Abstract
The coronavirus pandemic has changed the priorities of the whole medical society. During the clinical course of COVID-19, it has been observed that hepatic injury occurs in a significant proportion of patients, particularly in those with severe or critical illness. In this literature review, we summarize the most recent studies, which covered the pathophysiology of COVID-19 induced liver injury including; hepatic pathological findings, therapy related liver damage, and the effects of the viral infection on pre-existing liver diseasesin context of the most recent recommendations. Conclusions: This review sheds light on the impact of COVID-19 infection on the liver, as well as the prognostic effect of liver laboratory markers on disease outcome. Temporal variations in liver parameters during disease course as well as different patterns of derangement are depicted. More intensive surveillance and individualized therapeutic approaches should be tailored for immunocompromised patients with advanced liver disease, hepatocellular carcinoma, and liver transplant patients. Despite the limited studies on COVID-19 infected patients with preexisting liver disease, this comprehensive overview provides a perspective on the management of liver disease during COVID-19.
Keywords: Covid -19, Drug hepatotoxicity, Inflammatory response, Liver function, Liver pathology
In December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was initially identified in the city of Wuhan, China [1]. By the end of January 2020, the infection was confirmed by WHO as a Public Health Emergency of International Concern [2]. Coronavirus disease 2019 (COVID-19) has a wide range of clinical presentations, varying from asymptomatic carrier state to viral pneumonia in addition to various extra-pulmonary manifestations, including liver affection [3]. Many COVID-19 patients, especially those who have severe or critical disease experienced some form of liver injury [4]. COVID-19 associated liver injury usually occurs as a result of disease progression, or iatrogenic drug reactions from COVID-19 drug trials, regardless of the presence or absence of a pre-existing liver condition [5]. Till now, the etiological mechanisms for COVID-19 induced hepatic damage are unclear but may have significant consequences in the management and prognosis of the disease. Given the great burden of chronic liver disease globally [6], this pandemic can further disrupt the care of these patients as a result of the failure of the screening, and follow-up. Therefore, the interaction between pre-existing liver illness and COVID-19 requires additional studies. In this review we summarized the most recent studies, which have addressed the pathophysiology of COVID-19-induced liver injury including; the adverse reactions linked to the medications used in treatment, the hepatic pathological findings in COVID-19 patients, and the effect of this infection on the course of pre-existing liver diseases.
1. Pathophysiology of COVID-induced liver injury
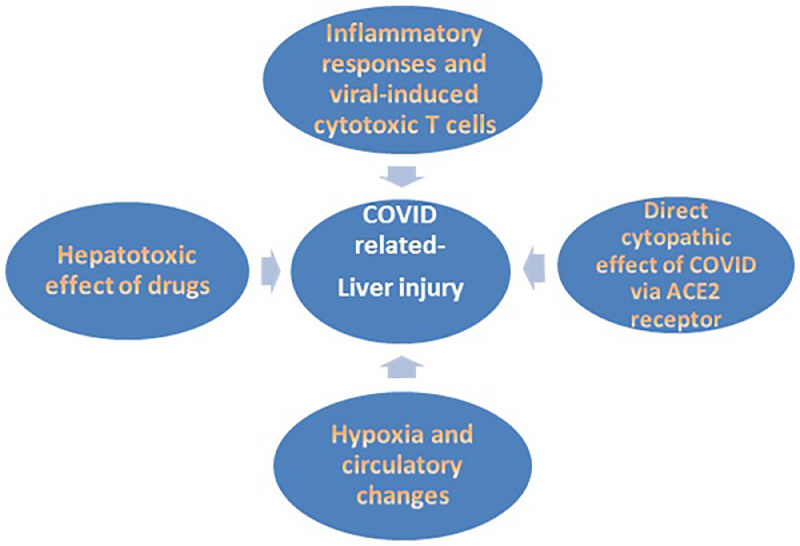
Several mechanisms are proposed to explain hepatic injury during COVID-19 infections (Fig. 1 ). Hypoxia and cardiac failure in critically affected COVID-19 cases can predispose to hypoxic hepatitis [4]. Likewise, the use of high levels of positive end-expiratory pressure (PEEP) may cause hepatic congestion by increasing right atrial pressure and hindering venous return. However, these mechanisms alone cannot explain liver affection in all patients, as liver laboratory test abnormalities are commonly encountered in stable patients who did not require mechanical ventilation. Furthermore, the pattern of aminotransferase rise in this set of patients is not consistent with that of hypoxic hepatitis [7].
Fig. 1.
Mechanisms of liver injury in COVID-19 infection.
ACE2 receptors, found on many cells including the lungs, heart, liver, kidney, and blood vessels, interact with SARS-CoV-2 by inducing direct cytopathic effects [8]. Recently, the single-cell RNA-seq data in healthy human hepatic samples suggest that ACE2 expression by cholangiocytes (59.7%) is considerably higher than its expression by hepatocytes(2.6%) [9]. Despite this major difference in ACE2 expression, a cholestatic pattern of injury is not a common feature of COVID-19 disease, also ALP and bilirubin were only elevated in a minority of cases [10,11]. Although some studies found GGT, a potential diagnostic marker for cholangiocyte injury, levels were increased by up to 72% in severely infected COVID-19 patients [12,13].
A recent study was conducted by Wang, et al. [14] using the ultrastructural examination of postpartum liver tissue biopsies of two deceased COVID-19 patients, detected typical coronavirus particles with their spikes in the cytoplasm of hepatocytes. Virus-related hepatocyte damage was recognized as mitochondrial swelling, endoplasmic reticulum dilatation, and cell membrane dysfunction. Moreover, this study had documented viral ability to replicate in hepatocytes. This is the first study to report SARS-CoV-2 cytopathic liver cell affection as a cause of liver function derangement.
Discordances of ACE2 level of expression in SARS-CoV affected organs are noted [15]. The affinity of SARS-CoV-2 to affect the liver cannot only be explained by the localization of ACE2 receptors. There is a likelihood that the expression levels of ACE2 in hepatocytes may be up-regulated upon viral entry. Another possibility is that extra- ACE2 receptors or co-receptors might exist [14]. In female tissue, ACE2 hepatic expression was markedly elevated, which may explain the better prognosis observed in COVID-19 infected females compared to males [16,17]. Also, TMPRSS2, a SARS-CoV2-interacting host receptor, which is expressed in cholangiocytes and hepatocytes, is essential for proteolytic activation and spread of virus particles [18]. Further studies are needed to explain why COVID-19-induced liver function abnormalities are mainly manifested as an elevation of serum aminotransferases, rather than increased alkaline phosphatase and bilirubin.
