder of study period | -0.610 | 1.45 | [-2.44, 3.95] | |
| Male/Female ratio | Pandemic Period | 0.068 | 0.48 | [-0.928, 1.01] |
| Remainder of study period | -0.032 | 0.182 | [-0.39, 0.327] | |
| Hospitalization rate: Total Stroke |
Pandemic Period | -1.035 | 0.269 | [-1.56, -0.49] |
| Remainder of study period | -0.479 | 0.244 | [-0.96, 0.004] | |
| Hospitalization rate: Ischemic stroke | Pandemic Period | -1.034 | 0.227 | [-1.48, -0.59] |
| Remainder of study period | -0.466 | 0.211 | [-0.88, -0.047] | |
| Hospitalization rate: Hemorrhagic stroke | Pandemic Period | 0.0003 | 0.12 | [-0.21, 0.21] |
| Remainder of study period | -0.0002 | 0.1 | [-0.23, 0.23] | |
| Intravenous Thrombolytic Therapy* | Pandemic Period | -0.125 | 0.057 | [-0.23,-0.01] |
| Remainder of study period | 0.042 | 0.06 | [-0.08, 0.174] | |
| Stroke Unit admission | Pandemic Period | -0.017 | 0.06 | [-0.15, 0.12] |
| Remainder of study period | 0.06 | 0.05 | [-0.04, 0.16] | |
| Presentation time (hrs) | Pandemic Period | -0.505 | 1.36 | [-3.23, 2.14] |
| Remainder of study period | -0.46 | 1.25 | [-2.8, 2] | |
| Door to Needle time (hrs) | Pandemic Period | 3.35 | 3.63 | [-0.19, 0.02] |
| Remainder of study period | -0.15 | 0.05 | [-0.25, -0.056] | |
| NIHSS: <5 at admission* | Pandemic Period | -0.50 | 0.10 | [-0.7, -0.29] |
| Remainder of study period | -0.269 | 0.09 | [-0.46, -0.08] | |
| NIHSS: 5–15 at admission* | Pandemic Period | 0.076 | 0.05 | [-0.04, 0.18] |
| Remainder of study period | 0.01 | 0.05 | [-0.09, 0.11] | |
| NIHSS: 16–20 at admission* |
Pandemic Period | 0.001 | 0.05 | [-0.1, 0.1] |
| Remainder of study period | 0.05 | 0.04 | [-0.05, 0.15] | |
| NIHSS: 21–42 at admission* | Pandemic Period | 0.0072 | 0.01 | [-0.02, 003] |
| Remainder of study period | 0.01 | 0.013 | [-0.01, 003] | |
| mRS ≥ 2 at admission** | Pandemic Period | -0.162 | 0.74 | [-0.30, -0.01] |
| Remainder of study period | -0.198 | 0.07 | [-0.34, -0.05] | |
| mRS ≥ 2 at discharge** | Pandemic Period | 0.486 | 0.22 | [0.01, 0.87] |
| Remainder of study period | -0.03 | 0.19 | [-0.43, 0.34] | |
| In-hospital mortality rate** | Pandemic Period | 0.007 | 0.03 | [-0.05, 0.07] |
| Remainder of study period | 0.02 | 0.03 | [-0.04, 0.085] | |
| Length of stay (day) | Pandemic Period | -0.385 | 0.715 | [-1.88, 0.924] |
| Remainder of study period | 0.359 | 0.66 | [-0.933, 1.63] |
Pandemic period: Feb 1, 2020 to June 31, 2020; Corresponding to COVID-19 in 2019: Feb 18, 2019, July 18, 2019 (Reference period); Remainder of study period: July 19, 2019 to February 18, 2020.
Abbreviations: NIHSS: The National Institutes of Health Stroke Scale; mRS: modified Rankin Scale.
* Proportion to ischemic stroke; ** Proportion to total stroke.
Table 3.
Changes in the stroke epidemiological trends after COVID-19 pandemic: The results of interrupted time series analysis
| Variable | Estimate | Standard Error | t | p |
|---|---|---|---|---|
| Age average (yrs) | 0.636 | 1.48 | 0.43 | 0.67 |
| Male/Female ratio | 0.085 | 0.36 | 0.231 | 0.818 |
| Hospitalization Rate: Total stroke | -1.03 | 0.24 | -4.19 | <0.0001 |
| Hospitalization rate: Ischemic stroke | -1.032 | 0.21 | -4.7 | <0.0001 |
| Hospitalization rate: Hemorrhagic stroke | -0.003 | 0.09 | -0.04 | 0.968 |
| Intravenous thrombolytic therapy* | -0.115 | 0.05 | -1.98 | 0.042 |
| Stroke Unit admission | 0.027 | 0.077 | 0.354 | 0.724 |
| Presentation time (hrs) | -0.355 | 0.49 | -0.712 | 0.478 |
| Door to Needle time (hrs) | 1.057 | 1.82 | 0.58 | 0.563 |
| NIHSS: <5 at admission* | -2.61 | 0.53 | -4.89 | <0.0001 |
| NIHSS: 5–15 at admission* | -0.322 | 0.15 | -2.02 | 0.047 |
| NIHSS: 16–20 at admission* | -0.216 | 0.04 | -0.526 | 0.601 |
| NIHSS: 21–42 at admission* | 0.041 | 0.02 | 1.8 | 0.069 |
| mRS ≥ 2 at admission | -0.16 | 0.06 | -2.64 | 0.010 |
| mRS ≥ 2 at discharge | 0.581 | 0.124 | 4.672 | <0.0001 |
| In-hospital mortality** | -0.057 | 0.07 | -0.808 | 0.421 |
| Length of Stay (day) | -0.47 | 0.47 | -0.993 | 0.324 |
Study period: February 18, 2019 to July 18, 2020; First confirmed case: Feb 18, 2020.
Abbreviations: NIHSS: The National Institutes of Health Stroke Scale; mRS: modified Rankin Scale.
* Proportion to ischemic stroke; ** Proportion to total stroke.
Stroke care during the pandemic
There were no significant differences in the presentation time to hospital and door-to-needle time (adjusted for age and sex). Using multiple logistic regression analysis, the number of those with NIHSS > 5 who presented later than 4.5 h during the pandemic were significantly higher than the corresponding months in 2019 [odds ratio (OR: 2.135, 95%CI: 1.03, 4.39). We did not observe any differences in door-to-needle time (>60 min) for patients with NIHSS > 5 during the pandemic (OR: 0.64, 95%CI: 0.065, 6.33). The stroke unit admission and the length-of-stay (adjusted for age, sex, NIHSS, mRS at admission) were reduced, but they were not statistically significant. The proportion of patients treated with IV tPA (adjusted for age, sex, mRS at admission and NIHSS) reduced significantly during the pandemic in the Bayesian analysis [Beta: -0.125; SE: 0.057, 95% CrI: (-0.23, -0.01)] and ITS analysis (estimate: -0.115, SE: 0.058, t: -1.98, p = 0.042).
Stroke severity, disability, and death
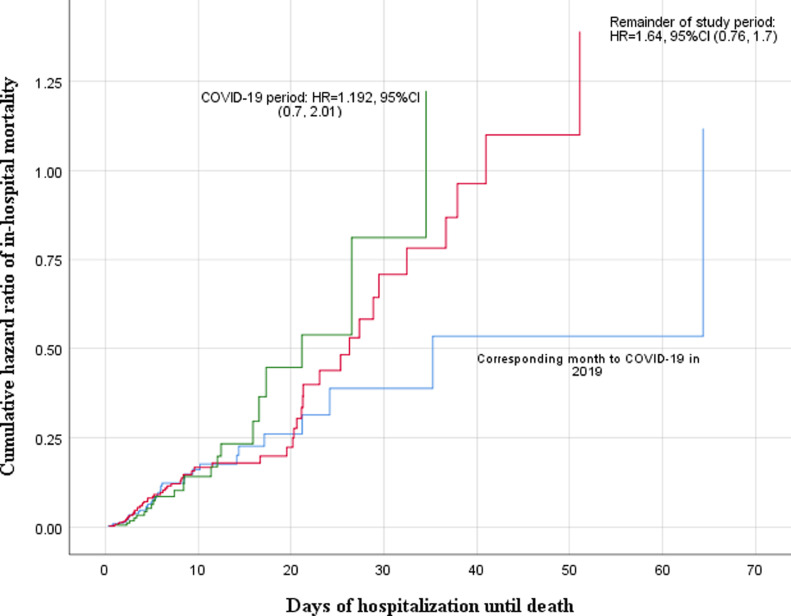
Patients with mild stroke (NIHSS < 5) presented less to the hospital during the pandemic (0.793 per week per 100,000 population in February 2019 as compared to 0.296 per week per 100,000 population in July 2020). In both the Bayesian and ITS analyses, the rate of admission of mild strokes reduced significantly (Tables 2 & 3). After adjusting for age, sex, mRS, and NIHSS at admission, in-hospital mortality rate per admission was not different before and during the pandemic (Tables 2 & 3). In the Cox proportional analysis (adjusted for age, sex, mRS at admission, NIHSS at admission), adjusted HR (aHR) of in-hospital mortality during the pandemic increased, though not significantly [aHR total stroke: 1.19, 95%CI: (0.597, 1.66); a HR ischemic stroke: 1.24, 95% CI (0.90, 1.70); aHR hemorrhagic stroke: 1.04, 95% CI: 0.312, 1.31); Fig. 2 ]. Finally, despite a decrease in the rate of pre-stroke disability at admission during the pandemic, we observed a significant increase of mRS scores at discharge for all strokes (adjusted for age and sex, mRS at admission, and length of stay), ischemic strokes (adjusted for age, sex, length of stay, NIHSS at admission, mRS at admission, and treatment with IV tPA) and hemorrhagic strokes (adjusted for age, sex, mRS at admission and length of stay; Table 2 & 3).
Fig. 2.
Cox proportional hazard analysis of in-hospital mortality model. Results from the CASCADE initiative. Recruiting centre Valiasr Hospital, Zanjan, Iran. HR: Hazard Ratio. The number of days of hospitalization until death was calculated based on the difference between date of death and date of admission.
Discussion
During the COVID-19 pandemic in Zanjan province, Iran, we observed a significant reduction in the total number of stroke admissions, particularly among those with mild strokes. While some aspects of stroke care and outcomes (stroke unit admission, length of stay, door-to-needle time and in-hospital mortality) did not change, a significantly lower percentage of patients received thrombolytic therapy. Disability at discharge increased significantly for all strokes during the COVID-19 pandemic.
The COVID-19 pandemic has dramatically affected the entire health system, in part due to the significant association with NCDs 3. While social distancing, widespread testing, and contact tracing surveillance are crucial in reducing the burden of COVID-19, they are not sufficient to reduce the dual burden of communicable and NCDs. In the current study, we observed a significant reduction in stroke hospitalization during the COVID-19 pandemic. Changes in vascular diseases have been reported during previous national crises and events such as the 2011 Great East Japan Earthquake and Tsunami disaster,15 as well as World and European Football Cup events.16 , 17 However, a global crisis like COVID-19 has not occurred since the 1918 influenza pandemic.18 In addition, the impact of COVID-19 on the healthcare system will be sustained and longer-lasting, in contrast to the immediate impact of an earthquake or a major sporting event. Therefore, it is important to closely monitor the changes in stroke rates during various phases of the pandemic. The CASCADE initiative will enable us to follow these trends across many centers worldwide.
We observed a lower rate of admission among those with mild ischemic stroke (defined as NIHSS < 5), which may be due to a delay in seeking treatment and fear of exposure to COVID-19 in hospitals. This is an alarming finding for health care authorities, as even mild strokes may result in poor clinical outcomes, if not treated.19 , 20 Therefore, many of the patients who are not admitted are at higher risk of disability, recurrent stroke, and/or death. These findings may have particular implications during the pandemic and may suggest a more important role of telemedicine for preventive or therapeutic care in stroke. This can particularly benefit mild stroke patients who may be hesitant to visit a hospital but are more agreeable to contacting a healthcare provider remotely.
The COVID-19 pandemic has the potential to significantly impact patient outcomes following stroke and vascular disease. Stroke care protocols in numerous countries have undergone changes in order to increase resources for COVID-19 patients.21 Despite the recent shift towards prioritization of COVID-19 care, it is equally important to ensure that non-COVID-19 patients, such as stroke patients, continue to receive timely access to care and adequate support. Of note, in our center, there were no observed changes in stroke unit admission and in-hospital mortality rate, despite the decrease in available resources for stroke care. Thus, it is still possible to provide sufficient care for patients, even in low- and middle-income countries, while working with limited resources.
Several factors have the potential to influence the reduction in the number of IV tPA cases. First, the limited experience of healthcare practitioners using IV tPA in settings of the SARS-CoV-2 infection could have influenced rates of administration. Second, healthcare professionals may have faced difficulties in the evaluation and management of acute stroke patients who were at risk of acquiring COVID-19 from suspected or confirmed COVID-19 cases who were asymptomatic during the prodromal period. Finally, healthcare professionals may have been concerned about possible CT scanner contamination before administering IV tPA. In the current study, we did not observe a significant change in door-to-needle time during the pandemic. Therefore, a reduction in the proportion of cases receiving IV tPA cannot be explained by changes in the chain of acute stroke care in our hospital. While presentation time to the hospital did not change during the COVID-19 pandemic, we observed a significant increase in the proportion of those with severe stroke who presented later than 4.5 h. This finding may be explained by a delay in the patient calling an ambulance, or due to changes in dispatch during the pandemic, and can be a major reason for a lower rate of thrombolytic therapy during the pandemic. Implementing comprehensive acute stroke management guidelines could aid in promoting the use of IV tPA in the pandemic. This approach should occur at a pre-hospital level to ensure the safety of the public and encourage patients to present to hospital in cases of possible stroke, and at an in-hospital level to provide adequate sources for stroke care.
We also found a significant increase in disability at discharge during the pandemic period. Increased disability at discharge during the pandemic in our report was independent of the severity of stroke and mRS at admission, the rate of IV tPA injection, and length of stay. Therefore, this increase in disability may be partially explained by delayed or limited access to early rehabilitation and early assisted discharge programs due to reorganization of these services.5 In addition, COVID-19 has resulted in a number of healthcare professional shortages across the country. As a result, hospital systems have redeployed physicians, nurses, and other members of the healthcare team from different specialties to satisfy these shortages. Consequently, specialists who are less experienced in neurological care were tasked to care for stroke patients. Thus, it is possible that this redeployment is associated with a decrease in the level of care given to patients, as compared to before the pandemic.
The increased disability rate upon discharge may be indicative of changes in stroke outcome, which may further amplify the overall burden of stroke. In addition, a lower rate of admission for mild stroke cases may lead to an increase in stroke recurrence, which may increase the potential for more severe strokes and death. These findings are particularly important in low- and middle-income countries,22 where stroke is frequently more prevalent than in many high-income countries,23 and with higher rates of recurrence,24 disability25 and death.26 Furthermore, lifestyle changes, such as physical inactivity, unhealthy diet, and mental health and psychosocial problems related to COVID-19, may cause a rise in the occurrence of chronic health conditions and worsen the existing ones.27
Our study has limitations. Particularly, at this time, we report the results of stroke hospitalization from one center. These results need to be confirmed with other CASCADE centres to determine generalizability. The CASCADE initiative provides a unique opportunity to share these results promptly with several centers worldwide. We performed the analysis using R and will make the relevant scripts available to other centers to standardize the reporting of data. Additionally, in the Valiasr center, we do not perform endovascular therapy and therefore, are not able to comment regarding changes in large artery occlusion and its management during the COVID-19 pandemic. We are also planning to perform subsequent studies comparing the rate of stroke hospitalization with changes in the trend of COVID-19 confirmed cases and death among CASCADE centers. In addition, we will also compare trends of other acute emergency admissions, such as acute coronary diseases, with stroke, in our centers.
In summary, the CASCADE initiative provides an opportunity to assess the cumulative changes in stroke epidemiology worldwide in a timely manner. The current report provides valuable information regarding the evolving changes in stroke epidemiological data, including hospitalization, management and outcomes during the COVID-19 pandemic, which can also be used as a prototype to enable the sharing of analytical models with other centers.
Acknowledgment
The authors have no conflict of interest related to this paper to declare. We would like to sincerely thank all centers participating in the CASCADE study during this extremely difficult period. The authors have not received any compensation for the current study. Valiasr Hospital is an active participating center in the Safe Implementation of Treatments in Stroke (SITS) registry, and we would like to thank SITS for its support.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jstrokecerebrovasdis.2020.105321.
Appendix. Supplementary materials
References
- 1.Adhanom Ghebreyesus T. We now have a name for the disease caused by the novel coronavirus: COVID-19 [Internet]. twitter . 2020 [cited 2020 Jul 22]. Available from: https://twitter.com/DrTedros/status/1227297754499764230. [Google Scholar]
- 2.Azarpazhooh MR, Morovatdar N, Avan A, Phan TG, Divani AA, Yassi N. COVID-19 pandemic and burden of non-communicable diseases: an ecological study on data of 185 countries. J Stroke Cerebrovasc Dis. 2020 doi: 10.1016/j.jstrokecerebrovasdis.2020.105089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azarpazhooh MR, Amiri A, Morovatdar N, Steinwender S, Rezaei Ardani A, Yassi N. Correlations between COVID-19 and burden of dementia: an ecological study and review of literature. J Neurol Sci. 2020;416 doi: 10.1016/j.jns.2020.117013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez R, Lloyd-Sherlock P, Soliz P, Ebrahim S, Vega E, Ordunez P. Trends in premature avertable mortality from non-communicable diseases for 195 countries and territories, 1990-2017: a population-based study. Lancet Glob Health. 2020;8(4):e511–e523. doi: 10.1016/S2214-109X(20)30035-8. [DOI] [PubMed] [Google Scholar]
- 5.Bersano A, Kraemer M, Touzé E, Weber R, Alamowitch S, Sibon I. Stroke care during the COVID-19 pandemic: experience from three large European countries. Eur J Neurol. 2020 doi: 10.1111/ene.14375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montaner J, Barragán-Prieto A, Pérez-Sánchez S, Escudero-Martínez I, Moniche F, Sánchez-Miura JA. Break in the stroke chain of survival due to COVID-19. Stroke. 2020;51(8):2307–2314. doi: 10.1161/STROKEAHA.120.030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Appleby J. What is happening to non-covid deaths? BMJ. 2020;24(369):m1607. doi: 10.1136/bmj.m1607. [DOI] [PubMed] [Google Scholar]
- 8.Abootalebi S, Aertker BM, Andalibi MS, Asdaghi N, Aykac O, Azarpazhooh MR. Call to Action: SARS-CoV-2 and Cerebrovascular DisordErs (CASCADE) J Stroke Cerebrovasc Dis. 2020 doi: 10.1016/j.jstrokecerebrovasdis.2020.104938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadighi A, Stanciu A, Banciu M, Abedi V, Andary NE, Holland N. Rate and associated factors of transient ischemic attack misdiagnosis. eNeurological Sci. 2019;15 doi: 10.1016/j.ensci.2019.100193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJB, Culebras A. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(7):2064–2089. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ministry of Health and Medical Education in Iran. Covid-19 In Iran [Internet]. https://behdasht.gov.ir. 2020 [cited 2020 Aug 9]. Available from: https://behdasht.gov.ir/News of COVID-19 in Iran.
