Abstract
Purpose
Chemotherapy of colon cancer needs improvement to mitigate the severe adverse effects (AEs) associated with the cytotoxic drugs. The aim of this study is to develop a novel targeted drug delivery system (TDDS) with practical application potential for colon cancer treatment.
Methods
The TDDS was built by loading docetaxel (DTX) in albumin nanoparticles (NPs) that were functionalized with nucleolin-targeted aptamers (AS1411).
Results
The TDDS (Apt-NPs-DTX) had an average size of 62 nm and was negatively charged with a zeta potential of −31.2 mV. DTX was released from the albumin NP with a typical sustained release profile. Aptamer-guided NPs were preferentially ingested by nucleolin-expressing CT26 colon cancer cells vs the control cells. In vitro cytotoxicity study showed that Apt-NPs-DTX significantly enhanced the killing of CT26 colon cancer cells. Importantly, compared with non-targeted drug delivery, Apt-NPs-DTX treatment significantly improved antitumor efficacy and prolonged the survival of CT26-bearing mice, without raising systemic toxicity.
Conclusion
The results suggest that Apt-NPs-DTX has potential in the targeted treatment of colon cancer.
Keywords: aptamer, nanoparticles, colon cancer, targeted drug delivery system
Introduction
Colorectal cancer (CRC) is a serious threat to human health. Globally, the incidence and the mortality rate of CRC rank the third among all malignancies.1 With the development of world economy, there is drastic change in dietary structure in many countries. The reduced dietary fiber intake is associated with an increase in CRC incidence.2 It has been reported that the age of CRC onset has shifted towards younger population in many countries in recent years.3 CRC now accounts for 10.2% of all new cancer cases.4 Although screening with colonoscopy improves the early detection of CRC, the clinical prognosis of late-stage disease remains grim, especially for those with distant metastases, whose 5-year survival rate is lower than 26% on average.5
While early-stage CRC can be cured by surgical resection, over 50% of cases are diagnosed at late stage and require chemotherapy, which is the standard treatment to prolong survival.6,7 The first-line chemotherapy for metastatic colon cancer is FOLFOX (5-fluorouracil (5-FU)/leucovorin/oxaliplatin) or FOLFIRI (5-FU/leucovorin/irinotecan).8 Because current chemotherapies use cytotoxic drugs that are not tumor-specific, the treatments are associated with severe adverse effects (AEs) and poor patient tolerance. The frequently used chemotherapeutics, 5-FU and oxaliplatin may cause severe AEs including myelosuppression, mucositis, diarrhea, malnutrition, and most notably neurotoxicity.9 These AEs seriously compromise the quality of life and treatment outcome, especially for elderly patients. As a result, novel strategy of chemotherapy needs to be developed to treat metastatic colon cancer.
A promising strategy for improving chemotherapy is the utilization of targeted drug delivery system (TDDS). The central idea of TDDS is to deliver cytotoxic drugs selectively to the tumor rather than normal tissue, thereby enhancing the therapeutic efficacy and reducing the AEs. Theoretically, this approach may prolong survival time and improve quality of life. A typical TDDS usually consists of three components: the tumor-targeting ligand, the anticancer drug, and the drug carrier. The most common drug carriers are nanoparticles (NPs), which can accumulate in solid tumors due to the enhanced permeability and retention effect (EPR).10 Moreover, drug-loaded NPs functionalized with tumor-targeting ligands may accumulate more efficiently in tumor tissue (active-targeting), allowing further enrichment of the anticancer drug at the target site.11 As a result, TDDS can channel anticancer drug to the tumor tissue, thereby mitigating the AEs and improving the therapeutic efficacy. In preclinical studies, Si et al designed a MUC-1 aptamer-guided and doxorubicin-loaded silica NP, which improved the efficacy of chemotherapy against breast cancer.12 Khayrani et al built a paclitaxel-loaded liposome conjugated with CD44 antibody, and enhanced the efficacy against ovarian cancer in vivo.13 In clinical studies, TDDS in the form of antibody-drug conjugates (ADCs) significantly improved the treatment outcome, with some ADCs now approved by FDA for treatment of hematological malignancies or HER2-positive breast cancer.14,15 These results underscore the great potential of TDDS in cancer chemotherapies. Given the urgent medical need to improve the chemotherapy for colon cancer, it is reasonable to develop a TDDS to treat the malignancy.
To improve the chance for clinical application, a TDDS for colon cancer treatment should have good biocompatibility, with NPs preferably made of FDA-approved excipients. Albumin is a water-soluble protein and an excipient approved by FDA for human use. It is the most abundant protein in blood and accounts for approximately half of the total proteins in plasma.16 Due to the endogenous nature of albumin, NPs made of albumin have excellent biocompatibility, with the inherent properties of non-toxicity, low immunogenicity, and appropriate biodegradability. Since albumin has three homologous domains that can negotiate with various drugs, it is considered to have great drug-binding capacity.17 Moreover, anticancer agents are usually released from albumin NPs with a typical sustained release profile, improving pharmacokinetics and drug utilization.18,19 Albumin-based formulation of paclitaxel (Abraxane®) has been approved by FDA for treatment of metastatic breast cancer, non-small cell lung cancer, and adenocarcinoma of the pancreas.20
In recent years, efforts have been made to utilize albumin-based drug delivery system to treat colon cancer. Thao et al developed an albumin NP for delivery of doxorubicin and TRAIL to colon cancer.21 Sharma et al constructed a PEG-coated albumin NP carrying 5-FU for colon cancer treatment.22 Kinoshita et al used S-nitrosated albumin dimmer to enhance the EPR effect of Abraxane in colon cancer treatment.23 So far, however, there is a paucity of research on utilizing albumin-based TDDS with active-targeting capability to treat colon cancer. An ideal TDDS should have the following features: active tumor-targeting capability, good biocompatibility, broad anticancer spectrum, and scalable production protocol. In an effort to achieve these goals, here in this study, we developed the first albumin-based TDDS (Apt-NPs-DTX) for targeted delivery of docetaxel (DTX) to colon cancer. The tumor-targeting ligand in the TDDS is a DNA aptamer (AS1411) that and can bind with nucleolin, which is expressed on the cell surface of colon cancer.24 DTX is an FDA-approved broad-spectrum anticancer agent that is more potent than paclitaxel.25,26 We now report that Apt-NPs-DTX is more efficacious against colon cancer cells vs non-targeted drug delivery system.
Materials and Methods
Cell Lines and Cell Culture
CT26 murine colon carcinoma cells and CHO cells were purchased from National Infrastructure of Cell Line Resource (Beijing, China), and cultured in DMEM that was supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% fetal bovine serum (FBS), at 37°C with 5% CO2.
Animals
BALB/c female mice were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). All mice were fed with standard diet and water. Mice of 6–8 weeks (18–22 g) were used for the experiment. All animal experiments were performed in accordance with guidelines approved by the Ethics Committee of Peking Union Medical College and the Chinese Academy of Medical Sciences (ACUC-A02-2018-029).
