Abstract
Continuous light can be used as a tool to understand the diurnal rhythm of plants and it can also be used to increase the plant production. In the present research, we aimed to investigate the photosynthetic performance of V. radiata under continuous light as compared with the plants grown under normal light duration. Chlorophyll a fluorescence transient (OJIP test) technique was used to understand the effect on various stages of photosynthesis and their consequences under continuous light condition. Various Chl a Fluorescence kinetic parameters such as Specific energy fluxes (per QA-reducing PSII reaction center (RC)) (ABS /RC; TR0/RC; ET0/RC; DI0/RC), phenomenological fluxes, leaf model, (ABS/CSm; TR/CSm; ETo/CSm), Quantum yields and efficiencies (φPo; φEo; Ψo) and Performance index (PIabs) was extracted and analysed in our investigation. Conclusively, our study has revealed that continuous light alters the photosynthetic performance of V. radiata at a different point but also improve plant productivity.
Keywords: Chlorophyll fluorescence, Continuous light, OJIP test, Performance index, Specific energy fluxes, Vigna radiata
Highlights
-
•
Effect of continuous light on V. radiata photosynthetic performance with comparison of plant grow under normal light period.
-
•
Chlorophyll a fluorescence kinetic (OJIP test) technique was used in present study.
-
•
Various technical fluorescence parameter were analysed using Handy PEA (Plant efficiency analyzer).
-
•
The study reveals that continuous light increase the density of active reaction centers and performance index in V. radiata.
1. Introduction
The plant interacts with various abiotic factors in their life cycle, among them light is also an important factor. The energy embedded in photon perceived by chlorophyll and converted into energy molecules ATP and NADPH. This energy consumed in fixation of CO2 and synthesize photosynthetic assimilate. Apart from, the light also up-regulates various processes such as seed germination, seedling development, plant growth, biomass production, flowering, reproduction, and circadian rhythms [1]. Nowadays, use of artificial light is enhanced in the greenhouse, plant factories and glasshouse to improve the crop production at the commercial scale to meet the demands of the growing population [2]. To achieve high production plant growers are using the different type of light based on their quality, quantity and duration. Extension of light period to 24 h light periods (continuous light) is also in trend for crop improvement [3]. In northern countries, supplementary artificial light is used in the greenhouse to gain higher production [4]. Velez-Ramirez et al. (2011) reported that cultivation of tomato under continuous light is more beneficial for production [5].
The circadian rhythm regulates the plant physiological processes by sensing the length of day/night periods. Nowadays, an extension of light period by using supplement lighting is a common method for the plant breeder to improve crop yield. However, plants respond in different ways to this increased photoperiod. Demers et al. (2002) reported that the continuous light can hasten the flowering in tomato initially but later on no change during 14 h and 24 h photoperiod [6]. In Ficus benjamina the dry weight progressively due to the lack of diurnal rhythm in photosynthesis under continuous light with increasing lighting periods at 16, 20 and 24 h/day [7]. It has been reported that in Brassica rapa L continuous light increases chlorophyll content [8], while in some plants such as soybean, wheat, barley, millet, pea, alfalfa, sunflower, kale etc have no chlorosis effect [9]. The negative effect of continuous light reported in the form of leaf injury in onion [10], cucumber [11]. In tomato species (Solanum lycopersicum) grown under continuous light with constant temperature decrease in the rate of photosynthesis and the maximum quantum efficiency of photosystem II (PSII) were found [5,12]. Dorais 1992 found that continuous light causes a reduction in the size and increased activity of PSII in pepper plants [13].
Chlorophyll fluorescence parameters are important tools to study the effects of different environmental stresses on photosynthesis [14]. It is one of the important methods to analyses the role of PSII and its response towards changes in the environment and growth conditions [15,16]. The PIabs and Fv/Fo are sensitive parameters related to PSII related damage and efficiency. As the above-mentioned fluorescence parameters, the effects of continuous light conditions on primary photochemistry of PSII of Vigna radiata and fate of dark reactions are also clearly reflected by chlorophyll fluorescence parameters. However, relevant research has not been reported yet. In the present study, the aim is to analyse the chlorophyll fluorescence characteristics of Vigna radiata grown under continuous light conditions, an effort is proposed to known about the effects of continuous light on photosystem efficiency.