Moreover, a hyper-inflammatory reaction to COVID-19 may add to liver injury. Laboratory tests reveal an increase in plasma cytokines and other inflammatory reactants, such as IL-1, IL-6, and tumor necrosis factor leading to the development of cytokine storm in some patients. This results in hepatocellular immune-mediated damage due to viral-induced cytotoxic (CD8) T cells, and the induction of a dysregulated innate immune response [19]. Another proposed mechanism is attributed to the gut vascular barrier and microbiota alterations. Additional studies are needed to prove the existence of a causal role played by the liver in COVID-19 disease pathogenesis by affecting the release of cytokines and coagulation factors, given the prothrombotic state in severe COVID-19 infections [20].
2. Liver pathology and COVID-19
Morphological studies concerning the description and interpretation of liver parenchymal alterations associated with COVID-19 infection are completely lacking, and most are post-mortem autopsies. Xu et al. performed the first post-mortem liver autopsy specimen examinations, which showed a moderate degree of microvesicular steatosis and both mild lobular and portal activity. This liver injury could be caused by either SARS-CoV-2 infection or drug-related liver injury [20]. Similar results were shown by Liu et al. [21] Another study suggested collateral liver damage from viral-induced cytotoxic T-cells [22]. A preliminary study, which included forty-nine COVID-19 positive patients, revealed widespread vascular involvement of portal intrahepatic system in the form of acute (thrombosis and luminal ectasia) or chronic changes (fibrous thickening of the vascular wall), with an abnormal configuration of intrahepatic blood vessels. These findings suggest that coagulation dysfunction or endothelial damage could be the main triggering mechanism in the pathogenesis of COVID-19 liver-related damage [23]. Furthermore, no signs of bile duct damage or histological features of hepatic failure in liver autopsies of critical COVID-19 cases were detected [24]. Many studies failed to detect viral inclusion bodies in liver tissue [20,25].
3. Abnormal liver functions during COVID-19 infection
Abnormal liver blood tests have been found in almost one-half of patients, throughout the SARS-CoV-2 infection course. A large systematic review of 11 studies evaluating the liver laboratory parameters of 2541 patients infected with SARS CoV-2, showed the following results; elevated AST and/or ALT (25%), increased LDH (20%), elevated bilirubin (3%), and normal ALP in almost all cases [26] which may indicate limited direct virus-related liver injury due to the overexpression pattern of ACE2 on cholangiocytes. The largest published study to date, which included 5700 patients found that AST and ALT were both commonly elevated (58.4% and 39.0% of patients, respectively) [27], Cai Q et al. found that GGT was elevated more than 3 × ULN in 41% of patients [12], and in another study, GGT was elevated in severe cases, but that was not associated with increased ALP [28]. Furthermore, COVID-19 presentation was not consistently associated with important alterations of liver function tests [29]. Only rare cases of acute liver failure were reported in infected patients [30]. Other causes for transaminitis should be evaluated including myositis, ischemia, and cytokine release syndrome, as it is not always exclusively originating from the liver [25].
Another large preprint meta-analysis that covers 20 retrospective studies with 3428 COVID-19 infected patients revealed that higher levels of ALT, AST, and bilirubin were associated with a significant increase in the severity of COVID-19 infection [31]. A large study estimated that the occurrence of liver injury during COVID-19 infection is associated with a 9-fold greater risk of severe infection [12]. Similarly, liver function tests have been reported as an important risk factor for severe outcome and death in SARS and MERS [32,33]. Many recent studies reported elevated levels of serum ALT, AST, and GGT in severe patients than non-severe or mild patients [34,35]. A recent meta-analysis linked elevated admission levels of these markers to patient mortality [36]. Other studies link the increase in those parameters to worse pulmonary CT score [37], increase in patients requiring ICU care [38], and longer hospital stay [39]. In COVID-19 mortality cases, the incidence of elevated liver parameters ranged between 58.06% to 78% [40,41].
AST was associated with the highest mortality according to the study conducted by Lei et al. [36], and it was the first marker found to be elevated upon hospital admission. A recent study by Guan et al., which enrolled nearly 1100 Chinese patients, demonstrated that elevated serum AST and ALT levels were observed in nearly 18% and 29% of patients with non-severe COVID-19 infection, compared to 56% and 28% of patients with severe COVID-19 disease respectively [35]. The previous findings suggest the critical role of immune-mediated systemic inflammation in liver impairment associated with severe cases of COVID-19 infection [36]. Recently Gordon et al. suggested the possibility of direct interaction of mitochondrial proteins with the virus, providing a probable mechanistic reason for the elevated AST-dominant liver profile [42]. A recent study by Wang et al. reported that liver enzyme abnormalities were associated with; disease severity, higher radiology scores as well as higher alveolar-arterial oxygen partial pressure difference, higher GGT, higher ferritin, lower albumin and decreased CD4+ T cells and B lymphocytes [14]. Cai et al. reported that in patients with abnormal liver markers of hepatocellular type or mixed, upon admission had greater odds of progressing to severe disease [12]. A recent study found that the AST/ALT ratio, total bilirubin, and ALT/ALP ratio helped predict survival in cirrhotic COVID-19 infected patients [43].
A recent meta-analysis revealed that, among 15,407 COVID-19 patients, the pooled incidence of elevated liver enzymes in COVID-19 was 23.1% at early presentation and 24.4%throughout the course of illness [44]. Another new study by Q Wang which described temporal variations during COVID-19 disease course showed that, the percentage of the patients with both elevated ALT and AST was 12.6% in mild cases vs. 46.2% in severe cases. The majority of the patients experienced ALT elevations between days 4 and 17 of their hospitalization, with a mean of (7.3 and 10.7 days) in severe and mild cases respectively. During treatment, 19% of patients had elevated liver function parameters but most patients had only mild and isolated elevations in ALT and AST. Most of the patients were discharged with normal liver markers [45]. Recent data from other studies suggest that liver enzymes become more frequently, and more severely deranged during the course of hospitalization [12,34].
