Abstract
The coronavirus disease 2019 (COVID-19) virus has spread all over the world. Scientists are trying to discover drugs as effective treatment for patients with COVID-19. So far about 30 drugs have been introduced that one of them is Tocilizumab. Recently Tocilizumab has been introduced to treat patients with COVID-19 and researchers are investigating further the efficacy of this drug for different are patients. In Iran and China, some reports showed a positive effect of Tocilizumab on Saturation of Peripheral Oxygen (SPO2) but results of CT scan in patients in different. In some patients, CT scan showed reduced infiltration, however in other no change was observed. Unfortunately, until now there has been no definitive and effective treatment for patients with COVID-19. Although Tocilizumab has been accepted by China Health Commission to treat infected patients, its positive effects still cannot be predicted in all patients. Based on evidence of the Tocilizumab’s effect on the SARS COV 2, researchers hope this drug will make effective and promising treatment to improve lung tissue inflammation in patients with the fatal COVID-19 virus. The present study provides an overview of respiratory inflammation with COVID-19 and probable effect of Tocilizumab on SARS-COV 2.
Keywords: Tocilizumab, COVID-19, Coronavirus disease, Actemra
1. Background
Two known zoonotic coronaviruses, SARS-CoV and MERS-CoV, have been reported to damage the respiratory tract and cause severe outbreaks in the past decade [1], [2], [3]. The coronavirus disease 2019 (COVID-19) virus, emerged in December 2019 in Wuhan, China [4]. On Jan 30, 2020, WHO declared the current novel coronavirus disease 2019 (COVID-19) as the sixth public health emergency epidemic [5] This virus has rapidly spread in china, japan, South Korea and with cases now confirmed in many countries [4], [6].
Common symptoms at the onset of the disease include fever, cough, myalgia, fatigue, dyspnea and diarrhea [7]. Most of the patients developed pneumonia, which can rapidly worsen into respiratory failure and develop Acute Respiratory Distress Syndrome (ARDS) [7], [8]. Higher susceptibility and mortality was observed in elderly and patients with low immune function [9]. According to a report, Yang X and colleague, the mortality for critical cases with ARDS increased and reached 60.5% [10].
Researchers are discovering new drugs to treat this emerging virus. So far about 30 drugs have been introduced to treat patients with COVID-19 such as Lopinavir, Ritonavir, Oseltamivir, and Ganciclovir [9]. There is a classification of used drugs to treat respiratory infections from COVID-19 and stratified into three categories: 1. immune system enhancement (Interfron,Glubolin), 2.Chloroquine phosphate, 3. Antiviral components [11]. Tocilizumab (a humanized anti-IL-6 receptor antibody) is one of drugs discussed for the treatment of these patients [12]. Xiaoling Xu and colleagues conducted a clinical trial study in china and demonstrated that Tocilizumab is effective treatment in patients with severe COVID-19 [13]. The current study reviews respiratory inflammation with COVID-19 and probable effect of Tocilizumab on SARS-COV 2.
2. What’s the mechanism of respiratory inflammation with COVID-19?
Although, the exact mechanism of immunology that reinforces the potential damages of the respiratory system with COVID-19 has not been elucidated, but based on SARS COV, we can describe it [13]. CD4+ T lymphocytes are rapidly activated and-differentiated into T helper and generate granulocyte-macrophage colony-stimulating factor (GM-CSF). The cytokine environment provides inflammatory monocytes with a high production of inflammatory cytokines such as IL-6 [14]. In the observations, large amounts of inflammatory cells infiltrate into the lungs of patients with COVID-19. This process may be responsible for an immune damaging and causing lung functional injuries and quick mortality [15].
According to the studies in ICU patients with COVID-19, higher plasma levels of cytokines including IL-6, IL-2, IL-7, IL-10, granulocyte-colony stimulating factor (G-CSF), interferon-γ-inducible protein (IP10), monocyte chemoattractant protein (MCP1), macrophage inflammatory protein 1 alpha (MIP1A), and tumor necrosis factor alpha (TNF-α) were found [7], [16]. This increase of inflammatory cytokine levels occurs as cytokine storm and is related to the severity and prognosis of the disease [16].
3. Role of IL-6 in lung fibrosis
IL-6 is produced in response to tissue injuries and various types of infections and contributes to host defense through activation of immune responses and stimulation of acute phase reactions. IL-6 is of great importance in the pathogenesis of various inflammatory diseases including infectious inflammations associated with tissue fibrosis and for this reason, tocilizumab, anti-IL-6 receptor antibody, has been developed. IL-6 binds to the membrane IL-6 receptor (IL-6R) which is only expressed on hepatocytes and some of the leukocytes and leads to classical signaling through the membrane-bound β-receptor glycoprotein 130 (gp130) [17], [18]. There is another pathway, called trans-signaling, such that IL-6 binds to soluble forms of the IL-6R (sIL-6R), and this complex, IL-6/sIL-6R, can activate all of the tissue cells due to the expression of gp130 on all cells [17], [19]. In this section, we review the role of IL-6 in lung inflammation and fibrosis.