Reagents
AS1411 aptamer with a 5ʹ polyA linker and 5′-SH modification and the sequence of 5′-SH-AAAAAA-GGTGGTGGTGGTTGTGGTGGTGGTGG was synthesized by Invitrogen (Shanghai, China). Docetaxel was purchased from Aladdin Chemical Co., Ltd. (Shanghai, China). Bovine serum albumin (BSA) was purchased from TBD Science Bio-engineering Co., Ltd. (Tianjin, China). Tris (2-carboxyethyl)phosphine (TCEP) and coumarin 6 were purchased from Sigma-Aldrich (Shanghai, China). Sulfosuccinimidyl 4-[N-maleimidomethyl]cyclohexane-1-carboxylate (Sulfo-SMCC) was purchased from Jinsui Bio-Technology Co., Ltd. (Shanghai, China).
Conjugation of Aptamer to Albumin
For preparation of Apt-BSA, thiol-modified AS1411 aptamer was linked to the amino groups of albumin via the commonly used linker Sulfo-SMCC.27,28 Briefly, 0.4 mg BSA and 0.72 mg Sulfo-SMCC reacted in 950 μL PBS (pH 7.2) ultrafiltrated with a device of 30 kD cut-off to remove unreacted Sulfo-SMCC, and resuspended in 200 μL PBS. Next, 480 μg thiol-modified AS1411 aptamer powder was dissolved in 150 μL Milli-Q water, and 10 μL 800 mM of TCEP solution was added to expose the sulfhydryl group for 30 min. The aptamer solution was mixed with the SMCC-treated BSA solution, put on a rotating mixer to react overnight. The product was ultrafiltrated with a device of 30KD cut-off, and resuspended in PBS, before being used for albumin NP production.
Preparation of Aptamer-Modified Nanoparticles
Apt-NPs-DTX was fabricated using a modified self-assembling method that was described previously.29,30 Briefly, 0.65 mL 15% disodium hydrogen phosphate was injected into 1 mL pure ethanol containing 2 mg DTX with intensive mixing. Next, 0.1 mL PBS containing 19.6 mg BSA and 0.4 mg Apt-BSA monomer was injected into this solution and incubated at 65°C for 10 min. The mixture was put on a rotating mixer and reacted for 20 min at room temperature, poured into 28.88 mL Milli-Q water heated to 65°C under rapid stirring for 20 min, and put into an ice bath for 10 min. Ethanol and disodium hydrogen phosphate were removed using an ultrafiltration column with cutoff of 30 kDa. The albumin NPs recovered from the column were freeze-dried without adding a cryoprotectant. The preparation of NPs-DTX was similar to that of Apt-NPs-DTX, except using BSA in place of Apt-BSA. Lyophilized albumin NPs were resuspended in solutions prior to biological experiments.
Apt-NPs-cou6 was prepared by a similar procedure as described above. Briefly, 0.65 mL 15% disodium hydrogen phosphate was injected into 1 mL coumarin6 ethanol solution (0.0078 mg/mL) with intensive mixing. The subsequent procedure was the same as the synthesis of Apt-NPs-DTX. The preparation of NPs-cou6 was similar to that of Apt-NPs-cou6, except using BSA in place of Apt-BSA.
Characterization of Nanoparticles
The morphology of nanoparticles was observed by transmission electron microscope (TEM) (JEM-200CX, JEOL, Tokyo, Japan) after the samples were deposited on copper grid and stained with 3% phosphotungstic acid.
The average particle size distribution, polydispersity index (PDI) and zeta potential of nanoparticles before and after the lyophilization were determined by dynamic light scattering (DLS) using Zeta Sizer Nano ZS90 (Malvern Instruments, Malvern, UK) at 25°C.
Assessment of DNA Conjugation to NPs
Agarose gel electrophoresis experiments were applied to evaluate whether aptamers were attached to the albumin NPs. Tris-Borate-EDTA 0.5 x (TBE) buffer solution with 2% (w/v) agarose containing the GelRed DNA dye (Invitrogen, Shanghai, China) was used as the gel. Free AS1411 aptamer, albumin NPs functionalized with AS1411 aptamer, or plain albumin NPs were loaded into the gel. The samples were subjected to 110 V for 20 min. The DNA was visualized by exposing the gel under UV light by an UV documentation device (Alliance, London, UK).
Measurement of Drug Encapsulation Efficiency and Drug-Loading Capacity
For estimation of the percentage of drug entrapped in NPs, the amount of DTX not encapsulated was measured with standard approach.31,32 Briefly, the lyophilized albumin NPs were suspended in 1.0 mL water. The NP solution was centrifuged at 3000 rpm for 10 min to separate the unencapsulated DTX from the NPs. The supernatant was discarded. The unentrapped DTX in the precipitate was dissolved in ethanol. The amount of DTX was measured by high-performance liquid chromatography (HPLC) with a UV-Vis detector at 229 nm, using a C18 column (Diamonsil C18 (2), 5 μm 100Å 250 × 4.6 mm). The mobile phase was composed of acetonitrile and water (55:45, v/v) with a flow rate of 1.0 mL/min. The calibration curve was linear in the range of 0.78–200 μg/mL with a correlation coefficient of R2=0.999. The drug encapsulation efficiency (EE) and drug-loading capacity (DL) were calculated by the following equations:
 |
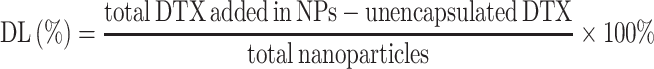 |
Drug Release Study
In vitro drug release experiment was performed using the standard dialysis method.33 Briefly, 2 mL of drug-loaded samples (2 mg of equivalent DTX) was placed in a bag made of dialysis membrane (1 kDa, Solarbio, Beijing, China). The dialysis bag was immersed in 50 mL of PBS (pH 7.4) at 37°C and placed in a shaker at 100 rpm. At specified intervals, 1 mL buffer was withdrawn and replaced with equal volume of fresh release buffer. The amount of drug released at each time point was determined by the HPLC method described above. The cumulative drug release rate (Q%) is calculated using the following equation:34
 |
Cellular Uptake of NPs
In order to visualize the cellular uptake of the NPs, CT26 or CHO cells (4 × 103/well) were seeded onto 96-well plates. After cultivation for 24 h, cells were treated with 10 μL of aptamer, NPs-cou6, or Apt-NPs-cou6 (cou6 concentration: 0.06 μg/mL). Cells were further incubated for 2 h to allow for NP uptake. The cell medium was discarded, and cells were washed thrice with PBS, and imaged using an Axio Vert A1 fluorescent microscope (Zeiss, Oberkochen, Germany).
Cellular uptake of the NPs was also evaluated by flow cytometry. CT26 and CHO cells (1.5 × 105/well) were seeded in 24-well plates and cultured overnight. The cells were treated with 10μL of aptamer, NPs-cou6, or Apt-NPs-cou6 (cou6 concentration: 0.06 μg/mL). Cells were further incubated for 2 h to allow for NP uptake. The cell medium was removed, and the cells washed with PBS for three times. After adding 0.05% trypsin/0.02% EDTA for 5 min to omit the cells from the wells, Accuri C6 Flow Cytometer (BD Biosciences, San Jose, CA, USA) was used to analyze the cellular fluorescent signals.
