Abstract
Objective
Increasing evidence points to endothelial dysfunction as a key pathophysiological factor in coronavirus disease-2019 (COVID-19). No specific methods have been identified to predict, detect and quantify the microvascular alterations during COVID-19. Our aim was to assess microvasculature through nailfold videocapillaroscopy (NVC) in COVID-19 patients.
Methods
We performed NVC in patients with a confirmed diagnosis of COVID-19 pneumonia. Elementary alterations were reported for each finger according to a semi-quantitative score. Capillary density, number of enlarged and giant capillaries, number of micro-hemorrhages and micro-thrombosis (NEMO score) were registered.
Results
We enrolled 82 patients (mean age 58.8 ± 13.2 years, male 68.3%) of whom 28 during the hospitalization and 54 after recovery and hospital discharge. At NVC examination we found abnormalities classifiable as non-specific pattern in 53 patients (64.6%). Common abnormalities were pericapillary edema (80.5%), enlarged capillaries (61.0%), sludge flow (53.7%), meandering capillaries and reduced capillary density (50.0%). No pictures suggestive of scleroderma pattern have been observed. Acute COVID-19 patients, compared to recovered patients, showed a higher prevalence of hemosiderin deposits as a result of micro-hemorrhages (P = .027) and micro-thrombosis (P < .016), sludge flow (P = .001), and pericapillary edema (P < .001), while recovered patients showed a higher prevalence of enlarged capillaries (P < .001), loss of capillaries (P = .002), meandering capillaries (P < .001), and empty dermal papillae (P = .006).
Conclusion
COVID-19 patients present microvascular abnormalities at NVC. Currently ill and recovered subjects are characterized by a different distribution of elementary capillaroscopic alterations, resembling acute and post-acute microvascular damage. Further studies are needed to assess the clinical relevance of NVC in COVID-19.
Keywords: Nailfold videocapillaroscopy, COVID-19, Microangiopathy, Micro-thrombosis
Highlights
-
•
We assessed and accurately described peripheral microvasculature in COVID-19 patients through nailfold videocapillaroscopy.
-
•
Capillary alterations at the nailfold bed suggest a broad COVID-related microvascular involvement.
-
•
Different alterations in acutely-ill and recovered patients resemble a transition from acute to post-acute injury.
1. Introduction
Coronavirus disease 2019 (COVID-19) is a newly identified respiratory syndrome sustained by SARS-CoV2 infection. The outbreak of this disease reached, during the last few months, pandemic dimensions, engaging healthcare professionals all over the world in an unprecedented challenge (https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports, n.d.).
An initial viremic phase - with fever, dry cough, alteration of the upper airways and mucous membranes and arthralgias sometimes accompanying viral pneumonia - could transit, usually in cases of particular virulence and poor clinical control, in a second phase of the disease, characterized by hyperactivation of the host's immune system, with a rapid deterioration of the respiratory function up to the acute respiratory distress and multiorgan failure (Siddiqi and Mehra, 2020). The observation of a state of hyper-inflammation has led clinicians to use targeted anti-cytokine therapies in severe COVID-19, in the attempt to avoid the progression of lung involvement and the damage related to uncontrolled systemic phlogosis (Gremese et al., 2020; Cavalli et al., 2020; Campochiaro et al., 2020; De Luca et al., 2020).
Dysfunctional endothelial activation is a phenomenon now recognized during COVID-19 (Sardu et al., 2020). The pathogenetic mechanisms of this process are still not clear and could depend both on a stimulus secondary to the hyperinflammatory state and a direct pathogenetic role of the viral agent, as suggested by the finding of indirect signs of endothelial activation already in the first days of the disease (Kaur et al., 2020). Even the first pathological evidence seems to support the centrality of these phenomena, and the histological finding of acral vasculitis with the presence of fibrin thrombus has been reported (Fox et al., 2020; Colonna et al., 2020).
Clinically, patients easily develop thromboembolism. The greatest evidence poses for a prevalent commitment at the level of the lung compartment, the target organ of the virus, while the association with thromboembolic phenomena at different districts - which have also been described - is more nuanced (Ackermann et al., 2020). Disseminated intravascular coagulation is mainly to refer to the severe cases with an advanced deterioration of the clinical picture.
As yet, no specific methods have been identified to predict, detect and quantify the endothelial alteration during COVID-19. In clinical practice, the dosage of humoral markers of fibrin degradation and systemic inflammation could be useful, also for the prognostic role (Zhou et al., 2020). Some case series have shown a significant prevalence in both acutely ill and convalescent patients of antibodies related to interference with the phospholipid-dependent coagulation pathways, and in particular of lupus anticoagulant (Harzallah et al., 2020; Paglionico et al., 2020). The anticoagulant treatment with heparin and derivatives, even in the absence of overt thromboembolism, is increasingly widespread in the therapeutic schemes for hospitalized and non-hospitalized patients, with promising results (Bikdeli et al., 2020).
Nailfold capillaroscopy (NVC) is an investigation capable of identifying the morphological alterations of microcirculation at the level of the capillaries of the nailfold bed. This method is widely used in the rheumatology field for the diagnostic classification of vascular acro-syndromes, thanks to its ability to discern typical and highly predictive patterns for underlying connective tissue diseases (CTDs), known as “scleroderma patterns” (Cutolo et al., 2000). In other clinical contexts, the evidence on specific patterns are less robust, but the method has nevertheless proved to be valid, sensitive and reproducible for the study and definition of elementary alterations of the microcirculation; it is also characterized by advantages such as economy, feasibility and non-invasiveness (Etehad Tavakol et al., 2015; Smith et al., 2020).