Saito and colleagues revealed the significance of IL-6 production in the infiltration of inflammatory cells in Broncho alveolar lavage fluid of wild-type and IL-6-deficient mice were treated with bleomycin. The number of neutrophils and macrophages decreased in BAL fluid in IL-6-deficient mice. In addition, lung pathology showed a decrease of the accumulation of inflammatory cells in IL-6-deficient mice compared with wild type. These findings revealed the significant role of IL-6 in the pathogenesis of bleomycin-induced lung injury and lung fibrosis [17].
Le and colleagues evaluated the importance of soluble IL-6Ra in idiopathic pulmonary fibrosis (IPF). The increase of soluble IL-6Ra was observed in mice during the onset and progression of fibrosis as well as patients with IPF. Neutralization of soluble IL-6Ra attenuated fibrosis in mice by a decrease in collagen, myofibroblasts and fibronectin in the lung. In addition, it has been shown that the production of soluble IL6Ra from macrophages interfere with IL-6 trans signaling and affect fibrosis in lung tissue. These results indicate the recovery of lung fibrosis by neutralization of soluble IL-6Ra [18]. Another study was performed by Kobayashi et al. who investigated the neutralizing effect of anti-IL-6 antibody on lung injury induced by bleomycin in mice. Inhibition of the early increase of IL-6 by IL-6 neutralizing antibody promoted apoptosis of type 2 pneumocytes and infiltration of neutrophils and presented lung fibrosis. However, inhibition of the second increase of IL-6 by IL-6-neutralizing antibody recovered fibrosis in the lung. The results of this study showed that IL-6 could have a bidirectional role in the pathogenesis of lung fibrosis in animal models [19].
4. Tocilizumab
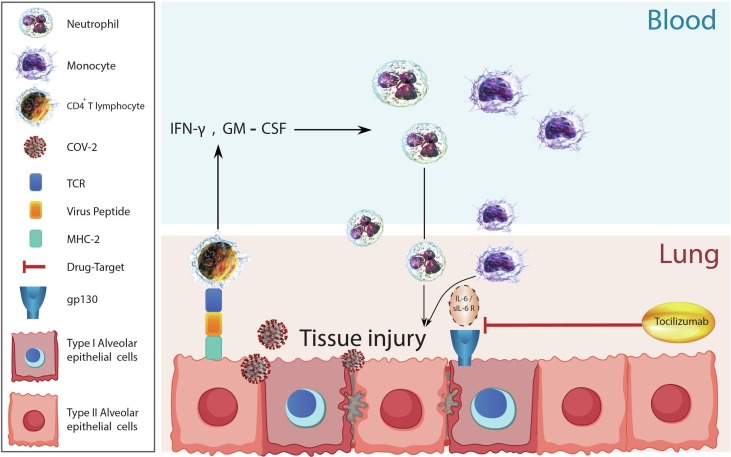
Tocilizumab known as traditional Actemra and Atlizumab is an immunosuppressive humanized monoclonal antibody drug [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20]. This drug is mainly used for the treatment of rheumatoid arthritis (RA) and systemic juvenile idiopathic arthritis [21], [22]. Tocilizumab selectively and competitively binds to soluble expressing the IL-6 receptor (IL-6) and then blocking the signaling caused by IL- 6 [21]. This drug displays dose-dependent, nonlinear pharmacokinetics and has a long elimination half-life [22]. Mechanism of Tocilizumab for inhibiting IL-6 receptors is shown in Fig. 1 .
Fig. 1.
Possible mechanism of lung tissue injury by Covid-2. Virus particles become phagocytosis by alveolar cells. These cells present the virus peptides through MHC-II to CD4+ T lymphocytes. CD4+ T lymphocytes activate and produce IFN-γ, GM-CSF and other inflammatory cytokines. These inflammatory cytokines recruit blood leukocytes such as neutrophils and monocytes into inflamed lung tissue. Neutrophils and monocytes activate and produce reactive oxygen species (not shown in the figure) and IL-6. IL-6 bind to sIL-6-R that form an IL-6/ sIL-6R complex. This complex bind to alveolar epithelial cells through gp130 and during the hierarchy happen tissue injury. Tocilizumab neuralizes binding of IL-6/ sIL-6R to alveolar epithelial cells. GM-CSF: granulocyte-macrophage colony-stimulating factor, gp: glycoprotein, IFN: interferon, IL: interleukin and MHC: major histocompatibility complex, sIL-6-R: soluble IL-6 receptor.