The cellular uptake of nanoparticles was also studied by confocal microscopy. The CT26 and CHO cells (5 × 103/well) were cultivated in Lab-Tek Chamber Slide System (ThermoFisher Scientific, Waltham, MA, USA). After 24 h, the cells were incubated with 10μL of NPs-cou6 or Apt-NPs-cou6 (cou6 concentration: 0.06 μg/mL). The cells were further cultured for 2 h and rinsed for three times in PBS. Hoechst 33342 (2 mg/mL; ApexBio Technology, Boston, MA, USA) was used to counterstain the cell nuclei. Afterward, 4% formaldehyde polymer was added. Fifteen min later, the sample was washed with ice-cold PBS for three times. Finally, the cells were analyzed by a confocal laser-scanning microscope (Perkin Elmer Ultraview, Perkin, Waltham, MA, USA).
Cytotoxicity Study
Cytotoxicities against CT26 or CHO cells by free DTX, Apt-NPs-DTX, or NPs-DTX were evaluated in vitro. The cells were seeded in 96-well plates at a density of 3×103 cells/100 μL/well. After 72 h, 10 μL of DTX, Apt-NPs-DTX, or NPs-DTX (of DTX concentration from 0.5 to 300 μg/mL) were added to the designated wells. After 2 h, the cells were washed twice with PBS and received 100 μL fresh medium per well. After another 48 h, MTS assay was applied to determine the cell viability according to the standard protocol as outlined by the manufacture. Absorbance was read by using a microplate reader at 492 nm.
Animal Study
A CT26 cell suspension (5 × 105 cells in 100 μL PBS) was injected into the right hind flank of the BALB/c mice. One week after the inoculation, the tumor-bearing mice were randomly divided into 3 groups (6 animals per group). The animal received treatment via intraperitoneal injection every three days for a total of three times. The control group received 100 μL saline injection. The two treatment groups received NPs-DTX and Apt-NPs-DTX suspended in 100μL saline, with a DTX dosage of 40 mg/kg. Tumor dimensions were measured periodically. The first day of injection was recorded as day zero. The tumor volume was calculated by the following formula: tumor volume (mm3) = (L × S2)/2, where “L” is the largest diameter and “S” is the smallest diameter. The body weight, the appetite, and the hair condition were recorded for each animal to evaluate the treatment toxicities. The survival time was also recorded.
Statistical Analysis
Statistical analysis was performed using one-way ANOVA and two-tailed Student’s t-test. All experimental data were presented as the mean value with its standard deviation (mean ± SD). P < 0.05 was considered statistically significant. Statistical analysis was conducted using commercially available software (SPSS 11.3).
Results
Characterization of Nanoparticles
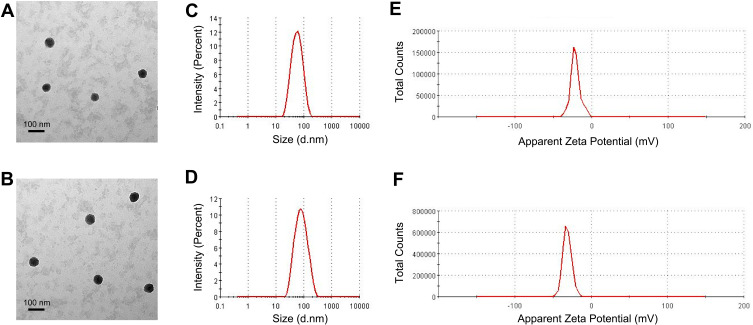
The size of a drug-loaded nanoparticle is critical for its therapeutic efficacy and EPR effect. If the NP size is smaller than 6 nm, the particles tend to be excreted from the kidneys; whereas if the NP size exceeds 300 nm, the particles may be easily captured and trapped by the reticuloendothelial system (RES).11 To characterize the nanoparticles in this study, TEM and DLS were performed. TEM images showed that Apt-NPs-DTX had a slightly larger size vs NPs-DTX (Figure 1A and B). DLS studies revealed that the average size of NPs-DTX was 48 nm (Figure 1C), while that of Apt-NPs-DTX was 62 nm (Figure 1D). The results indicated that the sizes of both NPs were appropriate for avoiding renal leakage and RES clearance. To further characterize the physical properties of the nanoparticles, we also measured zeta potential and PDI. The zeta potential of Apt-NPs-DTX was −31.2 mV (Figure 1F), whereas that of NPs-DTX was −21.1 mV (Figure 1E). The negative charges of the NPs might prevent the particles from agglomeration and maintain their stability.35 The PDIs of both nanoparticles were less than 0.3, indicating that the NP sizes were narrowly distributed (Table 1). The drug encapsulation efficiency was 92% for NPs-DTX and 90% for Apt-NPs-DTX, respectively.
Figure 1.
Characterization of nanoparticles. TEM images of (A) NPs-DTX and (B) Apt-NPs-DTX. Size distributions of (C) NPs-DTX and (D) Apt-NPs-DTX. Zeta potential distribution of (E) NPs-DTX and (F) Apt-NPs-DTX.
Table 1.
Properties of Nanoparticles
| Formulation | Z-Average (nm) | PDI | Zeta Potential (mV) | Drug Encapsulation Efficiency | Drug-Loading Capacity |
|---|---|---|---|---|---|
| NPs-DTX | 48 ± 0.4 | 0.22 ± 0.024 | −21.1 ± 0.24 | 92% ± 0.5 | 8.4%± 0.5 |
| Apt-NPs-DTX | 62 ± 0.6 | 0.28 ± 0.031 | −31.2 ± 0.18 | 90% ± 0.7 | 8.1%± 0.6 |
Note: Each value represents mean ± SD (n = 3).
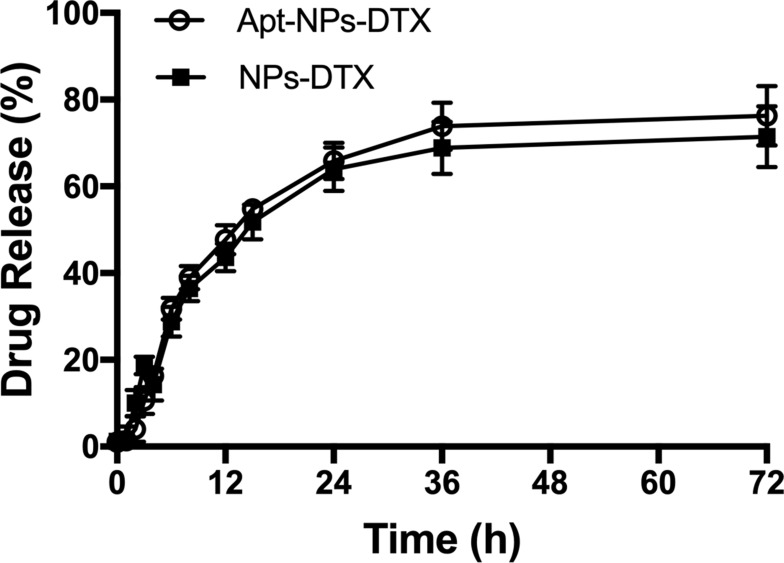
Release of DTX from Nanoparticles
Sustained release of drug from NP influences pharmacokinetics and therapeutic efficacy.36 The drug release profiles from NPs-DTX or Apt-NPs-DTX were evaluated using a dialysis bag with cutoff limit of 1000 Da. As shown in Figure 2, NP-encapsulated DTX was released relatively slowly, and there was no obvious difference in release profile between Apt-NPs-DTX and NPs-DTX. The results showed that both albumin NPs had a typical sustained drug release profile.
