Abstract
A patient with a prosthetic joint infection (PJI) complicated with deep surgical site infection due to vancomycin-susceptible Enterococcus faecalis. The initial treatment consisted of 10 days with daptomycin plus ampicillin. The hip prosthesis was retained and salvaged with six outpatient sequential doses of oritavancin 1200 mg every seven days without intra-articular irrigation or other surgical interventions. The patient was ambulating independently without symptoms after ten months of the last treatment of oritavancin.
Keywords: Prosthetic joint infection, Enterococcus, Oritavancin
Introduction
Enterococcal infections have presented a variety of treatment challenges for decades. Enterococcus sp., mostly E. faecalis and E. faecium, possess intrinsic characteristics that convey resistance to aminoglycosides, aminopenicillins, and cephalosporins. Enterococcus can develop resistance to glycopeptides through the acquisition of resistance genes located within plasmids and transposons. Enterococci may undergo spontaneous mutations by increasing the minimum inhibitory and bactericidal concentrations against some antimicrobials [1].
Enterococcus sp. has been considered a bacterium with low pathogenicity despite its known ability to cause endocarditis. An increased risk of enterococcal infection is associated with immunosuppression, previous surgery, indwelling urinary or vascular catheters, prolonged hospitalizations, and previous antibiotic therapy. Sites of enterococcal infection include surgical procedural areas like intravascular catheters or intrabdominal spaces. Bacteremia may cause a native heart valve infection.
The complications and failures in treatment are significant after the infection of a prosthetic joint in a total hip arthroplasty (THA). Salvage therapies are frequently attempted due to the limited available therapeutic options. The bacterium's ability to develop multiple mechanisms of resistance often carries unfavorable clinical outcomes, even if there is in vitro laboratory susceptibility.
Case report
A 62-year-old Hispanic female with essential hypertension, obesity (BMI 30.9 kg/m2) had severe osteoarthritis of both hips for six years. Progressive pain and debility in the right hip significantly impacted her quality of life and independence. There were no comorbidities, such as diabetes mellitus, autoimmune disorders, coagulopathy, peripheral vascular disease, cardiopulmonary disease, and renal disease. The patient had a left total hip arthroplasty performed in the past.
A right total hip replacement was performed without complications. The patient was discharged the next day with outpatient physical therapy and wound care. After seventeen days, most of the staples were removed with four staples remaining. There were no apparent signs of infection such as fever, erythema, warmth or tenderness, suppuration or swelling, or progressive decrease in the range of motion.
On postoperative day 21, the patient reported surgical wound dressing soaked with bloody drainage without symptoms. The dressing had bloody purulent drainage, changed by a home health care nurse. The patient was admitted for observation. The orthopedic surgery team noticed minimal drainage without erythema, pain, or swelling. The vital signs were normal. Pertinent emergency department (ED) laboratory findings included a white blood cell count (WBC) of 7.52 cells/mm3. There were no orders for Inflammatory markers, like erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP). There was no antibiotic prescription upon discharge. The patient was instructed to follow-up in three days with the orthopedic surgeon.
At her follow-up appointment with the orthopedic surgeon on postoperative day 24, the patient reported acute pain at the surgical site and right groin without systemic symptoms. Physical examination of the right hip revealed a well-healed surgical wound with mild serous drainage from the superior aspect of the wound. The wound area had erythema with fluctuance and tenderness to palpation. There was no evidence of a fistula tract. Radiography revealed a right total hip arthroplasty without signs of a hardware malfunction or loosening.
The patient had blood cultures after another admission to the hospital. The initial laboratory results indicated a white blood count (WBC) of 14.7 cells/mm3 with 57.8 % neutrophils, 27.9 % lymphocytes, 13.4 % monocytes, 0.30 % eosinophils, and 0.30 % basophils. Subsequently, the patient went to the operating room for an extensive revision and exploration of the wound.