The elimination of Tocilizumab has been relatively slow and dependent on concentration. After saturating the IL6 receptors, clearance of dependent begins by the mononuclear phagocyte system. It has been shown that increase of the dose leads to prolongation of the half-life, but it should be considered that the elimination of Tocilizumab is capacity limited [6]. In a review study of Sheppard and colleagues (2017), effects of gender, age, ethnicity, mild renal failure and treatment with methotrexate, NSAIDs or corticosteroids on the pharmacokinetics of Tocilizumab were unclear [22].
5. What is Tocilizumab medical use and side effect?
Tocilizumab is prescribed to treat moderate to severe active arthritis in adults, Giant cell arthritis, Polyarticular juvenile idiopathic arthritis and cytokine release syndrome in patients 2 years of age older with active disease [23], [24]. The recommended dose of Tocilizumab is 4–8 mg/kg administered as a single 60- minute intravenous infusion every 4 weeks. This drug should be stored refrigerated at 2 to 8c (36 to 46F) [24].
Individuals with active infections should not be treated with Tocilizumab. There are no adequate studies of Tocilizumab in the pregnant women. Also, it is not known whether Tocilizumab is excreted in breast milk [23].
We can categories side effects into 1) common side effects (respiratory tract infections, headache, hypertension, elevation in liver test), 2) reactions of injection site (rash, redness, swelling, itching), 3) associated serious infection (tuberculosis, sepsis, fungal infection), 4) side effects reported in studies (hyper sensitivity reactions, developed cancer, reactivation of herpes zoster, gastrointestinal perforation in patients with diverticulitis) [22], [24].
About side effects of Tocilizumab in infected patient with coronavirus, initial researches did not reveal any side effects. Although may be reported longer time side effects.
6. Tocilizumab; treatment of COVID-19 infection
Tocilizumab inhibits IL-6- receptor, witch as described above is a key cytokine leading to an inflammatory storm which may result in increased alveolar-capillary blood-gas exchange dysfunction, especially impaired oxygen diffusion, and eventually lead to pulmonary fibrosis and organ failure. [25], [27] Based on some reports, Tocilizumab can be a suitable and effective drug for COVID-19 patients. [28], [29], [30]
As mentioned in table 1 , to date some studies such as case reports, retrospective studies, and clinical trial about effectiveness of Tocilizumab in COVID-19 have been published in many affected countries i.e. China, France, Italy, Switzerland and Qatar Xiaoling, 2020; Michot et al., 2020; Luo et al., 2020; Mihai et al., 2020; Toniati P, Piva S, Cattalini M, Garrafa E, Regola F, Castelli F, Franceschini F, Focà E, Andreoli L, Latronico N, Research BI. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 19[13], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39]. However, still no evidence based study confirms efficacy of treatment with this drug. Some researchers have even criticized the use of this drug [40].
Table 1.
Clinical outcomes of patients with Covid-19 after Tocilizumab therapy.
| References cited in the text | Title | Country | Patient no | Study design | Dose of Tocilizumab | Other drug | Key results |
|---|---|---|---|---|---|---|---|
| 13 | Effective treatment of severe COVID-19 patients with tocilizumab | China | 21 Patients with severe COVID-19 ranged from 25 to 88 y | Clinical trial | 4–8 mg/kg (one dose) and 400 mg through an IV drip up to a maximum of 800 mg (3dose) | antiviral therapy of lopinavir/ ritonavir (200/50 mg per tablet for adults twice a day, IFN-α (5 million U each time for adults or equivalent dissolved in 2 mL of sterilized water and aerosol inhalation twice a day, ribavirin (recommended for use with IFN or lopinavir/ritonavir, 500 mg per dose for adultsglucocorticoid (use for a short period of time, range 3 to 5 d, as appropriate, at a dose not exceeding the equivalent of 1 to 2 mg/kg per day methylprednisolone | tocilizumab is an effective treatment to reduce mortality |
| 31 | Tocilizumab, an anti-IL6 receptor antibody, to treat Covid-19-related respiratoryfailure: a case report | France | 42 year patient with COVID-19 | Case report | 8 mg/kg IV for each dose, 8 h apart | lopinavir-ritonavir 400 mg-100 mg orally | tocilizumab is an effective treatment to reduce lung inflammationcytokine storm decreased from 225 mg/L to 33 mg/L |
| 32 | TocilizumabtreatmentinCOVID‐19:A single center experience | China | 15 patients with severe COVID-19 | Retrospective study | 80 to 600 mg per time | Unclear | Single dose of TCZ seems to fail to improve the disease activity in critically ill patients although it was used in combination with glucocorticoid. However, repeated doses (even repeated with a lower dose) of TCZ might improve the condition of critically ill patients |
| 33 | COVID-19 in a patient with systemic sclerosis treated with tocilizumab for SSc- ILD | Switzerland | 57- year- old woman with systemic sclerosis (SSc) and COVID-19 | Case report | 8 mg/kg IV every 4 weeks | Unclear | a patient with insulin- dependent type 2 diabetes mellitus and SSc- ILD treated with tocilizumab developed a mild form of COVID-19 |
| 34 | Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatorysyndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy | Italy | 100 patients with severe COVID-19 | Clinical trial | 8 mg/kg (max800mg) by two IV infusions 12 h apart | antiviral drugs (lopinavir 400 mg + ritonavir 100 mg twice a day or remdesivir 100 mg/day), antibiotic prophylaxis (azithromycin, ceftriaxone or piperacillin/tazobactam), hydroxychloroquine400mg/dayand dexamethasone20mg/ day | The response to TCZ was rapid, sustained, and associated with significant clinical improvement |
| 35 | Tocilizumab for the treatment of severe coronavirus disease 2019 | Qatar | 25 patients with severe COVID-19 | Retrospective study | 4.8 mg/kg (range, 2.7‐7.5 mg/kg)(one to three doses) | hydroxychloroquine, azithromycin, lopinavir/ritonavir, ribavirin, and/or interferon α2‐a | Tocilizumab was associated with dramatic decline in inflammatory markers, radiological improvement and reduced ventilator support requirements. |
| 36 | COVID-19 pneumonia in a kidney transplant recipient successfully treated with tocilizumab and hydroxychloroquine | Italy | 61-year-old man, who underwent kidney transplantation | Case report | 324 mg SC | Meropenem, Azithromycin was administered orally for 3 days, Hydroxychloroquine, IVIG | the infection was successfully managed with the use of hydroxychloroquine and a single administration of tocilizumab |
| 37 | Off‐label useoftocilizumabinpatientswithSARS‐CoV‐2 infection | Italy | Three patients with COVID-19 | Case series | 8 mg/kg IV with a second dose 12 h after the first and a possible third dose after further 24‐36 h (three dose) | Unclear | Rapid relief of respiratory symptoms, resolution of fever, and reduction in CRP and no adverse events were registered |
| 38 | Rapid and Severe Covid-19 Pneumonia with Severe Acute Chest Syndrome in a Sickle Cell PatientSuccessfully Treated with Tocilizumab | France | One patient with Severe Acute Chest Syndrome in a Sickle Cell | Case report | 8 mg/kg IV (one dose) | Hydroxychloroquine | Improvement in Patients condition and level of SPO2 after 3 days. |
| 39 | Favorable changes of CT findings in a patient with COVID-19 pneumonia after treatment with tocilizumab | Italy | A 64 old man with | Case report | 8 mg/kg (two doses) | Unclear | Improvement in chest CT finding after use of TCZ |
| 40 | Hydroxychloroquine and Tocilizumab Therapy in COVID-19 Patients - An Observational Study | US | 198 patients | Observational Study | 400 mg (96%), followed by 800 mg (1%), 8 mg/kg (1%), 4 mg/kg (1%) | Unclear | Tocilizumab demonstrated a trend association towards reduced mortality among ICU patients. |
| 41 | Impact of tocilizumab administration on mortality in severe COVID-19 | New Jersey | 132 patients | Cohort study | 10 patients (15.1%) received 800 mg of tocilizumab, 3 patients (4.5%) received 600 mg of tocilizumab, and 53 patients (80.3%) received 400 mg | Unclear | The current analysis does not support the use of tocilizumab for the management of cytokine storm in patients with COVID-19.Use of this therapeutic agent should be limited to the context of a clinical trial until more evidence is available |
| 46 | Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study | Italy | 32 patients | Cohort study | 400 mg IV | hydroxychloroquine 400 mg daily, lopinavir/ritonavir 400/100 mg twice daily, ceftriaxone 2 gr for 6 days, azithromycin 500 mg daily | This is the first study comparing tocilizumab to standard of care in severe COVID-19Tocilizumab in severe COVID-19 patients did influence 28-day clinical outcomesTocilizumab safety was satisfactory except for ICU-admitted patients |
| 47 | Outcomes in patients with severe COVID-19 disease treated with tocilizumab: a case–controlled study | USA | 96 patients | Case–control | Unclear | Hydroxychloroquine, Azithromycin, Corticosteroids, Remdesivir, Vitamin C, Zinc | non-statistically significant lower mortality in patients with severe to critical COVID-19 disease who received tocilizumabbut use of tocilizumab was associated with lower mortality in intubated patients |
| 48 | Tocilizumab for Treatment of Severe COVID-19 Patients: Preliminary Results from SMAtteo COvid19 REgistry (SMACORE) | Italy | 21 patients | Clinical trial | 8 mg/kg IV (up to a maximum 800 mg per dose) | hydroxychloroquine (200mgbid), azithromycin (500mgonce), prophylactic dose of low weight heparin, and methylprednisolone (a tapered dose of 1 mg/kg up to a maximum of 80 mg) | TCZ administration did not reduce ICU admission or mortality rate |
| 49 | Improved survival outcome in SARs-CoV-2 (COVID-19) Acute Respiratory Distress Syndrome patients with Tocilizumab administration | 44 patients | Case- control | Unclear | Hydroxychloroquine, Azithromycin, Steroids - hydrocortisone/ methylprednisolone/ dexamethasone | Tocilizumab group have improved survival outcome |
COVID-19: corona virus disease −19 , TCZ: Tocilizumab, CRP: C-reactive protein, IVIG: intravenous immune globulin, SPO2:Saturation of Peripheral Oxygen, CT: Computed tomography, SSc: systemic sclerosis, IV: Intra venous, SC: Subcutaneous.