The present study aimed to assess microvasculature and to characterize NVC abnormalities in acute and recovered COVID-19 patients.
2. Patients and methods
Eighty-two adult patients affected by COVID-19 pneumonia, diagnosed by laboratory test (positive nasopharyngeal-swab and/or SARS-CoV2 serology) and suggestive chest imaging, were enrolled at IRCCS San Raffaele Hospital (Milan, Italy) and Fondazione Policlinico Agostino Gemelli IRCCS (Rome, Italy).
In a first group of consecutive patients, the NVC was performed during hospitalization, while in a second group of consecutive patients was performed after discharge (i.e. patients' recovery based on clinical improvement and after repeated negative swabs confirmation) in an outpatient setting.
A comprehensive medical and pharmacological history was obtained for all patients at each center. Specifically, the following data were collected: COVID-19 onset symptoms, duration and outcome, clinical and radiologic characteristics of COVID-19 pneumonia, lung parameters and need for supplemental oxygen therapy, inflammatory and coagulation blood markers, thromboembolic events or other COVID-19 related complications, comorbidities, body mass index (BMI), concomitant therapies and COVID-19 specific treatments. The presence of pre-existing or recent acral symptoms with respect to COVID-19 was also investigated.
Patients with a positive history of traumatization or repeated micro-traumatization of the hands in the last two weeks (e.g. onychophagy, use of vibrating instruments and use of musical instrument), and patients with diagnosed or suspected CTDs were excluded from the study.
All patients provided written informed consent. The study was approved by the Ethics Committees at each Institution (Milan: approval no. 34/int/2020; Rome: approval no. 0024185/20).
A specialized VideoCap 3.0 tissue-contact type biomicroscope providing color images at 200× magnification (DS Medica, Milan, Italy) and dedicated software for image analysis (version 10.0) with standard settings for NVC was used at both sites. The procedure was conducted at each site by two physicians at the same level of experience, already practicing capillaroscopy (image acquisition and analysis) for many years in their routine clinical practice, and applying standard recommendations for patient preparation and execution of the exam (Etehad Tavakol et al., 2015). All fingers except the thumb were examined bilaterally. Four different images were consecutively recorded for each finger, in order to accurately characterize the central area of the nail bed (32 images per patient in total). The images were assessed dynamically and, at the end of the examination, statically by the executing physician. Considering the novelty of the pathology under study and the absence of specific standardization and previous data, to better distinguish all the possible microvascular alterations, we considered a variety of elemental alterations of capillaries at the distal row, as previously described or defined in details below:
-
1.
enlarged capillary: an increase in capillary diameter (homogeneous or irregular) >20 μm and <50 μm (Sulli et al., 2008);
-
2.
giant capillary: homogeneously enlarged loops with a diameter ≥50 μm (Sulli et al., 2008);
-
3.
hemosiderin deposit: dark mass due to micro-hemorrhage (single or multiple hemosiderin deposits of round form at variable distance from the distal row, as the result of blood extravasation) or micro-thrombosis (hemosiderin deposit that resembles the shape of a capillary loop, as the result of arrest of the blood column within the capillary) (Sambataro et al., 2014);
-
4.
lower capillary density: reduction of the capillary number below the average normal value of 9 capillaries per millimeter (an abnormally low value is usually considered below 7 capillaries per millimeter) (Smith et al., 2020);
-
5.
microvascular derangement: irregular capillary distribution and orientation (e.g. contiguous loops that do not develop parallel to each other), together with shape heterogeneity of the loops of the same finger (Sulli et al., 2008);
-
6.
capillary ramifications and bizarre morphology: branching, bushy or coiled capillaries, often originated from a single normal-sized capillary (Sulli et al., 2008);
-
7.
meandering capillary: limbs crossing upon themselves or with each other more than two times (if not meeting the definitions 5 and 6) (Bernardino et al., 2019);
-
8.
sludge flow: markedly slowed or discontinuous flow inside the capillary at the dynamic evaluation at the time of examination (Bernardino et al., 2019);
-
9.
pericapillary edema: foggy appearance around capillaries due to fluids build-up (Monticone et al., 2000);
-
10.
subpapillary plexus visibility: observation of large and linked arrangement of vessels under the distal row due to enlargement and congestion of venules and capillaries related to persistent opening of arteriovenous anastomoses (Ingegnoli et al., 2009);
-
11.
avascular area: distance greater than 500 μm between two adjacent capillary loops from the distal rows (Etehad Tavakol et al., 2015);
-
12.
empty dermal papilla: one or more missing capillary at the expected place inside dermal papilla which does not reach the extent to define an avascular area.
A widely used semi-quantitative scoring for features 1–7 was applied (Sulli et al., 2008). Specifically, for each capillary abnormality the score for each digit was calculated as follow: absence of alterations (0), less than 33% of examined capillaries altered (1), 33–66% of examined capillaries altered (2), more than 66% of examined capillaries altered (3). For the definition of lower capillary density, the percentage refers to reduction with respect to a normal mean value of 9 capillaries per linear millimeter. The average score values from the eight digits were added, and the final value divided for eight fingers; the resulting value represents the score for each parameter analyzed. Since the score was previously developed for scleroderma patients, in whom a high frequency of major alterations is expected, we decided to design and apply in parallel a further semi-quantitative scoring approach, to avoid losing sensitivity. Indeed, for each feature from 1 to 12, a synthetic score was then assigned according to the following criteria: absent (0), present at least one time in only one finger (1), present at least one time in 2 to 4 fingers (2), present at least one time in 5 to 8 fingers (3). Subpapillary plexus visualization was qualitatively scored as previously reported (Kabasakal et al., 1996), while neoangiogenesis was noted in the presence of a suggestive combination of elementary alterations, based on the physician's evaluation (e.g. several deranged or bizarre capillaries which develop inside the same dermal papilla accompanied by a reduction in capillary density). Regarding crude quantitative data, mean and minimum capillary density were registered for each patient, as per maximum diameter and number of enlarged and giant capillaries, number of micro-hemorrhages and micro-thrombosis (NEMO score) (Andracco et al., 2017), and length of representative capillaries. Finally, for each patient, an overall qualitative assessment was made by the clinician distinguishing between normal pictures and the presence of significant abnormalities, based on previous observations and definitions (Cutolo et al., 2000; Pavlov-Dolijanovic et al., 2012).
