Abstract
CD47 is overexpressed in various types of cancers and it can directly bind with SIRPα, which is mainly located on macrophages. The binding of CD47-SIRPα transmits a “don't eat me” signal, which can prevent cancer cells from immune clearance. Targeting the phagocytosis checkpoint of CD47-SIRPα axis has shown remarkable anticancer effect in preclinical and clinical research, which indicates the potential application of CD47-SIRPα blockade for cancer treatment. In this case, the comprehensive description of the regulation of CD47 in different types of cancer cells has significant implications for furthering our understanding of the role of CD47 in cancer. Based on the current reports, we summarized the regulatory factors, i.e., cytokines, oncogenes, microRNAs as well as enzymes, of CD47 expression in cancer cells. Accordingly, we also proposed several points needing further research, hoping to provide useful insights for the future investigation on the regulation of CD47 in cancers.
Keywords: CD47, Cytokine, Oncogene, MicroRNA, QPCTL
Graphical abstract
CD47 is overexpressed in various types of cancers and it can directly bind with SIRPα, which is mainly located on macrophages. The binding of CD47-SIRPα transmits a “don't eat me” signal, which can prevent cancer cells from immune clearance. In this case, the comprehensive understandings of the regulation of CD47 in cancer cells are essential for further developments. In this review, based on the current reports, we summarized that CD47 expression could be regulated by cytokines, oncogenes, microRNAs and enzymes in cancer cells. Likewise, CD47 expression could be regulated at the transcriptional level, post-transcriptional level, post-translational modification level, etc. However, further studies are required to determine other factors that regulate CD47 expression.
Highlights
-
•
Cytokines, oncogenes, microRNAs and enzymes regulate CD47 expression in cancer.
-
•
CD47 expression could be regulated at the transcriptional, post-transcriptional and post-translational modification level.
-
•
Further studies are required to determine other factors that regulate CD47 expression.
Introduction
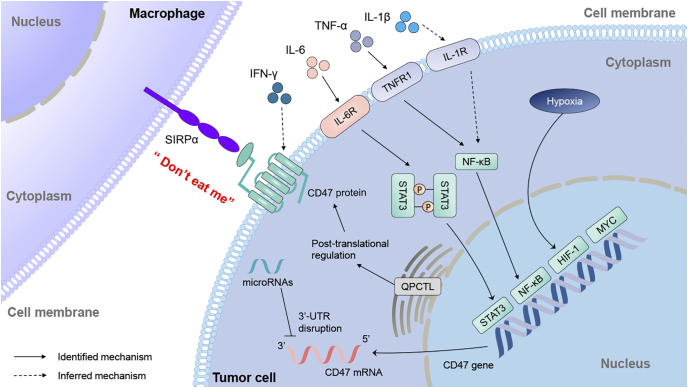
Cluster of differentiation 47 (CD47), also known as integrin-associated protein (IAP), is a 50-kDa transmembrane protein belonging to the immunoglobulin (Ig) superfamily, which is comprised of an extracellular amino-terminal Ig domain, five highly hydrophobic putative membrane-spanning segments, and a short cytoplasmic tail [1]. It is encoded by CD47 gene, which located on 3q13.12 region of chromosome in human. CD47 is originally identified as a membrane molecule involved in β3 integrin-mediated signaling on platelets and placenta in 1990s [1,2]. It was proved to interact with thrombospondin-1, signal regulatory protein-alpha (SIRPα) or others, leading to the regulation of different cellular behaviors including cell motility, cell apoptosis, T cell activation and phagocytosis. [[3], [4], [5]]. In 2000, CD47 was firstly identified as a “marker of self” on murine red blood cells, which interacted with SIRPα to prevent the clearance of red blood cells by splenic red pulp macrophages in bloodstream [6]. In 2009, CD47-SIRPα axis was considered as a tumor phagocytosis checkpoint signal, which transmits the “don't eat me” signal to macrophage and is utilized as an immune evasion mechanism by various cancers (Fig. 1) [7]. The binding of CD47 to SIRPα induces tyrosine phosphorylation of two tyrosine residues in the intracellular immunoreceptor tyrosine-based inhibitory motif of SIRPα and recruiting the protein tyrosine phosphatases Src Homology 2 (SH2)-containing protein tyrosine phosphatase 1 (SHP-1) and SHP-2. These signaling changes result in alterations of a variety of substrates and downstream signaling pathways, including inhibition of non-muscle myosin IIA, thereby restricting the phagocytic function of macrophage [[8], [9], [10]]. Blocking CD47 by monoclonal antibodies reactivated the phagocytosis function of macrophage and significantly decreased the tumor burden in mice models with hematologic neoplasms or solid tumors, dependent on the existing of macrophages rather than other immune cells [7,10].
Fig. 1.
Regulation of phagocytosis by CD47-SIRPα axis.
CD47 is overexpressed in majority of cancer cells and the binding of CD47-SIRPα send “don't eat me” signal, which enables cancer cells to escape macrophage phagocytosis.
Blocking of CD47 with an anti-CD47 antibody disables “don't eat me” signal from CD47-SIRPα axis, thereby stimulating phagocytosis of cancer cells.
Notably, although CD47 is ubiquitously expressed on normal tissue, evidence showed that high expression of CD47 was observed in wide range of human cancers including acute myeloid leukemia (AML), non-Hodgkin lymphoma (NHL), breast cancer and melanoma [[11], [12], [13], [14]]. Meanwhile, increased expression of CD47 is associated with poor prognosis in those patients with AML, NHL and breast cancer [11,13,15]. To date, targeting CD47-SIRPα axis becomes a focus of attention in cancer immunotherapy. Several CD47 blocking antibodies and SIRPα-Fc fusion proteins are studied in clinical trials among a wide range of cancer types, including CD47 blocking antibodies Hu5F9-G4 (Gilead Sciences, Inc.), SHR-1603 (Jiangsu Hengrui Medicine, Co., Ltd.), TJC4 (I-Mab Biopharma, Co., Ltd.), IBI188 (Innovent Biologics, Inc.) and AO-176 (Arch Oncology, Inc.), and SIRPα-Fc fusion protein TTI-621 (Trillium Therapeutics, Inc.). Recent clinical data (NCT02953509) exhibited that Hu5F9-G4, a humanized IgG4 monoclonal antibody blocking CD47-SIRPα interaction, combined with rituximab induced promising anti-cancer effect with a tolerate safety profile in patients with relapsed or refractory NHL [16], which further supported the future clinical application of CD47 blockade for cancer treatment. Collectively, the higher expression on tumor cells and more emerging clinical trials targeting CD47 highlights the requirement for a comprehensive understanding to the regulation of CD47 and imply targeting to CD47 expression as a strategy to block CD47-SIRPα axis.