Figure 2.
DTX release kinetics from Apt-NPs-DTX or NPs-DTX at pH 7.4 PBS. Data are shown as mean ± standard deviation (n = 3).
Evaluation of Aptamer Conjugation to Albumin NPs
Tumor-homing aptamer may facilitate targeted drug delivery to cancer cells by NPs. Thiol-modified aptamers were conjugated to the amino-groups in albumin via standard Sulfo-SMCC chemistry.27 Aptamer-modified albumin was subsequently used as the building material of the NPs.37 To evaluate whether the DNA aptamers were conjugated to albumin NPs, agarose gel electrophoresis of the free DNA aptamers, Apt-NPs, and NPs was performed. DNA electrophoresis is a commonly used method for evaluating the conjugation of DNA aptamer to NPs, because NP-conjugated DNA cannot move efficiently in the gel.38 As shown in Figure 3, free AS1411 aptamer moved efficiently in the gel (Lane 1), while a significant portion of DNA molecules in Apt-NPs-DTX remained in the loading well (Lane 2), suggesting that these DNA aptamers attached to the NPs and could not move in electrophoresis. Nanoparticles per se generated no signal for lack of DNA (Lane 3). The results indicated that at least some AS1411 aptamers were conjugated to the albumin NPs.
Figure 3.
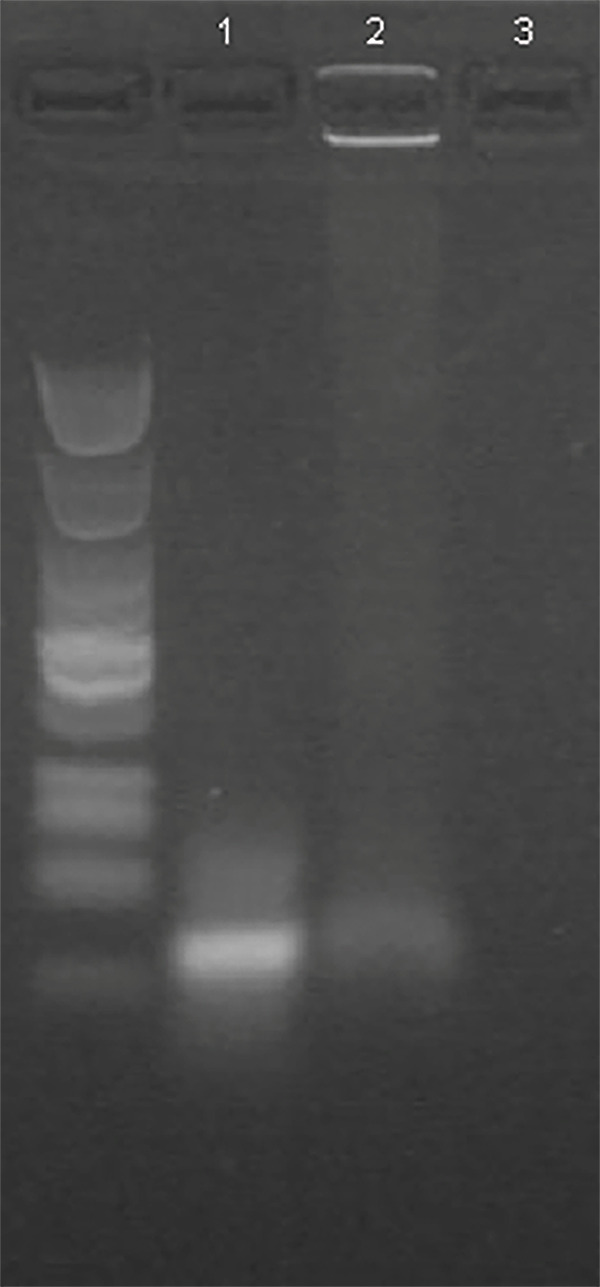
Evaluation of aptamer-NP conjugation by agarose gel electrophoresis. The agarose gel was stained for DNA by GelRed and photographed under UV light. Free AS1411 aptamer was in Lane 1. Aptamer conjugated with NP was in Lane 2. Albumin NP alone was in Lane3.
Cellular Uptake of Nanoparticles
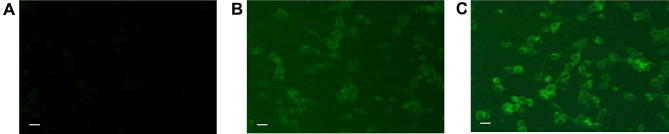
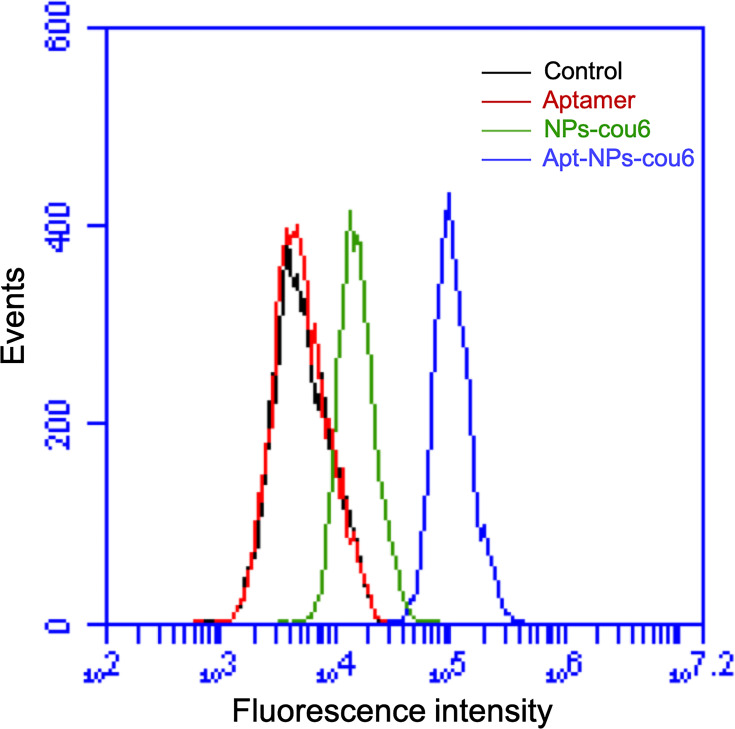
Many tumors, including CT26 colon cancer, express nucleolin in cell membrane.20,39,40 We next investigated whether modification of NPs with nucleolin aptamer would influence the NP uptake by CT26 cells. A fluorescent dye (Coumarin-6) was encapsulated by either aptamer-modified NPs (Apt-NPs-cou6) or plain albumin NPs (NPs-cou6).41 Fluorescence microscopy was applied to compare the cellular uptake of Apt-NPs-cou6 and NPs-cou6 by CT26 cells. As shown in Figure 4, stronger fluorescent signals were observed in cells treated with Apt-NPs-cou6 vs NPs-cou6. The images indicated that aptamer-modification of the NPs enhanced the NP ingestion by CT26 cells. To further validate the results, flow cytometry study was also conducted. As shown in Figure 5, CT26 cells treated with Apt-NPs-cou6 generated stronger fluorescence, again indicating that aptamer-modification of the NPs facilitated the NP uptake by CT26 cells.