2. Material and method
2.1. Collection of plant material and growth condition
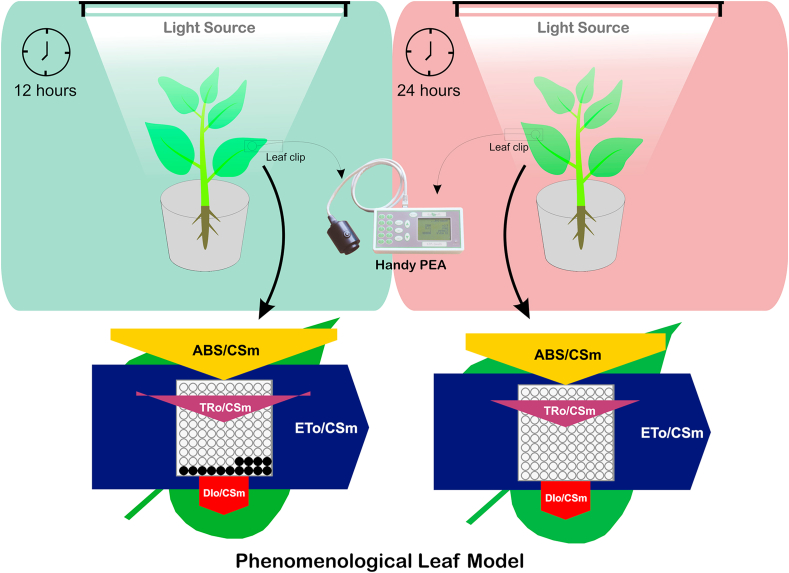
The seeds of the Vigna radiata were collected from the MPUAT Udaipur. Before the experiment seeds were disinfected by immersing in 1% (v/v) NaClO for 3–5 min, then washed thrice with double distilled water. The seeds were grown in pots (12 × 36cm) and labelled 12 h and 24 h artificial light treatment (Fig. 1, Fig. 2a). Both plants were grown under artificial light (maximum photosynthetically active radiation (PAR) 1800 μmol photons m− 2 s−1) for 12 h and 24 h of light period respectively and maintain the relative humidity 50–60% and temperature at 28 ± 2 °C.
Fig. 1.
(a) Schematic presentation of experiment design and effect of on V. radiata.
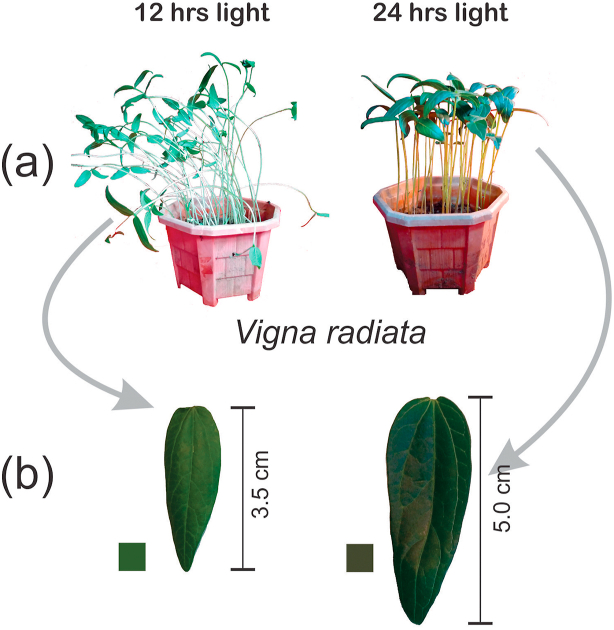
Fig. 2.
(a) Effect of continuous light and morphological differences on V. radiata. and (b) effect on leaf size of V. radiata.
2.2. Chlorophyll fluorescence measurement
Chlorophyll a fluorescence was measured using plant efficiency analyser (Handy PEA fluorimeter, Hansatech instruments Ltd. England) after 10 days of seedling emergence on the middle region of mature leaves. Before measurement mung seedling was dark-adapted for 50–60 min at 26 °C. Thereafter, Chl a fluorescence signals were analysed with the Biolyzer v.3.0.6 software (developed by Laboratory of Bioenergetics, University of Geneva, Switzerland). Appropriate numbers of replicates were taken and the experiment was repeated three times to ensure the results. Abbreviations, formulas, and definitions of the JIP-test parameters used in the current study are presented in Table 1, [17,18].
Table 1.
Abbreviations, formulas, and definitions of the JIP-test parameters used in the current study (Strasser et al., 2000, 2004).