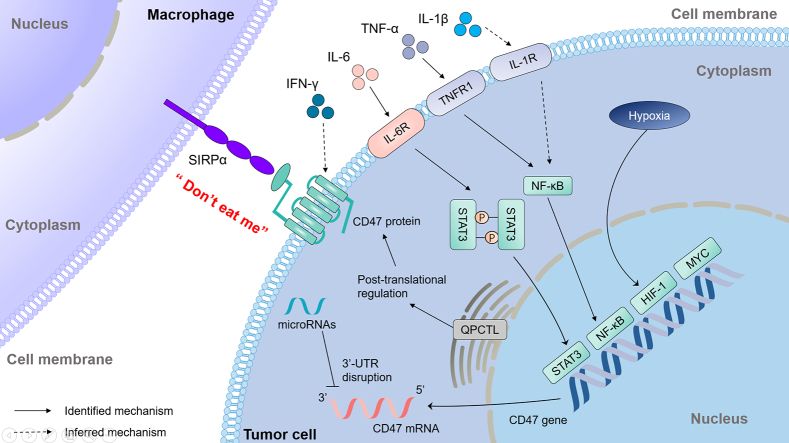
In this review, we mainly summarized the potential regulatory factors of CD47 expression in tumor tissue and divided them into four parts including cytokines, oncogenes, microRNAs as well as enzymes (Fig. 2). And we discussed the potential association between the information we collected and future research, hoping to provide an insight for the development of CD47 as a novel drug target.
Fig. 2.
The regulatory mechanisms of CD47 expression.
Regulators of CD47 expression in cancer cells includes cytokines, oncogenes, microRNAs as well as enzymes. IL-6 induces the expression of CD47 through activating STAT3 signaling pathway. Both TNF-α and IL-1β stimulate the expression of CD47 at the transcriptional level, which are mediated by the activation of NF-κB pathway and the increased binding of NF-κB to the CD47 promoter. IFN-γ increased the expression of CD47 and the specific mechanism need to be further confirmed. HIF-1, which is induced by hypoxia conditions, directly binds to CD47 promoter to increase the transcriptional expression of CD47. MYC directly binds to the CD47 promoter to transcriptionally up-regulate the expression of CD47. miR-708, miR-192, miR-222, miR-133a, miR-155 miR-200a and miR-340 suppress CD47 expression by directly targeting the 3′-UTR of CD47 mRNA. QPCTL modifies the N-terminal pyroglutamate formation of CD47 protein at the post-translational modification level, thereby influencing the binding of CD47-SIRPα. The solid arrows indicated that identified mechanism and dashed arrows indicated that inferred mechanism.
Regulation of CD47 expression
Cytokines
Tumor necrosis factor alpha (TNF-α) is a member of the TNF/TNFR cytokine superfamily [17], widely involved in promotion and progression of chronic inflammatory disease and malignant tumors [18,19]. TNF-α could regulate a range of cytokines in the tumor microenvironment such as CCL8, vascular endothelial growth factor (VEGF) [20,21]. Currently, TNF-α was reported as an inducer of CD47 expression in various types of cancer cells [12,22,23]. Treatment with 100 ng/ml of TNF-α for 8 h led to enhancement of almost four times CD47 transcriptional expression in MCF7 breast cancer cells and the same concentration of TNF-α increase nearly four times CD47 transcriptional expression in HepG2 hepatoma cancer cells after 48 h [12]. Upon TNF/TNFR1 pathway stimulation, NF-κB was released and entered the nucleus where it activated CD47 gene through binding to a CD47-associated specific super enhancer [12], which consists of multiple enhancers that are composed of a series of transcription factor proteins that bind together to drive the transcription of genes involved in cell identity. Meanwhile, blocking the TNF-α with infliximab, a monoclonal antibody that blocks the binding of TNF-α to cellular TNF receptors, decreased the expression of CD47 at MCF7 cell surface and had a greater effect of phagocytosis in the cancer cells-macrophage co-culture model when combined with an anti-CD47 blocking antibody (clone Hu5F9-G4) [12]. Similarly, treatment with 20 ng/ml of TNF-α increased cell surface CD47 localization in hepatocellular carcinoma (HCC) cells at the transcriptional level, which was mediated by the activation of TNF-α/NF-κB pathway and the increased binding of NF-κB to the CD47 promoter [23]. Additionally, VEGF blockade substantially enhanced TNF-α expression in relapsing non-small cell lung cancer (NSCLC) cells, leading to the enhancement of CD47 expression whereby TNF-α/NF-κB signaling pathway [22].
Interferon (IFN)-γ belongs to type II IFN, which is widely expressed cytokines that have potent immunomodulatory effects [24]. IFN-γ is generally served as a pro-inflammatory cytokine which is abundantly produced by activated T cells and NK cells, which thus has the potential antitumor effects [24,25]. However, IFN-γ also induces the expression of immune checkpoint programmed cell death 1 ligand 1 (PD-L1) via the JAK/STAT1/IRF1 pathway in various types of cancers, exerting immunosuppressive effects in tumor immune microenvironment [[26], [27], [28], [29]]. Meanwhile from our experiment data in non-small cell lung cancer cells (unpublished data) and reports from other articles [30,31], IFN-γ is strongly correlated with the expression of CD47. After 100 ng/ml of IFN-γ overnight treatment caused an increase in CD47 expression on B16F10 melanoma cell surface [30], but the specific mechanism of the IFN-γ-induced CD47 up-regulation is still unclear. Similarly, treatment with 200 ng/ml of IFN-γ for 72 h resulted in seven-fold up-regulation of CD47 in mRNA level in 92.1 uveal melanoma cells [31].
Interleukin-6 (IL-6) is a pleiotropic cytokine, which is produced and secreted by various types of cells including the tumor cells and immune cells [[32], [33], [34], [35]]. Recently, IL-6 was found strongly correlated with the upregulation of CD47 in HCC [36]. Treatment of the recombinant human IL-6 (20 ng/ml) for 48 h significantly induced approximately four-fold of CD47 expression at the transcriptional level in Huh7 and PLC hepatoma cancer cells, mechanistically activating the signal transducer and activator of transcription 3 (STAT3) signaling pathway [36]. Moreover, inhibiting CD47 or disrupting IL-6/STAT3 axis restored the macrophage-mediated phagocytosis [36]. Another interleukin 1 family member, interleukin-1β (IL-1β), was found to stimulate the expression of CD47 through NF-κB activation in cervical cancer cells. Treatment with IL-1β (10 ng/ml) for 8 h transcriptionally increased the CD47 expression in Ms751 and SiHa cervical cancer cells [37]. Besides, CD47 was also demonstrated with the regulation by other interleukins including interleukin-4, interleukin-7, interleukin-13 [37,38]. However, how these cytokines regulated the expression level of CD47 remains unclear.
Oncogenes
Uncontrolled tumor cell proliferation and abnormal blood vessel formation lead to the deprivation of oxygen in tumor tissue, inducing a hypoxic condition [39]. Hypoxia in tumors is correlated with the alteration of cellular metabolism, angiogenesis, drug resistance, mainly modulated by hypoxia-inducible factors 1 (HIF-1) [40]. Meanwhile, hypoxia also induced HIF-1-dependent immune evasion mechanisms including enhancing CD47 expression in the tumor microenvironment [[41], [42], [43]]. HIF-1 directly bound with the promoter of CD47 to activate gene transcription and increased cell surface CD47 in HCC1954 and SUM159 breast cancer cells [43]. Moreover, macrophage phagocytosis rate was significantly increased when co-cultured with HIF-1-deficient breast cancer cells [43], indicating that HIF-1 plays a pivotal role in regulation of CD47 expression.