Figure 4.
Fluorescent microscopy images of CT26 cells treated with (A) free aptamer, (B) NPs-cou6, or (C) Apt-NPs-cou6. Scale bar represents 25 μm.
Figure 5.
Flow cytometry analysis of CT26 cells treated with free aptamer (red), NPs-cou6 (green), or Apt-NPs-cou6 (blue). Signal generated by control cells is in black.
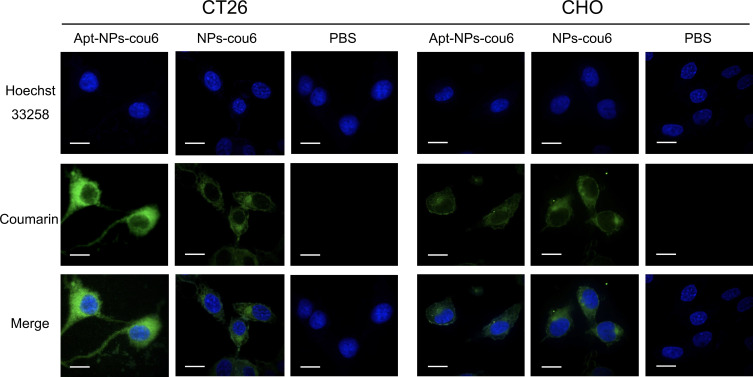
Nucleolin is highly expressed in the cell membrane of target cancer cells, but under-expressed on the surface of normal cells.39,40 The disparity in nucleolin expression between the two types of cells may influence the NP-ingestion behaviors. Next, we investigated whether aptamer-modified NPs could differentiate between the CT26 target cells (CT26) and a control cell line (CHO) that had low nucleolin expression.20,42 Apt-NPs-cou6 or NPs-cou6 were incubated with either the nucleolin-positive CT26 or the nucleolin-negative CHO cells, which were subsequently evaluated by confocal microscopy. In CT26 cells, the green fluorescence was much stronger when treated with Apt-NPs-cou6 vs NPs-cou6. In CHO cells, however, the green fluorescence was weak with both treatments (Figure 6). The results suggested that aptamer-modified NPs had a targeting preference for the nucleolin-positive CT26 cells vs the nucleolin-negative control cells.
Figure 6.
Confocal images of CT26 and CHO cells treated with either Apt-NPs-cou6 or NPs-cou6. The cell nuclei were stained blue with Hoechst 33258. Scale bar equals to 20 μm.
In vitro Cytotoxicity Study
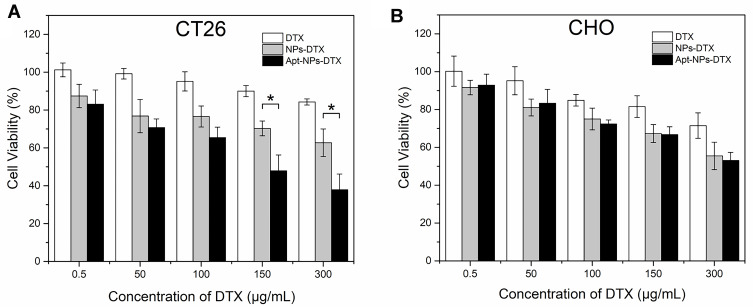
The above results showed that aptamer-modification of the NPs enhanced the particle ingestion by CT26 target cells. However, it was still unclear whether Apt-NPs-DTX could generate a targeted cytotoxicity against CT26 cells. To address this issue, targeted and non-targeted drug delivery systems were compared in cytotoxicity experiments. CT26 target cells and CHO control cells were treated with free DTX, NPs-DTX, or Apt-NPs-DTX of various concentrations. Cell viability was evaluated with standard MTS assay. In CT26 cells, Apt-NPs-DTX generated stronger cytotoxicity vs NPs-DTX at concentrations higher than 150 μg/mL (Figure 7A). In CHO cells, however, Apt-NPs-DTX and NPs-DTX produced similar cytotoxicity (Figure 7B). The data suggested that Apt-NPs-DTX selectively enhanced the efficacy against the CT26 target cells, but not the CHO control cells.
Figure 7.
In vitro evaluation of cytotoxicity against (A) the CT26 target cells or (B) the CHO control cells by various treatments. The cells were treated with free DTX, NP-DTX, or Apt-NP-DTX for 2h, washed, and evaluated with the MTS viability assay after 48h. Data represent mean ± standard deviation (n=6). The star (*) indicates statistically significant difference (p< 0.05).
In vivo Tumor Inhibition Study
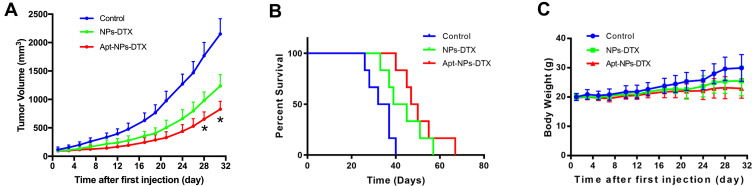
To investigate whether aptamer-modification of NP would improve its function as a drug carrier in vivo, therapeutic efficacies of Apt-NPs-DTX vs NPs-DTX were compared in CT26-bearing BALB/c mice. As illustrated in Figure 8A, both treatments inhibited tumor growth. However, Apt-NPs-DTX was more efficacious than NPs-DTX. The final tumor volumes for the control, the NPs-DTX, and the Apt-NPs-DTX groups were 2150 ± 270.15 mm3, 1236.61 ± 197.61 mm3, and 827.19 ± 140.71 mm3, respectively. The influences of Apt-NPs-DTX vs NPs-DTX on animal survival were also compared (Figure 8B). Both nanoparticles prolonged the survival, with the Apt-NPs-DTX being the most efficacious treatment. These results indicated that Apt-NPs-DTX had superior therapeutic efficacy than NPs-DTX.
Figure 8.
Animal studies in CT26-bearing BALB/c mice (n=6). (A) Tumor growth curves in mice treated with NPs-DTX or Apt-NPs-DTX. (B) Survival time of CT26-bearing BALB/c mice. (C) Body weights of CT26-bearing mice in various treatment groups. The star (*) indicates statistically significant difference (p< 0.05).
To evaluate the side effects generated by NPs-DTX or Apt-NPs-DTX, the body weights were measured every 3 days. As shown in Figure 8C, there was no significant difference in body weight between the two treatment groups. There was also no obvious difference in appetite or hair color between the treatment groups. The results suggested that, although Apt-NPs-DTX enhanced therapeutic efficacy, it did not generate extra toxicity in mice.