| Technical fluorescence parameter | Formulas | Definitions |
|---|---|---|
| Data extracted from the recorded fluorescence transient O-J-I-P | ||
| tFM | Time (in ms) to reach the maximal fluorescence intensity FM | |
| Area | total complementary area between the fluorescence induction curve and F = FM | |
| F0 | ≅ F50μs or ≅ F20μs | minimal fluorescence (all PSII RCs are assumed to be open) |
| FV | ≡ FM - F0 | maximal variable fluorescence |
| FM (= FP) | maximal fluorescence, when all PSII RCs are closed | |
| Specific energy flux | ||
| ABS /RC | = M0 (1/VJ)(1/ϕPo) | absorption flux (of antenna Chls) per RC |
| TR0/RC | = M0 (1/VJ) | trapped energy flux (leading to QA reduction) per RC |
| ET0/RC | = M0 (1/VJ)ψEo | electron transport flux (further than QA−) per RC |
| Quantum yields and efficiencies | ||
| ϕPo | ≡ TR0/ABS = [1-(F0/FM)] | maximum quantum yield for primary photochemistry |
| ψEo | ≡ ET0/TR0 = (1-VJ) | efficiency/probability for electron transport (ET), i.e. efficiency/probability that an electron moves further than QA− |
| ϕEo | ≡ ET0/ABS = [1-(F0/FM)]ψEo | quantum yield for electron transport (ET) |
| PIABS | ≡ | performance index (potential) for energy conservation from exciton to the reduction of intersystem electron acceptors |
| RC/ABS | = γRC/(1-γRC) = ϕPo (VJ/M0) | QA-reducing RCs per PSII antenna Chl (reciprocal of ABS/RC) |
| Sm | = Area/(FM - F0) | normalized total area above the OJIP curve |
| N | = (SM/SS) = SMM0 (1/VJ) | the number of turnovers |
| DIo/RC | = (ABS/RC) – (TR0/RC) | total energy dissipated per reaction center (RC) |
| FV/FO | ratio of rate constants for photochemical and nonphotochemical use of RC excitation energy |
|
| (dV/dt)0 | 4. (F300 – F0) /(FM – F0) | maximal rate of the accumulation of the fraction of closed reaction centers |
| kN | = (ABS/CS). kF. (1 /FM) | Non-photochemical rate constant |
| kP | = (ABS/CS). kF. {(1 /F0) – (1 /FM)} | photochemical rate constant |
| ABS /CSm | Fm | absorption flux (of antenna Chls) per RC |
| Phenomological energy flux | ||
| TR0 /CSm | (Fv/Fm) ·(ABS/CSm) | trapped energy flux (leading to QA reduction) per RC |
| ET0 /CSm | (Fv/Fm) · (1 − Vj) ·(ABS/CSm) | electron transport flux (further than QA−) per RC |
| DI0 /CSm | (ABS /CS0) – (TR0 /CSm) | total energy dissipated per reaction center (RC) |
| RC /CSm | φPo. (VJ /M0). Fm | Reaction centre per cross section |
3. Results and discussion
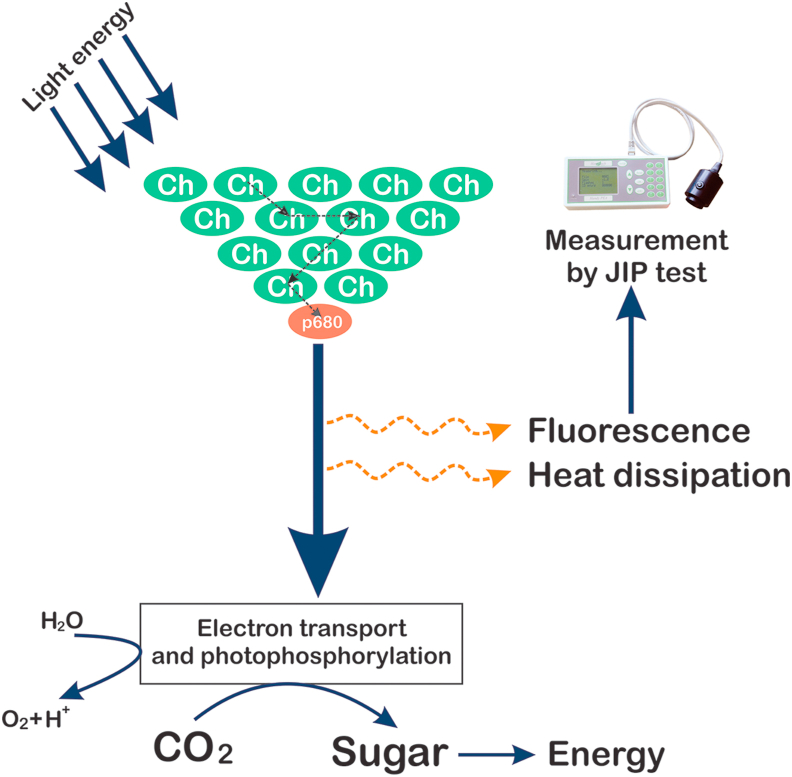
After emerging from seeds seedling has to start photosynthesis. In the natural environment, many abiotic and biotic factors affect the process of photosynthesis. The photosystem photochemistry is mostly affected by these factors. Plants have different mechanisms to manage with different environmental conditions by changes in photosystem II apparatus (Fig. 3). In our study effect of continuous light on V. radiata plant is revealed, by using OJIP curve, specific energy flux, phenomenological energy flux.
Fig. 3.
Hypothetical mechanism of fluorescence emission and photosynthetic electron transport in V. radiata. under continuous light. (Ch - Chlorophyll molecule).
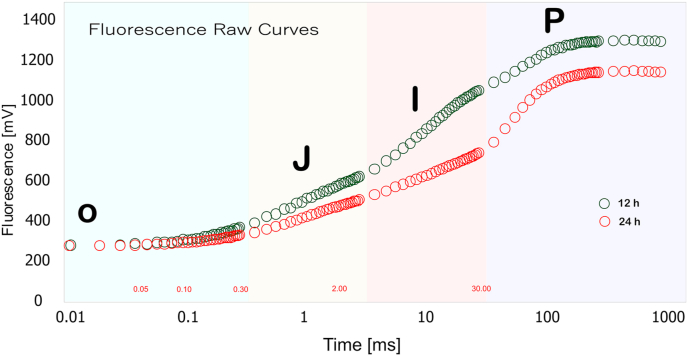
After continuous light treatment no change in the primary quinone acceptor of electrons QA in PSII (O-J phase), whereas quenching of the fluorescence controlled by the donor site of PSII and the characteristic activity of water splitting system (J-I phase) were slightly altered showed weaker I and P peaks compared with normal 12 h light treated plants. After reaching Fo the fluorescence yields continuously rise more slowly reaching a peak after 1–2 s. This fluorescence is variable (Fv) as it is responsive to changes in electron flow through photosystem, being regulated by the redox state of photosystem II electron acceptors, inhibition of a reaction on the photo oxidizing site of photosystem II decreases the Fv. The decrease in fluorescence at J, I, P (Fig. 4) may be due to two reasons, first by inhibition of electron transport at the donor site of the PS II which results in the accumulation of P680+ [19,20] and second due to a decrease in the pool size of QA−. The area over the fluorescence induction curve between Fo and Fm is proportional to the pool size of the electron acceptor QA on the reducing side of PS II.