Data were analyzed using IBM SPSS Statistics v26.0 (Armonk, NY, USA). Categorical variables were reported as number and percentage and continuous variables as mean ± standard deviation (SD) or median with interquartile range (IQR), according to the distribution of the data. The Fisher's exact test or the Mann-Whitney test was used for comparison between groups, as appropriate. Statistical significance was defined as a p-value (P) <0.05.
3. Results
We enrolled 82 patients: 28 during hospitalization, and 54 post-recovery. Considering the entire cohort, the mean age was 58.8 ± 13.2 years; 56 (68.3%) were male, 25 (30.5%) had hypertension, 50 (61.0%) were overweight or obese (BMI > 25 kg/m2; mean BMI 25.9 ± 3.5 kg/m2), 11 (13.4%) were current smoker and 9 patients (11.0%) had diagnosed diabetes. Four patients (4.9%) had a diagnosed rheumatic disease (2 rheumatoid arthritis, 1 crystal-induced arthritis and 1 granulomatosis with polyangiitis). None of the patients had active cancer at the time of evaluation. Eight patients (9.8%) reported acral symptoms temporally related to COVID-19 (1 Raynaud's phenomenon, 1 acrocyanosis, 1 “puffy” hands, 5 distal paresthesia/dysesthesia) and 3 reported long term Raynaud's phenomenon, not associated with other symptoms suggestive for a CTD. Population's characteristics are reported in Table 1 .
Table 1.
Population characteristics.
| All patients (n = 82) |
Acute (n = 28) |
Recovered (n = 54) |
P⁎ | |
|---|---|---|---|---|
| Age, years, mean ± SD | 58.8 ± 13.2 | 62.5 ± 13.6 | 57.3 ± 12.8 | .068 |
| Male, n (%) | 56 (68.3) | 20 (71.4) | 36 (66.7) | .8 |
| Duration from onset, days, mean ± SD | 37.3 ± 23.1 | 7.2 ± 5.0 | 52.2 ± 7.1 | <.001 |
| Duration from discharge, days, mean ± SD | NA | NA | 31.6 ± 9.3 | NA |
| Smoke (current), n (%) | 11 (13.4) | 7 (25.0) | 4 (7.4) | .039 |
| Hypertension, n (%) | 25 (30.5) | 10 (35.7) | 15 (27.8) | .48 |
| Diabetes, n (%) | 9 (11.0) | 7 (25.0) | 2 (3.7) | .006 |
| Rheumatic disease | 4 (4.9) | 3 (10.7) | 1 (1.8) | .11 |
| BMI >25 kg/m2, n (%) | 50 (61.0) | 16 (57.1) | 34 (63.0) | .92 |
| Acral symptomsa, n (%) | 8 (9.8) | 0 (0) | 8 (14.8) | .046 |
| Oxygen therapyb, n (%) | 47 (57.3) | 17 (60.7) | 30 (55.6) | .81 |
| ICU, n (%) | 5 (6.1) | 0 (0) | 5 (9.3) | .16 |
| Anti-IL6R, n (%) | 21 (25.6) | 7 (25.0) | 14 (25.9) | .93 |
| Enoxaparina, n (%) | 39 (47.5) | 24 (85.7) | 15 (25.9) | <.001 |
| PTE or DVT, n (%) | 8 (9.8) | 5 (17.9) | 3 (5.6) | .15 |
SD: standard deviation; BMI: body mass index; ICU: intensive care unit; IL6R: interleukin-6 receptor; PTE: pulmonary thrombo-embolism; DVT: deep vein thrombosis.
Bold is used to highlight a p-value <0.05 which is therefore considered statistically significant.
P comparisons between acute and recovered patients.
Temporally related to COVID-19.
During hospitalization.
Overall, at the NVC examination we found a high prevalence of abnormalities classifiable as non-specific pattern (53 patients, 64.6%), without pictures suggestive of scleroderma pattern. Common abnormalities, found in more than two fingers in at least 50.0% of cases, were pericapillary edema (80.5%), enlarged capillaries (61.0%), sludge flow (53.7%), meandering capillaries and capillary density below 9 capillaries per linear millimeter (50.0%) (Table 2 ).
Table 2.