The MYC oncogene is a transcription factor that regulates multitudinous gene products involved in cell proliferation, growth, differentiation, and apoptosis [44,45]. It is genetically activated and overexpressed in approximately 70% of human cancers and contributes to tumorigenesis [[44], [45], [46]]. MYC could directly bind to the CD47 promoter to transcriptionally up-regulate the expression of CD47 in murine and human leukemia and lymphomas and partially contribute to tumor cells evasion from the immune surveillance [47]. Knockdown of MYC or inhibiting MYC with bromodomain extra-terminal (BET) inhibitors such as JQ1 resulted in a rapid down-regulation of CD47 in cancer cells including T cell acute lymphoblastic leukemia cells, HCC cells, melanoma cells and NSCLC cells. Moreover, down-regulation of CD47 by MYC inactivation in the mouse tumor model repressed initiation and maintenance of MYC-driven tumorigenesis [47]. Similarly, suppression of MYC with JQ1 reduced the level of cell surface CD47 in double/triple-hit lymphomas cells [48].
MicroRNAs
MicroRNAs are small non-coding RNAs consisting of about 20–24 nucleotides and play a pivotal role in cell proliferation, differentiation and apoptosis [49,50]. MicroRNAs negatively control the expression of genes by mediating degradation of target mRNA and/or inhibiting their translation [51,52]. In human cancer, the role of microRNAs in regulating CD47 expression has been revealed. miR-708, functions as a tumor suppressor [53,54], directly bound the 3′-UTR of CD47 and inhibited CD47 expression in T cell acute lymphoblastic leukemia [55]. Meanwhile, in the presence of CD47 antibodies, the addition of miR-708 promoted almost two-fold phagocytosis index. Similarly, miR-708 was involved in metformin-mediated suppression of CD47 in breast cancer stem cells. Overexpression of miR-708 enhanced the phagocytosis of macrophages to breast cancer stem cells [56]. miR-155 attenuated cell surface CD47 level in myeloma cells, leading to extensive phagocytosis of myeloma cells by macrophages and inhibition of tumor growth in mice [57]. Another study demonstrated that miR-200a down-regulated CD47 expression, which leads to promote phagocytosis of macrophage to nasopharyngeal carcinoma cell [58]. Besides, miR-133a directly bound the 3′-UTR of CD47 and suppressed its protein expression in esophageal squamous cell carcinoma and laryngeal carcinoma [59,60]. Also, miR-192 bound the 3′-UTR of CD47 and repressed the expression of CD47 at the posttranscriptional level in medulloblastoma [61]. Recent study found that miR-222 directly decreased the expression of CD47 in human kidney carcinoma cells [62], and miR-340 down-regulated CD47 expression in pancreatic cancer cells [63]. Both of them regulated CD47 expression by targeting the 3′-UTR of its mRNA at the post-transcriptional level.
Enzymes
Various types of enzymes are involved in the regulation of a target protein synthesis. Glutaminyl-peptide cyclotransferase-like protein (QPCTL, isoQC) belongs to a family of enzymes which catalyze the formation of N-terminal glutamine and glutamic acid residues on target protein into an N-terminal pyroglutamate residue (pGlu) [64]. Notably, the presence of N-terminal pyroglutamate formation of CD47 protein, which is considered to be the post-translational modification of CD47, could be specifically recognized by SIRPα and contributed to the binding of CD47 to SIRPα [65,66]. QPCTL has been recently reported as an enzymatic modifier which can catalyze the pyroglutamate modification of CD47 protein [65,67,68]. Inhibiting the formation of CD47 N-terminal pGlu by the QPCTL inhibitor SEN177 or knockout of QPCTL significantly reduced the binding of CC2C6, an anti-CD47 antibody which has the same recognition site (pGlu) of SIRPα [65]. Similarly, the post-translational modification of CD47 by QPCTL had also been identified in DLD1 colorectal cancer and HCT116 colonic carcinoma cancer cells [68].
Discussion
Except depicting the regulation of CD47 from cytokines, oncogenes, microRNAs as well as enzymes, we could also conclude and discuss the regulation of CD47 from transcriptional level, post-transcriptional level and post-translational modification level. At the transcriptional level, the regulation of CD47 by cytokines is mediated by transcription factors, including NF-κB and STAT3 [12,22,23,36,37]. In the early 2000, researchers also found the transcriptional factor α-Pal/NRF-1 acted as a critical regulator to bind to CD47 promoter in human neuroblastoma and hepatoma cell lines [69]. Besides, the CD47 regulation by the same cytokine varies in different types of cancers. For instance, IL-6 up-regulates CD47 in HCC while no effect on Sézary cells [36,38]. This just indicates that the CD47 regulation by cytokines may be closely related with cell types due to the complexity of tumor microenvironment in different types of cancer cells. Since NF-κB activity could be induced by both TNF-α and IL-1β [12,37], then leading to the upregulation of CD47, which suggested that different kinds of cytokines in the tumor microenvironment may operate together with others, collaborating in the regulation of CD47. Likewise, oncogene MYC and HIF-1 directly bind to the CD47 promoter to increase the transcriptional expression of CD47 [43,47]. Additionally, increased NF-κB activity and hypoxia condition were observed in sorafenib-resistant HCC cells [23], which may indicate that NF-κB and HIF-1 exert a synergy effect in the regulation of CD47. Thus, further studies are required to determine whether oncogenes cooperate with other transcription factors, such as NF-κB or STAT3, co-regulating the transcriptional expression of CD47. At the posttranscriptional level, miR-708, miR-192, miR-222, miR-340 and miR-133a suppress CD47 expression by directly binding to the 3′-UTR of CD47 mRNA as a suppressor role [55,56,[59], [60], [61], [62], [63]]. Interestingly, miR-155 and miR-200a act as tumor oncogene to promote the proliferation, migration and invasion of human cancer cells [70,71]. However, both of them could inhibit CD47 expression by binding to the 3′-UTR of CD47 mRNA [57,58], indicating the oncogenic or tumor suppressor function of an microRNA depends on the role of the target mRNA. Enzymes are typically involved in the post-translational modification level of a target protein. To date, at the post-translational modification level, only QPCTL was validated and shown to modify the N-terminal pyroglutamate formation early in the CD47 protein life cycle, thereby influencing the binding of CD47-SIRPα [65,68].