Fig. 4.
OJIP fluorescence induction curve. Transient curves of each line represent the average of 10 measurements per treatment.
A decline in Fm is often associated with the protective interconversion of violaxanthin to zeaxanthin, in two independent reactions (the xanthophyll cycle), which leads to non-radiative (non-photochemical quenching, NPQ) energy dissipation [21].
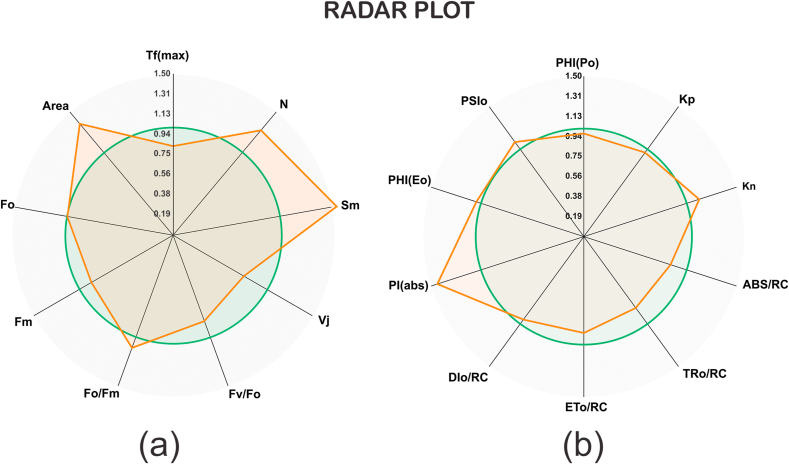
3.1. Tf (max)
Time to reach maximal fluorescence Tf (max) of mung plant treated with continuous light (24 h) decreases as compared to the plant growing under natural light condition (12 h). A decrease in the time to reach Fm (Tfm) (Fig. 3) by continuous light led to an increase in the average redox state of QA in the period from 0 to Tfm [18].
3.2. Area
Area parameter (the total complementary area between the fluorescence induction curve and Fm) was higher for 24 h light treated plant than 12 h light treated plant. The Area is relative to the pool size of the electron acceptors QA on the reducing side of PSII. Previously it was reported that a decrease in Area parameter will be due to electron transfer from the RCs to the quinone pool is blocked. Hence, improving the Area (Fig. 3) of mung bean by application of continuous light may be the result of increasing the electron transfer from the RC to the quinone pool [22].
3.3. FO and Fm
Fluorescence at 50μs is denoted as Fo when the all primary quinone acceptor (QA) is in the oxidized state (open). Plants exposed to CL shows no significant effect on Fo while a decrease in Fm meanwhile variations are also reported in value of Fv/Fo (Fig. 5a). Parameter Fo can be used as an indicator for irreversible damage in PSII, associated with LHCII dissociation and blocking of electron transference on the reductant side of PSII [23]. Decreasing Fm by application of CL may be associated with the less efficiency of PSII activity due to conformational changes in the D1 protein, causing alterations in the properties of PSII electron acceptors [24].
Fig. 5.
Radar Plot showing various Technical fluorescence parameter. Each line represents the average of 10 measurements per treatment.
3.4. Fv/Fo
Fv/Fo, a parameter that accounts for the simultaneous variations in Fm and Fo in determinations of the maximum quantum yield of PSII is higher in the plant under NL condition as compare than plants under CL. Fv/Fo is the most sensitive component in the photosynthetic electron transport chain [25]. The decrease in Fv/Fo is due to either by a decrease in Fv or increase in Fo. In the present study, there is no significant difference in Fo hance the decrement of Fv/Fo Is due to a decrease in variable fluorescence (Fv). The lower value of Fv/Fo (Fig. 5a) in treated leaves indicates the change in the rate of electron transport from PSII to the primary electron acceptors with the reduction in the number and the size of RC, which have also been reported in different plants exposed to the disease and the environmental stresses [26,27].
3.5. Variable fluorescence Vj
Relative variable fluorescence at the J-step (2 ms) was decreased in plants under CL condition. Vj was a measure of the fraction of the primary quinine electron acceptor of PSII that was in its reduced state [QA- /QA(total)] [28]. Fig. 5a shows that there is a decrease in value of Vj indicate that under continuous light the electron transfer at the donor side of PSII was affected.