Prevalence of elementary capillaroscopic abnormalities (frequency score 2–3) and comparisons in acute vs recovered vs patients (Fisher's exact).
| Abnormality | Frequency score 2–3 |
|||
|---|---|---|---|---|
| All patients (n = 82) |
Acute (n = 28) |
Recovered n = (54) | P⁎ | |
| Enlarged capillary, n (%) | 50 (61.0) | 4 (14.3) | 46 (85.2) | <.001 |
| Giant capillary, n (%) | 0 (0) | 0 (0) | 0 (0) | – |
| Hemosiderin deposit, n (%) | 19 (23.2) | 13 (46.4) | 6 (11.1) | <.001 |
| Micro-thrombosis, n (%) | 6 (7.3) | 5 (17.9) | 1 (1.9) | .016 |
| Micro-hemorrhage, n (%) | 13 (15.9) | 8 (28.6) | 5 (9.3) | .027 |
| Capillary density <9/mm, n (%) | 41 (50.0) | 7 (25.0) | 34 (63.0) | .002 |
| Microvascular derangement, n (%) | 39 (47.6) | 14 (50.0) | 25 (46.3) | .8 |
| Capillary ramification, n (%) | 21 (25.6) | 8 (28.6) | 13 (24.1) | .8 |
| Meandering capillary, n (%) | 44 (53.7) | 0 (0) | 44 (81.4) | <.001 |
| Sludge flow, n (%) | 44 (53.7) | 22 (78.6) | 22 (40.7) | .001 |
| Pericapillary edema, n (%) | 66 (80.5) | 28 (100) | 38 (70.4) | <.001 |
| Subpapillary plexus visibility, n (%) | 7 (8.5) | 1 (3.6) | 6 (11.1) | .4 |
| Empty dermal papilla, n (%) | 12 (14.6) | 0 (0) | 12 (22.2) | .006 |
| Avascular area, n (%) | 3 (3.7) | 0 (0) | 3 (5.6) | .05 |
| Neoangiogenesis, n (%) | 6 (7.3) | 0 (0) | 6 (11.1) | .09 |
Bold is used to highlight a p-value <0.05 which is therefore considered statistically significant.
P comparisons between acute and recovered patients.
In the patients evaluated during hospitalization (n = 28) the mean time from the onset of symptoms to the evaluation was 7.2 ± 5.0 days. Seventeen patients (60.7%) were on oxygen therapy (of which 5 in high flow oxygen therapy or non-invasive ventilation) but none was treated in an intensive care unit (ICU) with invasive ventilation at the time of NVC. Median SpO2/FiO2 ratio (Horowitz index) was of 373 (261–461). Two patients (7.1%) had evidence of pulmonary thromboembolism (PTE), in one case associated with deep vein thrombosis (DVT) while 4 (14.2%) had isolated DVT; none suffered from disseminated intravascular coagulation (DIC). All patients were on hydroxychloroquine therapy with (14, 50.0%) or without antivirals and 7 (25.0%) received anti-cytokine therapies; 24 (85.7%) were on enoxaparin, of which 16 (57.1%) at prophylactic dose and the remaining at therapeutic dose. Four patients also received antiplatelet therapy with acetylsalicylic acid (ASA). Median C-reactive protein (CRP) and D-dimer (DDU) levels at the time of evaluation were of 19.9 mg/L (6.8–38.4) and 1960 ng/mL (840–3380), respectively.
At the NVC examination, all acutely ill patients showed abnormalities. The most frequent, found in more than two fingers, were pericapillary edema (100% of patients), sludge flow (78.6% of patients) and microvascular derangement (50.0% of patients). According to the semi-quantitative evaluation, none of the alterations (1–7, see Patients and methods) involved a percentage greater than 33% of all the capillaries analyzed. Hemosiderin deposits as a result of micro-hemorrhage or micro-thrombosis were found in at least in one digit in 20 patients (71.4%, in 7 cases with micro-thrombotic aspect) of whom 13 (46.4%) had this alteration in more than one finger concurrently. The number of individual lesions ranged from 1 to 15 per patient; Detailed frequency and distribution of the considered capillaroscopic alterations are reported in Supplementary Table 1. Medium capillary density per linear millimeter in the acutely ill patients was of 8.7 ± 0.8 capillaries/mm, while the evaluation of the capillary length was not considered reliable due to the ongoing pericapillary edema.
In the patients evaluated after hospital discharge (n = 54), the mean time from the onset of symptoms to the NVC evaluation was 52.2 ± 7.1 days, while time from the discharge to the evaluation was 31.6 ± 9.3 days. During their hospitalization, 30 patients (55.6%) needed oxygen therapy and 5 (9.3%) needed to be treated in an ICU with mechanical ventilation. Three patients (5.6%) had evidence of PTE, in one case associated with DVT. None suffered from DIC. All patients received antivirals and hydroxychloroquine, 14 (25.9%) received anti-cytokine treatment and 15 (25.9) received enoxaparin, of which 10 (18.5%) at prophylactic dose and the remaining at therapeutic dose. At time of evaluation, 4 patients (7.4%) were on anticoagulants (warfarin, enoxaparin, edoxaban, apixaban, one patient each) and 4 were on ASA. Only one patient still needed low flow oxygen therapy at the time of evaluation and median CRP and DDU levels were of 0.7 mg/L (0.5–1.65) and 365 ng/mL (216–593), respectively.
At the NVC examination, 25 patients (46.3%) showed abnormalities classifiable as non-specific pattern. The most frequent abnormalities, found in more than two fingers, were enlarged capillaries (85.2% of patients), meandering capillaries (81.4% of patients), pericapillary edema (70.4% of patients) and capillary density below 9 capillaries per linear millimeter (63.0% of patients). Furthermore, according to the semi-quantitative evaluation, the involvement of a percentage greater than 33% of all the capillaries analyzed was found for the following alterations: meandering capillaries in 10 (18.5%), enlarged capillaries in 3 (5.6%), capillary density below 9 capillaries per linear millimeter in 3 (5.6%) and microvascular derangement in 2 (3.7%) of the patients. Giant capillaries and avascular areas were rare (both found in 3 cases) and always encountered as an isolated alteration (only one digit for each patient), therefore they did not delineate a scleroderma pattern. Hemosiderin deposits as a result of micro-hemorrhage or micro-thrombosis were found at least in one digit in 17 patients (31.5%, of whom 1 with micro-thrombotic aspect) and more than in one finger concurrently in 6 (11.1%) cases, with a number of individual lesions ranging from 1 to 11 per patient. Detailed frequency and distribution of the considered capillaroscopic alterations are reported in Supplementary Table 2. Medium capillary density per linear millimeter of recovered patients was of 8.8 ± 1.4 capillaries/mm while the medium length was of 265 ± 62 μm.