Collectively, there are still many questions that need to be investigated in future research. Firstly, despite of the regulation of CD47 by several kinds of cytokines has been reported, abundant cytokines exist in tumor microenvironment, such as IFN-α/β, transforming growth factor β (TGF-β) and IL-4/10/12/27, which enhance PD-L1 expression and promote tumor progression [26,[72], [73], [74], [75]]. Previously study had reported that the PD-1-PD-L1 axis was similar as the CD47-SIRPα axis, also functioned as a phagocytosis checkpoint which regulated the phagocytic ability of tumor associated macrophage [76,77]. Besides, many cytokines regulated CD47 we aforementioned was also a regulator to PD-L1. These common features indicated that there might be other cytokines control the expression of CD47. Secondly, although some articles have reported that oncogene MYC and HIF-1 are inducers of CD47 expression, several aberrant oncogenic pathways (EGFR, HER2, RAS, PI3K/AKT, ALK, etc.) may also participate in the process of inducing CD47 expression. Since these oncogenic pathways contribute to the multiple hallmarks of tumorigenesis, including initiation of intrinsic immune resistance to facilitate tumor-mediated immune evasion. At the same time, the CD47-SIRPα axis is crucial in tumor evasion from immune surveillance [[78], [79], [80], [81]]. Targeted therapy of these pathways including monoclonal antibody and tyrosine kinase inhibitors (TKIs) have been approved by the FDA or are currently under investigation in clinical trials such as Osimertinib (EGFR-targeted TKI), Neratinib (HER2-targeted TKI), Brigatinib (ALK-targeted TKI) and so on [[82], [83], [84]]. All data above indicate that investigating the relationship between other oncogenic pathways and CD47 expression also offers a rationale for future combination therapy consisting of immune checkpoint blockade antibodies and inhibitors targeting those oncogenic signaling pathways. Thirdly, although several researches have reported that microRNAs are involved in the regulation of CD47, non-coding RNAs (ncRNAs) beyond microRNA include intronic RNAs, circular RNAs, long non-coding RNAs and extracellular RNAs, which can affect the expression of other genes through a variety of mechanisms [85]. Due to the ability of ncRNAs to regulate gene expression, additional studies should be required to unleash the crucial roles of ncRNAs in regulatory of CD47 gene expression. Fourthly, apart from the regulation of QPCTL, glycosylation is the most abundant post-translational modification of the target protein. The potential N-linked glycosylation site of CD47 protein in cancer cells is distinct from red blood cells and platelets, and the difference of glycosylation site has been used in creating a novel CD47 antibody, TJC4 [86]. The successful development of TJC4 demonstrates that glycosylation of CD47 protein may serve as an important role in post-translational modifications of CD47. Thus, further studies are required to investigate the specific mechanism of glycosylation of CD47 protein. Apart from glycosylation, post-translational modifications (e.g., phosphorylation, ubiquitination, SUMOylation and acetylation) have emerged as important regulatory mechanisms of the target protein including PD-L1 [[87], [88], [89], [90], [91]]. Hence, elucidating the mechanisms controlling the stability of the CD47 protein may provide a complete understanding of the regulatory mechanisms of CD47 checkpoint. Making clear the questions mentioned above enhances our comprehension of the role of the CD47-SIRPα axis in tumor immune microenvironment, at the same time it gives some insight to unveil undiscovered regulating mechanism of other phagocytosis checkpoints, such as the PD-1-PD-L1 axis [76], the CD24-Siglec-10 axis [92] and the MHC-I–LILRB1 axis [93].
Besides the regulation of CD47 expression in cancer cells, its regulation on other types of cells is equally important. Recent study on immune response to pathogen recognition discovered CD47 up-regulation in human peripheral blood mononuclear cells after infection with Borrelia burgdorferi. In detail they speculated one reason of CD47 up-regulation may be the participation of inflammatory cytokines TNF-α, CXCL10, and IFN-α [94]. Specifically in fibrotic associated fibroblast, JUN was worked as an enhancer to CD47 [95]. Moreover, valid data had proved CD47 up-regulation on vascular smooth muscle cells was cause by TNF-α, probably explained why there is an impairment in macrophage phagocytosis within human atherosclerotic plaque [96]. All these findings demonstrated the regulation of CD47 expression was not only occurred in tumor immune microenvironment but also immune response in other cell types, which broadly strengthens the research significance of CD47 regulation.
In short, although there are plenty of questions waiting to be further research, integrating the regulation of CD47 provides a knowledge base for further uncovering the detailed mechanism of tumor-mediated immune evasion as well as the new therapeutic direction in targeting the CD47-SIRPα axis.
Consent
This review article does not contain studies with human participants or animals performed by any of the authors.
CRediT authorship contribution statement
Can-Yu Huang: Data curation, Writing - original draft. Zi-Han Ye: Writing - original draft, Writing - review & editing. Mu-Yang Huang: Data curation, Validation. Jin-Jian Lu: Supervision, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by The Science and Technology Development Fund, Macau SAR (File no. 0129/2019/A3).
References
- 1.Brown E.J., Frazier W.A. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130–135. doi: 10.1016/s0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- 2.Brown E., Hooper L., Ho T., Gresham H. Integrin-associated protein: a 50-kD plasma membrane antigen physically and functionally associated with integrins. J. Cell Biol. 1990;111:2785–2794. doi: 10.1083/jcb.111.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao A.G., Lindberg F.P., Finn M.B., Blystone S.D., Brown E.J., Frazier W.A. Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin. J. Biol. Chem. 1996;271:21–24. doi: 10.1074/jbc.271.1.21. [DOI] [PubMed] [Google Scholar]
- 4.Lindberg F.P., Bullard D.C., Caver T.E., Gresham H.D., Beaudet A.L., Brown E.J. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science. 1996;274:795–798. doi: 10.1126/science.274.5288.795. [DOI] [PubMed] [Google Scholar]