Toniati and colleagues in a single center study of 100 patients in Brescia, Italy prescribed 8 mg/kg (max800mg) by two consecutive intravenous infusions 12 h apart. Based on the results of this study Tocilizumab was rapid, with sustained response and was associated with significant clinical improvement in patients with COVID-19. Toniati P, Piva S, Cattalini M, Garrafa E, Regola F, Castelli F, Franceschini F, Focà E, Andreoli L, Latronico N, Research BI. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 19[33] In a retrospective study conducted by Alattar and colleagues in Qatar, 25 patients with COVID-19 received one to three median doses 4.8 mg/kg. Result of this study showed that Tocilizumab decreased the inflammatory markers, improved radiological outcomes and reduced needs for ventilator support [34].
In a case report study of 61-year-old man with COVID-19, who underwent kidney transplantation, it was observed that 324 mg Tocilizumab via subcutaneous with hydroxychloroquine can successfully manage the infection [35].
On the other hand, in another study that was recently published, new impact of Tocilizumab administration on mortality in severe COVID-19 was assessed. Patients received several doses of Tocilizumab including 400 mg (96%), followed by 800 mg (1%), 8 mg/kg (1%), and 4 mg/kg (1%). Based on the results of this study, current analysis does not support the use of Tocilizumab for the management of cytokine storm in patients with COVID-19 and use of this drug should be limited [40].
We estimate that many of studies haves mentioned positive effect of Tocilizumab in treatment of COVID-19 but recent studies doubt about its effects.
7. Expert opinion
The probable reason for the serious deterioration and the loss of some organs in coronavirus disease are cytokines, known as cytokine release syndrome (CRS) [1], [27]. Therefore, drugs used in the past for the CRS have been considered as effective drugs for treating patients with COVID-19 [13]. Recently Tocilizumab has been introduced to treat patients with COVID-19 and researchers are investigating further the efficacy of this drug for different patients [13].
There are multiple RCTs in progress to evaluate the efficacy of Tocilizumab include NCT04320615, NCT04317092 and NCT04363853. [41] Based on the results of reviewed studies, many patients observed positive effect after prescribing this drug Xiaoling, 2020; Michot et al., 2020; Luo et al., 2020; Mihai et al., 2020; Toniati P, Piva S, Cattalini M, Garrafa E, Regola F, Castelli F, Franceschini F, Focà E, Andreoli L, Latronico N, Research BI. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 19[13], [30], [31], [32], [33], [34], [35], [36], [37], [38]. However, we cannot claim that Tocilizumab has approved and confirmed positive therapeutic effect on this viral disease.
Initial studies has been shown that Tocilizumab had reduced mortality and improved clinical manifestation in patients infected with coronavirus. Michot et al., 2020; Luo et al., 2020; Mihai et al., 2020; Toniati P, Piva S, Cattalini M, Garrafa E, Regola F, Castelli F, Franceschini F, Focà E, Andreoli L, Latronico N, Research BI. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 19[30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40] But recently, Andrew Tsai and colleagues published an article that debates the use of Tocilizumab should be limited to the context of a clinical trial until more evidence is available [40]. Indeed, the researchers claimed there was no difference in mortality in patients treated with tocilizumab versus those receiving supportive care. However, a systematic review study conducted by Avi Kaye and Robert Siegel in Stanford University, investigating several other studies, concluded positive evidence for the potential efficacy of Tocilizumab resulting in less deaths. [42], [43], [44]. According to the authors of this article, notable limitations such as different data of geographies, resources, demographics, and TCZ dosing amount, number and timing can affect the result of main conclusion. In this regard different studies have different participants, complementary drugs such as hydroxychloroquine, azithromycin, lopinavir/ritonavir, ribavirin, and/or interferon α2‐a, Meropenem, Azithromycin and dexamethasone, different doses (4, 8 mg/kg), different time [1], [2], [3] and different route (IV, SQ). Therefore, we can assert that continuous accurate protocol and method of treatment with Tocilizumab is not found and physicians prescribe this drug with trial and error.