Antibiotics were held before surgery to avoid masking of microbiology results. Intraoperatively, an area of a cloudy sanguinolent fluid collection was in the deep tissue of periprosthetic space. The fluid collected was cultured for bacteria. The iliotibial band appeared to have dehisced and ruptured with multiple areas of the suture material, having pulled through the iliotibial band, musculature, and tendinous structures. Furthermore, the gluteus medius had avulsed from the greater trochanter. This area was debrided and repaired. The wound received irrigation with a gentamicin solution. The surgeon implanted Antibiotic beads (0.75 g gentamicin and methyl methacrylate) along the incision's length. The joint capsule was not breached during this procedure. The patient received daptomycin intraoperatively (300 mg, 4 mg/kg adjusted body weight of 65 kg) after collecting cultures. The surgeon covered the wound with a negative pressure vacuum assist device (VAC). The initial gram stain report from the specimens obtained intraoperatively revealed gram-positive cocci. Daptomycin was continued daily at the same dose (300 mg) until the organism's identification and phenotype were available.
Three days following the revision, the culture report revealed Enterococcus faecalis as the causative organism with susceptibilities shown in Table 3. The patient received two grams of intravenous ampicillin every 4 h for possible synergism with daptomycin until ten days after revision (34 days after THR). The patient continued to experience pain and swelling with the inability to bear weight on the right lower extremity. The medical-surgical team offered several options to the patient. The options included the following: surgical exploration of the joint with retention of the implant and antibiotics; perform a two-stage procedure (TSP); or salvage therapy with intravenous oritavancin 1200 mg weekly.
Table 3.
Antimicrobial Resistance. Organism: Enterococcus Faecalis.
| Antimicrobial | MIC | Systemic |
|---|---|---|
| Ampicillin | < = 2 | S |
| Gent. Synergy | < = 500 | S |
| Levofloxacin | 2 | S |
| Ceftriaxone | > 32 | R* |
| Ciprofloxacin | < = 1 | S |
| Clindamycin | > 4 | R* |
| Daptomycin | 2 | S |
| Penicillin | 2 | S |
| Tetracycline | < = 4 | S |
| Trimeth/Sulfa | < = 0.5/0.95 | R* |
| Vancomycin | 2 | S |
| Linezolid | 2 | S |
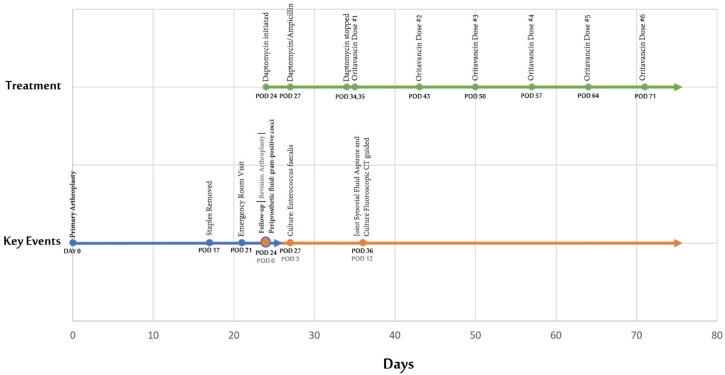
The patient opted for salvage therapy with oritavancin without further surgical intervention. The surgeon aspirated the joint with fluoroscopy guidance for palliation of pain before discharge from the hospital (Fig. 1). Approximately 7 mL of thick dark yellow with slight serosanguineous tinged fluid was aspirated. The synovial fluid analysis showed white blood count (WBC) 40,405 cells/mm3 with 92 % synovial neutrophils and 8 % synovial lymphocytes, as shown in Table 2. The first dose of oritavancin was administered eleven days after revision (35 days after THR), with additional treatments delivered an outpatient (Fig. 2).
Fig. 1.
Fluoroscopy-guided joint aspiration (POD 36).
Table 2.
Synovial Fluid Analysis.
| Synovial Fluid Analysis | Post-Operative Day 35 |
|---|---|
| Source | Synovial |
| Color | Red |
| Appearance | Cloudy |
| Viscosity | Highly Viscous |
| WBC (Auto) | 40,405 cells/mm3 |
| RBC (Auto) | 33,000 cells/mm3 |
| Neutrophils | 92 cells/μL (H) |
| Lymphocytes | 8 cells/L |
| Fibrin Clot | Not Present |
Fig. 2.