Discussion
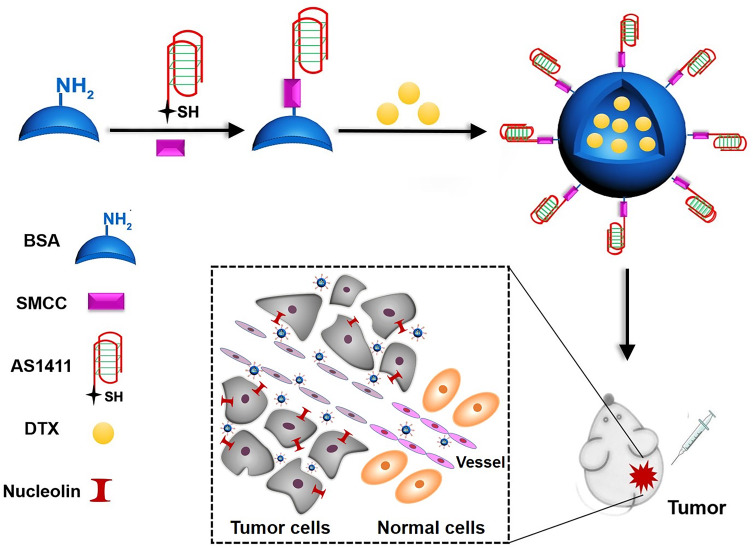
The primary purpose of this work was to develop a TDDS for colon cancer treatment with practical application potential. Ideally, such a TDDS should be made of biocompatible components approved for human use. In this study, albumin NPs encapsulating DTX were functionalized with a tumor-targeting aptamer (Figure 9). Apt-NPs-DTX had an average diameter of 62 nm, which was the appropriate size for EPR effects (Figure 1 and Table 1). DTX was released from Apt-NPs-DTX with a typical sustained release profile (Figure 2). Aptamer-modified NPs were preferentially ingested by CT26 colon cancer cells (Figures 4–6). Importantly, Apt-NPs-DTX increased the cytotoxicity against CT26 colon cancer cells in vitro vs NPs-DTX (Figure 7). Moreover, in vivo study showed that Apt-NPs-DTX enhanced inhibition of colon cancer and prolonged survival, without raising the systemic toxicity (Figure 8). These results suggest that Apt-NPs-DTX may have application potential in chemotherapy against colon cancer.
Figure 9.
Scheme of preparation of the Apt-NPs-DTX for colon cancer therapy. Thiol-modified AS1411 aptamer was conjugated to the amino-groups of albumin via SMCC chemistry. DTX was encapsulated by aptamer-modified albumin to form the Apt-NPs-DTX.
Metastatic colon cancer can be treated with either immunotherapy or chemotherapy. Immunotherapy with PD-1 or PD-L1 inhibitors is the latest modality to treat colon cancer. PD-1 or PD-L1 antibodies are usually efficacious in colon cancers with dMMR/MSI-H genotype.43–45 However, about 80% patients have colon cancer with MSS genotype, for which immunotherapy is largely ineffective.46,47 As a result, chemotherapy is nonetheless of value for many patients with metastatic diseases. FOLFOX and FOLFIRI are the first-line chemotherapies for metastatic colon cancer.8 Both chemotherapies frequently generate severe AEs, compromising the clinical outcome. Therefore, it is important to develop targeted chemotherapy for advanced colon cancer, in order to improve the therapeutic efficacy.
Targeted treatment of colon cancer can be achieved with either ADCs or NP-based TDDS. ADCs are made of cytotoxic drugs conjugated to tumor-targeting antibodies. ADCs have been approved by FDA for treatment of Hodgkin’s lymphoma, systemic anaplastic large cell lymphoma, and HER2-positive breast cancer.48 So far, however, no ADC has been approved by FDA for colon cancer treatment. Unlike nanoparticles, ADCs usually do not generate EPR effect, which facilitates drug accumulation in solid tumor. ADCs also have a relatively low drug-loading capacity and thus need to carry highly toxic drugs in order to achieve proper therapeutic efficacy.49,50 Compared with ADCs, NP-based TDDS has the advantages of higher drug-loading capacity, EPR effect, and more choices of tumor-targeting ligands (including antibody, peptide, or aptamer).51–53 Therefore, it is reasonable to explore NP-based TDDS for colon cancer treatment other than ADCs.
An ideal NP-based TDDS should have the following features: superb tumor-targeting capability (preferably with both active and passive targeting mechanisms), good biocompatibility, uncomplicated production process, and broad antitumor spectrum. In this study, the Apt-NPs-DTX was designed in an effort to meet these requirements. First, the nucleolin aptamer in TDDS enables active targeting of colon cancer. Moreover, NP size of 62 nm facilitates passive tumor-targeting with EPR effect and avoids NP capture by the reticuloendothelial system.11 Second, Apt-NPs-DTX has good biocompatibility, because all of its major components have been approved by FDA for human use. The nanoparticle is made of albumin, which is a component of Abraxane® approved by FDA for breast cancer treatment.20 AS1411 aptamer is a short DNA aptamer and has been evaluated in clinical trials.54 Aptamers as a class of molecules were first approved for clinical use by FDA in 2004. AS1411 Aptamer is conjugated to the NP via SMCC, which is a thioether linker approved by FDA for usage in T-DM1®, a HER2-targeting ADC.55 Third, in this study, the process of NP fabrication was relatively uncomplicated and potentially scalable.30 Moreover, the protocol did not involve the cross-linking reagent glutaraldehyde. Fourth, acting on microtubules,56 DTX has a remarkably broad anticancer spectrum and is used clinically to treat ovarian cancer, non-small-cell lung cancer, metastatic breast cancer, prostate cancer, neck cancer, etc.57 Due to the above features, Apt-NPs-DTX designed in this study may have potential for further development as a novel strategy to treat colon cancer.
Conclusion
In conclusion, we developed a novel biocompatible TDDS (Apt-NPs-DTX), which enhanced the antitumor therapeutic efficacy in vivo. The results suggest that, besides ADCs, aptamer-guided albumin NPs may also have potential for targeted treatment of colon cancer. Future work may focus on exploring the therapeutic efficacy of the TDDS against other malignancies in more animal models, and on refining the fabrication protocol to facilitate large-scale production of the TDDS.
Funding Statement
This work was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS 2016-I2M-3-004) and the Ministry of Science and Technology (2017YFA0205504).
Disclosure
The authors declare no competing interests.