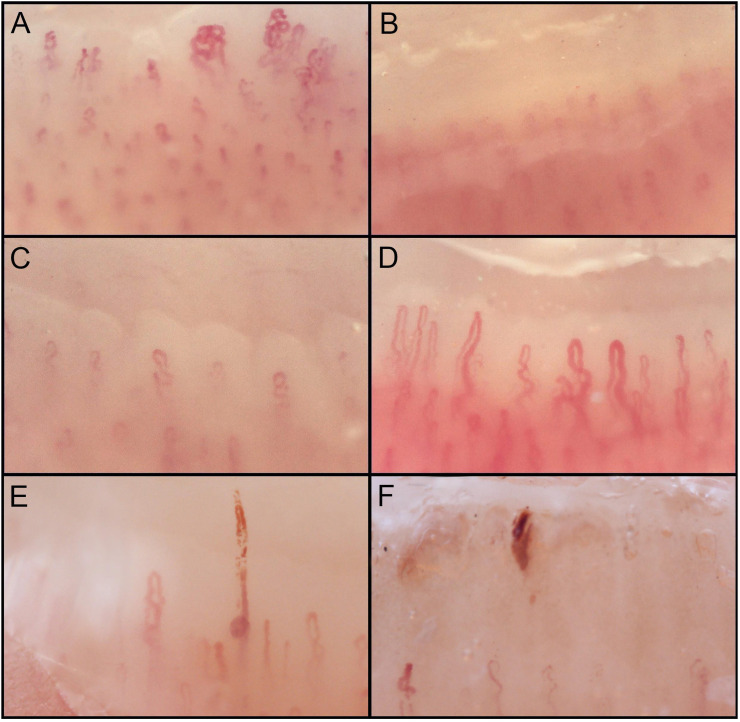
Acutely ill COVID-19 patients, compared to patients discharged after recovery, showed a higher prevalence of abnormal capillaroscopic findings (P < .001). Considering the prevalence of single alterations (found in at least 2 fingers), acute COVID-19 patients had more frequently hemosiderin deposits (P < .001), as a result of both micro-hemorrhages (P = .027) and micro-thrombosis (P = .016), sludge flow (P = .001), and pericapillary edema (P < .001), while recovered patients showed a higher prevalence of enlarged capillaries (P < .001), meandering capillaries (P < .001), capillary density below 9 capillaries per linear millimeter (P = .002), and empty dermal papillae (P = .006) (Table 2). Fig. 1 shows representative pictures of the alterations commonly found in acutely ill and recovered COVID-19 patients.
Fig. 1.
Nailfold capillaroscopy abnormalities found in COVID-19 patients (200× magnification). A) Meandering capillaries and microvascular derangement; B) pericapillary edema; C) low capillary density; D) capillary ectasia; E) micro-thrombosis; F) micro-hemorrhage.
In both groups, we have not found any meaningful association between available markers of inflammation and endothelial impairment and NVC findings. In particular, there was no correlation between NEMO score and CRP and DDU levels.
Patients evaluated during hospitalization and after discharge were not significantly different for mean age (P = .068) and proportion of male (P = .8), hypertensive (P = .48), as well as for the number of observed major thromboembolic events during COVID-19 pneumonia (P = .058) and need for ICU care (P = .16, considering the hospitalization period for recovered patients). Acute patients were more frequently diabetic (P = .006) and active smoker (P = .039). As expected levels of CRP and DDU were significantly higher in acute patients. Both groups were equally exposed to anti-cytokine drugs (P = .93) while the proportion of patients on anticoagulation therapy with enoxaparin was significantly lower in recovered patients (considering the hospitalization period) compared to acute patients (P < .001). All 8 patients that reported acral symptoms temporally related to COVID-19 were in the recovered group (P = .046).
4. Discussion
To the best of our knowledge, this is the first study investigating the presence of microvascular alterations evaluated by NVC in COVID-19 patients. We performed NVC and described in detail the morphological appearance in patients who needed hospital admission for COVID-19 pneumonia, in two different stages of the disease: patients still hospitalized with acute pneumonia, and patients already discharged and evaluated at outpatient clinics. The most striking result was that, while a clear scleroderma pattern was absent in both cohorts of patients, a significant number of microvascular abnormalities was depicted in the vast majority of both groups. Interestingly, the prevalent abnormalities that we observed were different according to the stage of the disease. Specifically, the prevalence of hemosiderin deposits was significantly higher in acute COVID-19 pneumonia patients compared to patients already discharged from hospital. Conversely, recovered patients out of the acute phase had a higher prevalence of altered capillary bed with enlarged capillaries, meandering capillaries and frequent capillary density below 9 per millimeter with empty dermal papillae.