3.6. Sm and N
Complementary Area (Sm) increased in continuous light condition than natural light. The parameters Sm, assessing of the electron transporter PQ pool between PS II and PSI. increase in Sm in continuous light displayed a reduced electron transport between these photosystems [29] (Fig. 5a). Turnover number (N) is higher in continuous light condition than natural light. N indicates how many times QA has been reduced in the period from 0 to Tfm [17]. However, an increase in N also shown by PSI cyclic electron transport, as a photoprotection method.
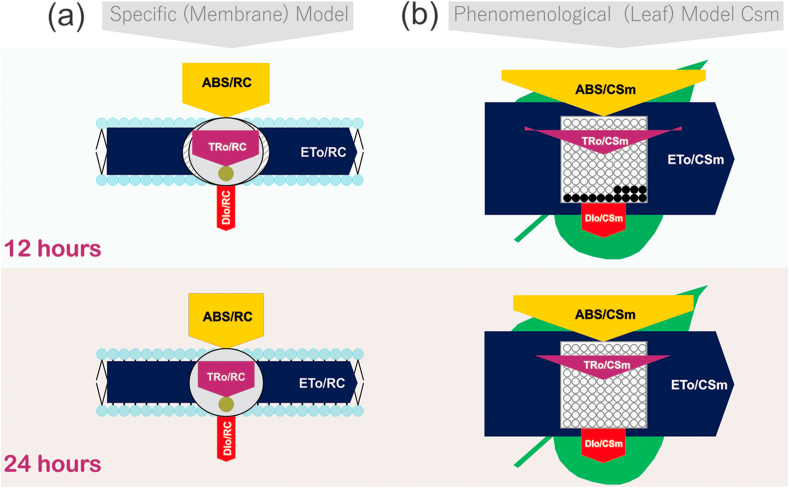
3.7. Specific energy flux (membrane model)
Specific energy flux i. e. ABs /RC, TRo/RC, ETo/RC and DIo/RC were lower in plants treated with CL as compare to NL (Fig. 6a). ABs/RC is lower in CL treated plants; TRo/RC and DIo/RC is equal in both conditions. According to leaf pipeline model in continuous light, there is more active RC but the lower value of specific energy flux (ABs /RC, TRo/RC, ETo/RC and DIo/RC) shows the decreased ability of RC to the reduction of plastoquinone The flux ratios ABS/RC, TRo/RC, ETo/RC and DIo/RC decreased under continuous light treatment. The ABS/RC represents the total number of photons absorbed by Chl molecules of all RCs divide by the total number of active RCs. It is influenced by the ratio of active/inactive RCs and as the number of active centers increased, the ratio ABS/RC decreased. TRo/RC represents the maximal rate by which an exciton is trapped by the RC resulting in the reduction of QA. A decrease in this ratio indicates that all the QA has not been reduced. Reduced ETo/RC describes that the reoxidation of reduced QA via electron transport in an active RC is decreased because of a greater number of the active RC, hence it only reflects the activity of active RCs. Fig. 6a shows a decreased per active RC but overall increased electron transport, due to more active RCs. DIo/RC represents the ratio of the total dissipation of untrapped excitation energy from all RCs concerning the number of active RCs. Dissipation occurs as heat, fluorescence and energy transfer to other systems. It is also influenced by the ratios of active/inactive RCs. The ratio of total dissipation to the number of active RCs not so affected (DIo/RC) due to the efficient use of energy by the active RCs [30].
Fig. 6.
Energy pipeline models (a) Specific energy flux (membrane model) of 12 h and 24 h light treatment and (b) Phenomenological energy flux (Leaf model) of 12 h and 24 h light treatment.
3.8. Phenomenological energy flux (leaf model)
Phenomenological energy fluxes i.e. ABS/CSm, TRo/CSm, ETo/CSm and DIo/CSm are shown in Fig. 6b. In phenomenological fluxes per cross-section and the leaf model, ABS/CSm,TR/CSm, ETo/CSm and DIo/CSm not significantly affected than control (Fig. 6b). Thus ABS/CSm reflects an increased density of active reaction centers in response to continuous light. Thus, TR0/CSm and ETo/CSm indicates that the efficiency of trapping and PSII activity are similar to control plants. An increase in the density of active RCs (indicated as open circles) and a decrease in the density of inactive RCs (indicated as filled circles) was observed in response to continuous light treatment [31,32]. CL light treated plants shows a broader leaf which can be proved by Boardman, (1977) [33] who reported that plants growing in full sunlight had increased leaf length, leaf size (Fig. 2b) and therefore increased leaf area. This can be assigned to the fact that leaves grown in full sunlight had higher stomatal conductance than shade leaves.
Continuous light-grown plants have more leaf greenness due to enhanced chlorophyll content. Synthesis of chlorophyll can occur only when a plant is irradiated since the reduction of protochlorophyllide to chlorophyllide is light-dependent [34,35]. Furthermore, other steps of chlorophyll synthesis or degradation are regulated by light and controlled by phytochrome and the blue light receptor [34].
Besides chlorophyll, the role of carotenoids and tocopherols are found in process of photoprotection. Carotenoids play a major role in photoprotection by shading the reaction and xanthophyll cycle [36].