References
- 1.Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–1953. doi: 10.1002/ijc.31937 [DOI] [PubMed] [Google Scholar]
- 2.Baena R, Salinas P. Diet and colorectal cancer. Maturitas. 2015;80(3):258–264. doi: 10.1016/j.maturitas.2014.12.017 [DOI] [PubMed] [Google Scholar]
- 3.Araghi M, Soerjomataram I, Bardot A, et al. Changes in colorectal cancer incidence in seven high-income countries: a population-based study. Lancet Gastroenterol Hepatol. 2019;4(7):511–518. doi: 10.1016/s2468-1253(19)30147-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Sauer AG, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145–164. doi: 10.3322/caac.21601 [DOI] [PubMed] [Google Scholar]
- 6.Gustavsson B, Carlsson G, Machover D, et al. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin Colorectal Cancer. 2015;14(1):1–10. doi: 10.1016/j.clcc.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 7.McQuade RM, Stojanovska V, Bornstein JC, Nurgali K. Colorectal cancer chemotherapy: the evolution of treatment and new approaches. Curr Med Chem. 2017;24(15):1537–1557. doi: 10.2174/0929867324666170111152436 [DOI] [PubMed] [Google Scholar]
- 8.Benson AB 3rd, Venook AP, Cederquist L, et al. Colon cancer, version 1.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(3):370–398. doi: 10.6004/jnccn.2017.0036 [DOI] [PubMed] [Google Scholar]
- 9.Lee JJ, Chu E. The adjuvant treatment of Stage III colon cancer: might less be more? Oncology (Williston Park, NY). 2018;32(9):437–442, 444. [PubMed] [Google Scholar]
- 10.Greish K. Enhanced permeability and retention of macromolecular drugs in solid tumors: a royal gate for targeted anticancer nanomedicines. J Drug Target. 2007;15(7–8):457–464. doi: 10.1080/10611860701539584 [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi H, Watanabe R, Choyke PL. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics. 2013;4(1):81–89. doi: 10.7150/thno.7193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Si P, Shi J, Zhang P, et al. MUC-1 recognition-based activated drug nanoplatform improves doxorubicin chemotherapy in breast cancer. Cancer Lett. 2020;472:165–174. doi: 10.1016/j.canlet.2019.12.019 [DOI] [PubMed] [Google Scholar]
- 13.Khayrani AC, Mahmud H, Oo AKK, et al. Targeting ovarian cancer cells overexpressing CD44 with Immunoliposomes encapsulating glycosylated paclitaxel. Int J Mol Sci. 2019;20(5):1042. doi: 10.3390/ijms20051042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hedrich WD, Fandy TE, Ashour HM, Wang H, Hassan HE. Antibody-drug conjugates: pharmacokinetic/pharmacodynamic modeling, preclinical characterization, clinical studies, and lessons learned. Clin Pharmacokinet. 2018;57(6):687–703. doi: 10.1007/s40262-017-0619-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wedam S, Fashoyin-Aje L, Gao X, et al. FDA approval summary: ado-trastuzumab emtansine for the adjuvant treatment of HER2-positive early breast cancer. Clin Cancer Res. 2020. doi: 10.1158/1078-0432.Ccr-19-3980 [DOI] [PubMed] [Google Scholar]
- 16.Farrugia A. Albumin usage in clinical medicine: tradition or therapeutic? Transfus Med Rev. 2010;24(1):53–63. doi: 10.1016/j.tmrv.2009.09.005 [DOI] [PubMed] [Google Scholar]
- 17.He XM, Carter DC. Atomic structure and chemistry of human serum albumin. Nature. 1992;358(6383):209–215. doi: 10.1038/358209a0 [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Wu J, Xu L, Xie K, Chen C, Dong Y. Albumin nanoparticle encapsulation of potent cytotoxic therapeutics shows sustained drug release and alleviates cancer drug toxicity. Chem Commun. 2017;53(17):2618–2621. doi: 10.1039/c6cc08978j [DOI] [PubMed] [Google Scholar]
- 19.Sleep D, Cameron J, Evans LR. Albumin as a versatile platform for drug half-life extension. Biochim Biophys Acta. 2013;1830(12):5526–5534. doi: 10.1016/j.bbagen.2013.04.023 [DOI] [PubMed] [Google Scholar]
- 20.Alibolandi M, Taghdisi SM, Ramezani P, et al. Smart AS1411-aptamer conjugated pegylated PAMAM dendrimer for the superior delivery of camptothecin to colon adenocarcinoma in vitro and in vivo. Int J Pharm. 2017;519(1–2):352–364. doi: 10.1016/j.ijpharm.2017.01.044 [DOI] [PubMed] [Google Scholar]
- 21.Thao LQ, Byeon HJ, Lee C, et al. Doxorubicin-bound albumin nanoparticles containing a TRAIL protein for targeted treatment of colon cancer. Pharm Res. 2016;33(3):615–626. doi: 10.1007/s11095-015-1814-z [DOI] [PubMed] [Google Scholar]
- 22.Sharma A, Kaur A, Jain UK, Chandra R, Madan J. Stealth recombinant human serum albumin nanoparticles conjugating 5-fluorouracil augmented drug delivery and cytotoxicity in human colon cancer, HT-29 cells. Colloids Surf B Biointerfaces. 2017;155:200–208. doi: 10.1016/j.colsurfb.2017.04.020 [DOI] [PubMed] [Google Scholar]
- 23.Kinoshita R, Ishima Y, Chuang VTG, et al. Improved anticancer effects of albumin-bound paclitaxel nanoparticle via augmentation of EPR effect and albumin-protein interactions using S-nitrosated human serum albumin dimer. Biomaterials. 2017;140:162–169. doi: 10.1016/j.biomaterials.2017.06.021 [DOI] [PubMed] [Google Scholar]
- 24.Chen Z, Xu X. Roles of nucleolin. Focus on cancer and anti-cancer therapy. Saudi Med J. 2016;37(12):1312–1318. doi: 10.15537/smj.2016.12.15972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi MG, Park S, Oh DK, et al. Effect of medical thoracoscopy-guided intrapleural docetaxel therapy to manage malignant pleural effusion in patients with non-small cell lung cancer: a pilot study. Thorac Cancer. 2019;10(10):1885–1892. doi: 10.1111/1759-7714.13158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones DR, Taylor MD, Petroni GR, et al. Phase I trial of intrapleural docetaxel administered through an implantable catheter in subjects with a malignant pleural effusion. J Thorac Oncol. 2010;5(1):75–81. doi: 10.1097/JTO.0b013e3181c07ddc [DOI] [PubMed] [Google Scholar]
- 27.Hu Z, He J, Gong W, et al. TLS11a aptamer/CD3 antibody anti-tumor system for liver cancer. J Biomed Nanotechnol. 2018;14(9):1645–1653. doi: 10.1166/jbn.2018.2619 [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Kim MY, Kim HS, Hah SS. Binding of uranyl ion by a DNA aptamer attached to a solid support. Bioorg Med Chem Lett. 2011;21(13):4020–4022. doi: 10.1016/j.bmcl.2011.04.139 [DOI] [PubMed] [Google Scholar]
- 29.Qu N, Lee RJ, Sun Y, et al. Cabazitaxel-loaded human serum albumin nanoparticles as a therapeutic agent against prostate cancer. Int J Nanomedicine. 2016;11:3451–3459. doi: 10.2147/ijn.S105420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qu N, Sun Y, Xie J, Teng L. Preparation and evaluation of in vitro self-assembling HSA nanoparticles for cabazitaxel. Anticancer Agents Med Chem. 2017;17(2):294–300. doi: 10.2174/1871520616666160526103102 [DOI] [PubMed] [Google Scholar]
