Abstract
Purpose
To report on a case of submacular choroiditis in a patient with common variable immunodeficiency (CVID).
Observations
An 80-year-old man was referred with a diagnosis of a central retinal vein occlusion with CME and later developed intraocular inflammation. History was notable for recurrent bacterial infections and myelodysplastic syndrome known to be due to CVID. Ophthalmic examination and multimodal imaging revealed mild intraocular inflammation, retinal vasculitis, submacular choroiditis, and CME. Genetic testing identified a point mutation in TNFRSF13B, a pathogenic variant in the tumor necrosis factor gene known to be associated with CVID, but not with CVID-associated uveitis.
Conclusions and importance
The diagnosis of CVID should be considered in patients with uveitis and a history of recurrent bacterial infections. Genetic testing can support the diagnosis.
Keywords: Common variable immunodeficiency, Submacular choroiditis, Uveitis
1. Introduction
Common variable immunodeficiency (CIVD) is the most common primary immunodeficiency disorder and is characterized by the occurrence of recurrent bacterial infections, malignancies, and autoimmune disorders, which are often granulomatous in nature.1 Linked to a number of specific mutations, CVID is pleotypic, with varied clinical presentations occurring even in patients with the same underlying genetic defects.2,3 We report a patient with CVID who developed intraocular inflammation, retinal vasculitis, submacular choroiditis, and cystoid macular edema (CME), and in whom genetic testing identified a point mutation in TNFRSF13B, a pathogenic variant in the tumor necrosis factor gene known to be associated with CVID.
2Case Report
An 80-year-old Caucasian man was referred for a central retinal vein occlusion (CRVO) with associated CME involving his left eye. He had a history of recurrent bacterial infections and longstanding anemia with ring sideroblasts related to myelodysplastic syndrome (MDS), both known complications of CVID.4 His initial examination was consistent with CRVO and his CME responded well to a series of intravitreal bevacizumab injections. A few months later he developed bilateral intraocular inflammation.
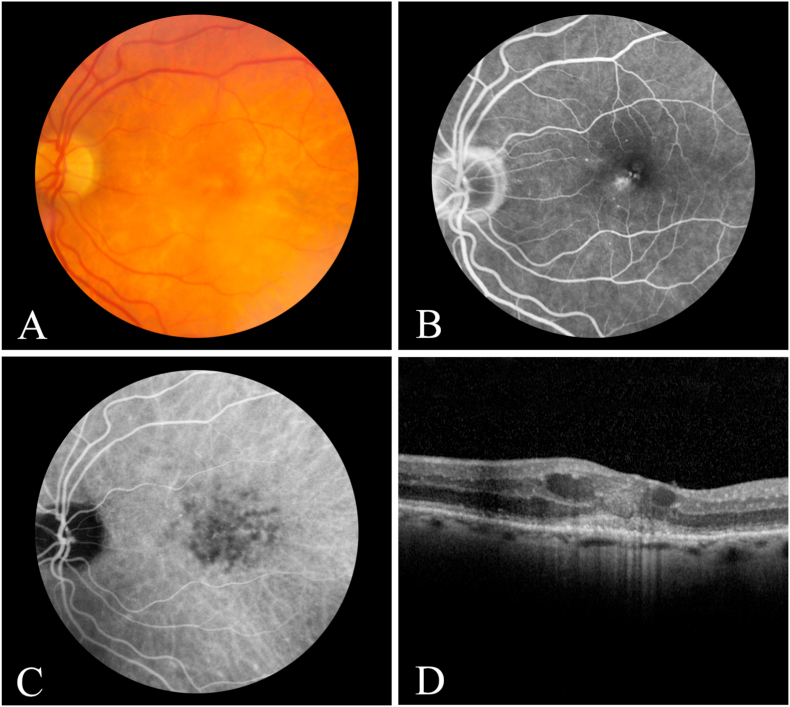
On examination, vision was 20/40 in the right eye and 20/160 in the left eye. The patient had fine keratic precipitates with rare cell in the anterior chamber of each eye. Posterior segment examination revealed mild vitritis as well as scattered peripheral chorioretinal scars in each eye, with macular retinal pigment epithelial (RPE) mottling in the left eye only. Optical coherence tomography (OCT) demonstrated CME in the left eye. Fluorescein angiography revealed mild foveal leakage in the left eye, and indocyanine green angiography showed small, sub- and para-foveal hypofluorescent choroidal spots in both eyes (Fig. 1). Serologic testing for rapid plasma reagin, fluorescent treponemal antibody absorption, angiotensin converting and lysozyme enzyme levels, and interferon gamma releasing assay were unrevealing. A carotid ultrasound was unremarkable. Magnetic resonance imaging of the brain demonstrated mild volume loss and mild microvascular changes. A repeat positron emission tomography scan showed patchy hypermetabolic activity in various bones throughout his body, which his hematologist thought was due to his longstanding MDS, which was stable. The patient was diagnosed with CVID-associated panuveitis with submacular choroiditis, retinal vasculitis, and CME and was given an intravitreal dexamethasone intravitreal implant (Ozurdex®) in the left eye, which lead to resolution of his CME and vasculitis. The small, submacular choroiditis spots remained in each eye, but the patient declined further treatment.