Serum albumin levels were also significantly lower in patients who died due to the infection [40]. Elevated serum levels of ferritin, IL-6, CRP, and procalcitonin have been described with a non-favorable course of liver injury in COVID-19 positive patients. The simultaneous increase in ferritin, IL-6, and ALT levels in addition to decreased albumin concentration and platelet count suggests more significant liver involvement in the course of COVID-19 [39,40]. Common factors associated with elevated indicators of liver affection include; declined lymphocyte count, neutrophil count increase, and male gender [36]. The degree of pulmonary lesions on CT imaging may be a predictor of liver damage. Thus, patients with aggressive pulmonary lesions need to undergo close monitoring of liver function to allow early identification of any liver insult [37]. Liver involvement in mild COVID-19 cases is usually transient and does not require specific treatment [13]. AASLD recommends that all COVID-19 patients with abnormal liver functions should be screened for HCV and HBV, and unnecessary imaging should be avoided [46].
4. Drug-induced liver injury
It was postulated that liver impairment in COVID-19 patients could also be drug-related, and this theory was supported by the detection of moderate microvesicular steatosis with mild hepatic inflammation in those patients [20]. Cai et al. showed that more than 10% of those patients experienced increased levels of liver enzymes during hospitalization, which could be attributed to the used medications [12]. In a meta-analysis done by Kulkarni et al. which involved 20 874 COVID-19 patients, the pooled incidence of drug-induced liver injury was 25.4% [44]. Currently prescribed medications for COVID-19 (e.g. oseltamivir, lopinavir/ritonavir, and chloroquines) are all metabolized in the liver [47]. For this reason, whenever any liver test abnormality occurs in COVID-19 patients, the drug-induced liver injury should first be confirmed or ruled out [48]. It was noticed that many patients suffering from COVID19 had a history of antipyretic use, commonly paracetamol, overdose of which is a well-established etiology for liver injury. Hydroxychloroquine (an anti-malarial drug) is one of the medications proposed for COVID-19 treatment regimens, relying on sparse evidence in small clinical settings [49]. Only two cases of acute liver failure attributed to hydroxychloroquine were reported [50]. Acute elevation of aminotransferases due to hydroxychloroquine is also rare, and only four cases were reported [51]. Such reactions may be attributed to hypersensitivity. However, there is no cross-reactivity between hydroxychloroquine and chloroquine, and it is reasonable to switch between them in case of hypersensitivity. Hydroxychloroquine should be used cautiously in patients with pre-existing liver diseases as it can concentrate in the liver [52].
Azithromycin (a macrolide antibiotic) is used in combined therapy with hydroxychloroquine, with little evidence to support its use. The azithromycin-induced liver injury occurs in rare cases within 1 to 3 weeks after azithromycin initiation. It is predominantly of a hepatocellular pattern, and most patients recover completely [53]. There is limited data available on the use of remdesivir (anti-Ebola drug), as phase III trials are still underway. In a paper describing the first 12 patients with COVID-19 in the United States, the three hospitalized patients, who received remdesivir at the time of clinical worsening, reported elevated liver enzymes [54]. In a case series (n = 53) which studied the use of remdesivir in COVID-19 treatment, 23% of patients had developed elevations in liver enzymes which resulted in premature discontinuation of treatment [55]. Another randomized, double-blind, placebo-controlled, multicenter study showed elevated serum bilirubin in 10% and elevated aminotransferases in 5% of the remdesivir group [56].
In the study conducted by Cao B and colleagues, lopinavir/ritonavir (anti-HIV medications) used for COVID-19 treatment, showed no significant increase in hepatotoxicity compared to the control group [57], while in the ELACOI trial, 4.8% showed a 2.5-fold elevation in liver enzymes [58]. Recovery from this drug reaction takes 1–2 months and re-challenging the drug is not recommended [52]. The two controlled studies published by Chen et al. [59] and Cai et al. [60]on favipiravir usage for COVID-19 patients showed elevated liver enzymes in 7.6% and 2.6% respectively. Certain drugs are used to control cytokine immune storm like methylprednisolone, however, it increases the risk of spontaneous bacterial peritonitis in patients with decompensated liver cirrhosis, also the risk of HBV reactivation should be taken into consideration. Tocilizumab (Humanized mAb targeting interleukin-6 receptor), showed frequent ALT elevation, but jaundice seems to be rare, and therefore, it should not be used in decompensated cirrhosis [19]. Liver functions should be monitored especially for those receiving lopinavir/ritonavir, remdesivir, or tocilizumab. The presence of abnormal liver biochemistries should not be a contraindication to using these drugs, although AST or ALT levels >5x ULN may exclude patients from investigational drugs [46].
5. NASH and COVID-19
Several studies have reported obesity as a significant predicting factor for mortality in COVID-19 patients. BMI was significantly higher in patients with severe COVID-19 infection. Moreover, obesity was correlated with the need for mechanical ventilation and overall survival [61,62]. The level of ACE2 expression in adipose tissue is higher than that of lung tissue. This finding may justify the vulnerability of adipose tissue to the COVID-19 invasion [63]. COVID-19 might infect adipose tissue then spread to other organs [64]. Obese patients are at high risk for NAFLD, which in turn have a higher risk of developing severe COVID-19, a higher likelihood of abnormal liver function from admission to discharge, and a longer viral shedding time. NAFLD was found to be associated with COVID-19 progression (defined by worsening respiratory distress or lung CT findings during hospitalization) [22].
NAFLD patients may suffer from comorbidities such as diabetes and hypertension, which increases the risk of severe COVID-19 infections. On the other hand, the synergistic effect of NAFLD on COVID-19 severity in young patients with no other co-morbidities was established by a multi-center study [65]. In NAFLD patients, the polarization states of macrophage might be skewed, thus affecting host inflammatory or tolerance response to SARS-CoV-2 signals generated from the gut-liver axis. Also, an imbalance between inflammation-promoting M1 macrophages and inflammation-suppressing M2 macrophages in NAFLD, will lead to the progression of COVID-19 [66].
NAFLD patients often present with elevated cytokine levels, making them more vulnerable to exaggerated cytokine production associated with COVID-19. Moreover, ACE2 expression was shown to increase in chronic liver damage and experimental diet-induced NAFLD [67]. It has also been demonstrated that patients suffering from COVID-19 exhibited increased serum levels of monocyte chemoattractant protein-1, which is a chemokine known to exacerbate steatohepatitis. Thus the virus may increase NAFLD progression to NASH in the long-term [49]. In short, identification and monitoring of patients with NAFLD who are infected with COVID-19 are advisable.