3.9. Kp and Kn
An increase in Kn (de-excitation rate constants for nonphotochemical reaction) whereas no significant effect on Kp (de-excitation rate constants for photochemical reaction). Under continuous light, the extra energy exceeds the capacity for utilization, then the extra Chl redials quenched by photoprotective processes and dissipation of extra energy as heat. These mechanisms are measured from the decrease of Chl fluorescence from PSII and are collectively referred to as non-photochemical quenching (NPQ) (Fig. 3). NPQ includes short-term responses to rapid fluctuations in light, as well as responses that occur over longer periods allowing for acclimation to high light exposure [37].
NPQ and antioxidant systems [38] have the protective roles, despite these the PSII centers chance to damage at all light intensities and hence, an efficient PSII repair cycle continues to resume the function of the PS II complexes. Besides the PSII repair cycle plant have another alternative cycle to cope with light stress, among them cyclic electron flow, the water cycle, photorespiration, mitochondrial respiration in excess light [39].
3.10. Maximum quantum yield
Besides, the quantum yield of primary photochemistry (φPo), the quantum yield for electron transport (φEo) and the efficiency per trapped excitation (Ψo) were not affected (Fig. 5b). These parameters can provide relevant information on electron transport activity at the PSII acceptor sites [18]. These results suggested that continuous light enhance electron transport at the PSII acceptor site in V. radiata.
3.11. PIabs
Performance index (PIabs) calculated on energy absorption basis and the value of PIabs is higher in the plant under CL (Fig. 5b). PIabs are increased due to increased activity of the RC so the overall activity of the RC is increased. The PI combines three independent functional steps of photosynthesis, the density of RCs in the chlorophyll bed((RC/ABS), excitation energy trapping (φPo) and conversion of excitation energy to electron transport (Ψo), into a single multi-parametric expression [18,40].
The efficiency with which a trapped exciton can move an electron into the electron transport chain further than QA-is Ψo. The PI increased in continuous light, compared to the control, because of a higher number of active RCs and overall energy use efficiency which indicates that the leaves were growing well in continuous light.
4. Conclusion
In the present research paper, the effect of continuous light on chlorophyll fluorescence kinetics of V. radiata and their comparative study against control was presented. Based on our findings and analyses, we concluded that continuous light improves the PSII efficiency by suppressing the light-induced PSII damage and by promoting the repair system and photoprotective strategies. Continuous light has a positive impact on plant morphology, photochemical efficiency of the plant. The non-photochemical quenching and decapitation of excess light in form of heat are the main processes which makes plant acclimated to continuous light. It was also found that continuous light-grown plants had an increased number of active RC and lowered absorption per RC, to avail the excess light. Plants treated with artificial continuous light has increase in leaf size and pigment concentration which gives evidence that plant develops protection strategy towards excessive light. Rise in the Performance index (PIabs) of the plant under continuous light shows that plants have high vitality and efficient use of excess light in electron transportation beyond PS II and carbon assimilation (Calvin cycle). An interruption in the oxygen-evolving complex (OEC) at the donor site of PS II can be managed by the various photoprotective strategy of the plant such as the use of alternative electron donor, antioxidant responses which were our future prospective.
Statistical analysis
The data analysis was done using Biolyzer v.3.0.6 software. All values presented in the paper are means of three independent replicates.
CRediT authorship contribution statement
Deepak Kumar: conceived the idea and designed the research plan, execute the experiment, Conceptualization, Writing - original draft, data analysis. Hanwant Singh: Conceptualization, Writing - original draft, data analysis, prepared all figures and graphical artworks. Shani Raj: execute the experiment. Vineet Soni: execute the experiment, supervised the complete work. All the authors contributed to discussing and reviewing the manuscript. Finally, all the authors read and approved the final version of the manuscript for publication.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors thank the Mohanlal Sukhadia University, India for providing laboratory facilities. Authors are also grateful to Prof. Reto J. Strasser, University of Geneva, Switzerland for his help in the analysis of chlorophyll fluorescence data.
References
- 1.Kaur R., Kaur G., Singh K., Singh B. Phyto-Microbiome Stress Regul. Springer; 2020. Plant growth and development under suboptimal light conditions; pp. 205–217. [Google Scholar]
- 2.Mitchell C.A., Sheibani F. Plant Fact. 2020. LED advancements for plant-factory artificial lighting. [DOI] [Google Scholar]
- 3.Ohyama K., Manabe K., Omura Y., Kozai T., Kubota C. Potential use of a 24-hour photoperiod (continuous light) with alternating air temperature for production of tomato plug transplants in a closed system. Hortscience. 2005 doi: 10.21273/hortsci.40.2.374. [DOI] [Google Scholar]
- 4.Heuvelink E., Bakker M.J., Hogendonk L., Janse J., Kaarsemaker R.C., Maaswinkel R.H.M. Is supplementary lighting worth it? Fruit Veg Tech. 2006;6:20–23. [Google Scholar]
- 5.Velez-Ramirez A.I., Van Ieperen W., Vreugdenhil D., Millenaar F.F. Plants under continuous light. Trends Plant Sci. 2011 doi: 10.1016/j.tplants.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Demers D.A., Gosselin A. Acta Hortic. 2002. Growing greenhouse tomato and sweet pepper under supplemental lighting: Optimal photoperiod, negative effects of long photoperiod and their causes. [DOI] [Google Scholar]
- 7.Mortensen L.M. Diurnal photosynthesis and transpiration of Ficus benjamina L. As affected by length of photoperiod, CO2 concentration and light level. Acta Agric. Scand. Sect. B Soil Plant Sci. 1992 doi: 10.1080/09064719209410206. [DOI] [Google Scholar]
- 8.Fukuda N., Suzuki K., Ikeda H. Effects of supplemental lighting from 23:00 to 7:00 on growth of vegetables cultured by NFT. J. Japanese Soc. Hortic. Sci. 2000 doi: 10.2503/jjshs.69.76. [DOI] [Google Scholar]
- 9.Lefsrud M.G., Kopsell D.A., Augé R.M., Both A.J. Biomass production and pigment accumulation in kale grown under increasing photoperiods. Hortscience. 2006 doi: 10.21273/hortsci.41.3.603. [DOI] [Google Scholar]
- 10.Van Gestel N.C., Nesbit A.D., Gordon E.P., Green C., Paré P.W., Thompson L., Peffley E.B., Tissue D.T. Continuous light may induce photosynthetic downregulation in onion - consequences for growth and biomass partitioning. Physiol. Plantarum. 2005 doi: 10.1111/j.1399-3054.2005.00560.x. [DOI] [Google Scholar]
- 11.Wolff S.A., Langerud A. Fruit yield, starch content and leaf chlorosis in cucumber exposed to continuous lighting. Eur. J. Hortic. Sci. 2006 [Google Scholar]
- 12.Matsuda R., Ozawa N., Fujiwara K. Sci. Hortic.; Amsterdam): 2014. Leaf Photosynthesis, Plant Growth, And Carbohydrate Accumulation Of Tomato Under Different Photoperiods And Diurnal Temperature Differences. [DOI] [Google Scholar]
- 13.Dorais M. Université Laval; 1992. Aspects Culturaux Et Physiologiques De La Tomate Et Du Poivron De Serre Soumis à Un éclairage d’appoint. [Google Scholar]
- 14.Allakhverdiev S.I., Murata N. Environmental stress inhibits the synthesis de novo of proteins involved in the photodamage-repair cycle of Photosystem II in Synechocystis sp. PCC 6803. Biochim. Biophys. Acta Bioenerg. 2004 doi: 10.1016/j.bbabio.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Kalaji M.H., Pietkiewicz S. Some physiological indices to be exploited as a crucial tool in plant breeding. Plant Breed. Seed Sci. 2004;49:19–39. [Google Scholar]
- 16.Bukhov N.G., Carpentier R. Chlorophyll a Fluoresc. Springer; 2004. Effects of water stress on the photosynthetic efficiency of plants; pp. 623–635. [Google Scholar]
- 17.Strasser R.J., Srivastava A., Tsimilli-Michael M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. Probing Photosynth. Mech. Regul. Adapt. 2000 [Google Scholar]
- 18.Strasser R.J., Tsimilli-Michael M., Srivastava A. Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou G.C., Govindjee, editors. Chlorophyll a Fluoresc. A Signat. Photosynth. Springer Netherlands; Dordrecht: 2004. pp. 321–362. [DOI] [Google Scholar]
- 19.Govindje e. Sixty-three years since kautsky: chlorophyll a fluorescence. Funct. Plant Biol. 1995 doi: 10.1071/pp9950131. [DOI] [Google Scholar]
- 20.Schreiber U., Neubauer C. The polyphasic rise of chlorophyll fluorescence upon onset of strong continuous illumination: II. partial control by the photosystem II donor side and possible ways of interpretation. Zeitschrift Fur Naturforsch. - Sect. C J. Biosci. 1987 doi: 10.1515/znc-1987-11-1218. [DOI] [Google Scholar]
- 21.Demmig-Adams B., Adams W.W., III, Barker D.H., Logan B.A., Bowling D.R., Verhoeven A.S. Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiol. Plantarum. 2008 doi: 10.1034/j.1399-3054.1996.980206.x. [DOI] [Google Scholar]
- 22.Ghassemi-Golezani K., Lotfi R. The impact of salicylic acid and silicon on chlorophyll a fluorescence in mung bean under salt stress. Russ. J. Plant Physiol. 2015 doi: 10.1134/S1021443715040081. [DOI] [Google Scholar]
- 23.Goltsev V.N., Kalaji H.M., Paunov M., Bąba W., Horaczek T., Mojski J., Kociel H., Allakhverdiev S.I. Variable chlorophyll fluorescence and its use for assessing physiological condition of plant photosynthetic apparatus. Russ. J. Plant Physiol. 2016 doi: 10.1134/S1021443716050058. [DOI] [Google Scholar]
- 24.Kalaji H.M., Schansker G., Ladle R.J., Goltsev V., Bosa K., Allakhverdiev S.I., Brestic M., Bussotti F., Calatayud A., Dąbrowski P., Elsheery N.I., Ferroni L., Guidi L., Hogewoning S.W., Jajoo A., Misra A.N., Nebauer S.G., Pancaldi S., Penella C., Poli D., Pollastrini M., Romanowska-Duda Z.B., Rutkowska B., Serôdio J., Suresh K., Szulc W., Tambussi E., Yanniccari M., Zivcak M. Frequently asked questions about in vivo chlorophyll fluorescence: practical issues. Photosynth. Res. 2014 doi: 10.1007/s11120-014-0024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohammed G.H., Zarco-Tejada P., Miller J.R. Applications of chlorophyll fluorescence in forestry and ecophysiology. In: DeEll J.R., Toivonen P.M.A., editors. Pract. Appl. Chlorophyll Fluoresc. Plant Biol. Springer US; Boston, MA: 2003. pp. 79–124. [DOI] [Google Scholar]
- 26.Martinazzo E.G., Ramm A., Bacarin M.A. The chlorophyll a fluorescence as an indicator of the temperature stress in the leaves of Prunus persica. Braz. J. Plant Physiol. 2012 doi: 10.1590/S1677-04202013005000001. [DOI] [Google Scholar]
- 27.Janka E., Körner O., Rosenqvist E., Ottosen C.O. High temperature stress monitoring and detection using chlorophyll a fluorescence and infrared thermography in chrysanthemum (Dendranthema grandiflora) Plant Physiol. Biochem. 2013 doi: 10.1016/j.plaphy.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 28.Strasserf R.J., Srivastava A. Govindjee, polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem. Photobiol. 1995 doi: 10.1111/j.1751-1097.1995.tb09240.x. [DOI] [Google Scholar]
- 29.Stirbet A. Govindjee, on the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B Biol. 2011 doi: 10.1016/j.jphotobiol.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Grieco M., Suorsa M., Jajoo A., Tikkanen M., Aro E.M. Light-harvesting II antenna trimers connect energetically the entire photosynthetic machinery - including both photosystems II and i. Biochim. Biophys. Acta Bioenerg. 2015 doi: 10.1016/j.bbabio.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Clark A.J., Landolt W., Bucher J.B., Strasser R.J. Beech (Fagus sylvatica) response to ozone exposure assessed with a chlorophyll a fluorescence performance index. Environ. Pollut. 2000 doi: 10.1016/S0269-7491(00)00053-1. [DOI] [PubMed] [Google Scholar]
- 32.Sunil B., Strasser R.J., Raghavendra A.S. Targets of nitric oxide (NO) during modulation of photosystems in pea mesophyll protoplasts: studies using chlorophyll A fluorescence. Photosynthetica. 2020 doi: 10.32615/ps.2019.183. [DOI] [Google Scholar]
- 33.Boardman N.K. Comparative photosynthesis of sun and shade plants. Annu. Rev. Plant Physiol. 1977 doi: 10.1146/annurev.pp.28.060177.002035. [DOI] [Google Scholar]
- 34.Thompson W.F., White M.J. Physiological and molecular studies of light-regulated nuclear genes in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991 doi: 10.1146/annurev.pp.42.060191.002231. [DOI] [Google Scholar]
- 35.Suzuki J.Y., Bauer C.E. A prokaryotic origin for light-dependent chlorophyll biosynthesis of plants. Proc. Natl. Acad. Sci. U. S. A. 1995 doi: 10.1073/pnas.92.9.3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Djediat C., Feilke K., Brochard A., Caramelle L., Kim Tiam S., Sétif P., Gauvrit T., Yéprémian C., Wilson A., Talbot L., Marie B., Kirilovsky D., Bernard C. Light stress in green and red Planktothrix strains: the orange carotenoid protein and its related photoprotective mechanism. Biochim. Biophys. Acta Bioenerg. 2020 doi: 10.1016/j.bbabio.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 37.Kalaji H.M., Carpentier R., Allakhverdiev S.I., Bosa K. Fluorescence parameters as early indicators of light stress in barley. J. Photochem. Photobiol. B Biol. 2012 doi: 10.1016/j.jphotobiol.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Singh H., Kumar D., Soni V. Copper and mercury induced oxidative stresses and antioxidant responses of Spirodela polyrhiza (L.) Schleid. Biochem. Biophys. Reports. 2020;23:100781. doi: 10.1016/j.bbrep.2020.100781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erickson E., Wakao S., Niyogi K.K. Light stress and photoprotection in Chlamydomonas reinhardtii. Plant J. 2015 doi: 10.1111/tpj.12825. [DOI] [PubMed] [Google Scholar]
- 40.Tsimilli-Michael M., Eggenberg P., Biro B., Köves-Pechy K., Vörös I., Strasser R.J. Synergistic and antagonistic effects of arbuscular mycorrhizal fungi and Azospirillum and Rhizobium nitrogen-fixers on the photosynthetic activity of alfalfa, probed by the polyphasic chlorophyll a fluorescence transient O-J-I-P. Appl. Soil Ecol. 2000 doi: 10.1016/S0929-1393(00)00093-7. [DOI] [Google Scholar]