Fig. 1.
Color fundus photograph (A), mid-phase fluorescein angiogram (B), late-phase indocyanine green angiogram (C), and horizontal optical coherence tomography scan through the central macula (D) of the left eye showing multiple, small hypofluorescent spots consistent with choroiditis (C) associated with by cystoid macular edema (B & D) and disruption of the central photoreceptors (D). Vision was 20/160.
Reevaluation by his immunologist confirmed decreased serum levels of several immunoglobulin G isotypes, lack of response to polysaccharide antigens, and a low number of B-cells, but with normal T-cell levels. These laboratory findings, in conjunction with a history of recurrent bacterial infections, confirmed his diagnosis of CVID. Genetic testing was performed and identified a pathogenic variant in the tumor necrosis factor gene- TNFRSF13B - a mutation known to be associated with CVID.1
3Discussion
Common variable immunodeficiency is an autoimmune condition that is characterized by low immunoglobulin levels. Since the initial report by van Meurs et al. of retinal vasculitis5 in a patient with CVID, several additional publications have appeared. A large population-based study in the Savoy area of France identified uveitis in four of 252 patients known to have CVID.6 Most reported cases have had granulomatous features, including conjunctival granulomas, choroidal granulomas, and multifocal choroiditis.7 Such granulomas are believed to develop as a result of CVID-associated immune dysregulation. While association of a single gene polymorphism is not proof for a causal relationship between that polymorphism and uveitis, it is noteworthy that TNFRSF13B variations are a contributing factor to the development of antibody deficiency. With a population-based prevalence of CVID of approximately 1:25,0008 and of uveitis of between 1:500 and 1:1000,9 it remains possible, however, that a random and completely independent association might occur in a very small number in the population.
There are a few unique aspects of our case. We recognize that most CVID patients with uveitis in the literature have been much younger. We believe the incidence of CVID-associated uveitis to be underappreciated; CVID is a lifelong condition that can be associated with CVID-associated complications at any age and our case suggests the same for accompanying uveitis. Additionally, a diagnosis of post injection uveitis was considered, however the intravitreal bevacizumab he received was only in his left eye.
4Conclusions
Eye care providers should consider the diagnosis of CVID in patients with uveitis, particularly when there is choroidal involvement and a history of recurrent bacterial infections.
Patient consent
Consent to publish the case was not obtained. This report does not contain any personal information that could lead to identification of the patient.
Funding
No funding or grant support.
Conflicts of interest
The authors have no disclosures.
Authorship
All authors attest that they meet current ICMJE criteria for authorship.
Acknowledgements
None.
References
- 1.Resnick E.S., Moshier E.L., Godbold J.H. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood. 2012;119(7):1650–1657. doi: 10.1182/blood-2011-09-377945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogaert D.J.A., Dullaers M., Lambrecht B.N. Genes associated with common variable immunodeficiency: one diagnosis to rule them all? J Med Genet. 2016;53(9):575–590. doi: 10.1136/jmedgenet-2015-103690. [DOI] [PubMed] [Google Scholar]
- 3.Shields C.L., Say E.A.T., Mashayekhi A. Assessment of CTLA-4 deficiency–related autoimmune choroidopathy response to abatacept. JAMA Ophthalmol. 2016;134(7):844–846. doi: 10.1001/jamaophthalmol.2016.1013. [DOI] [PubMed] [Google Scholar]
- 4.Toh J., Eisenberg R., Bakirhan K. Myelodysplastic syndrome and acute lymphocytic leukemia in common variable immunodeficiency (CVID) J Clin Immunol. 2016;36:366–369. doi: 10.1007/s10875-016-0269-2. [DOI] [PubMed] [Google Scholar]
- 5.van Meurs J.C., Lightman S., deWaard P.W. Retinal vasculitis occurring with common variable immunodeficiency syndrome. Am J Ophthalmol. 2000;129(2):269–270. doi: 10.1016/s0002-9394(99)00325-6. [DOI] [PubMed] [Google Scholar]
- 6.Pasquet F., Kodjikian L., Mura F. Uveitis and common variable immunodeficiency: data from the DEF-I study and literature review. Ocul Immunol Inflamm. 2012;20(3):163–170. doi: 10.3109/09273948.2012.674612. [DOI] [PubMed] [Google Scholar]
- 7.Rohart C., Badelon I., Fajnkuchen F. Ophthalmologic disease in sarcoid-like granulomatosis and true sarcoidosis in immunodeficiency: four case reports. J Fr Ophtalmol. 2008;31(7):683–691. doi: 10.1016/s0181-5512(08)74382-1. [DOI] [PubMed] [Google Scholar]
- 8.Salzer U., Bacchelli C., Buckridge S. Relevance of biallelic versus monoallelic TNFRSF13B mutations in distinguishing disease-causing from risk-increasing TNFRSF13B variants in antibody deficiency syndromes. Blood. 2009;113(9):1967–1976. doi: 10.1182/blood-2008-02-141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.González M.M., Solano M.M., Porco T.C. Epidemiology of uveitis in a US population-based study. J Ophthalmic Inflamm Infect. 2018;8(1):6. doi: 10.1186/s12348-018-0148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]