Among all abnormalities, the presence of hemosiderin deposits is particularly interesting in the context of acute patients as it suggests a higher prevalence of micro-hemorrhages and micro-thrombosis in this phase of the disease. Also, consistently with a pro-thrombotic alteration of the microcirculation in acute COVID-19, we observed more frequently a sludge flow in these patients. This is in keeping with the proposed cardinal role of diffuse lung micro-thrombosis in the physiopathology of SARS-CoV-2 infection (Ciceri et al., 2020); recent pathological studies (Fox et al., 2020; Colonna et al., 2020) have indeed shown how a diffuse microvascular thrombosis is a common finding in the lungs of COVID-19 patients at post-mortem examination. Moreover, coagulation abnormalities and increased risk of thrombosis have been widely recognized as peculiar clinical features of COVID-19, highlighting the use of anticoagulation as fundamental for the treatment strategy (Bikdeli et al., 2020). It is then not surprising that even the nailfold capillary bed is affected by microvascular abnormalities in the acute phase of the disease. In addition, the frequent finding of enlarged capillaries and capillary density at the lower limit in post-acute patients strengthens this concept as these findings could reasonably be the result of previous thrombotic capillary damage in the acute phase.
Our results suggest therefore a continuum spectrum of microvascular alterations in the nailfold capillary bed that goes from micro-thrombosis to altered capillary structure, according to COVID-19 phase. It is interesting to note that only in post-acute patients there was an involvement greater than the cut-off of 33% of the capillaries examined with regard to specific alterations (i.e. enlarged capillaries, capillary density below 9 per millimeter, microvascular derangement and meandering capillaries). This observation could be justified by the longer exposure time of post-acute patients to the detrimental effects of viral infection on microcirculation, which may have resulted in a greater accumulation of damage than in patients with acute disease, usually examined in the first days after onset of symptoms.
These observations pave the way to some important clinical questions on the role of the evaluation of the microcirculation and, specifically, of the use of the NVC in the management of COVID-19 patients. First, it would be important to further investigate whether the use of NVC in acute patients could rapidly detect those at risk of major microvascular complications. Second, it would be worth considering whether the detection of micro-thrombosis at NVC could be used, together with other clinical parameters, to individually tailor the extent of anticoagulation therapies. Remarkably, a recent study first highlighted the presence of capillaroscopic changes in a viral infectious disease, analyzing with univariate statistics the findings obtained from a cross-sectional cohort of patients with chronic hepatitis B and C compared with healthy controls (without any capillaroscopic alteration) with a low magnification technique. Similar to our observations, capillary tortuosity, capillary enlargement and alterations of the distribution were common findings in the chronic phase of both diseases, which have been well characterized by the presence of extrahepatic pathological manifestations on a micro- and macro-vascular basis. The authors also concluded that capillaroscopy could be able to identify the microvascular damage as a result of endothelial injury secondary to the viral disease, mainly through immune-mediated mechanisms (Pancar et al., 2020).
Given its exploratory nature, the present study nevertheless presents several shortcomings that deserve to be discussed. The main limitation of our study is the small number of patients evaluated, especially in the acute phase. Fortunately, this is due to the timing of our study (mid-May 2020) when the peak of new COVID-19 cases had already passed in Italy. Another major limitation is the cross-sectional design. Indeed, the evaluation of the same patients in the acute and post-acute phase, which would provide more solid and clear data on the possible evolution of microvascular damage, is missing. For these reasons, we cannot rule out the absence of correlation between the severity of the clinical status and the NVC results. Similarly, the impact of some inhomogeneities in clinical features such as the presence of diabetes and active smoking cannot be clearly defined.
Of note, in our cohort there were no patients with CTD classically linked to specific alterations of the microcirculation (i.e. scleroderma pattern), however, it should also be considered that non-specific capillaroscopic changes have been described in several non-rheumatological diseases such as arterial hypertension, diabetes, Alzheimer's disease, glaucoma, and interstitial lung disease, among others (Ciaffi et al., 2020; Faggioli et al., n.d.). Some of these comorbidities (e.g. arterial hypertension and diabetes) have been reported as risk factors for COVID-19 pneumonia (Zhou et al., 2020; Apicella et al., 2020) and therefore, having applied only a generic exclusion criterion (hand trauma), they are fairly represented in our cohort, representing a further variable to consider.
Regarding the influence of pharmacological treatments, the difference in exposure to enoxaparin in the two groups needs to be addressed: since the recovered patients had been hospitalized on average 5–8 weeks before the patients of acute group, should be emphasized that the observed difference probably reflects the change in clinical attitude in relation to the use of enoxaparin in patients with COVID-19 pneumonia, with use gradually increased during the pandemic. Exposure to other specific drugs such as antivirals, hydroxychloroquine and anti-IL6R and oxygen therapy during hospitalization was homogeneous as a result of defined treatment protocols within the clinics. In general, it should be emphasized that at present there is no clear evidence regarding the impact of these or other common treatments (e.g. lipid-lowering and antihypertensive drugs) on capillary morphology detected with NVC.
Longitudinal studies with a substantial number of patients, including both patients with asymptomatic or mild SARS-CoV2 infection (e.g. patients without pneumonia) and patients actively treated in ICU, are thus needed to make the application of more stringent exclusion criteria sensible and correct for possible confounding factors, such as known determinants of microvascular damage, and get further clues on the physio-pathogenetic implications of microvascular alterations seen at nail bed.
For the same purpose, the comparison between the capillaroscopic data of patients before and after COVID-19 would also be of great interest. Clearly, this kind of evaluation is difficult to implement since generally the capillaroscopy has restricted clinical indications. However, careful monitoring of patients followed up at rheumatology clinics, who regularly perform capillaroscopy for acral symptoms (e.g. primary RP without an underlying CTD) for re-evaluation of those with COVID-19, would be desirable. Another possibility would be to carry out capillaroscopic assessment in an at-risk population included in follow-up clinical studies.
