Abstract
Background
Disinfection of gloves can be used during a pandemic situation when performing various procedures on the same patient or when removing personal protective equipment. If performing glove disinfection, there is a need to check the compatibility of gloves with the disinfectant product used.
Aim
To test the resistance of nitrile gloves to various disinfectant solutions.
Methods
One hundred percent powder-free nitrile gloves, composed of nitrile butadiene rubber compounds, were exposed to various disinfectants to analyse resistance. The seven most commonly used disinfectant solutions in the healthcare field were selected for testing. The effects of each disinfectant were analysed in comparison with the control group (untreated glove). For tensile testing, the thickness of each test specimen was measured with a micrometer.
Findings
Bleach solution decreased the breaking load of gloves, although to a lesser extent than disinfectants that contained ethanol.
Conclusion
Disinfectants that contain alcohol decrease the breaking load of nitrile gloves.
Keywords: Coronavirus disease 2019, COVID-19, Disinfection, Gloves, Hand disinfectant solution, SARS-CoV-2
Introduction
The outbreak of coronavirus 2019 disease (COVID-19) in Wuhan, China, has rapidly developed into a worldwide health crisis [1]. According to the latest data from the World Health Organization (WHO) (7th September 2020) [2], there have been 26, 961, 795 confirmed cases and 880,955 confirmed deaths worldwide.
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is mainly spread through respiratory droplets and contact [1]. Therefore, the disinfection of objects and handwashing are essential to stop viral spread [3]. Studies have also shown the presence of SARS-CoV-2 in saliva and faeces of infected patients [4,5].
In this pandemic situation, the greatest risk is transmission of SARS-CoV-2 to healthcare workers (HCWs) from direct contact with infected patients [6]. HCWs may have to perform multiple tasks on an infected patient, and must be aware of the appropriate use of personal protective equipment (PPE), as well as the proper use and removal of gloves [1,7]. Regarding the proper use of gloves, it is important to remove them after a single use and avoid inappropriate use [8]. However, there are several clinical situations in which HCWs use gloves routinely to carry out various procedures on the same patient [9]. In order for HCWs to comply with international standards, gloves would have to be removed often, hands disinfected and a new pair of gloves used to carry out subsequent activities with the same patient [10]. This may not be realistic, particularly in times of material shortage and crisis, such as the current pandemic situation.
Faced with this situation, glove disinfection may be a viable alternative [9], as stated by the Commission for Hospital Hygiene and Infection Prevention at the Robert Koch Institute, Berlin [11]. According to the Commission, faced with the additional workload generated by COVID-19, gloves can be used for multiple procedures on the same patient if they are disinfected properly between each procedure.
Glove disinfection is not only useful for re-use for the same patient, but can also be considered as part of the process of removing PPE. The Centers for Disease Control and Prevention and the European Centre for Disease Prevention and Control (ECDC) recommend disinfecting gloves that have been used as part of PPE before removing the equipment in healthcare settings for the care of patients with suspected or confirmed COVID-19 due to the high risk of self-contamination [12,13]. For this reason, HCWs must be trained in the proper use of PPE according to hospital guidelines, including techniques to safely remove equipment that protects mucous membranes [7].
One important aspect to consider is that, prior to glove disinfection, it is necessary to check the compatibility of the glove with the disinfectant [9]. Two studies have analysed the integrity of glove material after repeated applications of disinfectant products [14,15]. Specifically, these studies found that tensile strength decreased with each alcohol-based hand rub, and combinations have relevant differences in the efficacy of disinfection. Further studies are required to clarify if the differences between the various disinfection products used are sufficient to exclude certain disinfectant solutions from glove decontamination activities in clinical settings [15]. As such, the objective of this study was to test the resistance of nitrile gloves to different disinfectant solutions.
Methods
This study used 100% powder-free nitrile gloves, composed of nitrile butadiene rubber compounds. The most commonly used disinfectant solutions in the healthcare field were selected for testing: (1) hand antiseptic (85 g ethanol/100 g) solution; (2) disinfectant for medical equipment surfaces (40.5 g ethanol + 9.20 g n-propanol/100 g); (3) water-based (2% chlorhexidine digluconate) antiseptic; (4) 1:10 bleach solution (sodium hypochlorite) (5000 ppm); (5) alcohol-based chlorhexidine digluconate antiseptic 2%; (6) hydrogen peroxide cutaneous solution (3 g/100 mL); (7) 96% ethanol; and (8) 70% alcohol.
To perform the tests, gloves were selected from a pack of 100 Sensiflex Lite gloves. The following procedure was followed for sampling. First, dumbbell-type specimens were cut according to UNE-EN ISO 527-3 standard, which specifies how trials and tests should be performed to determine tensile properties, into type 5 specimens (Figure 1 ) using a die. Four specimens were obtained from each glove. Specimens were cut from the palm and the back of the glove in the direction indicated by the fingers, ensuring that the edges of the specimens were smooth and had no raised edges, discarding those that presented edge imperfections (Figure 2 ). The samples obtained from each glove were divided into as many groups as tests to be carried out, so each group contained five samples and no group had more than one sample from the same glove. In this way, homogeneous and representative sampling was achieved.
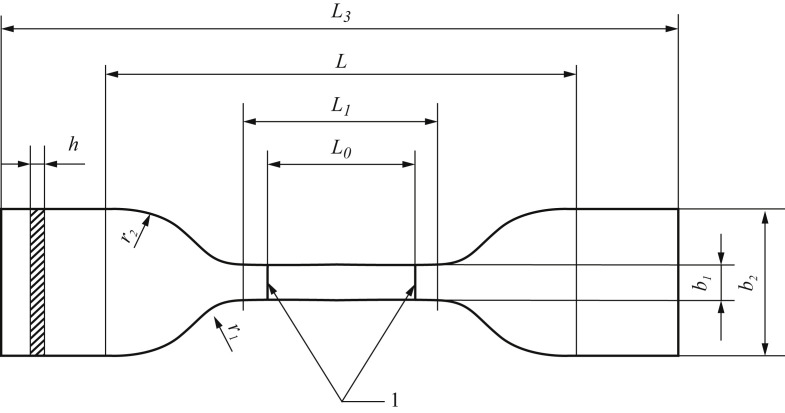
Figure 1.
Specifications of the type 5 dumbbell test specimens (UNE-EN ISO 527–3:2019). 1, indicators; b1, width of the narrow part (6 mm ± 0.4 mm); b2, width at the ends (25 mm ± 1 mm); h, thickness (≤1 mm); L0, distance between indicators (25 mm ± 0.25 mm); L1, length of the narrow part (33 mm ± 2 mm); L, initial length between jaws (80 mm ± 5 mm); L3, total length (≥115 mm); r1, smaller radius (14 mm ± 1 mm); r2, smaller radius (25 mm ± 2 mm).
Figure 2.
Test specimens obtained (UNE-EN ISO 527-3 Type 5).
Table I shows the thickness measurements of each of the test specimens by group. Once the groups were defined, the disinfectant was applied homogeneously to one face of each specimen (in order to simulate the glove disinfection process followed by health personnel). The visual effect it caused was noted. Specimens were allowed to dry for at least 2 min before performing the stress-strain test.
Table I.
Trial parameters
| Trial | Test | Thickness (μm) | Elongation at breakage (%) | Breakage traction (MPa) | Module (dryer 2%) (MPa) | Area under the curve (MPa) | Breakage load (cursor) (N) | |
|---|---|---|---|---|---|---|---|---|
| G1 | Untreated | 1 | 51 | 314 | 32.9 | 18.8 | 39.6 | 10.08 |
| 2 | 55 | 330 | 33.3 | 17.5 | 41.8 | 10.99 | ||
| 3 | 55 | 323 | 33.9 | 17.8 | 42.6 | 11.17 | ||
| 4 | 58 | 338 | 31.3 | 17.2 | 39 | 10.9 | ||
| 5 | 58 | 323 | 28.9 | 18.3 | 35.7 | 10.06 | ||
| M | 55 | 326 | 32.1 | 17.9 | 39.7 | 10.64 | ||
| SD | 2.9 | 9.1 | 2 | 0.6 | 27 | 0.5 | ||
| G2 | Hydrogen peroxide 3% | 1 | 56 | 344 | 33.2 | 13.8 | 41.3 | 11.17 |
| 2 | 57 | 338 | 32.4 | 16.4 | 38.5 | 11.08 | ||
| 3 | 55 | 334 | 30.6 | 17.7 | 37.9 | 10.1 | ||
| 4 | 54 | 339 | 35.8 | 21.7 | 45.6 | 11.59 | ||
| 5 | 53 | 335 | 31.5 | 18.2 | 38.8 | 10.02 | ||
| M | 55 | 338 | 32.7 | 18.2 | 40.4 | 10.79 | ||
| SD | 1.6 | 3.9 | 2 | 2.1 | 3.2 | 0.7 | ||
| G3 | Chlorhexidine alcohol | 1 | 53 | 336 | 23.1 | 16.1 | 29.2 | 7.35 |
| 2 | 56 | 314 | 20.9 | 17.7 | 25.7 | 7.02 | ||
| 3 | 55 | 330 | 23.4 | 16.8 | 29 | 7.72 | ||
| 4 | 54 | 342 | 25.6 | 18.2 | 33 | 8.28 | ||
| 5 | 51 | 323 | 27.1 | 20.4 | 33.6 | 8.28 | ||
| M | 54 | 329 | 24 | 17.9 | 30.1 | 7.73 | ||
| SD | 1.9 | 11.1 | 2.4 | 1.7 | 3.2 | 0.6 | ||
| G4 | 96% Ethanol | 1 | 59 | 354 | 16.2 | 14.1 | 22.5 | 5.75 |
| 2 | 53 | 338 | 17.3 | 16.4 | 24.3 | 5.5 | ||
| 3 | 57 | 350 | 21.4 | 16.2 | 29.1 | 7.33 | ||
| 4 | 54 | 323 | 21.2 | 17.2 | 27.5 | 6.88 | ||
| 5 | 57 | 318 | 21 | 15 | 25.5 | 7.18 | ||
| M | 56 | 337 | 19.4 | 15.8 | 25.8 | 6.53 | ||
| SD | 2.4 | 15.7 | 2.5 | 1.2 | 2.6 | 0.8 | ||
| G5 | Bleach solution 1:10 | 1 | 55 | 300 | 27 | 17 | 31.2 | 8.39 |
| 2 | 52 | 295 | 27.8 | 18.5 | 31.9 | 8.68 | ||
| 3 | 54 | 308 | 29.3 | 18.7 | 35.9 | 9.48 | ||
| 4 | 56 | 302 | 26.2 | 17.1 | 32.2 | 8.8 | ||
| 5 | 56 | 290 | 25.4 | 16.3 | 28.2 | 8.52 | ||
| M | 55 | 299 | 27.1 | 17.5 | 31.9 | 8.88 | ||
| SD | 1.7 | 6.9 | 1.5 | 1 | 2.8 | 0.4 | ||
| G6 | Alcohol-based disinfectant | 1 | 56 | 309 | 20.2 | 15.6 | 24.3 | 6.78 |
| 2 | 52 | 323 | 26.8 | 19.2 | 32.2 | 8.35 | ||
| 3 | 57 | 295 | 23.2 | 11.3 | 26.5 | 7.92 | ||
| 4 | 53 | 292 | 21.7 | 18.7 | 25 | 6.9 | ||
| 5 | 55 | 277 | 21.5 | 16.8 | 24.5 | 7.1 | ||
| M | 55 | 299 | 22.7 | 16.3 | 26.5 | 7.41 | ||
| SD | 2.1 | 17.8 | 2.5 | 3.2 | 3.3 | 0.7 | ||
| G7 | Hand sanitizer (85 g ethanol/100 g) | 1 | 54 | 324 | 27.4 | 15.7 | 33.1 | 8.87 |
| 2 | 56 | 304 | 21.9 | 15.9 | 25.3 | 7.36 | ||
| 3 | 53 | 305 | 24.9 | 16.6 | 28.8 | 7.9 | ||
| 4 | 57 | 315 | 24.8 | 16.5 | 30.7 | 8.47 | ||
| 5 | 56 | 286 | 17.1 | 15.2 | 20.2 | 5.75 | ||
| M | 55 | 307 | 23.2 | 16 | 27.6 | 7.67 | ||
| SD | 1.6 | 14.2 | 3.9 | 0.6 | 5 | 1.2 | ||
| G8 | Water-based antiseptic | 1 | 53 | 298 | 26.2 | 21.3 | 31.3 | 8.33 |
| 2 | 57 | 323 | 30.8 | 18.6 | 35.2 | 10.53 | ||
| 3 | 54 | 304 | 27.8 | 22.5 | 33.1 | 9.02 | ||
| 4 | 55 | 335 | 34.5 | 20.7 | 41.8 | 11.4 | ||
| 5 | 57 | 312 | 27.1 | 16.7 | 32.3 | 9.26 | ||
| M | 55 | 314 | 29.3 | 20 | 34.8 | 9.71 | ||
| SD | 1.8 | 15 | 3.4 | 2.3 | 4.2 | 1.2 | ||
| G8 | 70% Alcohol | 1 | 56 | 354 | 17.5 | 17.0 | 26.4 | 5.87 |
| 2 | 56 | 322 | 19.5 | 14.9 | ----- | 6.54 | ||
| 3 | 57 | 295 | 16.7 | 16.9 | 20.8 | 5.73 | ||
| 4 | 55 | 339 | 21.1 | 15.0 | 29.7 | 6.97 | ||
| 5 | 59 | 355 | 20.1 | 14.7 | 27.3 | 7.11 | ||
| M | 57 | 333 | 19.0 | 15.7 | 26.2 | 6.45 | ||
| SD | 1.5 | 25.1 | 1.8 | 1.1 | 3.8 | 0.6 | ||
M, mean; SD, standard deviation.
Exposure to the disinfectant was analysed by comparing each specimen with the control group (untreated glove) (Figure 3 ), following the UNE-EN ISO 527-3 standard. In order to do so, specimens were tensed along the longitudinal axis, at a constant rate, until they broke or until the strain (load) or deformation (elongation) reached a certain value, with both the strain and elongation values recorded throughout the procedure. ISO 7500–1 (tension/compression testing machines) and ISO 9513 (calibration of strain gauges used in uniaxial tests) standards were followed, as well as all of the specifications outlined in Sections 5.1.2–5.1.6 of the ISO 527–1:2012 standards.
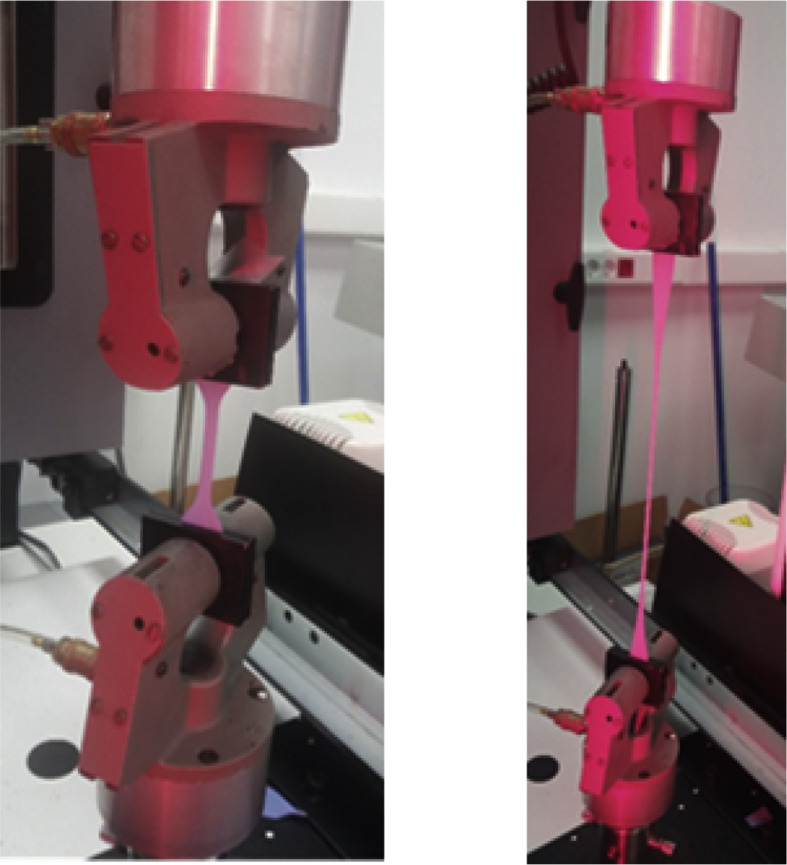
Figure 3.
Tensile deformation test.
For tensile testing, the thickness of each specimen was measured with a micrometer with sensitivity of ±1 μm. The distance between grips was adjusted to 65 mm, the test specimen was loaded correctly, and the separation speed between grips was set at 500 mm/min. Finally, load and elongation were monitored until the specimen broke.
Results
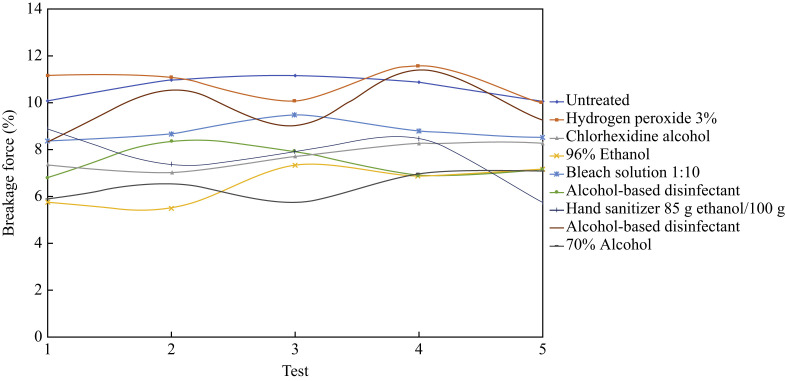
The results are based on the parameters specified in ISO 527 standard, where several other parameters of interest were recorded in addition to elongation and strain. Figure 4 shows a graphical representation of the breaking load percentage for the 45 test specimens by group.
Figure 4.
Breaking load for 45 test specimens by group.
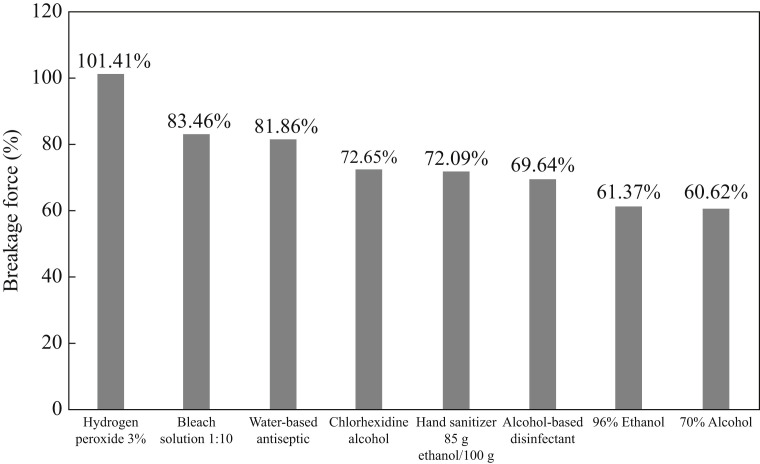
Elongation at breakage was not affected, although force at breakage decreased following the application of all disinfectants containing alcohol. The gloves disinfected with 70% alcohol broke using 60.62% of the effort required to break the untreated specimen, and this was found to be the most aggressive disinfectant (Figure 5 ). Specimens treated with any of the alcohol-based disinfectants broke more easily that the untreated specimen; they lost at least 25% of tensile strength, and this reached almost 40% in the case of 70% alcohol solution. Hydrogen peroxide 3% was the only disinfectant that maintained the glove's tensile strength properties.
Figure 5.
Strength at breakage following exposure to different solutions, in comparison with the untreated test specimen (control).
Discussion
The objective of this study was to test the resistance of nitrile gloves to various disinfectant solutions. Although WHO does not recommend re-using gloves, the current pandemic situation has led to a shortage of resources in the fight against COVID-19, which makes it necessary to reconsider the re-use of gloves with the same patient. In this situation, disinfection makes it safer to re-use gloves, ensuring that gloves are visibly clean and without perforations [9,16,17]. The disinfection of gloved hands by HCWs can reduce the risk of contagion considerably when the gloves are designated for the entire patient care process and multiple tasks are carried out on the same patient [9,18]. Specifically, it has been shown that the efficiency of disinfection is greater for different combinations of disinfectants/gloves than for bare hands [15]. For this reason, choosing an appropriate disinfectant solution for the type of glove used is a fundamental aspect during disinfection. Furthermore, differences in the effectiveness of disinfectant solutions seem to depend on the material of which the gloves are composed [15]. This study found that disinfectant solutions that contain alcohol do not affect elongation at breakage, but they do affect the force required to break the nitrile gloves. This may be due to the fact that, to achieve maximum elongation, the resistance that the material opposes is less, making it easier to break. A previous study found that tensile strength in nitrile gloves was reduced by 26% and 35.3% after using ethanol-based and isopropanol-based products, respectively [14]. In some types of nitrile gloves, elongation increased by 30.5%, while for other types of nitrile gloves, it decreased by 17.3% [14]. In addition, several studies have found that the treatment of gloved hands with alcohol-based disinfectants leads to higher rates of perforation [15,19,20].
The disinfectants tested in this study have various applications in the hospital environment. Sodium hypochlorite and hydrogen peroxide are used in the disinfection of surfaces and equipment against nosocomial infections [20,21]. The applications of 70% ethanol and chlorhexidine include the preparation and cleaning of intact skin prior to invasive surgical procedures, and performing antiseptic and surgical handwashing [[22], [23], [24], [25], [26], [27]].
Previous studies, such as that by Kocent et al. [28], have demonstrated the potential for disinfection of gloves using 70% alcohol and chlorhexidine 0.5%. Specifically, they found that washing gloved hands in antiseptic solution prior to central venous line insertion reduced contamination. In addition, a study by Kampf and Lemmen [9], which used alcohol-based solutions, found that disinfection of gloved hands may substantially reduce the risk of transmission when gloves are indicated for the entire episode of patient care.
Considering the findings, the use of bleach and disinfectants containing alcohol affected the breaking load of nitrile gloves, although bleach had less impact. Following treatment with all alcohol-based disinfectants, glove specimens required less effort to break. However, these disinfectants are widely recognized for their high disinfection capabilities [29]. Previous studies have reported the importance of exploring disinfection of gloves with alcohol solutions, as these types of solutions are widely used [15].
Limitations
This study had several limitations. Firstly, the experiments were only performed on nitrile gloves; therefore, there is a need for further testing with other materials. Furthermore, studies that have analysed the use of disinfectant with nitrile gloves are scarce, which makes it difficult to achieve an adequate discussion. However, this highlights the novel nature of the topic. Finally, due to the current healthcare crisis, a limited number of tests were performed, and there is a need to expand the research to include additional testing. Finally, it would be useful if glove manufacturers provided data about the compatibility of their gloves with different types of hand rubs and disinfectant solutions.
In conclusion, this study determined the influence of different disinfectants on nitrile gloves, and found that disinfectants that contain alcohol reduced the breaking load of gloves. Therefore, products that have ethyl components can weaken nitrile gloves, increasing the risk of breakage, which could increase the self-contamination of HCWs during clinical practice. There is a need for further research to analyse how disinfectants affect the breaking load or elongation of nitrile gloves, with the aim of minimizing the risk of spreading COVID-19 among HCWs.
Acknowledgements
The authors wish to thank the University of Almería, Diputacion of Almeria, and Sotrafa company. The authors also wish to thank Olga Fornieles Hernández, and all those who made this research possible.
Conflict of interest statement
None declared.
Funding sources
None.
References
- 1.Centers for Disease Control and Prevention . CDC; Atlanta, GA: 2019. Transmission of coronavirus disease 2010 (COVID-19)https://www.cdc.gov/coronavirus/2019ncov/about/transmission.html Available at: [last accessed April 2020] [Google Scholar]
- 2.World Health Organization . WHO; Geneva: 2020. Coronavirus disease (COVID-19) pandemic.https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Available at: [last accessed April 2020] [Google Scholar]
- 3.Lofti M., Hamblin M.R., Rezaei N. COVID-19: transmission, prevention, and potential therapeutic opportunities. Clin Chim Acta. 2020;508:254–266. doi: 10.1016/j.cca.2020.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.To K.K., Tsang O.T., Yip C.C., Chang K., Wu T., Chan J.M. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J., Wang S., Xue Y. Fecal specimen diagnosis 2019 novel coronavirus-infected pneumonia. J Med Virol. 2020 doi: 10.1002/jmv.25742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Mil Med Res. 2020;7:1–10. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chavez S., Long B., Koyfman A., Liang S.Y. Coronavirus disease (COVID-19): a primer for emergency physicians. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loveday H.P., Lynam S., Singleton J., Wilson J. Clinical glove use: healthcare workers' actions and perceptions. J Hosp Infect. 2014;86:110–116. doi: 10.1016/j.jhin.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Kampf G., Lemmen S. Disinfection of gloved hands for multiple activities with indicated glove use on the same patient. J Hosp Infect. 2017;97:3–10. doi: 10.1016/j.jhin.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Eveillard M., Rabjeau A., Pradelle M.T., Raymond F., Joly-Guillou M.L., Brunel P. Rates of adherence to hand hygiene and gloving practices in 2 French rehabilitation hospitals by differentiation between single contacts and series of successive contacts with patients or the environment. Infect Control Hosp Epidemiol. 2010;31:878–879. doi: 10.1086/655436. [DOI] [PubMed] [Google Scholar]
- 11.Kramer A., Briesch H., Christiansen B., Löffler H., Perlitz C., Reichardt C. Händehygiene in Einrichtungen des Gesundheitswesens. Empfehlung der Kommission für Krankenhaushygiene und Infektionsprävention (KRINKO) beim Robert Koch-Institut (RKI) Bundesgesundheitsblatt. 2016;59:1189–1220. doi: 10.1007/s00103-016-2416-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Diseases Control and Prevention . CDC; Atlanta, GA: 2014. Guidance for the selection and use of personal protective equipment (PPE) in healthcare settings.https://www.cdc.gov/hai/pdfs/ppe/ppeslide6-29-04.pdf Available at: [last accessed June 2020] [Google Scholar]
- 13.European Centre for Disease Prevention and Control . ECDC; Stockholm: 2020. Guidance for wearing and removing personal protective equipment in healthcare settings for the care of patients with suspected or confirmed COVID-19.https://www.ecdc.europa.eu/en/publications-data/guidance-wearing-and-removing-personal-protective-equipment-healthcare-settings Available at: [last accessed June 2020] [Google Scholar]
- 14.Gao P., Horvatin M., Niezgoda G., Weible R., Shaffer R. Effect of multiple alcohol-based hand rub applications on the tensile properties of thirteen brands of medical exam nitrile and latex gloves. J Occup Environ Hyg. 2016;13:905–914. doi: 10.1080/15459624.2016.1191640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheithauer S., Hafner H., Seef R., Seef S., Hilgers R.D., Lemmen S. Disinfection of gloves: feasible, but pay attention to the disinfectant/glove combination. J Hosp Infect. 2016;94:268–272. doi: 10.1016/j.jhin.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Fehling P., Hasenkamp J., Unkel S., Thalmann I., Hornig S., Trumper L. Effect of gloved hand disinfection on hand hygiene before infection-prone procedures on a stem cell ward. J Hosp Infect. 2019;103:321–327. doi: 10.1016/j.jhin.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Kampf G., Scheithauer S., Lemmen S., Saliou P., Suchomel M. COVID-19 associated shortage of alcohol-based hand rubs, face masks, medical gloves, and gowns: proposal for a risk-adapted approach to ensure patient and healthcare worker safety. J Hosp Infect. 2020;105:424–427. doi: 10.1016/j.jhin.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Assadian O., Humphreys P.N., Ousey K.J. Disinfection of artificially contaminated gloved hands reduces transmission of Staphylococcus epidermidis to catheter valves. J Hosp Infect. 2018;100:e57–e59. doi: 10.1016/j.jhin.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Pitten F.A., Muller P., Heeg P., Kramer A. The efficacy of repeated disinfection of disposable gloves during usage. Zentralbl Hyg Umweltmed. 1999;201:555–562. [PubMed] [Google Scholar]
- 20.Pitten F.-A., Kramer A. Desinfizierbarkeit medizinischer Handschuhe. Hygiene Medizin. 2001;26:10–12. [Google Scholar]
- 21.Bischofberger C., Gonzalez M.J., Herruzo R., Jaen F., García M.L., Sacristán A. Sociedad Española de Medicina Preventiva, Salud Pública e Higiene; Madrid: 2014. Guía de uso de desinfectantes en el ámbito hospitalario de la Sociedad Española de Medicina Preventiva de Salud Pública e Higiene. [Google Scholar]
- 22.de Andalucía Junta. Consejería de salud y familias. Dirección General de Salud Pública y Ordenación Farmacéutica; Andalucía: 2020. Procedimiento de limpieza y desinfección de superficies y espacios para la prevención del coronavirus en la comunidad autónoma de Andalucía. [Google Scholar]
- 23.Del Río-Carbajo L., Vidal-Cortés P. Types of antiseptics, presentations and rules of use. Med Intens. 2019;43:7–12. doi: 10.1016/j.medin.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Diomedi Pacheco A., Chacón E., Delpiano L., Hervé B., Jemenao M.I., Medel M. Antiseptics and disinfectants: aiming at rational use. Recommendations of the Advisory Committee on Healthcare Associated Infections. Sociedad Chilena de Infectología. Rev Chil Infectol. 2017;34:156–174. doi: 10.4067/S0716-10182017000200010. [DOI] [PubMed] [Google Scholar]
- 25.Ellingson K., Haas J.P., Aiello A.E., Kusek L., Maragakis L.L., Olmsted R.N. Strategies to prevent healthcare-associated infections through hand hygiene. Infect Control Hosp Epidemiol. 2014;35:937–960. doi: 10.1086/677145. [DOI] [PubMed] [Google Scholar]
- 26.Marschall J., Mermel L.A., Fakih M., Hadaway L., Kallen A., O’Grady N.P. Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35:753–771. doi: 10.1086/676533. [DOI] [PubMed] [Google Scholar]
- 27.Tanner J., Swarbrook S., Stuart J. Surgical hand antisepsis to reduce surgical site infection. Cochrane Database System Rev. 2008;1:CD004288. doi: 10.1002/14651858.CD004288.pub2. [DOI] [PubMed] [Google Scholar]
- 28.Kocent H., Corke C., Alajeel A., Graves S. Washing of gloved hands in antiseptic solution prior to central venous line insertion reduces contamination. Anaesth Intensive Care. 2002;30:338–340. doi: 10.1177/0310057X0203000312. [DOI] [PubMed] [Google Scholar]
- 29.European Committee for Standardization . European Committee for Standardization; Brussels: 2013. European Norm 1500: chemical disinfectants and antiseptics. Hygienic handrub. Test method and requirements (phase 2/step 2) [Google Scholar]