Timeline of Events.
During the following three days, the patient experienced significant improvement in pain and swelling, allowing her to begin weight-bearing activities. The patient completed six weekly dosages of intravenous oritavancin 1200 mg. Inflammatory markers were measured to follow up as an outpatient. CRP and ESR markers were within normal limits after completion of treatment. After ten months of completing therapy, the patient has been able to ambulate independently without a walker or associated pain.
Discussion
The annual prosthetic joint infection (PJI) incidence rate in the United States, expressed as a percentage of the total number of hip arthroplasties performed, increased from 1.99 % in 2001 to 2.18 % in 2009 [2]. Risk factors for PJI include the presence of comorbidities such as rheumatoid arthritis, diabetes mellitus, malignancy, chronic kidney disease, obesity, lymphedema, chronic use of steroids, immunosuppression, and prior infection at the surgical site [3]. Most early-onset PJIs present with acute onset of one or more of the following findings: joint pain, warmth, erythema, induration or edema at the incision site, wound drainage, or dehiscence, joint effusion, and fever.
In a 2013 consensus report of experts (including orthopedic surgeons, infectious disease specialists, and clinical researchers), success after PJI treatment was defined by three factors: (1) eradication of infection, (2) no further surgical intervention for infection, and (3) no mortality associated with PJI [4].
The management approach of prosthetic joint infections depends on the timing and microbiology of the infection, condition of the joint and implant, quality of the soft tissue envelope, and individual patient circumstances. Over the last three decades, there has been significant debate around the corrective surgical approach for PJI. The three most common surgical techniques include a one-stage procedure (OSE), a two-stage procedure (TSP), and most recently, a combined approach known as debridement, antibiotics and implant retention (DAIR). Each of these procedures has its advantages and disadvantages. A multicenter, retrospective cohort study performed by Kandel et al. reported a combined treatment failure rate of 24.8 % at two years following OSE or TSP; at four years, the failure rate for OSE was reported as 36 % and 32 % for TSP [5].
The surgical therapeutic approach involves the culture of the affected tissue before antimicrobial therapy. Targeted therapy should include a treatment course of 4–8 weeks with intravenous or oral treatment. Prosthetic joint infections with streptococci and enterococci occur in approximately 10 % of cases [2, Table 1 from 2]. Enterococcal prosthetic hip infections are rare, but their occurrence generally portends a poor prognosis. Failure of treatment correlates to the unique microbiological features of various enterococcal species, which restricts the selection of therapeutic options within in vivo activity against deep-seated infections. Factors to be considered to achieve successful outcomes are drug interactions, joint space and bone concentrations, and patient's allergic profiles.
Table 1.
Inflammatory Markers.
| Post-Operative Day (POD) | 21 | 24 | 25 | 26 | 27 | 28 | 29 | 31 | 35 | 37 | 38 | 48 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ESR | 18.00 | 25.00 | 26.00 | 39.00 | 39.00 | 31.00 | 28.00 | |||||
| CRP | 1.62 | 1.10 | 1.21 | 2.80 | 0.23 | |||||||
| Procal | <0.05 | <0.05 | ||||||||||
| WBC | 7.52 | 14.70 | 7.60 | 11.30 | 9.80 | 8.30 | 8.30 | 10.40 | 8.05 | 6.66 | ||
| NEU% | 57.80 | 66.20 | 46.20 | 29.00 | 33.00 | 28.00 | 37.00 | 55.00 | ||||
| BAND% | 0.00 | 0.00 | 0.00 | 0.00 | ||||||||
| LYM% | 27.90 | 26.40 | 42.30 | 58.00 | 42.00 | 50.00 | 37.00 | 33.80 | ||||
| MON% | 13.40 | 7.00 | 9.60 | 5.00 | 17.00 | 14.00 | 19.00 | 9.60 | ||||
| EOS% | 0.30 | 0.00 | 1.30 | 8.00 | 7.00 | 8.00 | 7.00 | 0.90 | ||||
| BAS% | 0.30 | 0.10 | 0.30 | 0.00 | 0.00 | 0.00 | 0.00 | 0.50 | ||||
| IG% | 0.30 | 0.30 | 0.30 | 0.30 | 0.40 | 0.40 | 0.40 | 0.20 |
The treatment of enterococcal infections depends on the complexity of the case. Some examples include infections of the urinary tract, the surgical site involving skin and soft tissue, and uncomplicated septicemia from a retained intravascular device. However, even for uncomplicated infections, failure rates with monotherapy could be as high as 60 %. The combination of daptomycin and ampicillin has been effective in small cohorts in patients with higher comorbidity index scores involving chronic kidney disease, renal replacement therapy, and renal transplant recipients [6]. However, published reports on the use of this antibiotic combination is rare on deep-seated infections associated with joint hip arthroplasty.
Oritavancin has been recently used successfully in bone and joint infections and in cases in which a hip prosthetic device was salvaged with sequential dosing. Two case reports of single-stage revision with antibiotic spacer and subsequent dosing of oritavancin have proven successful [7]. We used oritavancin as salvage therapy with excellent clinical outcomes.
Oritavancin is a long-acting lipoglycopeptide antibiotic initially approved for the treatment of acute bacterial skin and skin structure infection (ABSSSI) and supported by the phase 3 SOLO program [[8], [9], [10], [11]]. Its terminal half-life is 245 h. Dosage adjustments are unnecessary for patients with mild to severe renal or mild to moderate hepatic impairments [[8], [9], [10], [11], [12]]. Given its protein binding of 85 %, with a single dose of 1200 mg, the predicted free drug concentrations at seven days would exceed the breakpoints for labeled pathogens [12]. The pharmacokinetic profile has been unexplored in humans [13]. The pharmacokinetic profile of oritavancin in rabbits showed rapid distribution in bone tissues. Oritavancin exerts bactericidal activity against gram-positive bacteria, including E. faecalis and E. faecium, regardless of vancomycin susceptibility [14]. Phase 3 SOLO studies and real-world experience demonstrated the safety and tolerability of oritavancin [[8], [9], [10], [11],15].
Recent studies showed clinical success with multiple sequential doses of oritavancin when used as initial or salvage therapies in bone and joint infections caused by S. aureus [[16], [17], [18], [19], [20]]. However, data on the treatment of enterococcal infections using oritavancin are sparse. Our case provides additional information regarding the practical and well-tolerated administration of oritavancin as salvage therapy for a complicated enterococcal prosthetic device infection.
We conducted a literature review to identify similar cases involving prosthetic total hip infections with enterococci. Kheir et al. provide data of 87 patients with hip PJIs, of whom 33 of them had monomicrobial or mixed microbiology with Enterococci species. The cases tabulated by Kheir et al., include data on several patient indices, including age, gender, body-mass-index, age-adjusted Charlson Comorbidity Index (aCCI), medical comorbidities, presence or absence of a detectable sinus tract, surgical treatment procedure(s), antibiotic therapies, and subsequently clinical and microbiologic outcomes. With regards to surgical management, 24 % of patients underwent a 1-stage surgical procedure, 24 % experienced a 2-stage surgical procedure, and the remaining patients underwent incision and drainage of the joint without debridement. Approximately 47 % of patients undergoing a surgical procedure plus antibiotics with or without lavage were clinical failures.
Among the orthopedic literature, it is clear that infected prosthetic joints must undergo debridement and lavage in addition to one of the three recognized surgical approaches. Our case is different from those of Kheir et al. because the joint capsule was not lavaged surgically. The only intervention that involved the patient's joint capsule was the diagnostic CT-guided fluoroscopic arthrocentesis. We attribute this patient's success to multiple factors. First, daptomycin and ampicillin susceptibilities were identified early in the course with seven days of administration. Several studies suggest MICs ≤ 0.25 mg/L for vancomycin-susceptible E. faecalis is an accepted criterion for oritavancin use. The general well-being of the patient besides the PJI may have contributed to a favorable outcome.
Conclusion
Prosthetic joint infections treatment includes debridement, surgical lavage, local and systemic antimicrobial therapy. A literature review on enterococcal monomicrobial and mixed infections of prosthetic hip devices reveals microbiological eradication in 28 of 33 (85 %) patients treated with multiple antibiotic regimens, mostly ampicillin and vancomycin. The infecting pathogen in our patient showed susceptibility to ampicillin, vancomycin, and daptomycin, although borderline susceptibility to the latter. After an extensive review of the English literature, this case is unique. The hip prosthesis infected with Enterococcus faecalis was retained and salvaged with six sequential doses of oritavancin 1200 mg intravenously weekly without intra-articular irrigation or any other surgical intervention.
Disclosures
The patient signed informed consent to publish this article.
Funding
None.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
CRediT authorship contribution statement
Jullian P. Nguyen: Investigation, Writing - original draft, Data curation. Brian X. Contreras: Investigation, Writing - original draft. Miguel Sierra-Hoffman: Conceptualization, Writing - review & editing. Kim Saddler: Investigation, Writing - original draft. Mark L. Stevens: Methodology, Supervision, Project administration. Miriams T. Castro-Lainez: Supervision, Writing - review & editing. Brett Knox: Investigation, Writing - original draft. Rafael J. Deliz: Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors do not have any competition or financial conflicts of interest to disclose. The authors declare that the study was unfunded and was the result of volunteer work.
Contributor Information
Jullian P. Nguyen, Email: jullianpnguyen@gmail.com.
Brian X. Contreras, Email: bcx0727@gmail.com.
Miguel Sierra-Hoffman, Email: msh.xatracho@gmail.com.
Kim Saddler, Email: kim.saddler@detar.com.
Mark L. Stevens, Email: markmd205@gmail.com.
Miriams T. Castro-Lainez, Email: Mtcastrolainez@gmail.com.
Brett Knox, Email: bknox@pharmacy.tamhsc.edu.
Rafael J. Deliz, Email: rdeliz@uiwtx.edu.
References
- 1.Sava I.G., Heikens E., Huebner J. Pathogenesis and immunity in enterococcal infections. Clin Microbiol Infect. 2010;16:533–540. doi: 10.1111/j.1469-0691.2010.03213.x. [DOI] [PubMed] [Google Scholar]
- 2.Tande A.J., Patel R. Prosthetic joint infection. Clin Microbiol Rev. 2014;27:302–345. doi: 10.1128/CMR.00111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lenguerrand E., Whitehouse M.R., Beswick A.D., Kunutsor S.K., Burston B., Porter M. Risk factors associated with revision for prosthetic joint infection after hip replacement: a prospective observational cohort study. Lancet Infect Dis. 2018;18:1004–1014. doi: 10.1016/S1473-3099(18)30345-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diaz-Ledezma C., Higuera C.A., Parvizi J. Success after treatment of periprosthetic joint infection: a Delphi-based international multidisciplinary consensus. Clin Orthop Relat Res. 2013;471(7):2374–2382. doi: 10.1007/s11999-013-2866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kandel C.E., Jenkinson R., Daneman N., Backstein D., Hansen B.E., Mueller M.P. Predictors of treatment failure for hip and knee prosthetic joint infections in the setting of 1- and 2-stage exchange arthroplasty: a multicenter retrospective cohort. Open Forum Infect Dis. 2019:6. doi: 10.1093/ofid/ofz452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sierra-Hoffman M., Iznaola O., Lamp K. Daptomycin and ampicillin combination for treatment of enterococcus faecalis endocarditis. Infect Dis Clin Pract. 2015;23:198–201. [Google Scholar]
- 7.Antony S.J., Cooper L.G. Use of oritavancin (Novel New Lipoglycopeptide) in the treatment of prosthetic joint infections (PJI): a possible alternative novel approach to a difficult problem. Infect Disord Drug Targets. 2017;17:77–80. doi: 10.2174/1871526517666161108130148. [DOI] [PubMed] [Google Scholar]