Finally, some technical limitations inherent to the NVC must be considered. Considerable efforts for the standardization of the methodology and the definition of capillaroscopic changes have been made recently (Smith et al., 2019), providing a fundamental framework for the diagnosis and follow-up of CTDs; however, a part of the alterations considered in this work, even though previously described, could have uncertain reliability (Smith et al., 2020). Specifically, the frequent finding of pericapillary edema, particularly in acute patients, may have marginally influenced the finding of milder alterations such as meandering capillaries, contributing in part to the difference observed between the two groups. Furthermore, bearing in mind that NVC is not the examination of choice for the evaluation of the flow, the reported assessments on rheological aspects of the microcirculation (i.e. sludge flow), must be taken cautiously, and future studies should include dedicated methods such as sidestream dark field imaging (Eriksson et al., 2014) for better peripheral perfusion measurements along with the NVC morphological assessment.
5. Conclusion
COVID-19 patients present microvascular abnormalities at direct assessment through NVC. Acutely ill and recovered subjects are characterized by a different distribution of elementary capillaroscopic alterations, resembling an acute and post-acute type of microvascular damage. These preliminary results could pave the way to further longitudinal studies in larger cohorts.
CRediT authorship contribution statement
Gerlando Natalello: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data Curation, Writing - Original Draft, Writing - Review & Editing, Visualization; Giacomo De Luca: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data Curation, Writing - Original Draft, Writing - Review & Editing, Visualization; Laura Gigante: Methodology, Investigation, Writing - Review & Editing; Corrado Campochiaro: Methodology, Investigation, Writing - Review & Editing; Enrico De Lorenzis: Formal analysis, Investigation, Writing - Review & Editing; Lucrezia Verardi: Investigation, Writing - Review & Editing; Annamaria Paglionico: Investigation, Writing - Review & Editing; Luca Petricca: Investigation, Writing - Review & Editing; Anna Maria Martone: Investigation, Writing - Review & Editing; Stefania Calvisi: Investigation, Writing - Review & Editing; Marco Ripa: Investigation, Writing - Review & Editing; Giulio Cavalli: Investigation, Writing - Review & Editing; Emanuel Della-Torre: Investigation, Writing - Review & Editing; Moreno Tresoldi: Investigation, Writing - Review & Editing; Francesco Landi: Resources, Supervision, Project administration, Writing - Review & Editing; Silvia Laura Bosello: Conceptualization, Methodology, Formal analysis, Supervision, Project administration, Writing - Original Draft, Writing - Review & Editing; Elisa Gremese: Conceptualization, Resources, Supervision, Project administration, Writing - Review & Editing; Lorenzo Dagna: Conceptualization, Resources, Supervision, Project administration, Writing - Review & Editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors want to thank the members of the Gemelli Against COVID-19 Post-Acute Care Study Group for their dedication in taking care of patients sadly affected by the COVID-19. All members and their specific roles are listed below.
Steering committee: Landi F, Gremese E. Coordination: Bernabei R, Fantoni M, Gasbarrini A. Field investigators: Gastroenterology team: Settanni CR; Geriatric team: Benvenuto F, Bramato G, Carfì A, Ciciarello F, Lo Monaco MR, Martone AM, Marzetti E, Napolitano C, Pagano F, Rocchi S, Rota E, Salerno A, Tosato M, Tritto M, Calvani R, Catalano L, Picca A, Savera G; Infectious disease team: Cauda R, Tamburrini E, Borghetti A, Di Gianbenedetto S, Murri R, Cingolani A, Ventura G, Taddei E, Moschese D, Ciccullo A; Internal Medicine team: Stella L, Addolorato G, Franceschi F, Mingrone G, Zocco MA; Microbiology team: Sanguinetti M, Cattani P, Marchetti S, Posteraro B, Sali M; Neurology team: Bizzarro A, Lauria A; Ophthalmology team: Rizzo S, Savastano MC, Gambini G, Cozzupoli GM, Culiersi C; Otolaryngology team: Passali GC, Paludetti G, Galli J, Crudo F, Di Cintio G, Longobardi Y, Tricarico L, Santantonio M; Pediatric team: Buonsenso D, Valentini P, Pata D, Sinatti D, De Rose C; Pneumology team: Richeldi L, Lombardi F, Calabrese A; Psychiatric team: Sani G, Janiri D, Giuseppin G, Molinaro M, Modica M; Radiology team: Natale L, Larici AR, Marano R; Rheumatology team: Paglionico A, Petricca L, Gigante L, Natalello G, Fedele AL, Lizzio MM, Tolusso B, Alivernini S; Vascular team: Santoliquido A, Santoro L, Nesci A, Popolla V.
Footnotes
The authors received no financial support for this research.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mvr.2020.104071.