- 12.The No-U-Turn Sampler: Adaptively Setting Path Lengths in Hamiltonian Monte Carlo [Internet]. [cited 2020 Jul 22]. Available from: https://arxiv.org/abs/1111.4246
- 13.Gelman A, Lee D, Guo J. Stan: a probabilistic programming language for bayesian inference and optimization. J Educ Behav Stat. 2015;40(5):530–543. [Google Scholar]
- 14.Institute for Statistics and Mathematics of WU. The Comprehensive R Archive Network [Internet]. 2020 [cited 2020 Aug 15]. Available from: https://cran.r-project.org
- 15.Nozaki E, Nakamura A, Abe A, Kagaya Y, Kohzu K, Sato K. Occurrence of cardiovascular events after the 2011 Great East Japan Earthquake and tsunami disaster. Int Heart J. 2013;54(5):247–253. doi: 10.1536/ihj.54.247. [DOI] [PubMed] [Google Scholar]
- 16.Wilbert-Lampen U, Leistner D, Greven S, Pohl T, Sper S, Völker C. Cardiovascular events during World Cup soccer. N Engl J Med. 2008;358(5):475–483. doi: 10.1056/NEJMoa0707427. [DOI] [PubMed] [Google Scholar]
- 17.Aboa-Eboulé C, Béjot Y, Cottenet J, Khellaf M, Jacquin A, Durier J. The impact of World and European Football Cups on stroke in the population of Dijon, France: a longitudinal study from 1986 to 2006. J Stroke Cerebrovasc Dis. 2014;23(3):e229–e235. doi: 10.1016/j.jstrokecerebrovasdis.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Morens DM, Taubenberger JK, Harvey HA, Memoli MJ. The 1918 influenza pandemic: lessons for 2009 and the future. Crit Care Med. 2010;38(4 Suppl):e10–e20. doi: 10.1097/CCM.0b013e3181ceb25b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amarenco P, Benavente O. Express transient ischemic attack study: speed the process! Stroke. 2008;39(8):2400–2401. doi: 10.1161/STROKEAHA.108.514166. [DOI] [PubMed] [Google Scholar]
- 20.Lavallée PC, Meseguer E, Abboud H, Cabrejo L, Olivot J-M, Simon O. A transient ischaemic attack clinic with round-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol. 2007;6(11):953–960. doi: 10.1016/S1474-4422(07)70248-X. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen TN, Abdalkader M, Jovin TG, Nogueira RG, Jadhav AP, Haussen DC. Mechanical thrombectomy in the era of the COVID-19 pandemic: emergency preparedness for neuroscience teams: a guidance statement from the society of vascular and interventional neurology. Stroke. 2020;51(6):1896–1901. doi: 10.1161/STROKEAHA.120.030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avan A, Digaleh H, Di Napoli M, Stranges S, Behrouz R, Shojaeianbabaei G. Socioeconomic status and stroke incidence, prevalence, mortality, and worldwide burden: an ecological analysis from the Global Burden of Disease Study 2017. BMC Med. 2019;17(1):191. doi: 10.1186/s12916-019-1397-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azarpazhooh MR, Etemadi MM, Donnan GA, Mokhber N, Majdi MR, Ghayour-Mobarhan M. Excessive incidence of stroke in Iran: evidence from the Mashhad Stroke Incidence Study (MSIS), a population-based study of stroke in the Middle East. Stroke. 2010;41(1):e3–10. doi: 10.1161/STROKEAHA.109.559708. [DOI] [PubMed] [Google Scholar]
- 24.Salehi M, Amiri A, Thrift AG, Kapral MK, Sposato L, Behrouz R. Five-year recurrence rate and the predictors following stroke in the Mashhad stroke incidence study: a population-based cohort study of stroke in the middle east. Neuroepidemiology. 2018;50(1–2):18–22. doi: 10.1159/000485509. [DOI] [PubMed] [Google Scholar]
- 25.Farzadfard MT, Sheikh Andalibi MS, Thrift AG, Morovatdar N, Stranges S, Amiri A. Long-term disability after stroke in Iran: evidence from the Mashhad stroke incidence study. Int J Stroke. 2019;14(1):44–47. doi: 10.1177/1747493018789839. [DOI] [PubMed] [Google Scholar]
- 26.Farzadfard MT, Thrift AG, Amiri A, Kapral MK, Hashemi P, Sposato LA. Five-year case fatality following first-ever stroke in the Mashhad stroke incidence study: a population-based study of stroke in the middle east. J Stroke Cerebrovasc Dis. 2018;27(4):1085–1089. doi: 10.1016/j.jstrokecerebrovasdis.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 27.Owen N, Sparling PB, Healy GN, Dunstan DW, Matthews CE. Sedentary behavior: emerging evidence for a new health risk. Mayo Clin Proc. 2010;85(12):1138–1141. doi: 10.4065/mcp.2010.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.