- 5.Reinhold M.I., Lindberg F.P., Kersh G.J., Allen P.M., Brown E.J. Costimulation of T cell activation by integrin-associated protein (CD47) is an adhesion-dependent, CD28-independent signaling pathway. J. Exp. Med. 1997;185:1–11. doi: 10.1084/jem.185.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oldenborg P.A., Zheleznyak A., Fang Y.F., Lagenaur C.F., Gresham H.D., Lindberg F.P. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 7.Majeti R., Chao M.P., Alizadeh A.A., Pang W.W., Jaiswal S., Gibbs K.D., Jr., van Rooijen N., Weissman I.L. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barclay A.N., Van den Berg T.K. The interaction between signal regulatory protein alpha (SIRPalpha) and CD47: structure, function, and therapeutic target. Annu. Rev. Immunol. 2014;32:25–50. doi: 10.1146/annurev-immunol-032713-120142. [DOI] [PubMed] [Google Scholar]
- 9.Matlung H.L., Szilagyi K., Barclay N.A., van den Berg T.K. The CD47-SIRPalpha signaling axis as an innate immune checkpoint in cancer. Immunol. Rev. 2017;276:145–164. doi: 10.1111/imr.12527. [DOI] [PubMed] [Google Scholar]
- 10.Willingham S.B., Volkmer J.P., Gentles A.J., Sahoo D., Dalerba P., Mitra S.S., Wang J., Contreras-Trujillo H., Martin R., Cohen J.D., Lovelace P., Scheeren F.A., Chao M.P., Weiskopf K., Tang C., Volkmer A.K., Naik T.J., Storm T.A., Mosley A.R., Edris B., Schmid S.M., Sun C.K., Chua M.S., Murillo O., Rajendran P., Cha A.C., Chin R.K., Kim D., Adorno M., Raveh T., Tseng D., Jaiswal S., Enger P.O., Steinberg G.K., Li G., So S.K., Majeti R., Harsh G.R., van de Rijn M., Teng N.N., Sunwoo J.B., Alizadeh A.A., Clarke M.F., Weissman I.L. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl. Acad. Sci. U. S. A. 2012;109:6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaiswal S., Jamieson C.H., Pang W.W., Park C.Y., Chao M.P., Majeti R., Traver D., van Rooijen N., Weissman I.L. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Betancur P.A., Abraham B.J., Yiu Y.Y., Willingham S.B., Khameneh F., Zarnegar M., Kuo A.H., McKenna K., Kojima Y., Leeper N.J., Ho P., Gip P., Swigut T., Sherwood R.I., Clarke M.F., Somlo G., Young R.A., Weissman I.L. A CD47-associated super-enhancer links pro-inflammatory signalling to CD47 upregulation in breast cancer. Nat. Commun. 2017;8:14802. doi: 10.1038/ncomms14802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chao M.P., Alizadeh A.A., Tang C., Myklebust J.H., Varghese B., Gill S., Jan M., Cha A.C., Chan C.K., Tan B.T., Park C.Y., Zhao F., Kohrt H.E., Malumbres R., Briones J., Gascoyne R.D., Lossos I.S., Levy R., Weissman I.L., Majeti R. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y., Xu Z., Guo S., Zhang L., Sharma A., Robertson G.P., Huang L. Intravenous delivery of siRNA targeting CD47 effectively inhibits melanoma tumor growth and lung metastasis. Mol. Ther. 2013;21:1919–1929. doi: 10.1038/mt.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manna P.P., Frazier W.A. CD47 mediates killing of breast tumor cells via Gi-dependent inhibition of protein kinase A. Cancer Res. 2004;64:1026–1036. doi: 10.1158/0008-5472.can-03-1708. [DOI] [PubMed] [Google Scholar]
- 16.Advani R., Flinn I., Popplewell L., Forero A., Bartlett N.L., Ghosh N., Kline J., Roschewski M., LaCasce A., Collins G.P., Tran T., Lynn J., Chen J.Y., Volkmer J.P., Agoram B., Huang J., Majeti R., Weissman I.L., Takimoto C.H., Chao M.P., Smith S.M. CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin's lymphoma. N. Engl. J. Med. 2018;379:1711–1721. doi: 10.1056/NEJMoa1807315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Locksley R.M., Killeen N., Lenardo M.J. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 18.Balkwill F., Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 19.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006;25:409–416. doi: 10.1007/s10555-006-9005-3. [DOI] [PubMed] [Google Scholar]
- 21.Nabors L.B., Suswam E., Huang Y., Yang X., Johnson M.J., King P.H. Tumor necrosis factor alpha induces angiogenic factor up-regulation in malignant glioma cells: a role for RNA stabilization and HuR. Cancer Res. 2003;63:4181–4187. [PubMed] [Google Scholar]
- 22.Zhang X., Wang Y., Fan J., Chen W., Luan J., Mei X., Wang S., Li Y., Ye L., Li S., Tian W., Yin K., Ju D. Blocking CD47 efficiently potentiated therapeutic effects of anti-angiogenic therapy in non-small cell lung cancer. J. Immunother. Cancer. 2019;7:346. doi: 10.1186/s40425-019-0812-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lo J., Lau E.Y., Ching R.H., Cheng B.Y., Ma M.K., Ng I.O., Lee T.K. Nuclear factor kappa B-mediated CD47 up-regulation promotes sorafenib resistance and its blockade synergizes the effect of sorafenib in hepatocellular carcinoma in mice. Hepatology. 2015;62:534–545. doi: 10.1002/hep.27859. [DOI] [PubMed] [Google Scholar]
- 24.Platanias L.C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 25.Parker B.S., Rautela J., Hertzog P.J. Antitumour actions of interferons: implications for cancer therapy. Nat. Rev. Cancer. 2016;16:131–144. doi: 10.1038/nrc.2016.14. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Diaz A., Shin D.S., Moreno B.H., Saco J., Escuin-Ordinas H., Rodriguez G.A., Zaretsky J.M., Sun L., Hugo W., Wang X., Parisi G., Saus C.P., Torrejon D.Y., Graeber T.G., Comin-Anduix B., Hu-Lieskovan S., Damoiseaux R., Lo R.S., Ribas A. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 2017;19:1189–1201. doi: 10.1016/j.celrep.2017.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li N., Wang J., Zhang N., Zhuang M., Zong Z., Zou J., Li G., Wang X., Zhou H., Zhang L., Shi Y. Cross-talk between TNF-alpha and IFN-gamma signaling in induction of B7-H1 expression in hepatocellular carcinoma cells. Cancer Immunol. Immunother. 2018;67:271–283. doi: 10.1007/s00262-017-2086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moon J.W., Kong S.K., Kim B.S., Kim H.J., Lim H., Noh K., Kim Y., Choi J.W., Lee J.H., Kim Y.S. IFNgamma induces PD-L1 overexpression by JAK2/STAT1/IRF-1 signaling in EBV-positive gastric carcinoma. Sci. Rep. 2017;7:17810. doi: 10.1038/s41598-017-18132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Concha-Benavente F., Srivastava R.M., Trivedi S., Lei Y., Chandran U., Seethala R.R., Freeman G.J., Ferris R.L. Identification of the cell-intrinsic and -extrinsic pathways downstream of EGFR and IFNgamma that induce PD-L1 expression in head and neck cancer. Cancer Res. 2016;76:1031–1043. doi: 10.1158/0008-5472.CAN-15-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sockolosky J.T., Dougan M., Ingram J.R., Ho C.C., Kauke M.J., Almo S.C., Ploegh H.L., Garcia K.C. Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E2646–E2654. doi: 10.1073/pnas.1604268113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basile M.S., Mazzon E., Russo A., Mammana S., Longo A., Bonfiglio V., Fallico M., Caltabiano R., Fagone P., Nicoletti F., Avitabile T., Reibaldi M. Differential modulation and prognostic values of immune-escape genes in uveal melanoma. PLoS One. 2019;14 doi: 10.1371/journal.pone.0210276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumari N., Dwarakanath B.S., Das A., Bhatt A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016;37:11553–11572. doi: 10.1007/s13277-016-5098-7. [DOI] [PubMed] [Google Scholar]
- 33.Liu Q., Yu S., Li A., Xu H., Han X., Wu K. Targeting interlukin-6 to relieve immunosuppression in tumor microenvironment. Tumour Biol. 2017;39 doi: 10.1177/1010428317712445. (1010428317712445) [DOI] [PubMed] [Google Scholar]
- 34.Waldner M.J., Foersch S., Neurath M.F. Interleukin-6—a key regulator of colorectal cancer development. Int. J. Biol. Sci. 2012;8:1248–1253. doi: 10.7150/ijbs.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dethlefsen C., Hojfeldt G., Hojman P. The role of intratumoral and systemic IL-6 in breast cancer. Breast Cancer Res. Treat. 2013;138:657–664. doi: 10.1007/s10549-013-2488-z. [DOI] [PubMed] [Google Scholar]
- 36.Chen J., Zheng D.X., Yu X.J., Sun H.W., Xu Y.T., Zhang Y.J., Xu J. Macrophages induce CD47 upregulation via IL-6 and correlate with poor survival in hepatocellular carcinoma patients. Oncoimmunology. 2019;8 doi: 10.1080/2162402X.2019.1652540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu F., Dai M., Xu Q., Zhu X., Zhou Y., Jiang S., Wang Y., Ai Z., Ma L., Zhang Y., Hu L., Yang Q., Li J., Zhao S., Zhang Z., Teng Y. SRSF10-mediated IL1RAP alternative splicing regulates cervical cancer oncogenesis via mIL1RAP-NF-kappaB-CD47 axis. Oncogene. 2018;37:2394–2409. doi: 10.1038/s41388-017-0119-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson L.D.S., Banerjee S., Kruglov O., Viller N.N., Horwitz S.M., Lesokhin A., Zain J., Querfeld C., Chen R., Okada C., Sawas A., O'Connor O.A., Sievers E.L., Shou Y., Uger R.A., Wong M., Akilov O.E. Targeting CD47 in Sezary syndrome with SIRPalphaFc. Blood Adv. 2019;3:1145–1153. doi: 10.1182/bloodadvances.2018030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown J.M., Giaccia A.J. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998;58:1408–1416. [PubMed] [Google Scholar]
- 40.Bertout J.A., Patel S.A., Simon M.C. The impact of O2 availability on human cancer. Nat. Rev. Cancer. 2008;8:967–975. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barsoum I.B., Smallwood C.A., Siemens D.R., Graham C.H. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014;74:665–674. doi: 10.1158/0008-5472.CAN-13-0992. [DOI] [PubMed] [Google Scholar]
- 42.Denko N.C. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat. Rev. Cancer. 2008;8:705–713. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- 43.Zhang H., Lu H., Xiang L., Bullen J.W., Zhang C., Samanta D., Gilkes D.M., He J., Semenza G.L. HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E6215–E6223. doi: 10.1073/pnas.1520032112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shachaf C.M., Kopelman A.M., Arvanitis C., Karlsson A., Beer S., Mandl S., Bachmann M.H., Borowsky A.D., Ruebner B., Cardiff R.D., Yang Q., Bishop J.M., Contag C.H., Felsher D.W. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature. 2004;431:1112–1117. doi: 10.1038/nature03043. [DOI] [PubMed] [Google Scholar]
- 45.Jain M., Arvanitis C., Chu K., Dewey W., Leonhardt E., Trinh M., Sundberg C.D., Bishop J.M., Felsher D.W. Sustained loss of a neoplastic phenotype by brief inactivation of MYC. Science. 2002;297:102–104. doi: 10.1126/science.1071489. [DOI] [PubMed] [Google Scholar]
- 46.Dang C.V. MYC on the path to cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Casey S.C., Tong L., Li Y., Do R., Walz S., Fitzgerald K.N., Gouw A.M., Baylot V., Gutgemann I., Eilers M., Felsher D.W. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352:227–231. doi: 10.1126/science.aac9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li W., Gupta S.K., Han W., Kundson R.A., Nelson S., Knutson D., Greipp P.T., Elsawa S.F., Sotomayor E.M., Gupta M. Targeting MYC activity in double-hit lymphoma with MYC and BCL2 and/or BCL6 rearrangements with epigenetic bromodomain inhibitors. J. Hematol. Oncol. 2019;12:73. doi: 10.1186/s13045-019-0761-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 50.Wang Q., Lin W., Tang X., Li S., Guo L., Lin Y., Kwok H.F. The roles of microRNAs in regulating the expression of PD-1/PD-L1 immune checkpoint. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18122540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun C., Mezzadra R., Schumacher T.N. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48:434–452. doi: 10.1016/j.immuni.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 53.Sun S., Hang T., Zhang B., Zhu L., Wu Y., Lv X., Huang Q., Yao H. miRNA-708 functions as a tumor suppressor in colorectal cancer by targeting ZEB1 through Akt/mTOR signaling pathway. Am. J. Transl. Res. 2019;11:5338–5356. [PMC free article] [PubMed] [Google Scholar]
- 54.Schneider E., Pochert N., Ruess C., MacPhee L., Escano L., Miller C., Krowiorz K., Delsing Malmberg E., Heravi-Moussavi A., Lorzadeh A., Ashouri A., Grasedieck S., Sperb N., Kumar Kopparapu P., Iben S., Staffas A., Xiang P., Rosler R., Kanduri M., Larsson E., Fogelstrand L., Dohner H., Dohner K., Wiese S., Hirst M., Keith Humphries R., Palmqvist L., Kuchenbauer F., Rouhi A. MicroRNA-708 is a novel regulator of the Hoxa9 program in myeloid cells. Leukemia. 2020;34:1253–1265. doi: 10.1038/s41375-019-0651-1. [DOI] [PubMed] [Google Scholar]
- 55.Huang W., Wang W.T., Fang K., Chen Z.H., Sun Y.M., Han C., Sun L.Y., Luo X.Q., Chen Y.Q. MIR-708 promotes phagocytosis to eradicate T-ALL cells by targeting CD47. Mol. Cancer. 2018;17:12. doi: 10.1186/s12943-018-0768-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tan W., Tang H., Jiang X., Ye F., Huang L., Shi D., Li L., Huang X., Li L., Xie X., Xie X. Metformin mediates induction of miR-708 to inhibit self-renewal and chemoresistance of breast cancer stem cells through targeting CD47. J. Cell. Mol. Med. 2019;23:5994–6004. doi: 10.1111/jcmm.14462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rastgoo N., Wu J., Liu M., Pourabdollah M., Atenafu E.G., Reece D., Chen W., Chang H. Targeting CD47/TNFAIP8 by miR-155 overcomes drug resistance and inhibits tumor growth through induction of phagocytosis and apoptosis in multiple myeloma. Haematologica. 2019;2019:227579. doi: 10.3324/haematol.2019.227579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao Y., Yu X., Tang H., Han R., Wang X., Wang J., Wang K., Li G. MicroRNA-200a promotes phagocytosis of macrophages and suppresses cell proliferation, migration, and invasion in nasopharyngeal carcinoma by targeting CD47. Biomed. Res. Int. 2020;2020:3723781. doi: 10.1155/2020/3723781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suzuki S., Yokobori T., Tanaka N., Sakai M., Sano A., Inose T., Sohda M., Nakajima M., Miyazaki T., Kato H., Kuwano H. CD47 expression regulated by the miR-133a tumor suppressor is a novel prognostic marker in esophageal squamous cell carcinoma. Oncol. Rep. 2012;28:465–472. doi: 10.3892/or.2012.1831. [DOI] [PubMed] [Google Scholar]
- 60.Li H., Wang Y., Li Y.Z. MicroRNA-133a suppresses the proliferation, migration, and invasion of laryngeal carcinoma cells by targeting CD47. Tumour Biol. 2016;37:16103–16113. doi: 10.1007/s13277-016-5451-x. [DOI] [PubMed] [Google Scholar]
- 61.Yang S.Y., Choi S.A., Lee J.Y., Park A.K., Wang K.C., Phi J.H., Koh E.J., Park W.Y., Park S.H., Hwang D.W., Jung H.W., Kim S.K. miR-192 suppresses leptomeningeal dissemination of medulloblastoma by modulating cell proliferation and anchoring through the regulation of DHFR, integrins, and CD47. Oncotarget. 2015;6:43712–43730. doi: 10.18632/oncotarget.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi L., Wang X., Hu B., Wang D., Ren Z. miR-222 enhances radiosensitivity of cancer cells by inhibiting the expression of CD47. Int. J. Clin. Exp. Pathol. 2019;12:4204–4213. [PMC free article] [PubMed] [Google Scholar]
- 63.Xi Q., Zhang J., Yang G., Zhang L., Chen Y., Wang C., Zhang Z., Guo X., Zhao J., Xue Z., Li Y., Zhang Q., Da Y., Liu L., Yao Z., Zhang R. Restoration of miR-340 controls pancreatic cancer cell CD47 expression to promote macrophage phagocytosis and enhance antitumor immunity. J. Immunother. Cancer. 2020;8 doi: 10.1136/jitc-2019-000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cynis H., Rahfeld J.U., Stephan A., Kehlen A., Koch B., Wermann M., Demuth H.U., Schilling S. Isolation of an isoenzyme of human glutaminyl cyclase: retention in the Golgi complex suggests involvement in the protein maturation machinery. J. Mol. Biol. 2008;379:966–980. doi: 10.1016/j.jmb.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 65.Logtenberg M.E.W., Jansen J.H.M., Raaben M., Toebes M., Franke K., Brandsma A.M., Matlung H.L., Fauster A., Gomez-Eerland R., Bakker N.A.M., van der Schot S., Marijt K.A., Verdoes M., Haanen J., van den Berg J.H., Neefjes J., van den Berg T.K., Brummelkamp T.R., Leusen J.H.W., Scheeren F.A., Schumacher T.N. Glutaminyl cyclase is an enzymatic modifier of the CD47- SIRPalpha axis and a target for cancer immunotherapy. Nat. Med. 2019;25:612–619. doi: 10.1038/s41591-019-0356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hatherley D., Graham S.C., Turner J., Harlos K., Stuart D.I., Barclay A.N. Paired receptor specificity explained by structures of signal regulatory proteins alone and complexed with CD47. Mol. Cell. 2008;31:266–277. doi: 10.1016/j.molcel.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 67.Schilling S., Niestroj A.J., Rahfeld J.U., Hoffmann T., Wermann M., Zunkel K., Wasternack C., Demuth H.U. Identification of human glutaminyl cyclase as a metalloenzyme. Potent inhibition by imidazole derivatives and heterocyclic chelators. J. Biol. Chem. 2003;278:49773–49779. doi: 10.1074/jbc.M309077200. [DOI] [PubMed] [Google Scholar]
- 68.Wu Z., Weng L., Zhang T., Tian H., Fang L., Teng H., Zhang W., Gao J., Hao Y., Li Y., Zhou H., Wang P. Identification of glutaminyl cyclase isoenzyme isoQC as a regulator of SIRPalpha-CD47 axis. Cell Res. 2019;29:502–505. doi: 10.1038/s41422-019-0177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chang W.T., Huang A.M. Alpha-Pal/NRF-1 regulates the promoter of the human integrin-associated protein/CD47 gene. J. Biol. Chem. 2004;279:14542–14550. doi: 10.1074/jbc.M309825200. [DOI] [PubMed] [Google Scholar]
- 70.Xia H., Zhao Y. miR-155 is high-expressed in polycystic ovarian syndrome and promotes cell proliferation and migration through targeting PDCD4 in KGN cells. Artif. Cells Nanomed. Biotechnol. 2020;48:197–205. doi: 10.1080/21691401.2019.1699826. [DOI] [PubMed] [Google Scholar]
- 71.Yang R., Xu J., Hua X., Tian Z., Xie Q., Li J., Jiang G., Cohen M., Sun H., Huang C. Overexpressed miR-200a promotes bladder cancer invasion through direct regulating Dicer/miR-16/JNK2/MMP-2 axis. Oncogene. 2020;39:1983–1996. doi: 10.1038/s41388-019-1120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baas M., Besancon A., Goncalves T., Valette F., Yagita H., Sawitzki B., Volk H.D., Waeckel-Enee E., Rocha B., Chatenoud L., You S. TGFbeta-dependent expression of PD-1 and PD-L1 controls CD8(+) T cell anergy in transplant tolerance. Elife. 2016;5 doi: 10.7554/eLife.08133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Quandt D., Jasinski-Bergner S., Muller U., Schulze B., Seliger B. Synergistic effects of IL-4 and TNFalpha on the induction of B7-H1 in renal cell carcinoma cells inhibiting allogeneic T cell proliferation. J. Transl. Med. 2014;12:151. doi: 10.1186/1479-5876-12-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carbotti G., Barisione G., Airoldi I., Mezzanzanica D., Bagnoli M., Ferrero S., Petretto A., Fabbi M., Ferrini S. IL-27 induces the expression of IDO and PD-L1 in human cancer cells. Oncotarget. 2015;6:43267–43280. doi: 10.18632/oncotarget.6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiong H.Y., Ma T.T., Wu B.T., Lin Y., Tu Z.G. IL-12 regulates B7-H1 expression in ovarian cancer-associated macrophages by effects on NF-kappaB signalling. Asian Pac. J. Cancer Prev. 2014;15:5767–5772. doi: 10.7314/apjcp.2014.15.14.5767. [DOI] [PubMed] [Google Scholar]
- 76.Gordon S.R., Maute R.L., Dulken B.W., Hutter G., George B.M., McCracken M.N., Gupta R., Tsai J.M., Sinha R., Corey D., Ring A.M., Connolly A.J., Weissman I.L. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495–499. doi: 10.1038/nature22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feng M., Jiang W., Kim B.Y.S., Zhang C.C., Fu Y.X., Weissman I.L. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat. Rev. Cancer. 2019;19:568–586. doi: 10.1038/s41568-019-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 79.Arteaga C.L., Engelman J.A. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell. 2014;25:282–303. doi: 10.1016/j.ccr.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ryan M.B., Corcoran R.B. Therapeutic strategies to target RAS-mutant cancers. Nat. Rev. Clin. Oncol. 2018;15:709–720. doi: 10.1038/s41571-018-0105-0. [DOI] [PubMed] [Google Scholar]
- 81.Kaur S., Sassano A., Dolniak B., Joshi S., Majchrzak-Kita B., Baker D.P., Hay N., Fish E.N., Platanias L.C. Role of the Akt pathway in mRNA translation of interferon-stimulated genes. Proc. Natl. Acad. Sci. U. S. A. 2008;105:4808–4813. doi: 10.1073/pnas.0710907105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Soria J.C., Ohe Y., Vansteenkiste J., Reungwetwattana T., Chewaskulyong B., Lee K.H., Dechaphunkul A., Imamura F., Nogami N., Kurata T., Okamoto I., Zhou C., Cho B.C., Cheng Y., Cho E.K., Voon P.J., Planchard D., Su W.C., Gray J.E., Lee S.M., Hodge R., Marotti M., Rukazenkov Y., Ramalingam S.S., Investigators F. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 83.Chan A., Delaloge S., Holmes F.A., Moy B., Iwata H., Harvey V.J., Robert N.J., Silovski T., Gokmen E., von Minckwitz G., Ejlertsen B., Chia S.K.L., Mansi J., Barrios C.H., Gnant M., Buyse M., Gore I., Smith J., 2nd, Harker G., Masuda N., Petrakova K., Zotano A.G., Iannotti N., Rodriguez G., Tassone P., Wong A., Bryce R., Ye Y., Yao B., Martin M., N.E.T.S.G. Exte Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17:367–377. doi: 10.1016/S1470-2045(15)00551-3. [DOI] [PubMed] [Google Scholar]
- 84.Camidge D.R., Kim H.R., Ahn M.J., Yang J.C., Han J.Y., Lee J.S., Hochmair M.J., Li J.Y., Chang G.C., Lee K.H., Gridelli C., Delmonte A., Garcia Campelo R., Kim D.W., Bearz A., Griesinger F., Morabito A., Felip E., Califano R., Ghosh S., Spira A., Gettinger S.N., Tiseo M., Gupta N., Haney J., Kerstein D., Popat S. Brigatinib versus Crizotinib in ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 2018;379:2027–2039. doi: 10.1056/NEJMoa1810171. [DOI] [PubMed] [Google Scholar]
- 85.Matsui M., Corey D.R. Non-coding RNAs as drug targets. Nat. Rev. Drug Discov. 2017;16:167–179. doi: 10.1038/nrd.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Meng Z., Wang Z., Guo B., Cao W., Shen H. American Society of Hematology; Washington, DC: 2019. TJC4, a Differentiated Anti-CD47 Antibody With Novel Epitope and RBC Sparing Properties. [Google Scholar]
- 87.Lim S.O., Li C.W., Xia W., Cha J.H., Chan L.C., Wu Y., Chang S.S., Lin W.C., Hsu J.M., Hsu Y.H., Kim T., Chang W.C., Hsu J.L., Yamaguchi H., Ding Q., Wang Y., Yang Y., Chen C.H., Sahin A.A., Yu D., Hortobagyi G.N., Hung M.C. Deubiquitination and stabilization of PD-L1 by CSN5. Cancer Cell. 2016;30:925–939. doi: 10.1016/j.ccell.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li C.W., Lim S.O., Xia W., Lee H.H., Chan L.C., Kuo C.W., Khoo K.H., Chang S.S., Cha J.H., Kim T., Hsu J.L., Wu Y., Hsu J.M., Yamaguchi H., Ding Q., Wang Y., Yao J., Lee C.C., Wu H.J., Sahin A.A., Allison J.P., Yu D., Hortobagyi G.N., Hung M.C. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat. Commun. 2016;7:12632. doi: 10.1038/ncomms12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li H., Li C.W., Li X., Ding Q., Guo L., Liu S., Liu C., Lai C.C., Hsu J.M., Dong Q., Xia W., Hsu J.L., Yamaguchi H., Du Y., Lai Y.J., Sun X., Koller P.B., Ye Q., Hung M.C. MET inhibitors promote liver tumor evasion of the immune response by stabilizing PDL1. Gastroenterology. 2019;156:1849–1861. doi: 10.1053/j.gastro.2019.01.252. (e1813) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang Y., Hsu J.M., Sun L., Chan L.C., Li C.W., Hsu J.L., Wei Y., Xia W., Hou J., Qiu Y., Hung M.C. Palmitoylation stabilizes PD-L1 to promote breast tumor growth. Cell Res. 2019;29:83–86. doi: 10.1038/s41422-018-0124-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Horita H., Law A., Hong S., Middleton K. Identifying regulatory posttranslational modifications of PD-L1: a focus on monoubiquitinaton. Neoplasia. 2017;19:346–353. doi: 10.1016/j.neo.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barkal A.A., Brewer R.E., Markovic M., Kowarsky M., Barkal S.A., Zaro B.W., Krishnan V., Hatakeyama J., Dorigo O., Barkal L.J., Weissman I.L. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature. 2019;572:392–396. doi: 10.1038/s41586-019-1456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Barkal A.A., Weiskopf K., Kao K.S., Gordon S.R., Rosental B., Yiu Y.Y., George B.M., Markovic M., Ring N.G., Tsai J.M., McKenna K.M., Ho P.Y., Cheng R.Z., Chen J.Y., Barkal L.J., Ring A.M., Weissman I.L., Maute R.L. Engagement of MHC class I by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy. Nat. Immunol. 2018;19:76–84. doi: 10.1038/s41590-017-0004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tal M.C., Dulgeroff L.B. Torrez, Myers L., Cham L.B., Mayer-Barber K.D., Bohrer A.C., Castro E., Yiu Y.Y., Angel C. Lopez, Pham E., Carmody A.B., Messer R.J., Gars E., Kortmann J., Markovic M., Hasenkrug M., Peterson K.E., Winkler C.W., Woods T.A., Hansen P., Galloway S., Wagh D., Fram B.J., Nguyen T., Corey D., Kalluru R.S., Banaei N., Rajadas J., Monack D.M., Ahmed A., Sahoo D., Davis M.M., Glenn J.S., Adomati T., Lang K.S., Weissman I.L., Hasenkrug K.J. Upregulation of CD47 is a host checkpoint response to pathogen recognition. mBio. 2020;11 doi: 10.1128/mBio.01293-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cui L., Chen S.Y., Lerbs T., Lee J.W., Domizi P., Gordon S., Kim Y.H., Nolan G., Betancur P., Wernig G. Activation of JUN in fibroblasts promotes pro-fibrotic programme and modulates protective immunity. Nat. Commun. 2020;11:2795. doi: 10.1038/s41467-020-16466-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kojima Y., Volkmer J.P., McKenna K., Civelek M., Lusis A.J., Miller C.L., Direnzo D., Nanda V., Ye J., Connolly A.J., Schadt E.E., Quertermous T., Betancur P., Maegdefessel L., Matic L.P., Hedin U., Weissman I.L., Leeper N.J. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature. 2016;536:86–90. doi: 10.1038/nature18935. [DOI] [PMC free article] [PubMed] [Google Scholar]