Appendix A. Supplementary data
Supplementary tables
References
- Ackermann Maximilian. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2015432. 21 May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andracco Romina. The cumulative number of micro-haemorrhages and micro-thromboses in nailfold videocapillaroscopy is a good indicator of disease activity in systemic sclerosis: a validation study of the NEMO score. Arthritis research & therapy. 2017;19(1):133. doi: 10.1186/s13075-017-1354-5. 13 Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella Matteo. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. The lancet. Diabetes & endocrinology. 2020;8(9):782–792. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardino Vera. The impact of nailfold capillaroscopy in the approach of microcirculation. IntechOpen. 2019 doi: 10.5772/intechopen.90525. https://www.intechopen.com/online-first/the-impact-of-nailfold-capillaroscopy-in-the-approach-of-microcirculation December 20th. Available from. [DOI] [Google Scholar]
- Bikdeli Behnood. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J. Am. Coll. Cardiol. 2020;S0735-1097(20) doi: 10.1016/j.jacc.2020.04.031. 15 Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campochiaro Corrado. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. European journal of internal medicine. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli Giulio. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. The Lancet Rheumatology. 2020;2(6):e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaffi Jacopo. Nailfold capillaroscopy in common non-rheumatic conditions: a systematic review and applications for clinical practice. Microvasc. Res. 2020;131 doi: 10.1016/j.mvr.2020.104036. [DOI] [PubMed] [Google Scholar]
- Ciceri Fabio. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Critical care and resuscitation. 2020;22(2):95–97. doi: 10.51893/2020.2.pov2. Epub ahead of print. PMID: 32294809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna Cristiana. Chilblain-like lesions in children following suspected COVID-19 infection. Pediatr. Dermatol. 2020 doi: 10.1111/pde.14210. 6 May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutolo Maurizio. Nailfold videocapillaroscopy assessment of microvascular damage in systemic sclerosis. J. Rheumatol. 2000;27(1):155–160. [PubMed] [Google Scholar]
- De Luca Giacomo. GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a single-centre, prospective cohort study. Lancet Rheumatology. 2020 doi: 10.1016/S2665-9913(20)30170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson Sam. Non-invasive imaging of microcirculation: a technology review. Medical devices (Auckland, N.Z.) 2014;7:445–452. doi: 10.2147/MDER.S51426. 9 Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etehad Tavakol Mahnaz. Nailfold capillaroscopy in rheumatic diseases: which parameters should be evaluated? Biomed. Res. Int. 2015;2015 doi: 10.1155/2015/974530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faggioli, Paola et al. “Nailfold videocapillaroscopy in internal medicine.” Italian Journal of Medicine, 9(3), 234–242. doi: 10.4081/itjm.2015.548. [DOI]
- Fox Sharon E. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir. Med. 2020;S2213-2600(20):30243–30245. doi: 10.1016/S2213-2600(20)30243-5. 27 May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremese, Elisa et al. “Sarilumab use in severe SARS-CoV-2 pneumonia.” Preprint on medRxiv 2020.05.14.20094144; doi: 10.1101/2020.05.14.20094144. [DOI]
- Harzallah Inès. Lupus anticoagulant is frequent in patients with Covid-19. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14867. 23 Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
- Ingegnoli Francesca. Feasibility of different capillaroscopic measures for identifying nailfold microvascular alterations. Semin. Arthritis Rheum. 2009;38(4):289–295. doi: 10.1016/j.semarthrit.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Kabasakal Yasemin. Quantitative nailfold capillaroscopy findings in a population with connective tissue disease and in normal healthy controls. Ann. Rheum. Dis. 1996;55(8):507–512. doi: 10.1136/ard.55.8.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur Savneet. The enigma of endothelium in COVID-19. Front. Physiol. 2020;11:989. doi: 10.3389/fphys.2020.00989. 4 Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticone Giovanna. Quantitative nailfold capillary microscopy findings in patients with acrocyanosis compared with patients having systemic sclerosis and control subjects. J. Am. Acad. Dermatol. 2000;42(5 Pt 1):787–790. doi: 10.1067/mjd.2000.103046. [DOI] [PubMed] [Google Scholar]
- Paglionico Annamaria, on behalf of Gemelli Against COVID-19 Post-Acute Care Study Group . CO0002 Annals of the Rheumatic Diseases. Vol. 79. 2020. Loss of self-tolerance in SARS-COV-2 infection: immunological assessment of a convalescent cohort; p. 213. [Google Scholar]
- Pancar G. Sefika. Nailfold capillaroscopic changes in patients with chronic viral hepatitis. Microvasc. Res. 2020;129:103970. doi: 10.1016/j.mvr.2019.103970. [DOI] [PubMed] [Google Scholar]
- Pavlov-Dolijanovic Slavica. Scleroderma pattern of nailfold capillary changes as predictive value for the development of a connective tissue disease: a follow-up study of 3,029 patients with primary Raynaud’s phenomenon. Rheumatol. Int. 2012;32(10):3039–3045. doi: 10.1007/s00296-011-2109-2. [DOI] [PubMed] [Google Scholar]
- Sambataro Domenico. Nailfold videocapillaroscopy micro-haemorrhage and giant capillary counting as an accurate approach for a steady state definition of disease activity in systemic sclerosis. Arthritis research & therapy. 2014;16(5):462. doi: 10.1186/s13075-014-0462-8. 9 Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardu Celestino. Hypertension, thrombosis, kidney failure, and diabetes: is COVID-19 an endothelial disease? A comprehensive evaluation of clinical and basic evidence. J. Clin. Med. 2020;9(5) doi: 10.3390/jcm9051417. 11 May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi Hasan K., Mehra Mandeep R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J. Heart Lung Transplant. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Vanessa. Fast track algorithm: how to differentiate a "scleroderma pattern" from a "non-scleroderma pattern". Autoimmun. Rev. 2019;18(11) doi: 10.1016/j.autrev.2019.102394. [DOI] [PubMed] [Google Scholar]
- Smith Vanessa. Standardisation of nailfold capillaroscopy for the assessment of patients with Raynaud's phenomenon and systemic sclerosis. Autoimmun. Rev. 2020;19(3) doi: 10.1016/j.autrev.2020.102458. [DOI] [PubMed] [Google Scholar]
- Sulli Alberto. Scoring the nailfold microvascular changes during the capillaroscopic analysis in systemic sclerosis patients. Ann. Rheum. Dis. 2008;67(6):885–887. doi: 10.1136/ard.2007.079756. [DOI] [PubMed] [Google Scholar]
- Zhou Fei. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables