Abstract
Purpose
We report a case of corneal keloid occurring 30 years after pterygium surgery and 3 years after cataract surgery.
Observations
The case of a 72-year-old man was referred because of blurred vision and corneal opacity in the right eye. Pterygium surgery had been performed on the right eye 30 years earlier, and bilateral cataract surgery had been done uneventfully via a temporal corneal incision 3 years ago. Deterioration of vision occurred in the right eye from 2 years ago. At the initial visit, his best corrected visual acuity (BCVA) was 20/2000 on the right. A white nodule that was well demarcated from the underlying stroma was seen on the right cornea. The nodule was excised by superficial keratectomy, with BCVA being 180/200 at 1 week after surgery. Pathological examination of the resected specimen revealed proliferation of fibroblasts and haphazard arrangement of collagen bundles, leading to a diagnosis of corneal keloid. Keloid-like lesion was also later noted in temporal corneal incision site of cataract surgery.
Conclusions and importance
This rare case of corneal keloid occurred as a late complication of pterygium surgery.
Keywords: Corneal keloid, Superficial keratectomy, Pterygium surgery
1. Introduction
Corneal keloid is a rare disorder that is characterized by proliferation of fibrous tissue in the cornea and presents as an enlarging, white, elevated, well-circumscribed corneal lesion.1 Most corneal keloids develop following trauma or disease of the cornea, although several cases of primary corneal keloid have been reported in patients without a history of surgery or trauma.2,3 Surgical removal is required for visually significant corneal keloid, but the lesion tends to recur.4 Fewer than 100 cases of corneal keloid have been reported and, to our knowledge, there have been no reports of corneal keloid after pterygium surgery. Here, we report a rare case of corneal keloid occurring 30 years after pterygium surgery.
2. Case report
A 72-year-old man was referred to our hospital for improvement of visual acuity and corneal opacity in the right eye. He had a history of pterygium removal from the nasal canthus of the right eye 30 years earlier and bilateral cataract surgery 3 years ago. Cataract surgery had been performed via a 2.4 mm temporal corneal incision with a side port at 90° to the main incision (at 12 o'clock in the right eye and 6 o'clock in the left eye). Phacoemulsification was uneventful, and a foldable acrylic hydrophilic intraocular lens was implanted in each eye. For the past 2 years, he had noted gradual progression of blurred vision in the right eye, and corneal opacification was noted in the local clinic (Fig. 1A).
Fig. 1.
-
(A)A year after the cataract surgery, corneal opacification located around the pupil zone of right eye was noted at the visit of the local clinic.
-
(B)At the initial visit of our hospital, slit-lamp microscopy identified a pearly white, elevated lesion with a smooth surface on the central right cornea.
-
(C)The left cornea was clear.
-
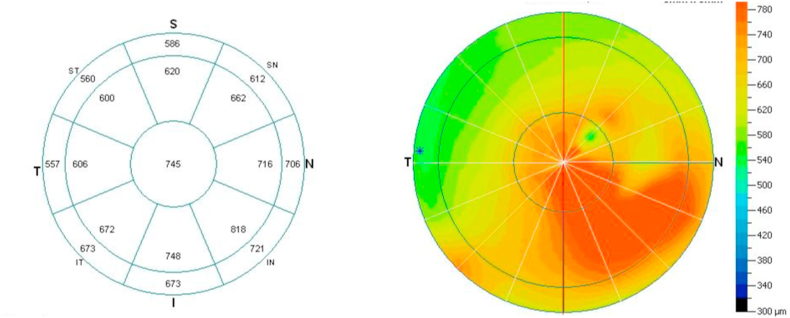
(D)Anterior segment optical coherence tomography (OCT) demonstrated well-demarcated lesions in the subepithelial region and anterior corneal stroma, with clear underlying posterior stroma, as well as a continuous high-intensity subepithelial lesion located around the main lesion and extending inferiorly toward the nasal limbus.
-
(E)Measurement of corneal thickness by anterior segment OCT revealed thickening of the cornea from the center to the inferior nasal region.
On initial examination in our hospital, the best corrected visual acuity (BCVA) was 20/2000 for the right eye and 20/20 for the left eye. Slit-lamp microscopy revealed a pearly white, elevated lesion with a smooth surface in the central right cornea (Fig. 1B). Similar pearly white lesions were also noted at the corneal limbus in the inferior, temporal, and nasal regions, at the sites of the temporal corneal incision for cataract surgery and the site of pterygium surgery, respectively. New vessels were seen on parts of the lesions adjacent to the limbus. The left cornea was clear (Fig. 1C). At the initial visit, the corneal endothelial cell density (ECD) was 2390 cells/mm2 in the right eye and 2547 cells/mm2 in the left eye. Anterior segment optical coherence tomography (OCT) demonstrated well-demarcated lesions that were located in the subepithelial to anterior corneal stroma, with clear underlying posterior stroma (Fig. 1D). OCT also displayed a continuous high-intensity lesion located in the subepithelial region around the main lesion, and extending inferiorly to the nasal limbus. Measurement of corneal thickness by anterior segment OCT identified thickening of the cornea from the center to the inferior nasal region (Fig. 1E).
2.1. Surgery and pathology
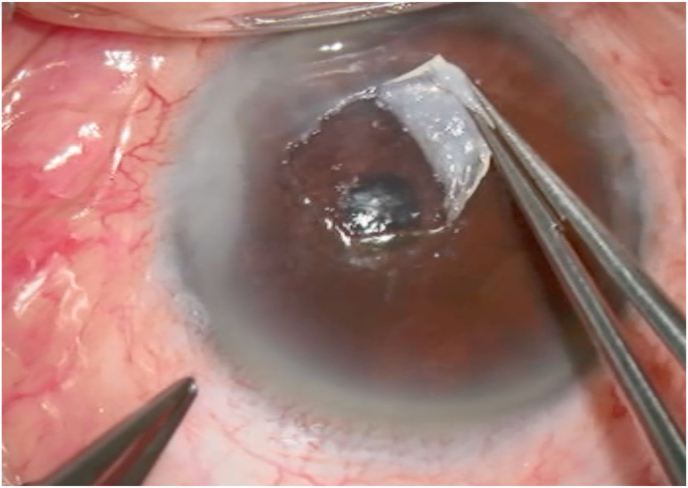
One month after the initial visit, superficial keratectomy was performed. The central lesion was easily dissected from the corneal stroma (Fig. 2A). In addition, superficial scraping with an ophthalmic surgical blade was performed from the central cornea towards the nasal limbus to remove fibrous tissue that had proliferated after pterygium surgery.
Fig. 2.
-
(A)During superficial keratectomy, the central lesion was easily dissected from the corneal stroma.
-
(B)Light microscopic examination of the resected specimen revealed hyperplastic epithelium, absence of Bowman's layer, irregular collagen fibrils, and infiltration of fibroblasts, findings which were consistent with a diagnosis of corneal keloid.
-
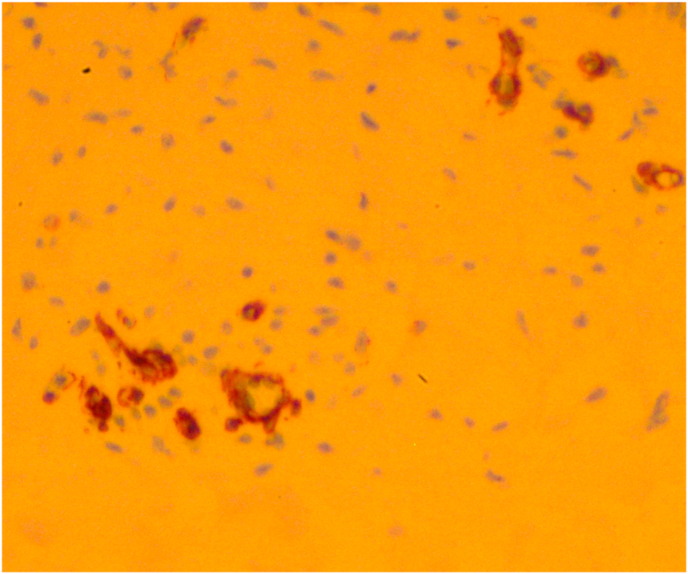
(C)Immunohistochemistry using alpha-smooth muscle actin antibody revealed that fibroblast-like cells noted in corneal stroma were myofibroblasts.
The resected specimen was fixed in 10% formalin and subjected to histopathological examination. Light microscopy revealed hyperplastic epithelium. The basal layer showed an irregular arrangement of cells and Bowman's layer was not visible. There was loss of the normal lamellar pattern in the stroma and it was comprised of irregular collagen fibrils with prominent infiltration of fibroblasts (Fig. 2B). Furthermore, these fibroblast-like cells were found by immunohistochemistry to express alpha-smooth muscle actin, which suggests that they were myofibroblasts (Fig. 2C). These findings were consistent with a diagnosis of corneal keloid.
2.2. Clinical course
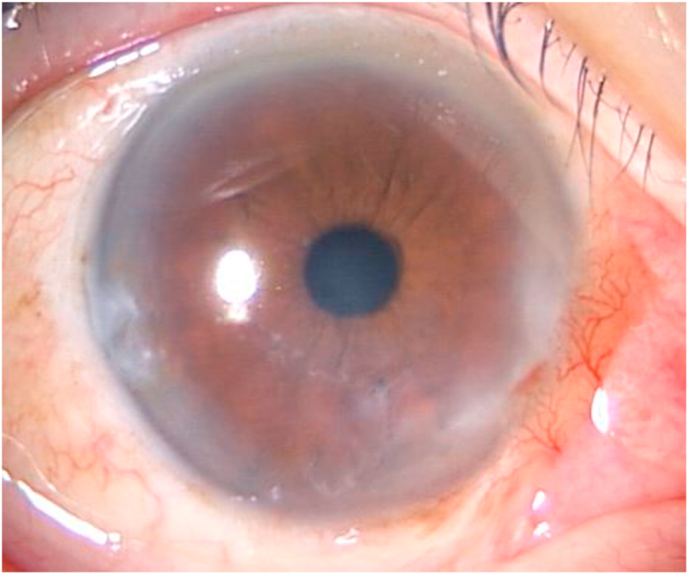
One week after surgery, BCVA was 180/200 in the right eye and epithelialization was noted at the site of surgery (Fig. 3A). Thereafter, the patient was followed at his local clinic. Although a small recurrent white opacity was noted on the inferior cornea at 6 months after superficial keratectomy, BCVA was still 180/200 (Fig. 3B). One and a half year after the superficial keratectomy, most of the surgical site of right cornea remained clear, and BCVA was still 180/200. However, worsening of pearly white lesion in temporal corneal incision site of cataract surgery 5 years ago was noted (Fig. 3C), while contralateral cornea remained clear (Fig. 3D).
Fig. 3.
-
(A)Epithelialization at 1 week after superficial keratectomy.
-
(B)A small recurrent white opacity on the inferior cornea at 6 months after superficial keratectomy.
-
(C)Worsening of pearly white lesion in temporal corneal incision site of cataract surgery 5 years ago was noted.
-
(D)While contralateral cornea remained clear.
3. Discussion
Corneal keloid is a rare disorder that is characterized by thickened keratinized epithelium, absence of Bowman's membrane, irregular collagen bundles, and proliferation of fibroblasts.1 Similar to skin keloid, this disorder probably arises due to excessive production of collagen by activated corneal fibroblasts. In this report, we described a case of corneal keloid that occurred 30 years after pterygium surgery.
Corneal keloid is categorized as primary or secondary. Primary corneal keloid is caused by congenital diseases, such as Lowe's syndrome,5 or may be of unknown etiology.3,4 Therefore, primary corneal keloid is commonly seen earlier in life, and is sometimes present at birth. In contrast, secondary corneal keloid usually develops after penetrating trauma or surgery,1 and secondary keloid thus sometimes needs to be distinguished from a hypertrophic scar. Unlike a hypertrophic scar, a secondary keloid usually appears from months to years after the initial trauma and enlarges over time, and it may extend beyond the border of the initial trauma.6 Regarding the relation with ocular surgery, corneal keloid most commonly occurs after penetrating keratoplasty. Therefore, some authors have suggested that there must be a penetrating corneal wound with incarceration of the iris for a corneal keloid to form,7,8 and that inflammatory cells derived from the iris are responsible for cellular proliferation that creates the keloid. On the other hand, it has been proposed that keloids may arise from the corneal stroma itself.9
In the present patient, the corneal keloid was presumed to be secondary because he was elderly and the lesion was unilateral. However, there was no history of a penetrating wound, except for that made to perform small incision cataract surgery. Instead, the patient had a thick, firm, and white lesion at the center of the cornea. Therefore, we hypothesized that residual fibroblasts persisting after pterygium surgery were activated for some reason and formed the corneal keloid in this patient. Furthermore, we speculate that the small incision cataract surgery also had the possibility to accelerate the progression of corneal keloid, because the keloid-like lesion was also noted in temporal corneal incision site of cataract surgery. Indeed, the slit-lamp microscopy findings suggested that the keloid had developed from fibrotic proliferation after pterygium surgery because smaller whitish opacities were also noted on the inferior nasal cornea, presumably the site of the original pterygium. Furthermore, anterior segment OCT revealed a continuous high-intensity subepithelial lesion around the main corneal lesion that extended inferiorly toward the nasal limbus. In patients with corneal keloid, recurrence has been reported after superficial keratectomy, lamellar keratoplasty, and penetrating keratoplasty.2 It was previously suggested that corneal myofibroblasts are involved in the recurrence of keloid and stromal opacification, based on successful management of keloid by deep anterior lamellar keratoplasty to achieve complete removal of stromal fibroblasts.2,10 It can be said that this speculation of other authors might support our hypothesis. Furthermore, as mentioned above, previous cataract surgery could also have stimulated the development of corneal keloid in our patient.11 Although the details regarding corneal keloid caused by small incision cataract surgery are not described in this case report from Singh et al., it can be speculated that penetrating wound during cataract surgery, even small and uneventful incision, are capable to activate keloid formation. Other studies also mentioned about the application of mitomycin C (MMC) to suppress the activity of corneal myofibroblast.2,4 The usage of MMC should be considered if the corneal keloid of this patient progresses. Further investigation will be needed to determine the pathophysiology of corneal keloid. Additionally, because the keloid formation potentially affect corneal surface irregularity and thus visual acuity, it is quite interesting to assess corneal higher-order aberrations and corrected visual acuity with rigid gas permeable contact lenses in further examinations.
4. Conclusion
In conclusion, we report a rare case of corneal keloid as a late complication of pterygium surgery. Uneventful small incision cataract surgery also had the possibility to accelerate the progression of corneal keloid. To our knowledge, this is the first report of corneal keloid arising after resection of a pterygium, and the mechanism is thought to involve postoperative activation of residual fibroblasts from the pterygium.
Patient consent
Written consent to publish personal information and case details has been obtained from the patient.
Funding
No funding or grant support.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing of interest
None.
Acknowledgements
None.
References
- 1.Vanathi M., Panda A., Kai S., Sen S. Corneal keloid. Ocul Surf. 2008;6(4):186–197. doi: 10.1016/s1542-0124(12)70179-9. [DOI] [PubMed] [Google Scholar]
- 2.Bakhtiari P., Agarwal D.R., Fernandez A.A. Corneal keloid: report of natural history and outcome of surgical management in two cases. Cornea. 2013;32(12):1621–1624. doi: 10.1097/ICO.0b013e3182a73a10. [DOI] [PubMed] [Google Scholar]
- 3.Fukuda K., Chikama T., Takahashi M., Nishida T. Long-term follow-up after lamellar keratoplasty in a patient with bilateral idiopathic corneal keloid. Cornea. 2011;30(12):1491–1494. doi: 10.1097/ICO.0b013e31822018f2. [DOI] [PubMed] [Google Scholar]
- 4.Lee H.K., Choi H.J., Kim M.K. Corneal keloid: four case reports of clinicopathological features and surgical outcome. BMC Ophthalmol. 2016;16(1):198. doi: 10.1186/s12886-016-0372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cibis G.W., Tripathi R.C., Tripathi B.J. Corneal keloid in Lowe's syndrome. Arch Ophthalmol. 1982;100(11):1795–1799. doi: 10.1001/archopht.1982.01030040775013. [DOI] [PubMed] [Google Scholar]
- 6.McGrouther D.A. Hypertrophic or keloid scars? Eye. 1994;8(pt 2):200–203. doi: 10.1038/eye.1994.46. [DOI] [PubMed] [Google Scholar]
- 7.Mejía L.F., Acosta C., Santamaría J.P. Clinical, surgical, and histopathologic characteristics of corneal keloid. Cornea. 2001;20(4):421–424. doi: 10.1097/00003226-200105000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Farkas T.G., Znajda J.P. Keloid of the cornea. Am J Ophthalmol. 1968;66(2):319–323. doi: 10.1016/0002-9394(68)92081-3. [DOI] [PubMed] [Google Scholar]
- 9.Fenton R.H., Tredici T.J. Hypertrophic corneal scars (keloids) Surv Ophthalmol. 1964;9:561–566. [PubMed] [Google Scholar]
- 10.Ashar J.N., Pahuja S., Ramappa M. Deep anterior lamellar keratoplasty in children. Am J Ophthalmol. 2013;155(3):570–574. doi: 10.1016/j.ajo.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 11.Singh A., Sen S., Vanathi M., Tandon R. Corneal keloid with cystoid cicatrix:post-small-incision cataract surgery. Can J Ophthalmol. 2017;52(3):e93–e95. doi: 10.1016/j.jcjo.2016.11.013. [DOI] [PubMed] [Google Scholar]