In some studies observed positive effect of Tocilizumab with combination of antiviral drugs such as lopinavir/ritonavir (400 mg/100 mg twice a day) or remdesivir 100 mg/day and corticosteroids able to improve clinical manifestations of patients. In this studies other drugs were different, for example in chinies and Italian clinical trials some drugs such as glucocorticoid, interferon α2, and antibiotic were used. Therefore, maybe antiviral drug, especially lopinavir/ritonavir (400 mg/100 mg twice a day) with Tocilizumab reduced mortality in severe COVID-19 patients.
Timing for prescribing Tocilizumab and selecting suitable patients for treatment is also an important question. All of the reviewed studies included patients in severe phase of disease. Some researchers claim that the optimal time to prescribing Tocilizumab is in beginning of inflammation and first steps of dropping O2 saturation. But still accurate time and infection stage to start this drug is unclear.
Comparison the clinical differences between effective Michot et al., 2020; Luo et al., 2020; Mihai et al., 2020; Toniati P, Piva S, Cattalini M, Garrafa E, Regola F, Castelli F, Franceschini F, Focà E, Andreoli L, Latronico N, Research BI. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 19 [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40] and ineffective [47] treatments revealed the importance of time of administration and combination therapy with antivirals and Tocilizumab. In one clinical trial show ineffective treatment, Tocilizumab administer before the progression of respiratory failure and in patients who met the aforementioned criteria for disease severity. In this trial patient didn’t received any antiviral drug [47]. Administration of Tocilizumab in the studies with effective results were combined with an antiviral drug and for patients with severe COVID-19.
There have been few reports about side effects after use of Tocilizumab. For example study of Campochiaro and colleagues Bacterial/fungal infection showed side effects only in 13% of patients who received this drug [42].In another study, Rojas-Marte and colleagues, reported bacteremia, fever, cough and shortness of breath [45]. Majority of studies did not find any side effects after use of Tocilizumab in patients affected with COVID-19 until with twice prescribing [46], [48]. Due to the current pandemic situation and three speed of resource dissemination, there may be side effects but they are not reported. On the other hand, taking drugs at the same time makes it difficult to judge the side effects.
We should know that Tocilizumab is not an anti-viral drug and may only be effective in a group of patients with inflammation and lung damage caused by the coronavirus. Another important point is that excessive production and activity of Tocilizumab can cause autoimmune diseases and damage body tissues [26], [27], [28], [29]. This drug is very sensitive and can be used in a specific age and certain patients. As a result of review of the published data and based on the mechanism of action of Tocilizumab, we may be able to claim that this drug can be a better or more suitable choice to be used for in patients with higher IL-6 level than normal.
Although Tocilizumab has been accepted by China Health Commission and also recommended by some other health commissions all over the world, its positive effects cannot be predicted in all patients.
8. Conclusion
Tocilizumab may have a positive effect on improving immune damaging, lung functional injuries and arterial oxygen saturation. Researchers who had the successful experience of using this drug for treating inflammation lungs diseases, hope it will make effective and promising treatment to improve lung tissue inflammation in patients with fatal COVID-19 virus. However, further accurate clinical trial studies are needed to determine its efficacy in patients with specific characteristics such as age, level of IL-6, and different clinical symptoms.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2020.107018.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Hu B., Zeng L.-P., Yang X.-L., et al. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog. 2017;13(11) doi: 10.1371/journal.ppat.1006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song H-D, Tu C-C, Zhang G-W, et al. Cross-host evolution of severe acute respiratory syndrome coronavirus in palm civet and human. Proc Natl Acad Sci U S A. 102 (7) (2005) Feb 15 2430-5.10.1073/pnas.0409608102. [DOI] [PMC free article] [PubMed]
- 3.Haagmans BL, Al Dhahiry SHS, Reusken CBEM, et al. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect Dis 2014; 14:140-5. 24 (5) (2018) 926-928. 10.3201/eid2405.171192. [DOI] [PMC free article] [PubMed]
- 4.Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle. J Med Virol 2020 Jan 16 [Epub ahead of print]. 10.1002/jmv.25678 .J Med Virol. 92 (4) (2020) 401-402. 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed]
- 5.WHO Emergency Committee. Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (COVID-19). Geneva: WHO, 2020. https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international healthregulations-(2005)-emergency-committee-regarding-the-outbreakof-novel coronavirus-(COVID-19) (accessed Feb 1, 2020).
- 6.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl. J. Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D., Hu B., Hu C., et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11) doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.She J., Jiang J., Bai C., Song Y. Novel coronavirus of pneumonia in Wuhan, China: emerging attack and management strategies. Clin. Transl. Med. 2020;9(1):19. doi: 10.1186/s40169-020-00271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frey N., Grange S., Woodworth T. Relationship between serum concentrations of the interleukin-6 receptor inhibitor tocilizumab and C-reactive protein reduction in RA patients: 6 months’ data from a phase 3 study. Arthritis Rheum. 2007;56:148–149. [Google Scholar]
- 13.X Xiaoling, et al. Effective Treatment of Severe COVID-19 Patients with Tocilizumab. 2020. Proc Natl Acad Sci U S A. 117(20) (2020) 10970-10975. 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed]
- 14.Zhou Y., et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. National Sci. Rev. 2020:998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.-Y. Pulmonary pathology of early phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J. Thorac. Oncol. 2020;15(5):700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saito F., Tasaka S., Inoue K., Miyamoto K., Nakano Y., Ogawa Y., Yamada W., Shiraishi Y., Hasegawa N., Fujishima S., Takano H., Ishizaka A. Role of interleukin-6 in bleomycin-induced lung inflammatory changes in mice. Am. J. Respir. Cell Mol. Biol. 2008;38(5):566–571. doi: 10.1165/rcmb.2007-0299OC. [DOI] [PubMed] [Google Scholar]
- 18.Le T.T., Karmouty-Quintana H., Melicoff E., Le T.T., Weng T., Chen N.Y., Pedroza M., Zhou Y., Davies J., Philip K., Molina J., Luo F., George A.T., Garcia-Morales L.J., Bunge R.R., Bruckner B.A., Loebe M., Seethamraju H., Agarwal S.K., Blackburn M.R. Blockade of IL-6 Trans signaling attenuates pulmonary fibrosis. J. Immunol. 2014;193(7):3755–3768. doi: 10.4049/jimmunol.1302470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi T., Tanaka K., Fujita T., Umezawa H., Amano H., Yoshioka K., Naito Y., Hatano M., Kimura S., Tatsumi K., Kasuya Y. Bidirectional role of IL-6 signal in pathogenesis of lung fibrosis. Respir. Res. 2015;16(1):99. doi: 10.1186/s12931-015-0261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishimoto N., Kishimato T. Humanized antihuman IL-6 receptor antibody, tocilizumab. Handb. Exp. Pharmacol. 2008;181:151–160. doi: 10.1007/978-3-540-73259-4_7. [DOI] [PubMed] [Google Scholar]
- 21.Song S.N., Yoshizaki K. Tocilizumab for treating rheumatoid arthritis: an evaluation of pharmacokinetics/pharmacodynamics and clinical efficacy. Expert Opin. Drug. Metab. Toxicol. 2015;11(2):307–316. doi: 10.1517/17425255.2015.992779. [DOI] [PubMed] [Google Scholar]
- 22.Oldfield V., Dhillon S., Plosker G.L. Tocilizumab. Drugs. 2009;69(5):609–632. doi: 10.2165/00003495-200969050-00007. [DOI] [PubMed] [Google Scholar]
- 23.Frey N., Grange S., Woodworth T. Population pharmacokinetic analysis of tocilizumab in patients with rheumatoid arthritis. J. Clin. Pharmacol. 2010;50(7):754–766. doi: 10.1177/0091270009350623. [DOI] [PubMed] [Google Scholar]
- 24.Sebba A. Tocilizumab: the first interleukin -6 receptor inhibitor. Am J Health Syst Pharm. 2008;65(15):1413–1418. doi: 10.2146/ajhp070449. [DOI] [PubMed] [Google Scholar]
- 25.Medical reviewed by John P. Cunha, DO, FACOPE, FDA prescribing information for Actemra.