- 8.Redell M., Sierra-Hoffman M., Assi M., Buchan M., Chansolme D., Gandhi A. The CHROME study, a real-world experience of single- and multiple-dose oritavancin for treatment of gram-positive infections. Open Forum Infect Dis. 2019:6. doi: 10.1093/ofid/ofz479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corey G.R., Kabler H., Mehra P., Gupta S., Overcash J.S., Porwal A. Single-dose oritavancin in the treatment of acute bacterial skin infections. N Engl J Med. 2014;370:2180–2190. doi: 10.1056/NEJMoa1310422. [DOI] [PubMed] [Google Scholar]
- 10.Corey G.R., Good S., Jiang H., Moeck G., Wikler M., Green S. Single-dose oritavancin versus 7-10 days of vancomycin in the treatment of gram-positive acute bacterial skin and skin structure infections: the SOLO II noninferiority study. Clin Infect Dis. 2015;60:254–262. doi: 10.1093/cid/ciu778. [DOI] [PubMed] [Google Scholar]
- 11.Corey Gr, Arhin Ff, Wikler Ma, Sahm D.F., Kreiswirth B.N., Mediavilla J.R. Pooled analysis of single-dose oritavancin in the treatment of acute bacterial skin and skin-structure infections caused by Gram-positive pathogens, including a large patient subset with methicillin-resistant Staphylococcus aureus. Int J Antimicrob Agents. 2016;48:528–534. doi: 10.1016/j.ijantimicag.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 12.Rubino C.M., Bhavnani S.M., Moeck G., Bellibas S.E., Ambrose P.G. Population pharmacokinetic analysis for a single 1,200-milligram dose of oritavancin using data from two pivotal phase 3 clinical trials. Antimicrob Agents Chemother. 2015;59:3365–3372. doi: 10.1128/AAC.00176-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehoux D., Ostiguy V., Cadieux C., Malouin M., Belanger O., Far A.R. Oritavancin pharmacokinetics and bone penetration in rabbits. Antimicrob Agents Chemother. 2015;59:6501–6505. doi: 10.1128/AAC.00981-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendes R.E., Castanheira M., Farrell D.J., Flamm R.K., Sader H.S., Jones R.V. Longitudinal (2001-14) analysis of enterococci and VRE causing invasive infections in European and US hospitals, including a contemporary (2010-13) analysis of oritavancin in vitro potency. J Antimicrob Chemother. 2016;71:3453–3458. doi: 10.1093/jac/dkw319. [DOI] [PubMed] [Google Scholar]
- 15.Redell M., Moeck G., Lucasti C., Durso S., Kennedy C., Fusaro K. A real-world patient registry for oritavancin demonstrates efficacy and safety consistent with phase 3 SOLO program. Open Forum Infect Dis. 2018;5:ofy051. doi: 10.1093/ofid/ofy051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrisette T., Miller M.A., Montague B., Barber G.R., McQueen R.B., Krsak M. Long-acting lipoglycopeptides: “lineless antibiotics” for serious infections in persons who use drugs. Open Forum Infect Dis. 2019;6 doi: 10.1093/ofid/ofz274. ofz274–ofz274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart C.L., Turner M.S., Frens J.J., Snider C.B., Smith J.R. Real-world experience with oritavancin therapy in invasive gram-positive infections. Infect Dis Ther. 2017;6:277–289. doi: 10.1007/s40121-017-0156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulz L.T., Dworkin E., Dela-Pena J., Rose W.E. Multiple-dose oritavancin evaluation in a retrospective cohort of patients with complicated infections. Pharmacotherapy. 2018;38:152–159. doi: 10.1002/phar.2057. [DOI] [PubMed] [Google Scholar]
- 19.Foster R., Philavong K., Weissman S., Tang X., Bookstaver P. Oritavancin for the treatment of daptomycin nonsusceptible vancomycin-resistant enterococci osteomyelitis. Infect Dis Clin Pract. 2017;26:1. doi: 10.1097/IPC.0000000000000517. [DOI] [Google Scholar]
- 20.Dahesh S., Wong B., Nizet V., Sakoulas G., Tran T.T., Aitken S.L. Treatment of multidrug-resistant vancomycin-resistant enterococcus faecium hardware-associated vertebral osteomyelitis with oritavancin plus ampicillin. Antimicrob Agents Chemother. 2019:63. doi: 10.1128/AAC.02622-18. [DOI] [PMC free article] [PubMed] [Google Scholar]