- 31.Meghani NM, Amin H, Park C, et al. Combinatory interpretation of protein corona and shear stress for active cancer targeting of bioorthogonally clickable gelatin-oleic nanoparticles. (1873-0191 (Electronic)). Mater Sci Eng C. 2020;111:110760. [DOI] [PubMed] [Google Scholar]
- 32.da Rocha MCO, da Silva PB, Radicchi MA, et al. Docetaxel-loaded solid lipid nanoparticles prevent tumor growth and lung metastasis of 4T1 murine mammary carcinoma cells. J Nanobiotechnology. 2020;18(1):43. doi: 10.1186/s12951-020-00604-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sonali S, Agrawal P, Singh RP, et al. Transferrin receptor-targeted vitamin E TPGS micelles for brain cancer therapy: preparation, characterization and brain distribution in rats. Drug Deliv. 2016;23(5):1788–1798. doi: 10.3109/10717544.2015.1094681 [DOI] [PubMed] [Google Scholar]
- 34.Kong WQ, Gao CD, Hu SF, Ren JL, Zhao LH, Sun RC. Xylan-modified-based hydrogels with temperature/pH dual sensitivity and controllable drug delivery behavior. Materials (Basel). 2017;10(3):304. doi: 10.3390/ma10030304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang J, Oberdorster G, Biswas P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J Nanopart Res. 2009;11(1):77–89. doi: 10.1007/s11051-008-9446-4 [DOI] [Google Scholar]
- 36.Gallego-Yerga L, Posadas I, de la Torre C, et al. Docetaxel-loaded nanoparticles assembled from β-cyclodextrin/calixarene giant surfactants: physicochemical properties and cytotoxic effect in prostate cancer and glioblastoma cells. Front Pharmacol. 2017;8:249. doi: 10.3389/fphar.2017.00249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y, Wang J, Wang J, et al. Aptamer functionalized cisplatin-albumin nanoparticles for targeted delivery to epidermal growth factor receptor positive cervical cancer. J Biomed Nanotechnol. 2016;12(4):656–666. doi: 10.1166/jbn.2016.2203 [DOI] [PubMed] [Google Scholar]
- 38.Moosavian SA, Abnous K, Badiee A, Jaafari MR. Improvement in the drug delivery and anti-tumor efficacy of PEGylated liposomal doxorubicin by targeting RNA aptamers in mice bearing breast tumor model. Colloids Surf B Biointerfaces. 2016;139:228–236. doi: 10.1016/j.colsurfb.2015.12.009 [DOI] [PubMed] [Google Scholar]
- 39.Rangiah K, Tippornwong M, Sangar V, et al. Differential secreted proteome approach in murine model for candidate biomarker discovery in colon cancer. J Proteome Res. 2009;8(11):5153–5164. doi: 10.1021/pr900518v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berger CM, Gaume X, Bouvet P. The roles of nucleolin subcellular localization in cancer. Biochimie. 2015;113:78–85. doi: 10.1016/j.biochi.2015.03.023 [DOI] [PubMed] [Google Scholar]
- 41.Miranda MA, Silva LB, Carvalho IPS, et al. Targeted uptake of folic acid-functionalized polymeric nanoparticles loading glycoalkaloidic extract in vitro and in vivo assays. Colloids Surf B Biointerfaces. 2020;192:111106. doi: 10.1016/j.colsurfb.2020.111106 [DOI] [PubMed] [Google Scholar]
- 42.Taghdisi SM, Danesh NM, Ramezani M, Yazdian-Robati R, Abnous K. A novel AS1411 aptamer-based three-way junction pocket DNA nanostructure loaded with doxorubicin for targeting cancer cells in vitro and in vivo. Mol Pharm. 2018;15(5):1972–1978. doi: 10.1021/acs.molpharmaceut.8b00124 [DOI] [PubMed] [Google Scholar]
- 43.Sclafani F. PD-1 inhibition in metastatic dMMR/MSI-H colorectal cancer. Lancet Oncol. 2017;18(9):1141–1142. doi: 10.1016/s1470-2045(17)30512-0 [DOI] [PubMed] [Google Scholar]
- 44.Yaghoubi N, Soltani A, Ghazvini K, Hassanian SM, Hashemy SI. PD-1/PD-L1 blockade as a novel treatment for colorectal cancer. Biomed Pharmacother. 2019;110:312–318. doi: 10.1016/j.biopha.2018.11.105 [DOI] [PubMed] [Google Scholar]
- 45.Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, Phase 2 study. Lancet Oncol. 2017;18(9):1182–1191. doi: 10.1016/s1470-2045(17)30422-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diaz LA Jr, Le DT. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;373(20):1979. doi: 10.1056/NEJMc1510353 [DOI] [PubMed] [Google Scholar]
- 47.Le DT, Hubbard-Lucey VM, Morse MA, et al. A blueprint to advance colorectal cancer immunotherapies. Cancer Immunol Res. 2017;5(11):942–949. doi: 10.1158/2326-6066.Cir-17-0375 [DOI] [PubMed] [Google Scholar]
- 48.Lambert JM, Berkenblit A. Antibody-drug conjugates for cancer treatment. Annu Rev Med. 2018;69:191–207. doi: 10.1146/annurev-med-061516-121357 [DOI] [PubMed] [Google Scholar]
- 49.Perez HL, Cardarelli PM, Deshpande S, et al. Antibody-drug conjugates: current status and future directions. Drug Discov Today. 2014;19(7):869–881. doi: 10.1016/j.drudis.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 50.Wu AM, Senter PD. Arming antibodies: prospects and challenges for immunoconjugates. Nat Biotechnol. 2005;23(9):1137–1146. doi: 10.1038/nbt1141 [DOI] [PubMed] [Google Scholar]
- 51.Maeda H, Tsukigawa K, Fang J. A retrospective 30 years after discovery of the enhanced permeability and retention effect of solid tumors: next-generation chemotherapeutics and photodynamic therapy–problems, solutions, and prospects. Microcirculation. 2016;23(3):173–182. doi: 10.1111/micc.12228 [DOI] [PubMed] [Google Scholar]
- 52.Yhee JY, Son S, Lee H, Kim K. Nanoparticle-based combination therapy for cancer treatment. Curr Pharm Des. 2015;21(22):3158–3166. doi: 10.2174/1381612821666150531165059 [DOI] [PubMed] [Google Scholar]
- 53.Bi Y, Hao F, Yan G, Teng L, Lee RJ, Xie J. Actively targeted nanoparticles for drug delivery to tumor. Curr Drug Metab. 2016;17(8):763–782. doi: 10.2174/1389200217666160619191853 [DOI] [PubMed] [Google Scholar]
- 54.Carvalho J, Mergny JL, Salgado GF, Queiroz JA, Cruz C. G-quadruplex, friend or foe: the role of the G-quartet in anticancer strategies. Trends Mol Med. 2020. doi: 10.1016/j.molmed.2020.05.002 [DOI] [PubMed] [Google Scholar]
- 55.Rinnerthaler G, Gampenrieder SP, Greil R. HER2 directed antibody-drug-conjugates beyond T-DM1 in breast cancer. Int J Mol Sci. 2019;20(5):1115. doi: 10.3390/ijms20051115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cortes JE, Pazdur R. Docetaxel. J Clin Oncol. 1995;13(10):2643–2655. doi: 10.1200/jco.1995.13.10.2643 [DOI] [PubMed] [Google Scholar]
- 57.Sternberg CN, Ten Bokkel Huinink WW, Smyth JF, et al. Docetaxel (Taxotere), a novel taxoid, in the treatment of advanced colorectal carcinoma: an EORTC early clinical trials group study. Br J Cancer. 1994;70(2):376–379. doi: 10.1038/bjc.1994.309 [DOI] [PMC free article] [PubMed] [Google Scholar]