- 26.Velazquez-Salinas L., Verdugo-Rodriguez A., Rodriguez L.L., Borca M.V. The Role of Interleukin 6 during Viral Infection. Front. Microbiol. 2019;10:1057. doi: 10.3389/fmicb.2019.01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV2 Pneumonia in Wuhan, China: A single-centered, retrospective,observational study. Lancet Respir Med 2020; Published Online February 10.1016/S2213-2600 (20)30079-5. [DOI] [PMC free article] [PubMed]
- 28.Sheppared M., et al. Tocilizumab. Human Vacc. Immunotherapeut. J. 2017;13(9):1972–1988. doi: 10.1080/21645515.2017.1316909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu B., Zeng L.-P., Yang X.-L., et al. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog. 2017;13(11):13. doi: 10.1371/journal.ppat. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michot J.M., Albiges L., Chaput N., Saada V., Pommeret F., Griscelli F., Balleyguier C., Besse B., Marabelle A., Netzer F., Merad M. Tocilizumab, an anti-IL-6 receptor antibody, to treat COVID-19-related respiratory failure: a case report. Ann Oncol. 2020;31(7):961–964. doi: 10.1016/j.annonc.2020.03.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: A single center experience. J. Med. Virol. 2020;92(7):814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mihai C., Dobrota R., Schröder M., Garaiman A., Jordan S., Becker M.O., Maurer B., Distler O. COVID-19 in a patient with systemic sclerosis treated with tocilizumab for SSc-ILD. Ann Rheum Dis. 2020;79(5):668–669. doi: 10.1136/annrheumdis-2020-217442. [DOI] [PubMed] [Google Scholar]
- 33.Toniati P, Piva S, Cattalini M, Garrafa E, Regola F, Castelli F, Franceschini F, Focà E, Andreoli L, Latronico N, Research BI. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 19(7) (2020) 102568.10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed]
- 34.Alattar R., et al. Tocilizumab for the treatment of severe coronavirus diseas 2019. J Med Virol. 2020;10 doi: 10.1002/jmv.25964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fontana F., Alfano G., Mori G., Amurri A., Tei L., Ballestri M., Leonelli M., Facchini F., Damiano F., Magistroni R., Cappelli G. Covid-19 pneumonia in a kidney transplant recipient successfully treated with Tocilizumab and Hydroxychloroquine. Am. J. Transplant. American J. Transplant. 2020;20(7) doi: 10.1111/ajt.15935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Giambenedetto S., Ciccullo A., Borghetti A., Gambassi G., Landi F., Visconti E., Zileri Dal Verme L., Bernabei R., Tamburrini E., Cauda R., Gasbarrini A. Off-label use of tocilizumab in patients with SARS-CoV-2 infection. J. Med. Virol. 2020;10 doi: 10.1002/jmv.25897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Luna G., Habibi A., Deux J.F., Colard M., d'Orengiani P.H., Schlemmer F., Joher N., Kassasseya C., Pawlotsky J.M., Ourghanlian C., Michel M. Rapid and severe Covid-19 pneumonia with severe acute chest syndrome in a sickle cell patient successfully treated with tocilizumab. Am. J. Hematol. 2020;95(7):876–878. doi: 10.1002/ajh.25833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cellina M., Orsi M., Bombaci F., Sala M., Marino P., Oliva G. Favorable changes of CT findings in a patient with COVID-19 pneumonia after treatment with tocilizumab. Diagn Interv Imaging. 2020;101(5):323–324. doi: 10.1016/j.diii.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ip A., Berry D.A., Hansen E., Goy A.H., Pecora A.L., Sinclaire B.A., Bednarz U., Marafelias M., Berry S.M., Berry N.S., Mathura S. Hydroxychloroquine and Tocilizumab Therapy in COVID-19 Patients-An Observational Study. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsai A., Diawara O., Nahass R.G., Brunetti L. Impact of tocilizumab administration on mortality in severe COVID-19. medRxiv. 2020 doi: 10.1101/2020.07.30.20114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.“Sanofi and Regeneron’s Kevzara fails in Phase III Covid-19 trial.” Clinical Trials Arena. (July 3, 2020). Retrieved from https://www.clinicaltrialsarena.com/news/kevzara-us-covid19-trial-data/.
- 42.Kay A., Siegel R. The Efficacy of IL-6 Inhibitor Tocilizumab in Reducing Severe COVID-19 Mortality: A Systematic Review. medRxiv. 2020 doi: 10.1101/2020.07.10.20150938doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klopfenstein T, Zayet S, Lohse A, Balblanc JC, Badie J, Royer PY, Toko L, Mezher C, Bossert M, Bozgan AM, Charpentier A. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Médecine et Maladies Infectieuses. (2020). Med Mal Infect. 50 (5) (2020) 397-400. 10.1016/j.medmal.2020.05.001. [DOI] [PMC free article] [PubMed]
- 44.Capra R., Rossi N., Mattioli F., et al. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur. J. Intern. Med. 2020;76:31–35. doi: 10.1016/j.ejim.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campochiaro C., Della-Torre E., Cavalli G., et al. Efficacy and safety of tocilizumab in severe COVID19 patients: a single-centre retrospective cohort study. Eur. J. Intern. Med. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rojas-Marte G., Khalid M., Mukhtar O., et al. Outcomes in Patients with Severe COVID-19 Disease Treated with Tocilizumab - A Case- Controlled Study. QJM. 2020;113(8):546–550. doi: 10.1093/qjmed/hcaa206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colaneri M., Bogliolo L., Valsecchi P., et al. Tocilizumab for Treatment of Severe COVID-19 Patients: Preliminary Results from SMAtteao COvid19 Registry (SMACORE) Microorganisms. 2020;8(5):695. doi: 10.3390/microorganisms8050695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wadud N., Ahmed N., Shergil M., et al. Improved survival outcome in SARs-CoV-2 (COVID-19) Acute Respiratory Distress Syndrome patients with Tocilizumab administration. medRxiv. 2020 doi: 10.1101/2020.05.13.20100081. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.