Parkinson's disease or parkinsonism have been described after infections by viruses, such as influenza A, Epstein-Barr virus, varicella zoster, hepatitis C virus, HIV, Japanese encephalitis virus, or West Nile virus.1 We report a patient with probable Parkinson's disease, who was diagnosed after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
A 45 year old Ashkenazi-Jewish man was hospitalised in Samson Assuta Ashdod University Hospital (Ashdod, Israel) on March 17, 2020, because of dry cough and muscle pain. A few days before admission, he had also noticed a loss of smell. His symptoms had started on March 11, 2 days after returning to Israel from a week-long trip to the USA. He might have been exposed to the virus on the flight back to Israel, since he recalled that a passenger sitting behind him was coughing repeatedly. His previous medical history included hypertension, treated daily with 200 mg labetalol, 80 mg valsartan, and 5 mg amlodipine, and asthma, treated with salbutamol sporadically and at admission. He was found positive for SARS-CoV-2 by use of a real-time RT-PCR test after a nasopharyngeal swab was done on the day of admission. His complete blood count and CRP measures were normal (CRP 1·5 mg/L).
During his hospitalisation in the COVID-19 ward, the patient had fatigue, shortness of breath, and chest pain without fever, and was treated for 3 days as an inpatient, mostly with salbutamol inhalations as needed for mild asthma symptoms, with no need for systemic medications, oxygen supplementation, or mechanical ventilation. The patient was then isolated on March 20 in a COVID-19 facility. He tested negatively on nasopharyngeal swabs done on March 25 and March 30. However, during the isolation period of 3 weeks, he noticed that his handwriting had changed and become smaller and less readable than previously. He started having difficulties speaking and writing text messages on his mobile phone. He also had episodes of tremor in his right hand. After returning home, he continued to have these symptoms and was eventually admitted to the Department of Neurology, at Shaare Zedek Medical Center (Jerusalem, Israel) about 2 months after initially testing positive for SARS-CoV-2 infection.
On examination, the patient had hypomimia and hypophonic fluent speech. He had moderate cogwheel rigidity in the neck and in the right arm, mild cogwheel rigidity in the left arm, moderate bradykinesia in the right extremities, mild bradykinesia in the left extremities, and no tremor. His gait was slightly slow, with no right arm swing, and the elbow appeared to be in flexion during walking but with normal step length and height. No retropulsion was found on a pull test. He did not have cognitive decline, shown by a Montreal Cognitive Assessment score of 28 of 30, but his subjective impression was that his cognitive performance was lower than usual. He did not have constipation, depression, or rapid eye movement behaviour disorder. He did not report a previous family history of Parkinson's disease, nor had he been exposed to neurotoxins or recreational drugs. The routine blood tests were unremarkable and CSF measures showed 6 white blood cells (83% mononuclear cells), with normal glucose (62 mg/dL) and protein (43 mg/dL) concentrations (appendix p 1). Anti-SARS-CoV-2 IgG was detected in the serum but not in the CSF, and real-time RT-PCR of the CSF was negative for SARS-CoV-2. CSF and serum were also negative for common neuronal antibodies, including for GABA type B receptors, NMDA receptors, CASPR2, AMPA receptor type 1, AMPA receptor type 2, and LGI1.
A brain CT, diffusion and fluid-attenuated inversion recovery sequences on MRI, and an EEG were all normal. But a 18F-fluorodopa (18F-FDOPA) PET scan showed decreased 18F-FDOPA uptake in both putamens, more apparent on the left side. Additionally, mild decreased uptake in the left caudate was also suspected (figure ). Genetic testing for mutations in common hotspots of the LRRK2 gene and full gene sequencing of GBA variants were negative. Next Generation Sequencing was done to screen for other genes related to Parkinson's disease (appendix pp 2–4), but this was also negative. We diagnosed parkinsonism, meeting the Movement Disorders Society Unified Parkinson's Disease Rating Scale criteria for the diagnosis of probable Parkinson's disease.2 We initiated treatment with 0·375 mg extended release pramipexole, once daily, which resulted in a quick improvement according to the patient's subjective impression, as well as in clinical signs.
Figure.
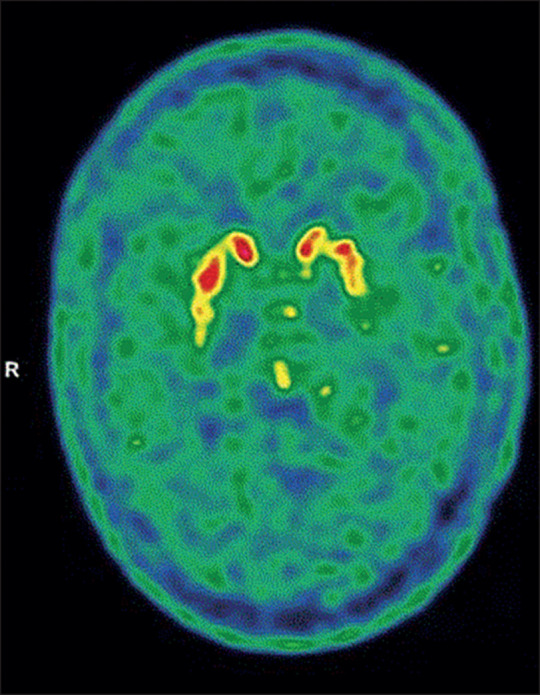
18F-FDOPA PET brain scan of our patient
Neuroimaging showed decreased 18F-FDOPA uptake in both putamens, more apparent on the left side. 18F-FDOPA=18F-fluorodopa. R=right.
During his 9 days of hospitalisation, the patient started complaining of tremor in both legs, more on the right side than the left, and of increased urinary frequency. On discharge, he still had unreadable handwriting, hypomimia, bradykinesia, and cogwheel rigidity, mostly on the right side. He was empirically treated with a course of 5 days intravenous high-dose methylprednisolone, without any consistent effect. Because of the worsening of tremor in his right extremities, in a follow-up visit on June 29, biperiden was added at a dose of 2 mg daily, and increased to 4 mg daily after 1 week, which resulted in improvement of the tremor.
The mechanism that led to the presumed degeneration of nigrostriatal dopaminergic nerve terminals is unclear. Perhaps a susceptible genetic makeup made our patient vulnerable to immunologically mediated mitochondrial injury and neuronal oxidative stress. Another hypothesis could be that the virus causes inflammation via microglial activation, contributing to protein aggregation and neurodegeneration.3 However, the short time interval between the acute infection and the parkinsonian symptoms makes this hypothesis unlikely. Other researchers have proposed the so-called multiple hit hypothesis, by which the combination of toxic stress and an inhibition of neuroprotective responses can lead to neuronal death.4
Parkinson's disease is often preceded by anosmia, which is a common feature of SARS-CoV-2 infection.5 Immune activation in the olfactory system might eventually lead to the misfolding of α-synuclein and the development of Parkinson's disease.6 This mechanism is supported by post-mortem studies, showing increased levels of TNF,7 IL1, and IL6.8 Moreover, patients with Parkinson's disease had an elevated CSF antibody response to seasonal coronaviruses, compared with age-matched healthy controls.9
In Ashkenazi-Jewish people with Parkinson's disease, about a third are carriers of either a GBA or a LRRK2 mutation.10 A genetic analysis for these mutations and 62 other mutations associated with the disease was negative and our patient had no previous family history of Parkinson's disease. However, we cannot exclude an interaction between other, less frequent mutations and SARS-CoV-2. The temporal association between the episode of SARS-CoV-2 infection and parkinsonian symptoms, which appeared during the acute infection, is intriguing. Before his admission to the Department of Neurology, the patient had tested negative for SARS-CoV-2 on real-time RT-PCR on two occasions; however, he was then found positive for anti-SARS-CoV-2 IgG antibodies in serum, but negative for these antibodies in CSF. Nonetheless, we cannot exclude the possibility that SARS-CoV-2 entered the CNS, particularly in view of the olfactory involvement and borderline pleocytosis.
Acknowledgments
We declare no competing interests.
Supplementary Material
References
- 1.Limphaibool N, Iwanowski P, Holstad MJV, Kobylarek D, Kozubski W. Infectious etiologies of parkinsonism: pathomechanisms and clinical implications. Front Neurol. 2019;10:652. doi: 10.3389/fneur.2019.00652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Postuma RB, Berg D, Stern M. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord. 2015;30:1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 3.Sadasivan S, Zanin M, O'Brien K, Schultz-Cherry S, Smeyne RJ. Induction of microglia activation after infection with the non-neurotropic A/CA/04/2009 H1N1 influenza virus. PLoS One. 2015;10 doi: 10.1371/journal.pone.0124047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sulzer D. Multiple hit hypotheses for dopamine neuron loss in Parkinson's disease. Trends Neurosci. 2007;30:244–250. doi: 10.1016/j.tins.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020;130 doi: 10.1002/lary.28692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lema Tomé CM, Tyson T, Rey NL. Inflammation and α-synuclein's prion-like behavior in Parkinson's disease—is there a link? Mol Neurobiol. 2013;47:561–574. doi: 10.1007/s12035-012-8267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boka G, Anglade P, Wallach D. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson's disease. Neurosci Lett. 1994;172:151–154. doi: 10.1016/0304-3940(94)90684-x. [DOI] [PubMed] [Google Scholar]
- 8.Blum-Degen D, Muller T, Kuhn W. Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer's and de novo Parkinson's disease patients. Neurosci Lett. 1995;202:17–20. doi: 10.1016/0304-3940(95)12192-7. [DOI] [PubMed] [Google Scholar]
- 9.Fazzini E, Fleming J, Fahn S. Cerebrospinal fluid antibodies to coronavirus in patients with Parkinson's disease. Mov Disord. 1992;7:153–158. doi: 10.1002/mds.870070210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inzelberg R, Hassin-Baer S, Jankovic J. Genetic movement disorders in patients of Jewish ancestry. JAMA Neurol. 2014;71:1567–1572. doi: 10.1001/jamaneurol.2014.1364. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