6. COVID-19 in patients with preexisting chronic hepatitis or cirrhosis
Chronic liver disease represents a major disease burden globally. Generally, patients with pre-existing chronic liver disease may be more susceptible to liver damage from SARS-CoV-2 [29]. A meta-analysis of 11 observational studies enrolling 2043 COVID-19 positive patients, showed that the prevalence of the chronic liver disease among them ranged between 3% and 11% [29,39]. Oyelade et al. conducted a meta-analysis revealing that patients with a pre-existing hepatic disease have increased risk for severe COVID-19 infection (57.33%), and higher mortality (17.65%) [68]. This could be correlated with low platelets and lymphocytes in those patients [69]. This may be due to cirrhosis-associated immune dysfunction; so strict precautions against SARS-CoV-2 infection should be exerted in that set of patients [70]. On the contrary, pooled analysis by Lippi et al., found that chronic liver disease may not be associated with severity or mortality [71].
Chen et al. found that patients with chronic hepatitis B are more vulnerable to COVID-19 [72], but other studies revealed that chronic viral hepatitis does not appear to be proportional to the severity of COVID-19 [13,35]. Regarding viral hepatitis treatment in patients co-infected with COVID-19, AASLD recommends continuing treatment for hepatitis B and hepatitis C if already initiated before acquiring COVID-19, and to consider initiating hepatitis B treatment in case of suspicion of hepatitis B flare; however, not enough studies are available. Initiating hepatitis C treatment in a patient with COVID-19 is not routinely warranted. Regarding patients with autoimmune liver disease, immunosuppressive therapy makes those patients at greater risk for severe infection and should be prioritized for early testing. If AIH patients infected with COVID-19 developed elevated liver enzymes, disease flare should not be presumed without confirmation by biopsy [46]. European Association for the Study of the Liver (EASL) currently advises against reducing immunosuppressive therapy in those patients. Reductions, particularly for antimetabolites, should only be considered after consultation of an expert and under special circumstances (e.g. in case of severe COVID-19 with medication-induced lymphopenia, or bacterial/fungal superinfection) [25].
Cirrhotic patients are at potentially increased risk for SARS-CoV-2 infection, higher risk for severe disease, and increased risk for hepatic decompensation [73]. Stress and sepsis are particularly problematic in patients with decompensated liver cirrhosis, as either can trigger acute-on-chronic liver failure [74]. A large multicenter cohort showed that hepatic decompensation was strongly associated with COVID-19 infection, increasing the risk of death from 26.2% to 63.2%. Remarkably, 24.3% of patients with new hepatic decompensation had no respiratory symptoms of COVID-19 at the time of diagnosis [75]. COVID-19 is characterized by significant cytokine activation, which induces hepatocyte apoptosis and necrosis, which in the setting of the diminished liver reserve, may lead to hepatic decompensation [76]. It appears that the cause of death in most of these patients is not due to progressive liver disease, but rather a pulmonary disease [69]. A recent multi-center study by Jagjag et al. [77], found that patients with cirrhosis plus COVID-19 had similar mortality compared to those suffering from cirrhosis alone, but higher than patients with COVID-19 alone. Moreover, a recent work by Hashemi et al. proposed the presence of cirrhosis as an independent predictor of mortality [78]. Similarly, 30-days mortality rate was higher in cirrhotics with COVID-19 [79].
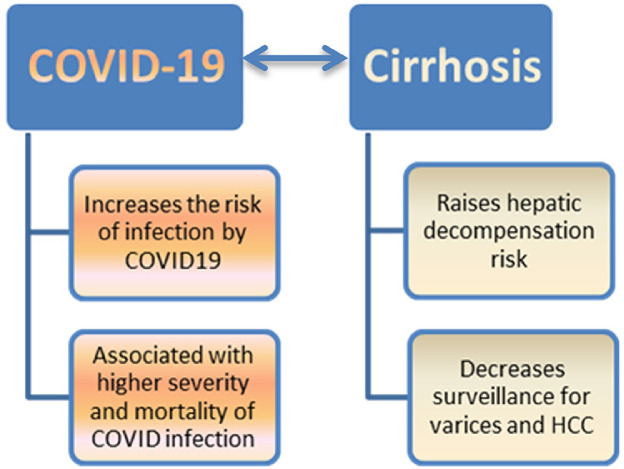
EASL recommends that care should be maintained according to guidelines with minimal exposure to medical staff; telemedicine is the preferred method. Listing for liver transplantation should be restricted only to patients with poor short-term prognosis. Guidelines on prophylaxis of spontaneous bacterial peritonitis and hepatic encephalopathy should be followed closely to prevent decompensation and the need for the hospital admission. Testing for SARS-CoV-2 is advisable in patients with acute decompensation or ACLF [25]. Also, a study by Iavarone et al., found no major adverse events related to thromboprophylaxis with heparin given to 80% of cirrhotic patients [79]. Urgent procedures (i.e. paracentesis) should be organized using a COVID-19-free path in the hospital or home care [80]. American Association for the Study of Liver Diseases (AASLD) recommends in-person new patient visits only for those with significant liver diseases, such as jaundice, elevated transaminases of >500 U/L, or recent decompensation [81]. COVID-19 infection increases the risk of hepatic decompensation, and it reschedules screening for both varices and HCC in cirrhotic patients. On the other hand, pre-existing cirrhosis increases the risk of COVID-19 infection and is associated with higher severity and mortality. The impact of COVID-19 infection on cirrhosis and vice versa is shown in (Fig. 2 ).
Fig. 2.
The impact of COVID-19 infection on cirrhosis and vice versa (COVID −19 – cirrhosis interrelationship).
7. COVID-19 and HCC
During the pandemic of COVID-19, patients suffering from any malignancy are at an increased risk of infection and more prone to developing more severe clinical outcomes [82]. In a retrospective study by Zhang et al. [83] on 28 cancer patients, two of them (7%) with HCC, infected with COVID-19, reported that patients suffering from different malignancies had poorer outcomes when compared to the general population. This was attributed to the age of these patients in addition to the associated comorbidities and underlying cirrhosis. Furthermore, these patients have anemia and hypoproteinemia associated with poor nutritional status, which compromises their immunity, making them more vulnerable to severe infection. According to a prospective nationwide cohort study in China, researchers identified that 1% of patients had both, COVID-19 and a history of cancer. These patients were more vulnerable to severe disease and had an increased chance of mortality, as well as ICU admission. Recent chemotherapy within 1 month also increased the risk of severe disease [84].
Iavarone et al. [85], based on EASL guidelines, recommended the use of telemedicine to replace clinic visits, as well as to replace multidisciplinary team meetings to decrease the risk of spread of COVID-19. The indications of liver transplantation (LT) and locoregional therapy (LRT) in HCC patients have not changed according to these recommendations, but they reserved LT for highly progressive cases because of the shortage of ICU beds and decline in the number of donors. They used LRT as a salvage procedure to decrease the risk of HCC progression during the waiting period. Boettler et al. [25] stated that stereotactic body radiotherapy (SBRT) is another alternative ablative option that can be offered to patients eligible for TACE, especially those with lesions near vascular structures, whom their procedures have been canceled because of COVID-19 [86].
AASLD [46] also recommends restricting healthcare facility visits but at the same time stressed the importance of continuing surveillance imaging of HCC patients with an acceptable delay of a maximum of 2 months. All local ablative therapies are advised to be done without any delay for eligible patients. Regarding TACE, short term use of corticosteroids is recommended to manage post embolization syndrome to minimize hospital stay, even in patients proven to have COVID-19 except if there is any other contraindication. TACE was preferred over SBRT as the latter requires separate workup and treatment visits while TACE sessions can be separated by intervals ranging from 4 to 12 weeks, according to the response and characteristics of the lesion. For patients with resectable BCLC stage HCC whose surgical procedures have been canceled, transarterial therapies as a bridge to definitive treatment are recommended [87]. Finally, sorafenib is recommended to be continued without any change in its dose [88]. EASL recommendations suggest that immunotherapy with nivolumab might have to be temporarily suspended to avoid exposure to COVID-19 at the infusion center [81].
8. Liver transplantation and COVID-19
There is a need for an international consensus on liver transplantation protocols during pandemics, due to the shortage of resources and the increased need for liver transplantation. Little is known on the transmission of the virus from donor to recipient but if this happens, it depends on the level and duration of donor viremia, as well as the incubation period and viability of the virus in different organs [89]. Liver transplant recipients are in an immune-compromised state, which makes them at higher risk of contracting COVID-19 infection. This makes them a source of dissemination of infection to others (super spreaders), especially healthcare workers [90]. Contrarily, immunosuppression was postulated to be protective against cytokine storm, which is responsible for severe COVID-19 illness [91].
There were some reported cases of liver transplant recipients confirmed to have COVID-19 pneumonia and required hospital admission. All of the cases showed improvement by symptomatic treatment, together with the reduction or withdrawal of their immune-suppression therapy, except for one case who received a single dose of tocilizumab [92], [93], [94]. A Swiss Transplant Cohort prospective observational multicenter Study (STCS), which included 21 solid organ transplant patients, 5 of which had LT, concluded that the only treatment intervention was temporary discontinuation or reduction of anti-metabolite drugs, and maintaining the same doses of steroids and calcineurin inhibitors. They concluded that the clinical course and degree of severity of COVID-19 among organ transplant recipients do not differ from the general population [95].
Seventeen liver transplant centers from different countries participated in an international multi-center open survey, and [96] concluded that the majority of the centers adopted a “sickest first” strategy, otherwise a case by case is discussed. Almost all centers advised for COVID-19 screening for donor and recipient. Donors with positive contact history, or compromising respiratory tract disease, were excluded by most centers. The survey revealed that transplantation protocols did not change at the beginning of the pandemic except in Singapore. Due to their experience with SARS-CoV in 2003, Singapore restricted liver transplantation to medically urgent living donor liver transplant (LDLT), and deceased donor liver transplant (DDLT). After the WHO declaration of the viral pandemic, 10 out of the 17 centers limited their transplantation activity.
AASLD recommends against delaying LT wherever possible depending on local availability of resources such as ICU beds, ventilators, and blood donations [46]. It also recommends COVID-19 screening for both donors and recipients listed for liver transplantation, emphasizing that negative results do not exclude infection. All consents for the procedure should include the possible risk of nosocomial COVID-19. After transplantation, they discourage the reduction of immunosuppressive therapy, except in severe cases of bacterial or fungal infections superadded to COVID-19, or associated lymphopenia [25]. If a patient tests positive for COVID-19, the dose of steroids should be decreased to a minimum to avoid adrenal insufficiency [25,46]. Drug levels of CNI and mammalian Target Of Rapamycin (mTOR) inhibitors, should be closely monitored when they are administered together with other drugs, such as hydroxychloroquine or azithromycin [81]. All stable patients are advised to receive influenza and streptococcal pneumonia vaccine [25].
9. Interventional procedures
Upper endoscopy is an aerosol-generating procedure that carries a high risk of transmission of infection to medical personnel. In the first case of COVID-19 in the USA, the virus RNA was detected in the patient's stool on the 7th day of illness suggesting a possible feco-oral transmission making the upper endoscopy or colonoscopy a high-risk procedure [97,98]. An Italian multi-center survey included 41 endoscopy units aimed to investigate the burden of COVID-19 on endoscopy units, and to assess the possibility of viral transmission from these units. Most of the centers limited their activity to urgent and high-risk patients, such as gastrointestinal bleeding and bacterial cholangitis. In their survey, there were no reported cases of healthcare staff infection associated with endoscopic procedures performed to COVID-19 positive patients. Most of the units provided protective personal equipment (PPE) for both endoscopy unit personnel and patients [99].
For variceal screening, EASL recommends risk assessments, such as applying the Baveno VI criteria, whereas AASLD even suggested primary prophylaxis with beta-blockers as an alternative for screening endoscopy, in those with clinically significant portal hypertension or those at risk of decompensation [81]. The Asian Pacific Society for Digestive Endoscopy (APSDE) advised performing upper endoscopy in negative pressure rooms if the patient is suspected to be COVID-19 positive [100,101]. American Gastroenterology Association (AGA) recommends N95 masks over surgical masks, and the use of double gloves as part of appropriate PPE [102]. Pre-screening of patients before the procedure is recommended by the American Society of Gastrointestinal Endoscopy [101]. As regards to liver biopsy, the EASL recommends deferring it for NAFLD, chronic viral hepatitis, and only to perform it for strongly elevated transaminases of unknown etiology, and liver masses suspicious of malignancy [25].
10. Conclusions
This review sheds light on the impact of COVID-19 infection on the liver, as well as the prognostic effect of liver laboratory markers on disease outcome. Further studies are needed to elucidate the mechanisms of COVID-19 infection and drug-induced hepatic pathologies. More intensive surveillance and individualized therapeutic approaches should be tailored for those immunocompromised patients with advanced liver disease, HCC, and liver transplant patients. Despite the current lack of knowledge regarding the effect of COVID-19 in patients with preexisting liver disease, this review introduces a comprehensive overview, providing a perspective on the management of liver disease during COVID-19. Improving care pathways for chronic liver disease patients infected with SARS-CoV-2, will be of great importance to improve disease outcomes of both liver disease and COVID-19.
Author's contribution
Marwa Ibrahim Metawea: Conception and design, drafting of the article.
Walid Ibrahim Yousif: Collection of data, analysis, interpretation of data and drafting of the article.
Islam Moheb: Editing and reviewing of the article.
Conflict of Interest
None declared.
Footnotes
Postal address: Internal medicine department, Faculty of Medicine, Champo`llion Street, El-Khartoum Square, El Azareeta Medical Campus,
References
- 1.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haimovich A, Warner F, Young H.P, et al. Patient factors associated with SARS-CoV-2 in an admitted emergency department population. J Am Coll Emerg Physicians Open202;1–9. [DOI] [PMC free article] [PubMed]
- 3.Cascella M., Rajnik M., Cuomo A., et al. StatPearls; 2020. Features, evaluation and treatment Coronavirus (COVID-19) [PubMed] [Google Scholar]
- 4.Li Y., Xiao S. Hepatic involvement in COVID-19 patients: pathology, pathogenesis, and clinical implications [Review] J Med Virol. 2020:1–4. doi: 10.1002/jmv.25973. [DOI] [PubMed] [Google Scholar]
- 5.Sun J., Aghemo A., Forner A., Valenti L. COVID-19, and liver disease. Liver Int. 2020;40:1278–1281. doi: 10.1111/liv.14470. [DOI] [PubMed] [Google Scholar]
- 6.Musa S. Hepatic and gastrointestinal involvement in coronavirus disease 2019 (COVID-19): what do we know till now? Arab J Gastroenterol. 2020;21:3–8. doi: 10.1016/j.ajg.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bangash M.N., Patel J., Parekh D. COVID-19, and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529–530. doi: 10.1016/S2468-1253(20)30084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kai H., Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors—Lessons from available evidence and insights into COVID-19. Hypertens Res. 2020;43:648–654. doi: 10.1038/s41440-020-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chai X., Hu L., Zhang Y., et al. bioRxiv; 2020. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. [Google Scholar]
- 10.Ong J., Young B.E., Ong S. COVID-19 in gastroenterology: a clinical perspective. Gut. 2020;69:1144–1145. doi: 10.1136/gutjnl-2020-321051. [DOI] [PubMed] [Google Scholar]
- 11.Yao N., Wang S.N., Lian J.Q., et al. Clinical characteristics and influencing factors of patients with novel coronavirus pneumonia combined with liver injury in Shaanxi region. Chin J Hepatol. 2020;28:234–239. doi: 10.3760/cma.j.cn501113-20200226-00070. [DOI] [PubMed] [Google Scholar]
- 12.Cai Q., Huang D., Yu H., et al. COVID-19: abnormal liver function tests. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y., Liu S., Liu H., et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamming I., Timens W., Bulthuis M.L.C., et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tukiainen T., Villani A.C., Yen A., et al. Landscape of X chromosome inactivation across human tissues. Nature. 2017;550:244–248. doi: 10.1038/nature24265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin J.-.M., Bai P., He W., et al. Gender Differences in Patients With COVID-19: focus on Severity and Mortality. Front Public Heal. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bestle D., Heindl M.R., Limburg H., et al. bioRxiv; 2020. TMPRSS2 and furin are both essential for proteolytic activation and spread of SARS-CoV-2 in human airway epithelial cells and provide promising drug targets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta P., McAuley D.F., Brown M., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet North Am Ed. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Q., Wang R.S., Qu G.Q., et al. Gross examination report of a COVID -19 death autopsy. Fa Yi Xue Za Zhi. 2020;36:21–23. doi: 10.12116/j.issn.1004-5619.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Ji D., Qin E., Xu J., Zhang D., Cheng G., Wang Y., et al. Non-alcoholic fatty liver diseases in patients with COVID-19: a retrospective study. J Hepatol. 2020;73:451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sonzogni A, Previtali G, Seghezzi M, et al. Liver and COVID 19 infection: a very preliminary lesson learnt from histological post-mortem findings in 48 patients. Preprints.org2020.
- 24.Liu Q., Wang R., Qu G., et al. General anatomy report of novel coronavirus pneumonia death corpse. J Forensic Med. 2020;36:19–21. [Google Scholar]
- 25.Boettler T., Newsome P.N., Mondelli M.U., et al. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID Position Paper. JHEP Rep. 2020;2 doi: 10.1016/j.jhepr.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kukla M., Skonieczna-Żydecka K., Kotfis K., et al. COVID-19, MERS and SARS with concomitant liver injury—systematic review of the existing literature. J Clin Med. 2020;9:1420. doi: 10.3390/jcm9051420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA - J Am Med Assoc. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu L., Liu J., Lu M., Yang D., Zheng X. Liver injury during highly pathogenic human coronavirus infections. Vol. 40. Liver Int. 2020;40:998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mantovani A., Beatrice G., Dalbeni A. Coronavirus disease 2019 and prevalence of chronic liver disease: a meta-analysis. Liver Int. 2020;40:1316–1320. doi: 10.1111/liv.14465. [DOI] [PubMed] [Google Scholar]
- 30.Weber S., Mayerle J., Irlbeck M., Gerbes A.L. Severe liver failure during SARS-CoV-2 infection. Gut. 2020;69:1365–1367. doi: 10.1136/gutjnl-2020-321350. [DOI] [PubMed] [Google Scholar]
- 31.Parohan M., Yaghoubi S., Seraj A. Liver injury is associated with severe Coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of retrospective studies. Hepatol Res. 2020;10 doi: 10.1111/hepr.13510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang H.L., Chen K.T., Lai S.K., et al. Hematological and biochemical factors predicting SARS fatality in Taiwan. J Formos Med Assoc. 2006;105:439–450. doi: 10.1016/S0929-6646(09)60183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bloom P.P., Meyerowitz E.A., Reinus Z., et al. Liver Biochemistries in Hospitalized Patients With COVID-19. Hepatology. 2020 doi: 10.1002/hep.31326. [DOI] [PubMed] [Google Scholar]
- 35.Guan W., Ni Z., Hu Y., et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lei F., Liu Y.-.M., Zhou F., et al. Longitudinal association between markers of liver injury and mortality in COVID-19 in China. Hepatology. 2020 doi: 10.1002/hep.31301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie H., Zhao J., Lian N., et al. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: a retrospective study. Liver Int. 2020;40:321–326. doi: 10.1111/liv.14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang D., Hu B., Hu C., et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA - J Am Med Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan Z., Chen L., Li J., et al. Clinical features of COVID-19-related liver damage. Clin Gastroenterol Hepatol. 2020;18 doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang B., Zhou X., Qiu Y., et al. medRxiv; 2020. Clinical characteristics of 82 death cases with COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gordon D.E., Jang G.M., Bouhaddou M., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarin S.K., Choudhury A., Lau G.K., et al. Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection,The APCOLIS Study. Hepatol Int. 2020 doi: 10.1007/s12072-020-10072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kulkarni A.V., Kumar P., Tevethia H.V., et al. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52:584–599. doi: 10.1111/apt.15916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Q., Wang Q., Zhao H., et al. Pattern of liver injury in adult patients with COVID-19: a retrospective analysis of 105 patients. Mil Med Res. 2020;7:28. doi: 10.1186/s40779-020-00256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fix O.K., Hameed B., Fontana R.J., et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID-19 pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020;72:287–304. doi: 10.1002/hep.31281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rismanbaf A., Zarei S. Liver and kidney injuries in COVID-19 and their effects on drug therapy, a letter to editor. Arch Acad Emerg Med. 2020;8:e17. [PMC free article] [PubMed] [Google Scholar]
- 48.Li J., Fan J.-.G. Characteristics and Mechanism of Liver Injury in 2019 Coronavirus Disease. J Clin Transl Hepatol. 2020;8:1–5. doi: 10.14218/JCTH.2020.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boeckmans J., Rodrigues R.M., Demuyser T., et al. COVID-19 and drug-induced liver injury: a problem of plenty or a petty point? Arch Toxicol. 2020;94:1367–1369. doi: 10.1007/s00204-020-02734-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Makin A.J., Wendon J., Fitt S., et al. Fulminant hepatic failure secondary to hydroxychloroquine. Gut. 1994;35:569–570. doi: 10.1136/gut.35.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheema B., Triplett D., Krishnamurthy P. Hydroxychloroquine-induced acute liver injury. Am J Gastroenterol. 2019;114:S1286. [Google Scholar]
- 52.Sultan S., Altayar O., Siddique S.M., et al. AGA institute rapid review of the gi and liver manifestations of COVID-19, meta-analysis of international data, and recommendations for the consultative management of patients with COVID-19. Gastroenterology. 2020;159:320–334. doi: 10.1053/j.gastro.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martinez M.A., Vuppalanchi R., Fontana R.J., et al. Clinical and histologic features of azithromycin-induced liver injury. Clin Gastroenterol Hepatol. 2015;13:369–376. doi: 10.1016/j.cgh.2014.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kujawski S.A., Wong K.K., Collins J.P., et al. First 12 patients with coronavirus disease 2019 (COVID-19) in the United States. 2020 doi: 10.1038/s41591-020-0877-5. [DOI] [PubMed] [Google Scholar]
- 55.Grein J., Ohmagari N., Shin D., et al. Compassionate use of Remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y., Zhang D., Du G., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cao B., Wang Y., Wen D., et al. A trial of Lopinavir–Ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Y., Xie Z., Lin W., et al. medRxiv; 2020. An exploratory randomized, controlled study on the efficacy and safety of lopinavir/ritonavir or arbidol treating adult patients hospitalized with mild/moderate COVID-19 (ELACOI) [Google Scholar]
- 59.Chen X., Zheng F., Qing Y., et al. medRxiv; 2020. Epidemiological and clinical features of 291 cases with coronavirus disease 2019 in areas adjacent to Hubei, China: a double-center observational study. [Google Scholar]
- 60.Cai Q., Yang M., Liu D., et al. Experimental treatment with Favipiravir for COVID-19: an open-label control study. Engineering. 2020 doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kassir R. Risk of COVID-19 for patients with obesity. Obes Rev. 2020;21 doi: 10.1111/obr.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Caussy C., Wallet F., Laville M., Disse E. Obesity is associated with severe forms of COVID-19. Obesity. 2020;28:1175. doi: 10.1002/oby.22842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jia X., Yin C., Lu S., Chen Y., Liu Q., Bai J., et al. Two things about COVID-19 might need attention. Preprints. 2020 [Google Scholar]
- 64.Bourgeois C., Gorwood J., Barrail-Tran A., et al. Specific biological features of adipose tissue, and their impact on HIV persistence. Front Microbiol. 2019;10:2837. doi: 10.3389/fmicb.2019.02837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou Y., Zheng K.I., Wang X., et al. Younger patients with MAFLD are at increased risk of severe COVID-19 illness: a multicenter preliminary analysis. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lefere S., Tacke F. Macrophages in obesity and non-alcoholic fatty liver disease: crosstalk with metabolism. JHEP Rep. 2019;1:30–43. doi: 10.1016/j.jhepr.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paizis G., Tikellis C., Cooper M.E., et al. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790–1796. doi: 10.1136/gut.2004.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oyelade T., Alqahtani J., Canciani G. Prognosis of COVID-19 in patients with liver and kidney diseases: an early systematic review and meta-analysis. Trop Med Infect Dis. 2020;5:80. doi: 10.3390/tropicalmed5020080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qi X., Liu Y., Wang J., et al. Clinical course and risk factors for mortality of COVID-19 patients with pre-existing cirrhosis: a multicentre cohort study. Gut. 2020 doi: 10.1136/gutjnl-2020-321666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Albillos A., Lario M., Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385–1396. doi: 10.1016/j.jhep.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 71.Lippi G., de Oliveira M.H.S., Henry B.M. Chronic liver disease is not associated with severity or mortality in Coronavirus disease 2019 (COVID-19): a pooled analysis. Eur J Gastroenterol Hepatol. 2020 doi: 10.1097/MEG.0000000000001742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen X., Jiang Q., Ma Z., et al. medRxiv; 2020. Clinical Characteristics Hospitalized Patients with SARS-Cov-2 and HBV Co-infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kushner T., Cafardi J. Chronic liver disease and COVID-19: alcohol Use disorder/alcohol-associated liver disease, nonalcoholic fatty liver disease/nonalcoholic steatohepatitis, autoimmune liver disease, and compensated cirrhosis. Clin Liver Dis. 2020;15:195–199. doi: 10.1002/cld.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Strnad P., Tacke F., Koch A., Trautwein C. Liver-guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol. 2017;14:55–66. doi: 10.1038/nrgastro.2016.168. [DOI] [PubMed] [Google Scholar]
- 75.Moon A.M., Webb G.J., Aloman C., et al. High mortality Rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: preliminary results from an international registry. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bonnel A.R., Bunchorntavakul C., Reddy K.R. Immune Dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:727–738. doi: 10.1016/j.cgh.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 77.Bajaj J.S., Garcia-Tsao G., Biggins S., et al. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut. 2020 doi: 10.1136/gutjnl-2020-322118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hashemi N., Viveiros K., Redd W.D., et al. Impact of chronic liver disease on outcomes of hospitalized patients with COVID-19: a multicentre United States experience. Liver Int. 2020;00:1–7. doi: 10.1111/liv.14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iavarone M., D'Ambrosio R., Soria A., et al. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lleo A., Invernizzi P., Lohse A.W., Aghemo A., Carbone M. Highlights for management of patients with Autoimmune Liver Disease during COVID-19 pandemic. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bollipo S., Kapuria D., Rabiee A., et al. One world, one pandemic, many guidelines: management of liver diseases during COVID-19. Gut. 2020 doi: 10.1136/gutjnl-2020-321553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsamakis K., Gavriatopoulou M., Schizas D., et al. Oncology during the COVID-19 pandemic: challenges, dilemmas and the psychosocial impact on cancer patients (Review) Oncol Lett. 2020;20:441–447. doi: 10.3892/ol.2020.11599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang L., Zhu F., Xie L., et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31:894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liang W., Guan W., Chen R., Wang W., Li J., Xu K., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;17:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iavarone M., Sangiovanni A., Carrafiello G., Rossi G., Lampertico P. Annals of Oncology. Elsevier Ltd; 2020. Management of hepatocellular carcinoma in the time of COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tchelebi L.T., Haustermans K., Scorsetti M., et al. Recommendations for the use of radiation therapy in managing patients with gastrointestinal malignancies in the era of COVID-19. Radiother Oncol. 2020;148:194–200. doi: 10.1016/j.radonc.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kudo M., Kurosaki M., Ikeda M., et al. Treatment of hepatocellular carcinoma during the COVID-19 outbreak: the Working Group report of JAMTT-HCC. Hepatol Res. 2020 doi: 10.1111/hepr.13541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Denys A., Guiu B., Chevallier P., et al. Interventional oncology at the time of COVID-19 pandemic: problems and solutions. Diagn Interv Imaging. 2020 doi: 10.1016/j.diii.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Michaels M.G., La Hoz R.M., Danziger-Isakov L., et al. Coronavirus disease 2019: implications of emerging infections for transplantation. Am J Transpl. 2020;20:1768–1772. doi: 10.1111/ajt.15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saigal S., Gupta S., Sudhindran S., et al. Liver transplantation and COVID-19 (Coronavirus) infection: guidelines of the liver transplant Society of India (LTSI) Hepatol Int. 2020;14:429–431. doi: 10.1007/s12072-020-10041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.El Kassas M., Alboraie M., Al Balakosy A., et al. Liver transplantation in the era of COVID-19. Arab Journal of Gastroenterol. 2020;21:69–75. doi: 10.1016/j.ajg.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morand A., Roquelaure B., Colson P., et al. Child with liver transplant recovers from COVID-19 infection. A case report. Arch Pediatr. 2020;27:275–276. doi: 10.1016/j.arcped.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hammami M.B., Garibaldi B., Shah P., et al. Clinical course of COVID-19 in a liver transplant recipient on hemodialysis and response to tocilizumab therapy: a case report. Am J Transpl. 2020;20:2254–2259. doi: 10.1111/ajt.15985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu B., Wang Y., Zhao Y., et al. Successful treatment of severe COVID-19 pneumonia in a liver transplant recipient. Am J Transpl. 2020;20:1891–1895. doi: 10.1111/ajt.15901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tschopp J., L'Huillier A., Mombelli M., et al. First experience of SARS-CoV-2 infections in solid organ transplant recipients in the Swiss Transplant Cohort Study. Am J Transplant. 2020;00:1–7. doi: 10.1111/ajt.16062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chew C.A., Iyer S.G., Chieh Kow A.W., et al. An international multicentre study of protocols for liver transplantation during a pandemic: a case for quadripartite equipoise. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.D'Amico F., Baumgart D.C., Danese S., et al. Diarrhea During COVID-19 Infection: pathogenesis, Epidemiology, Prevention, and Management. Clin Gastroenterol Hepatol. 2020;18:1663–1672. doi: 10.1016/j.cgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rana S.S. Risk of COVID-19 Transmission During Gastrointestinal Endoscopy. J Dig Endosc. 2020;11:27–30. [Google Scholar]
- 99.Repici A., Pace F., Gabbiadini R., et al. Endoscopy units and the Coronavirus disease 2019 outbreak: a multicenter experience from italy. Gastroenterology. 2020;159:363–366. doi: 10.1053/j.gastro.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chiu P.W.Y., Ng S.C., Inoue H., et al. Practice of endoscopy during COVID-19 pandemic: position statements of the Asian Pacific Society for Digestive Endoscopy (APSDE-COVID statements) Gut. 2020;69:991–996. doi: 10.1136/gutjnl-2020-321185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Repici A., Maselli R., Colombo M., et al. Coronavirus (COVID-19) outbreak: what the department of endoscopy should know. Gastrointest Endosc. 2020;92:192–197. doi: 10.1016/j.gie.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sultan S., Lim J.K., Altayar O., et al. AGA Institute Rapid Recommendations for Gastrointestinal Procedures During the COVID-19 Pandemic. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]